One-Step Synthesis of Ag@TiO2 Nanoparticles for Enhanced Photocatalytic Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photocatalysts
2.2.1. Preparation of Pure TiO2 Nanoparticles
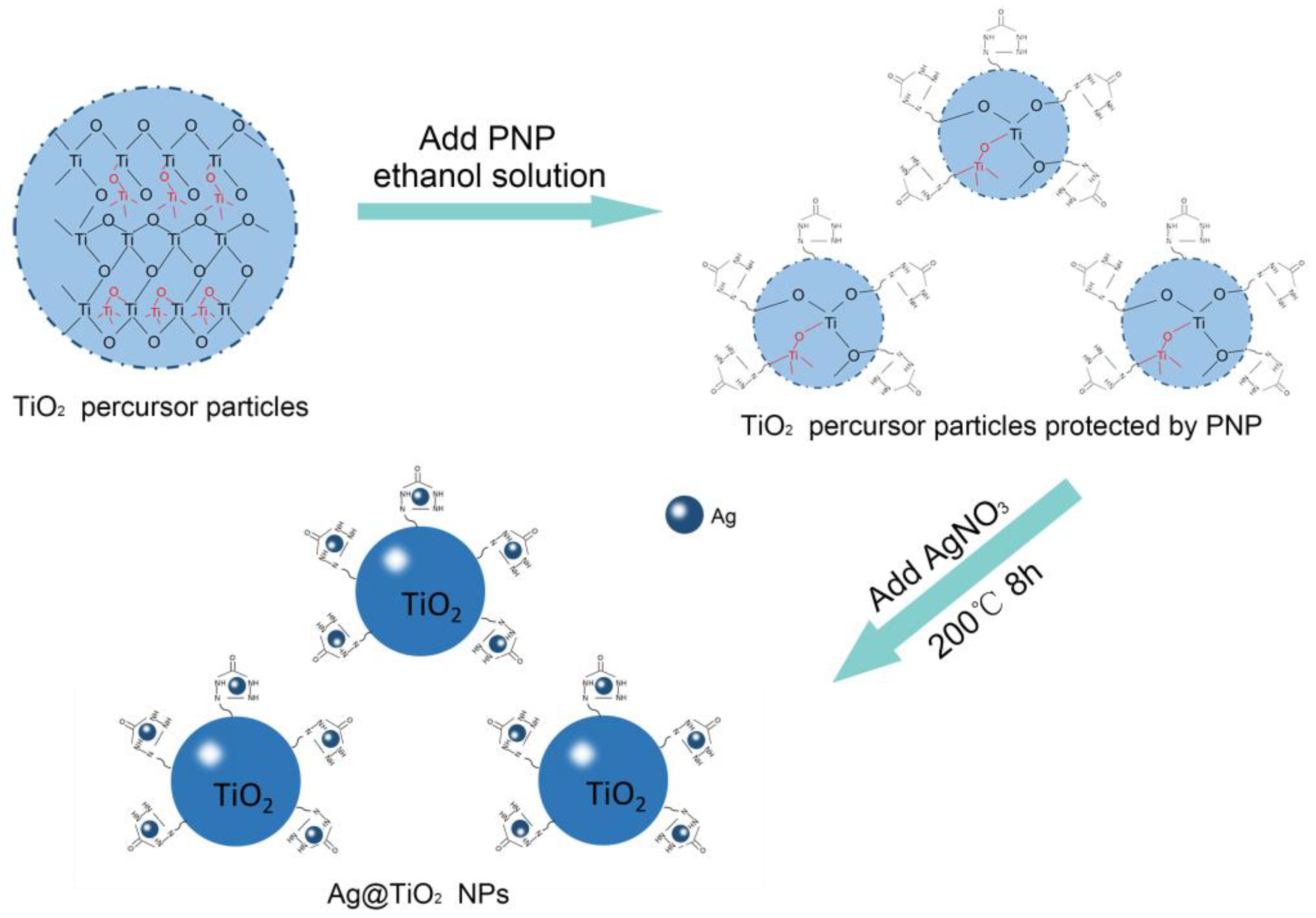
2.2.2. Preparation of Ag@ TiO2 Nanoparticles
2.3. Materials Characterization
2.4. Photoelectrochemical Measurements
2.5. Photocatalytic Activity Measurements
3. Results and Discussion
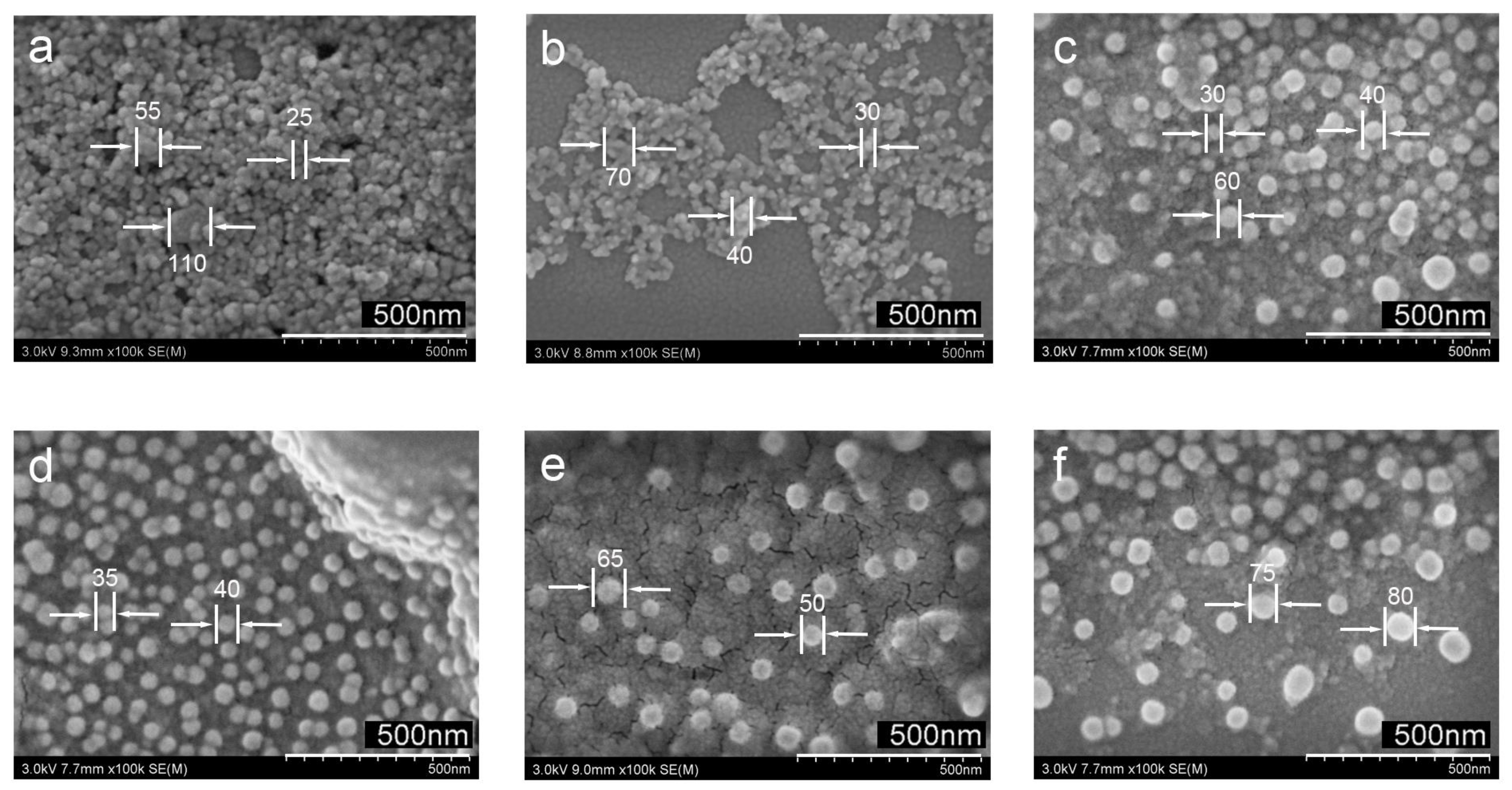
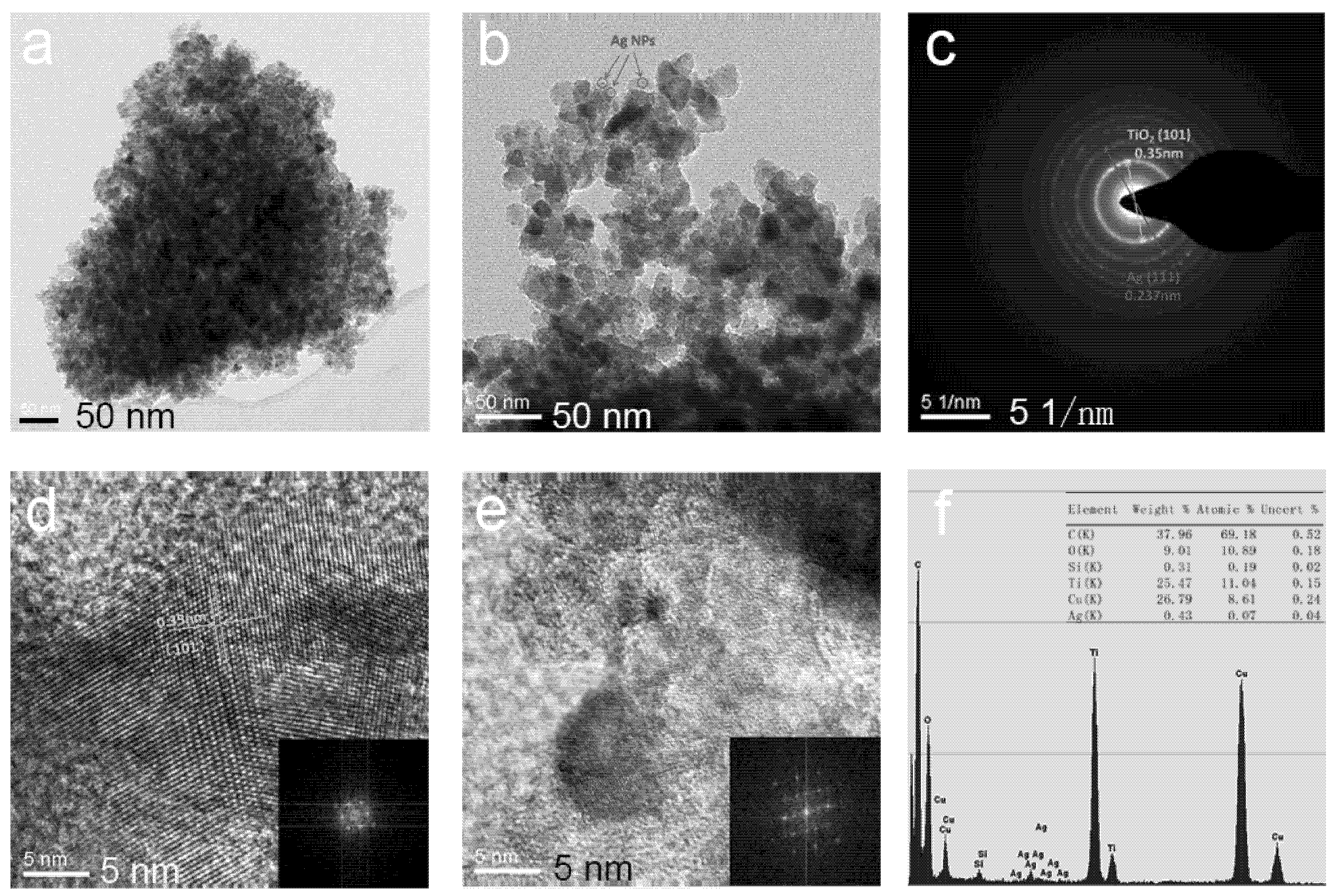
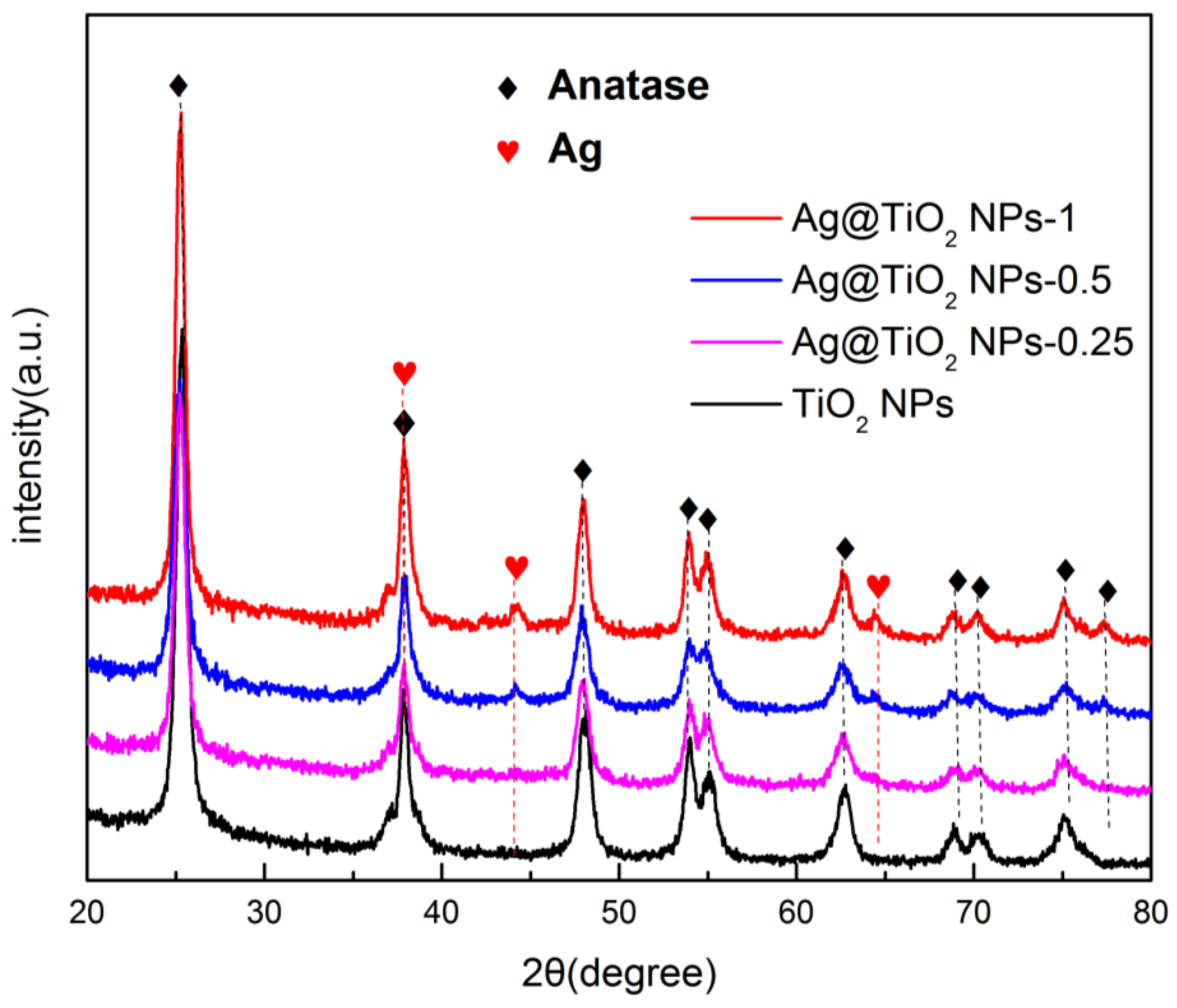
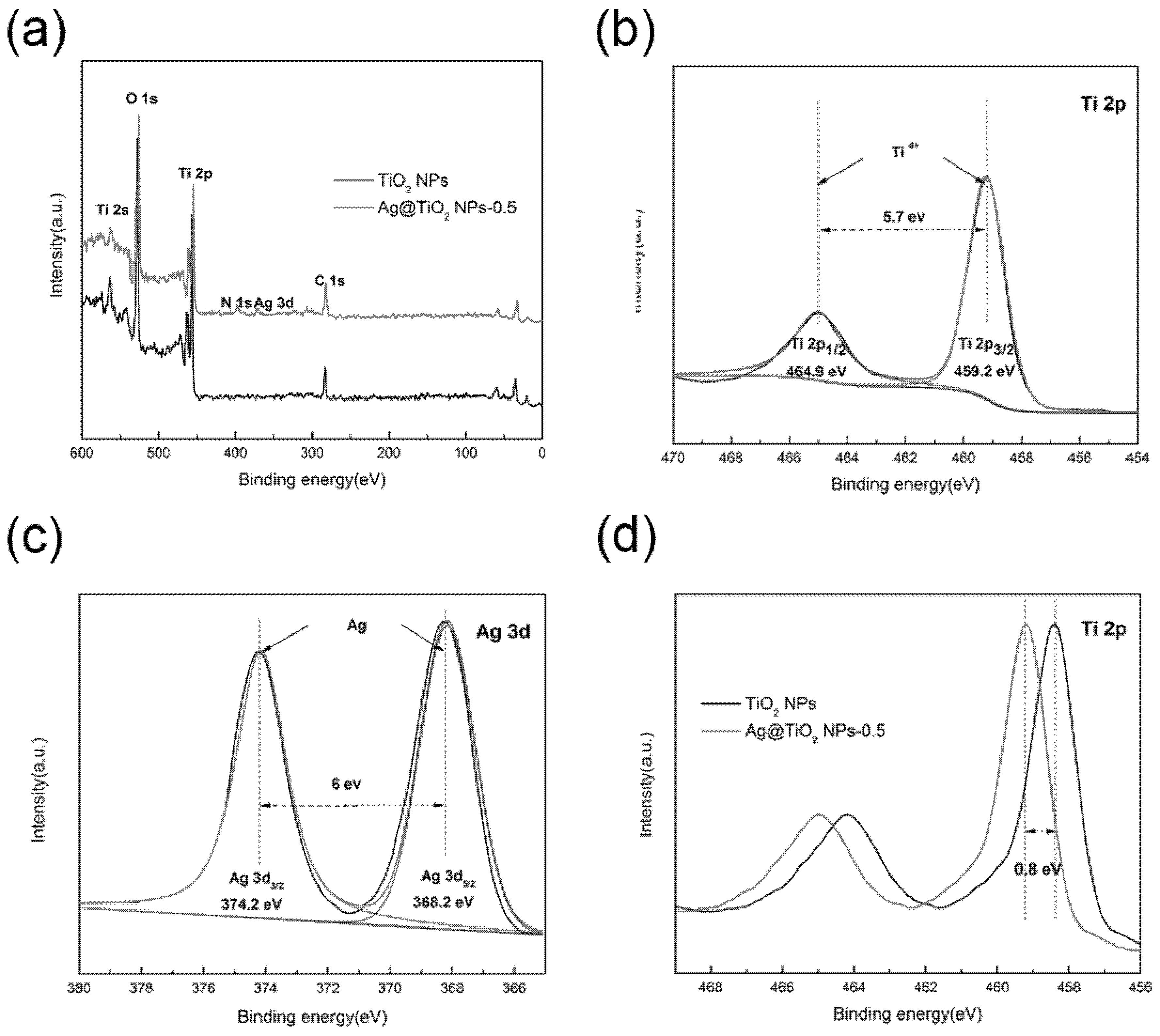
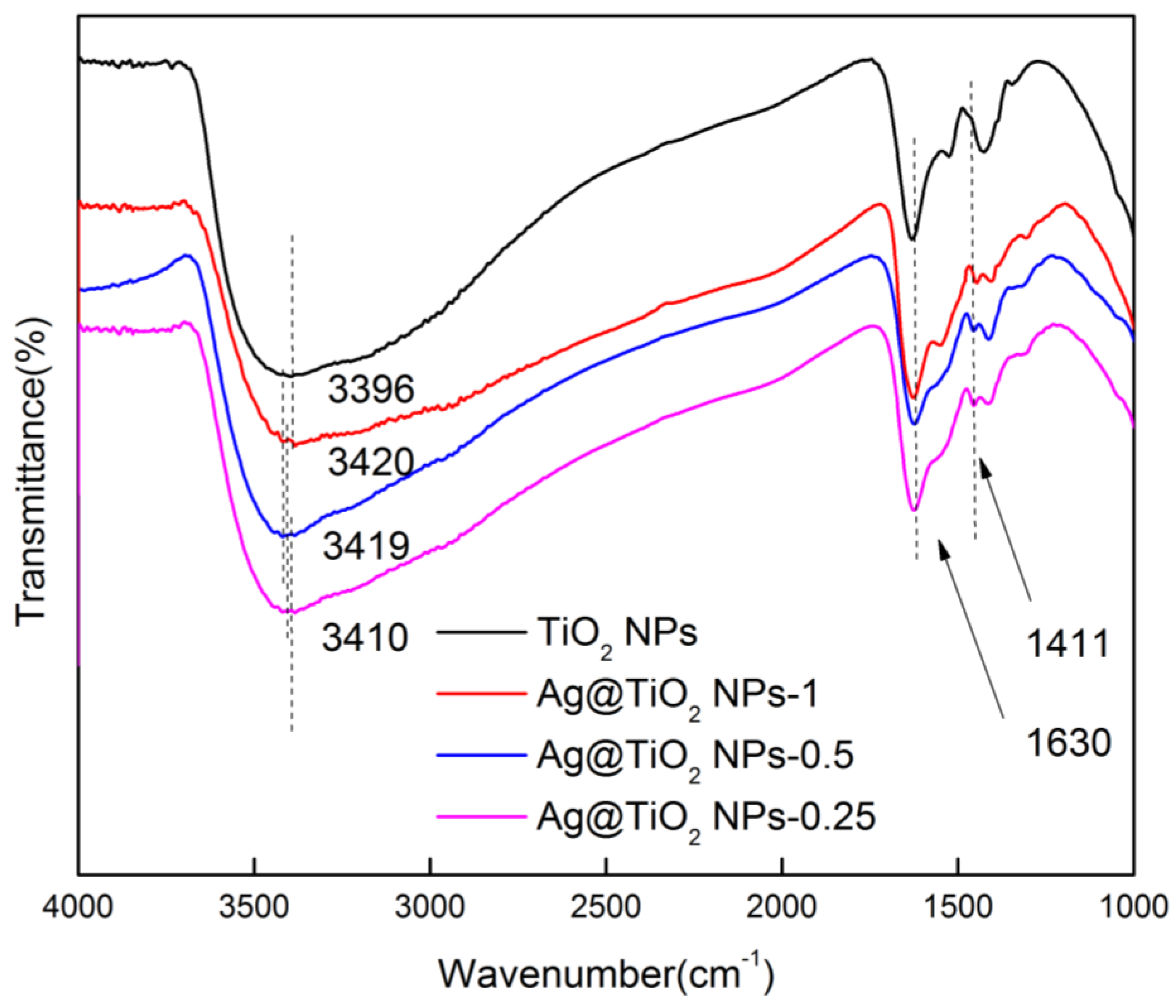
3.1. Preparation of Ag@TiO2 NPs
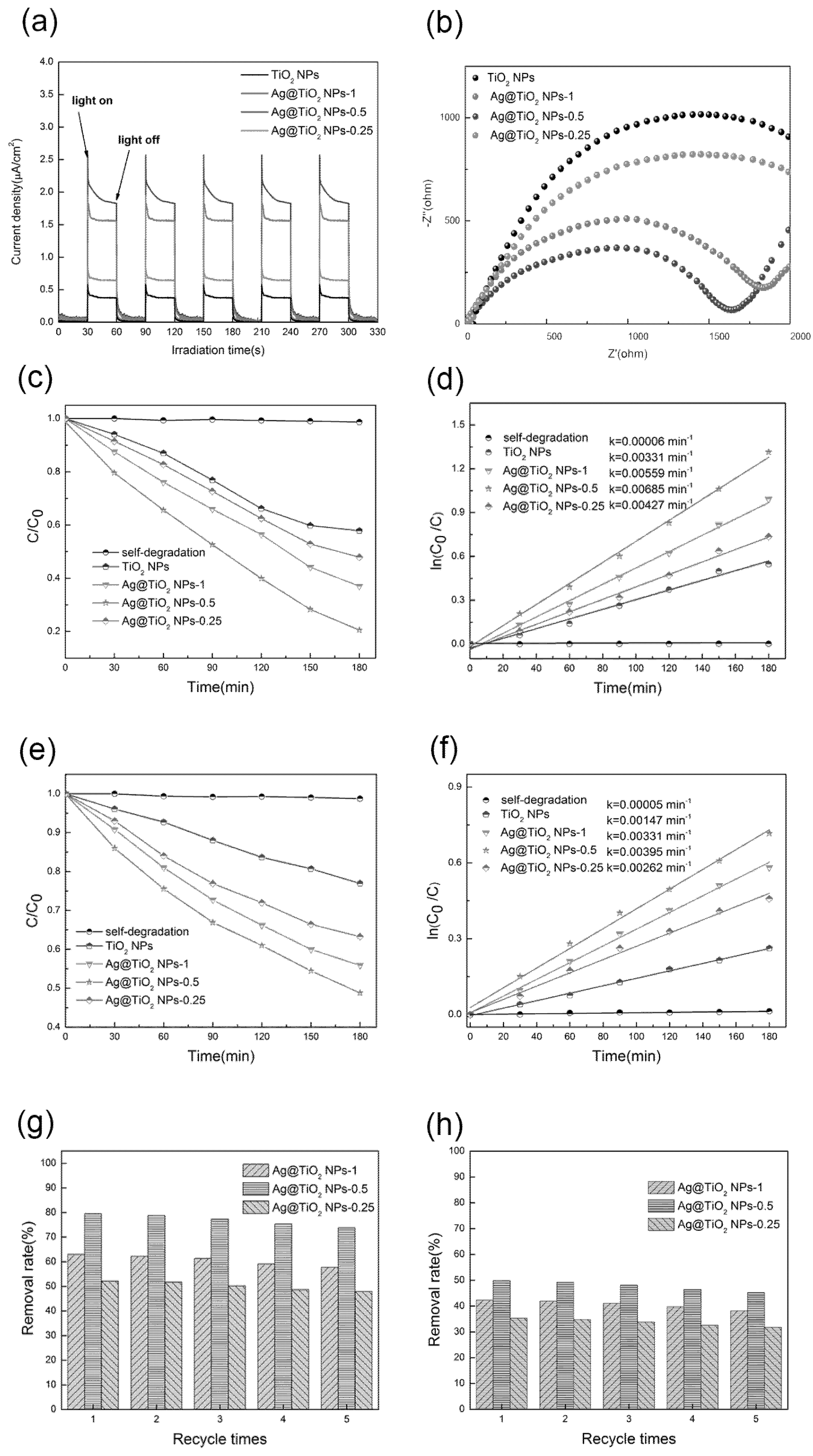
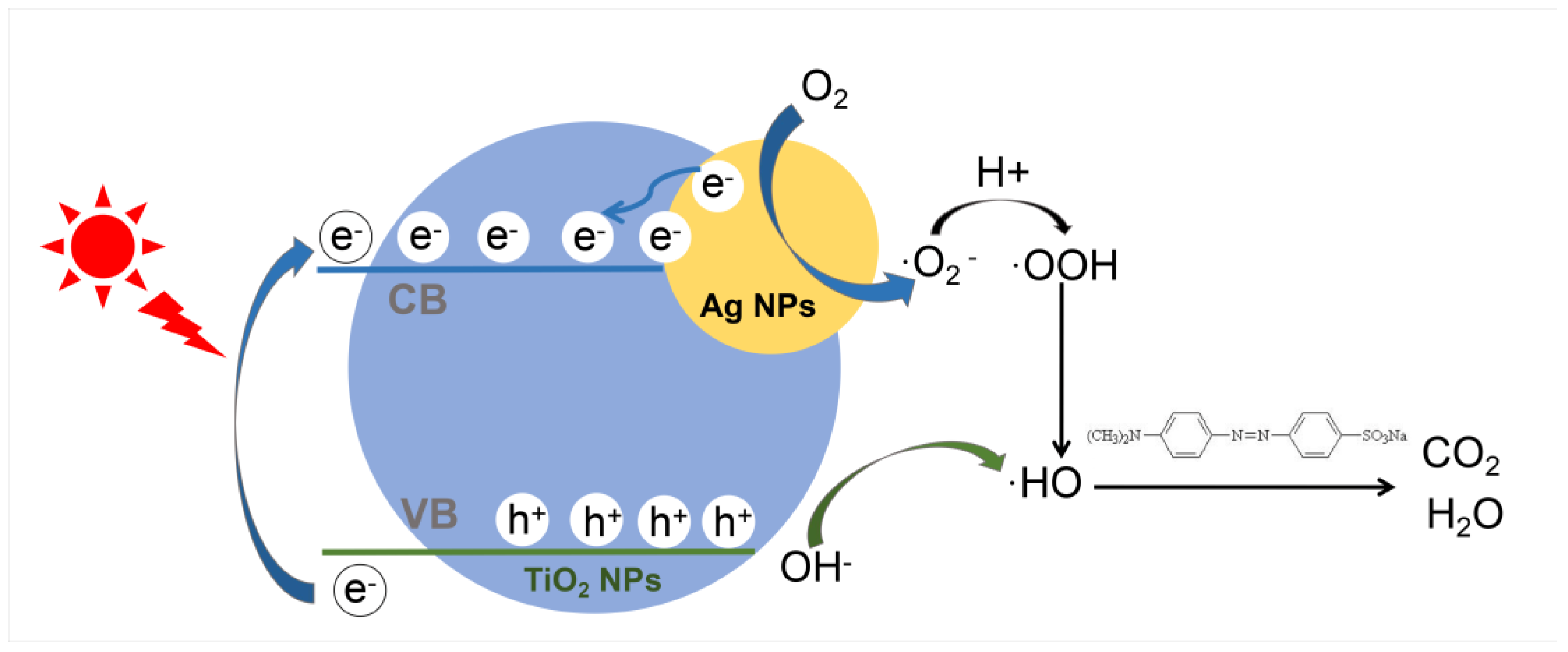
3.2. Photocatalytic Activity of Ag@TiO2 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, R.; Hashimot, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Xu, X.; Randorn, C.; Efstathiou, P.; Irvine, J.T. A red metallic oxide photocatalyst. Nat. Mater. 2012, 11, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Arul, N.S.; Mangalaraj, D.; Chen, P.C.; Ponpandian, N.; Meena, P.; Masuda, Y. Enhanced photocatalytic activity of cobalt-doped CeO2, nanorods. J. Sol-Gel Sci. Technol. 2012, 64, 515–523. [Google Scholar] [CrossRef]
- Kansal, S.K.; Kundu, P.; Sood, S.; Lamba, R.; Umar, A.; Mehta, S.K. Photocatalytic degradation of the antibiotic levofloxacin using highly crystalline TiO2 nanoparticles. New J. Chem. 2014, 38, 3220–3226. [Google Scholar] [CrossRef]
- Chan, S.C.; Barteau, M.A. Preparation of Highly Uniform Ag/TiO2 and Au/TiO2 Supported Nanoparticle Catalysts by Photodeposition. Langmuir 2005, 21, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. Trac-Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, L.; Liu, G.; Yu, H.; Tang, H. Enhanced Photocatalytic Degradation and Selective Removal of Nitrophenols by Using Surface Molecular Imprinted Titania. Environ. Sci. Technol. 2008, 42, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Y.; Han, J.; Guo, R.; Xiong, H.; Yin, Y. TiO2/NiO hybrid shells: P-n junction photocatalysts with enhanced activity under visible light. J. Mater. Chem. A 2015, 3, 20727–20735. [Google Scholar] [CrossRef]
- An, H.; Park, S.Y.; Huh, J.Y.; Kim, H.; Lee, Y.C.; Lee, Y.B.; Hong, Y.C.; Lee, H.U. Nanoporous hydrogenated TiO2 photocatalysts generated by underwater discharge plasma treatment for solar photocatalytic applications. Appl. Catal. B 2017, 211, 126–136. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B 2017, 209, 311–319. [Google Scholar] [CrossRef]
- Chai, X.; Zhang, H.; Cheng, C. 3D FTO inverse opals@hematite@TiO2 hierarchically structured photoanode for photoelectrochemical water splitting. Semicond Sci. Technol. 2017, 32, 4003. [Google Scholar] [CrossRef]
- Monfort, O.; Raptis, D.; Satrapinskyy, L.; Roch, T.; Plesch, G.; Lianos, P. Production of hydrogen by water splitting in a photoelectrochemical cell using a BiVO4/TiO2 layered photoanode. Electrochim. Acta 2017, 251, 244–249. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Luo, S.; Liu, C.; Li, Y. TiO2 nanotube arrays co-loaded with Au nanoparticles and reduced graphene oxide: Facile synthesis and promising photocatalytic application. J. Alloys Compd. 2013, 578, 242–248. [Google Scholar] [CrossRef]
- Green, I.X.; Tang, W.; Neurock, M.; Yates, J.T. Localized partial oxidation of acetic acid at the dual perimeter sites of the Au/TiO2 catalyst-formation of gold ketenylidene. J. Am. Chem. Soc. 2012, 134, 13569–13572. [Google Scholar] [CrossRef] [PubMed]
- Pandikumar, A.; Sivaranjani, K.; Gopinath, C.S.; Ramaraj, R. Aminosilicate sol-gel stabilized N-doped TiO2-Au nanocomposite materials and their potential environmental remediation applications. RSC Adv. 2013, 3, 13390–13398. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.; Liu, J.; Wu, J. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Yu, Y.; Wen, W.; Wu, J. Construction of hierarchical Ag@TiO2@ZnO nanowires with high photocatalytic activity. New J. Chem. 2017, 42, 265–271. [Google Scholar] [CrossRef]
- Sun, T.; Liu, E.; Fan, J.; Hu, X.; Wu, F.; Hou, W.; Yang, Y.; Kang, L.; Hou, W.; Yang, Y.; et al. High photocatalytic activity of hydrogen production from water over Fe doped and Ag deposited anatase TiO2 catalyst synthesized by solvothermal method. Chem. Eng. J. 2013, 228, 896–906. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, E.; Dai, H.; Kang, L.; Wu, H.; Fan, J.; Hu, X.; Liu, H. Photocatalytic activity of Ag-TiO2-graphene ternary nanocomposites and application in hydrogen evolution by water splitting. Int. J. Hydrogen Energy 2014, 39, 7664–7671. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, X.; Li, J.; Xu, H.; Lin, H.; Chen, Y. Design and fabrication of a new class of nano hybrid materials based on reactive polymeric molecular cages. Langmuir 2013, 29, 11498–11505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xiong, J.; Li, C.; Zhang, Y.; Toh, G.W.; Lin, H.; Chen, Y. Synthesis of size tunable gold nanoparticles polymeric hybrid based on molecular nanocages. Micro Nano Lett. 2014, 9, 235–238. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Sun, H. Decolorizing of azo dye Reactive red 24 aqueous solution using exfoliated graphite and H2O2 under ultrasound irradiation. Ultrason. Sonochem. 2008, 15, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Yeo, I.S.; Kim, H.C.; Ki, H.C.; Gu, H.B. Surface plasmon resonance effect of silver nanoparticles on a TiO2, electrode for dye-sensitized solar cells. Appl. Surf. Sci. 2017, 432, 266–271. [Google Scholar] [CrossRef]
- Fu, F.; Wang, F.; Li, T.; Jiao, C.; Zhang, Y.; Chen, Y. Synthesis of TiO2NWS@AuNPS Composite Catalyst for Methylene Blue Removal. Materials 2018, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Wang, X.; Wang, R. Controlling nanoparticle formation via sizable cages of supramolecular soft materials. Langmuir 2011, 27, 7820–7827. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, K.; Takagi, R.; Kishimoto, N.; Yamashita, I. A size controlled synthesis of CuS nano-particles in the protein cage, apoferritin. Mater. Lett. 2011, 65, 3245–3247. [Google Scholar] [CrossRef]
- Adachi, M.; Murata, Y.; Takao, J.; Jiu, J.; Sakamoto, M.; Wang, F. Highly Efficient Dye-Sensitized Solar Cells with a Titania Thin-Film Electrode Composed of a Network Structure of Single-Crystal-like TiO2 Nanowires Made by the “Oriented Attachment” Mechanism. J. Am. Chem. Soc. 2004, 126, 14943–14949. [Google Scholar] [CrossRef]
- Wang, P.; Han, L.; Zhu, C.; Zhai, Y.; Dong, S. Aqueous-phase synthesis of Ag-TiO2-reduced graphene oxide and Pt-TiO2-reduced graphene oxide hybrid nanostructures and their catalytic properties. Nano Res. 2011, 4, 1153–1162. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Song, W.; Li, G.; Luo, H.; Cooper, W.J. Mechanistic Considerations for the Advanced Oxidation Treatment of Fluoroquinolone Pharmaceutical Compounds using TiO2 Heterogeneous Catalysis. J. Phys. Chem. A 2010, 114, 2569–2575. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Malladi, R.S.; Hanagadakar, M.S.; Doddamani, M.R.; Santhakumari, B.; Kulkarni, S.D. Ru-TiO2, semiconducting nanoparticles for the photo-catalytic degradation of bromothymol blue. J. Mater. Sci. Mater. Electron. 2016, 27, 1–10. [Google Scholar] [CrossRef]
- Leshuk, T.; Parviz, R.; Everett, P.; Krishnakumar, H.; Varin, R.A.; Gu, F. Photocatalytic activity of hydrogenated TiO2. ACS Appl. Mater. Interfaces 2013, 5, 1892–1895. [Google Scholar] [CrossRef]
- Yu, D.; Yu, X.; Wang, C.; Liu, X.; Xing, Y. Synthesis of Natural Cellulose-Templated TiO2/Ag Nanosponge Composites and Photocatalytic Properties. ACS Appl. Mater. Interfaces 2012, 4, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, Y.; Huang, T.; Liu, M. Oxygen vacancies contained TiO2 spheres: Facile fabrication and enhanced ferromagnetism. J. Nanopart. Res. 2012, 14, 1030. [Google Scholar] [CrossRef]
- Xin, B.; Jing, L.; Ren, Z.; Wang, B.; Fu, H. Effects of simultaneously doped and deposited Ag on the photocatalytic activity and surface states of TiO2. J. Phys. Chem. B 2005, 109, 2805–2809. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, H.; Zhang, R.; Pan, W. Enhanced Photocatalysis of Electrospun Ag–ZnO Heterostructured Nanofibers. Chem Mater. 2009, 21, 3479–3484. [Google Scholar] [CrossRef]
- Mino, L.; Spoto, G.; Bordiga, S.; Zecchina, A. Particles Morphology and Surface Properties As Investigated by HRTEM, FTIR, and Periodic DFT Calculations: From Pyrogenic TiO2 (P25) to Nanoanatase. J. Phys. Chem. C 2012, 116, 17008–17018. [Google Scholar] [CrossRef]
- Mino, L.; Spoto, G.; Bordiga, S.; Zecchina, A. Rutile Surface Properties beyond the Single Crystal Approach: New Insights from the Experimental Investigation of Different Polycrystalline Samples and Periodic DFT Calculations. J. Phys. Chem. C 2013, 117, 11186–11196. [Google Scholar] [CrossRef]
- Mino, L.; Zecchina, A.; Martra, G.; Rossi, A.M.; Spoto, G. A surface science approach to TiO2 P25 photocatalysis: An in situ FTIR study of phenol photodegradation at controlled water coverages from sub-monolayer to multilayer. Appl. Catal. B-Environ. 2016, 196, 135–141. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z. The enhancement of photodegradation efficiency using Pt–TiO2 catalyst. Chemosphere 2002, 21, 1103–1111. [Google Scholar] [CrossRef]
- Colthup, N.B. Spectra-Structure Correlations in the Infra-Red Region. J. Opt. Soc. Am. 1950, 4, 421. [Google Scholar] [CrossRef]
- Reddy, D.A.; Lee, S.; Choi, J.; Park, S.; Ma, R.; Yang, H.; Kim, T.K. Green synthesis of AgI-reduced graphene oxide nanocomposites: Toward enhanced visible-light photocatalytic activity for organic dye removal. Appl. Surf. Sci. 2015, 341, 175–184. [Google Scholar] [CrossRef]
- Reddy, D.A.; Choi, J.; Lee, S.; Ma, R.; Kim, T.K. Green synthesis of AgI nanoparticle-functionalized reduced graphene oxide aerogels with enhanced catalytic performance and facile recycling. RSC Adv. 2015, 5, 67394–67404. [Google Scholar] [CrossRef]
- Choi, J.; Reddy, D.A.; Kim, T.K. Enhanced photocatalytic activity and anti-photocorrosion of AgI nanostructures by coupling with graphene-analogue boron nitride nanosheets. Ceram. Int. 2015, 41, 13793–13803. [Google Scholar] [CrossRef]
- Islam, M.J.; Reddy, D.A.; Ma, R.; Kim, Y.; Kim, T.K. Reduced-graphene-oxide-wrapped BiOI-AgI heterostructured nanocomposite as a high-performance photocatalyst for dye degradation under solar light irradiation. Solid State Sci. 2016, 61, 32–39. [Google Scholar] [CrossRef]
- Ma, R.; Islam, M.J.; Reddy, D.A.; Kim, T.K. Transformation of CeO2 into a mixed phase CeO2/Ce2O3 nanohybrid by liquid phase pulsed laser ablation for enhanced photocatalytic activity through Z-scheme pattern. Ceram. Int. 2016, 42, 18495–18502. [Google Scholar] [CrossRef]
- Lee, S.; Reddy, D.A.; Kim, T.K. Well-wrapped reduced graphene oxide nanosheets on Nb3O7(OH) nanostructures as good electron collectors and transporters for efficient photocatalytic degradation of rhodamine B and phenol. RSC Adv. 2016, 6, 37180–37188. [Google Scholar] [CrossRef]
- Reddy, D.A.; Reddy, D.A.; Ma, R.; Choi, M.Y.; Kim, T.K. Reduced graphene oxide wrapped ZnS–Ag2S ternary composites synthesized via hydrothermal method: Applications in photocatalyst degradation of organic pollutants. Appl. Surf. Sci. 2015, 324, 725–735. [Google Scholar] [CrossRef]
- Sun, C.; Xu, Q.; Xie, Y.; Ling, Y.; Hou, Y. Designed synthesis of anatase-TiO2 (B) biphase nanowires/ZnO nanoparticles heterojunction for enhanced photocatalysis. J. Mater. Chem. A 2018, 6, 8289–8298. [Google Scholar] [CrossRef]
- Islam, M.J.; Kim, H.K.; Reddy, D.A.; Kim, Y.; Ma, R.; Baek, H.; Kim, J.; Kim, T.K. Hierarchical BiOI nanostructures supported on a metal organic framework as efficient photocatalysts for degradation of organic pollutants in water. Dalton Trans. 2017, 46, 6013–6023. [Google Scholar] [CrossRef]
- Reddy, D.A.; Choi, J.; Lee, S.; Kim, T.K. Controlled synthesis of heterostructured Ag@AgI/ZnS microspheres with enhanced photocatalytic activity and selective separation of methylene blue from mixture dyes. J. Taiwan Inst. Chem. Eng. 2016, 66, 200–209. [Google Scholar] [CrossRef]
- Islam, M.J.; Reddy, D.A.; Han, N.S.; Choi, J.; Song, J.K.; Kim, T.K. An oxygen-vacancy rich 3D novel hierarchical MoS2/BiOI/AgI ternary nanocomposite: Enhanced photocatalytic activity through photogenerated electron shuttling in a Z-scheme manner. Phys. Chem. Chem. Phys. 2016, 18, 24984–24993. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavinia, F.; Amiri, M.J.; Faraji, A.R. Adsorption of methyl orange and salicylic acid on a nano-transition metal composite: Kinetics, thermodynamic and electrochemical studies. J. Colloid Interface Sci. 2016, 483, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Reddy, D.A.; Choi, J.; Kim, T.K. Surface oxygen vacancy assisted electron transfer and shuttling for enhanced photocatalytic activity of a Z-scheme CeO2–AgI nanocomposite. RSC Adv. 2016, 6, 19341–19350. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Tan, S.; Yu, X.; Zhu, Q.; Zhou, W. Fabrication of 3D flower-like black N-TiO2x@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl. Catal. B-Environ. 2017, 201, 119–127. [Google Scholar] [CrossRef]
- Ma, J.; Guo, S.; Guo, X.; Ge, H. A mild synthetic route to Fe3O4@TiO2-Au composites: Preparation, characterization and photocatalytic activity. Appl. Surf. Sci. 2015, 353, 1117–1125. [Google Scholar] [CrossRef]
- Choi, J.; Reddy, D.A.; Islam, M.J.; Ma, R.; Kim, T.K. Self-assembly of CeO2 nanostructures/reduced graphene oxide composite aerogels for efficient photocatalytic degradation of organic pollutants in water. J. Alloys Compd. 2016, 688, 527–536. [Google Scholar] [CrossRef]
- Yang, B.; He, D.; Wang, W.; Zhuo, Z.; Wang, Y. Gold-plasmon enhanced photocatalytic performance of anatase titania nanotubes under visible-light irradiation. Mater. Res. Bull. 2016, 74, 278–283. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Fu, F.; Li, Y.; Zhang, D.; Chen, Y. One-Step Synthesis of Ag@TiO2 Nanoparticles for Enhanced Photocatalytic Performance. Nanomaterials 2018, 8, 1032. https://doi.org/10.3390/nano8121032
Zhang Y, Fu F, Li Y, Zhang D, Chen Y. One-Step Synthesis of Ag@TiO2 Nanoparticles for Enhanced Photocatalytic Performance. Nanomaterials. 2018; 8(12):1032. https://doi.org/10.3390/nano8121032
Chicago/Turabian StyleZhang, Yufan, Fan Fu, Yuzhou Li, Desuo Zhang, and Yuyue Chen. 2018. "One-Step Synthesis of Ag@TiO2 Nanoparticles for Enhanced Photocatalytic Performance" Nanomaterials 8, no. 12: 1032. https://doi.org/10.3390/nano8121032
APA StyleZhang, Y., Fu, F., Li, Y., Zhang, D., & Chen, Y. (2018). One-Step Synthesis of Ag@TiO2 Nanoparticles for Enhanced Photocatalytic Performance. Nanomaterials, 8(12), 1032. https://doi.org/10.3390/nano8121032





