Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
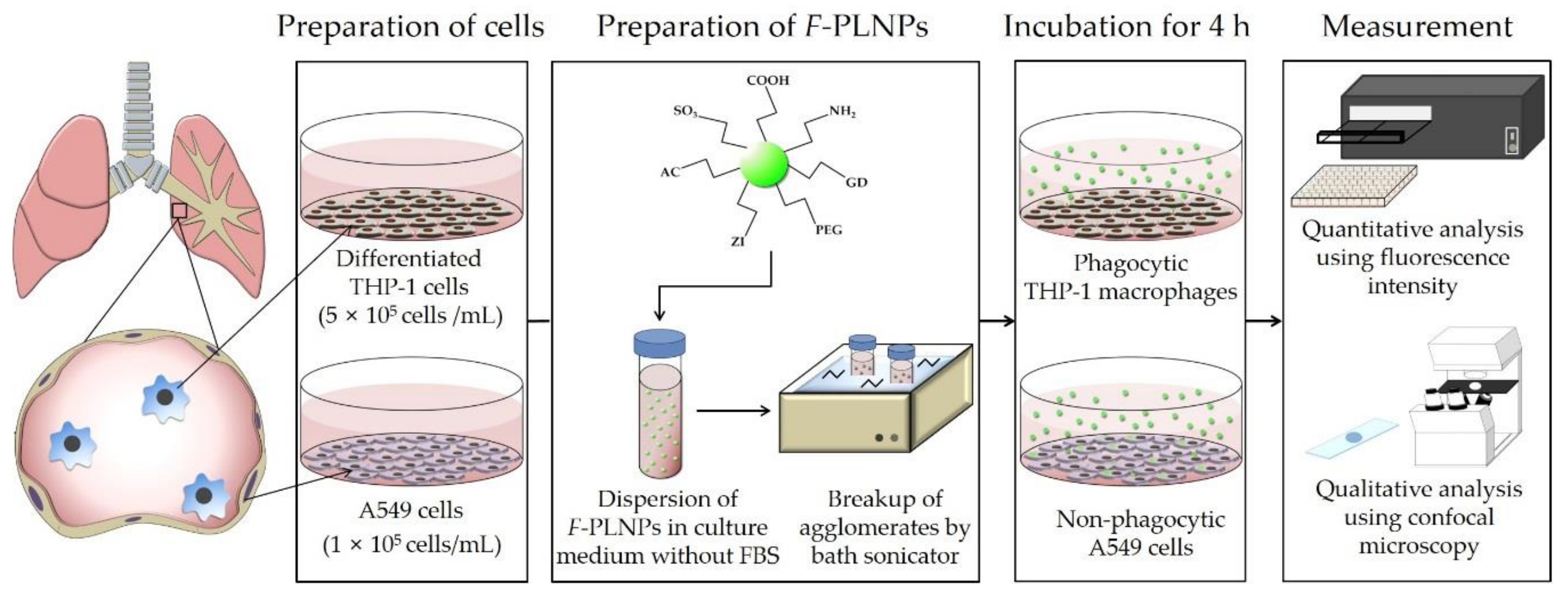
2.1. Synthesis of Fluorescein-Conjugated Polystyrene Nanoparticles (F-PLNPs)
2.2. Characterization of NPs
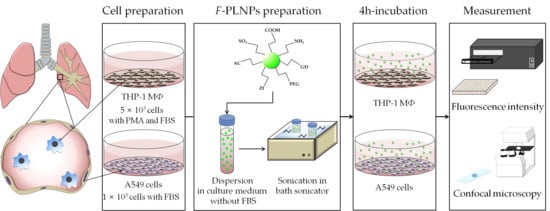
2.3. Study Design for Cellular Uptake Experiments
2.4. Cellular Uptake Assay Using Macrophage-Like THP-1 Cells
2.5. Cellular Uptake Assay Using Lung Epithelial A549 Cells
2.6. Statistical Analysis
3. Results and Discussion
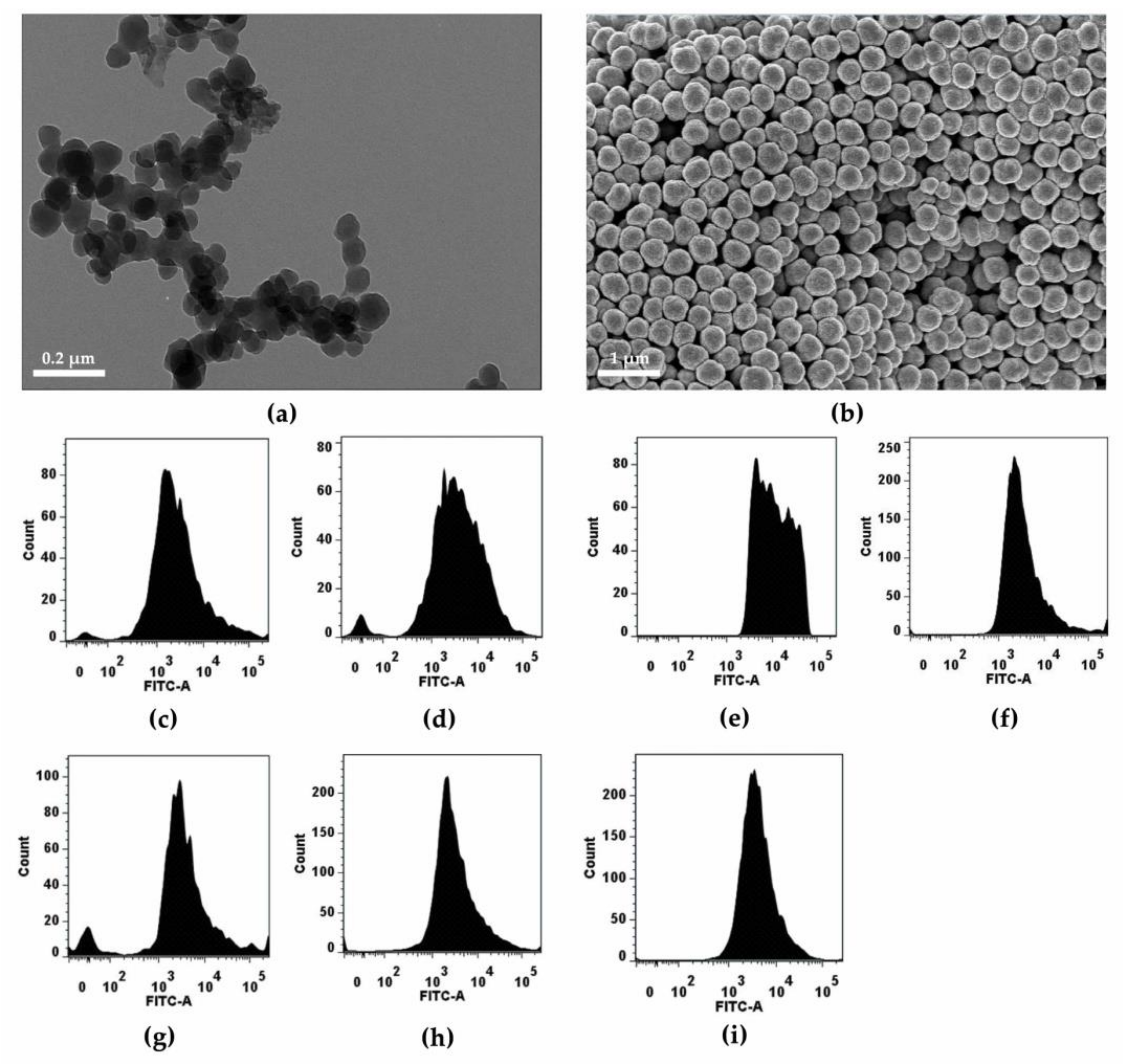
3.1. Physicochemical Properties of F-PLNPs
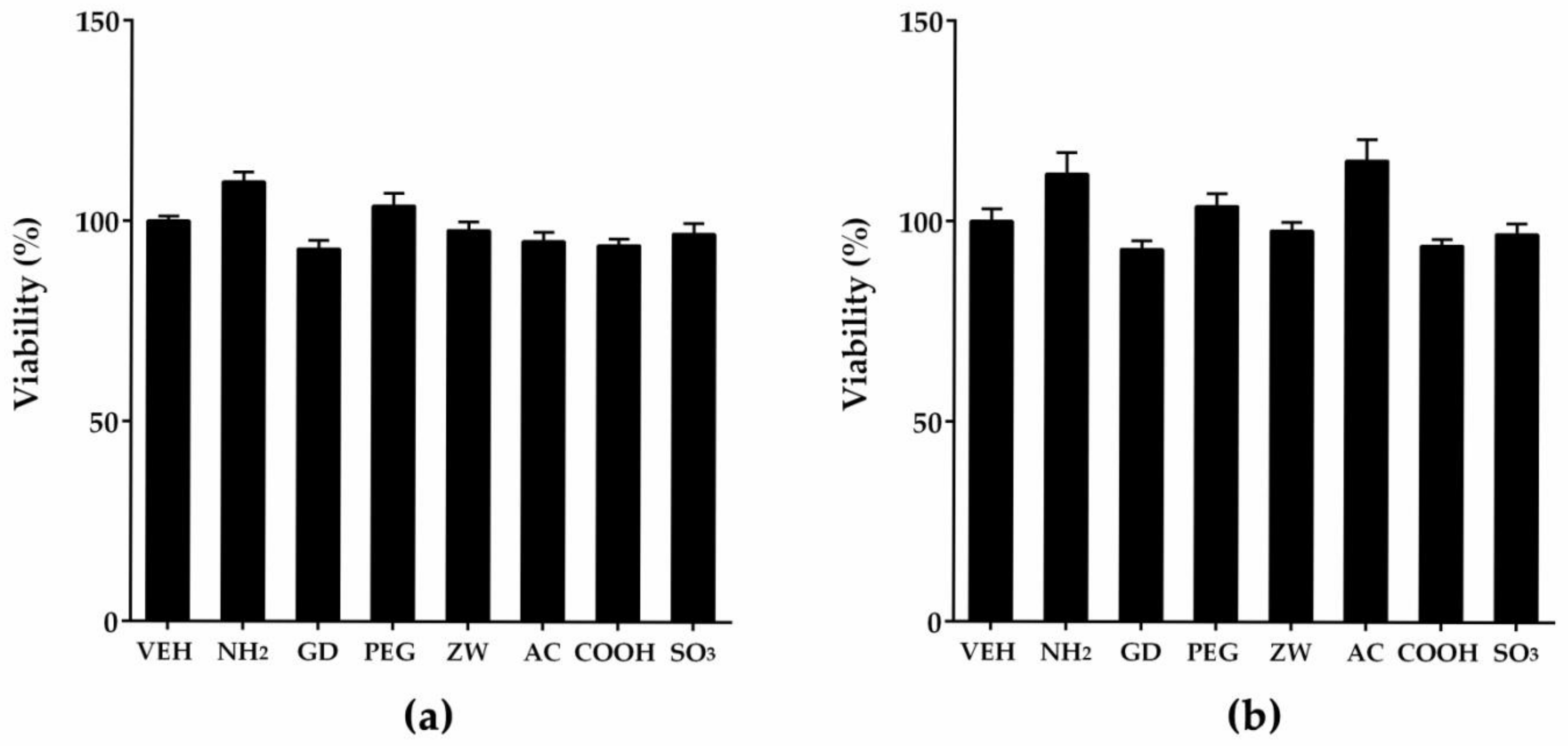
3.2. Cytotoxicity Assay of Nanoparticles Against THP-1 Macrophages and A549 Cells
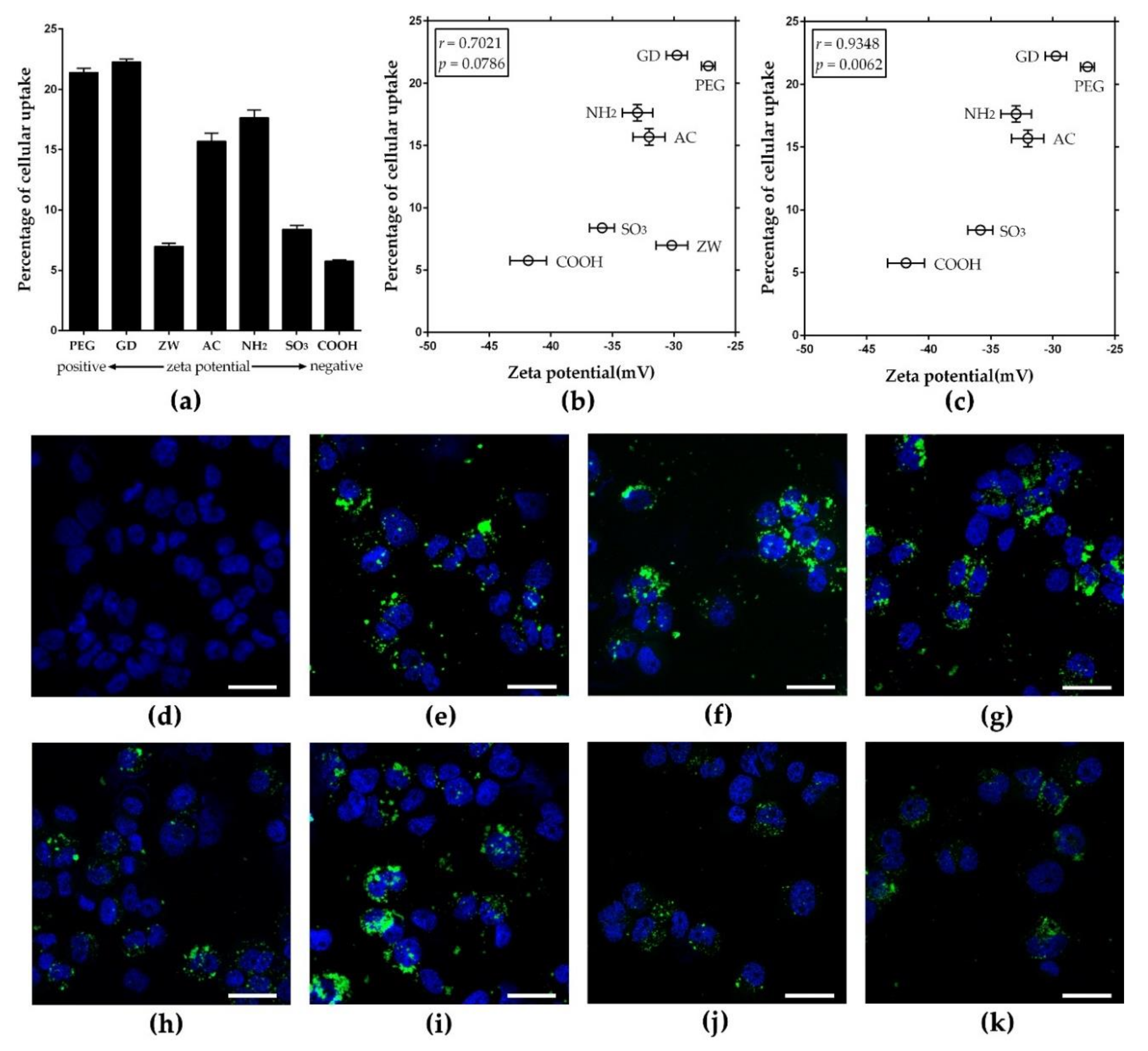
3.3. Zeta Potential-Dependent Cellular Uptake of Nanoparticles in Phagocytic THP-1 Macrophages
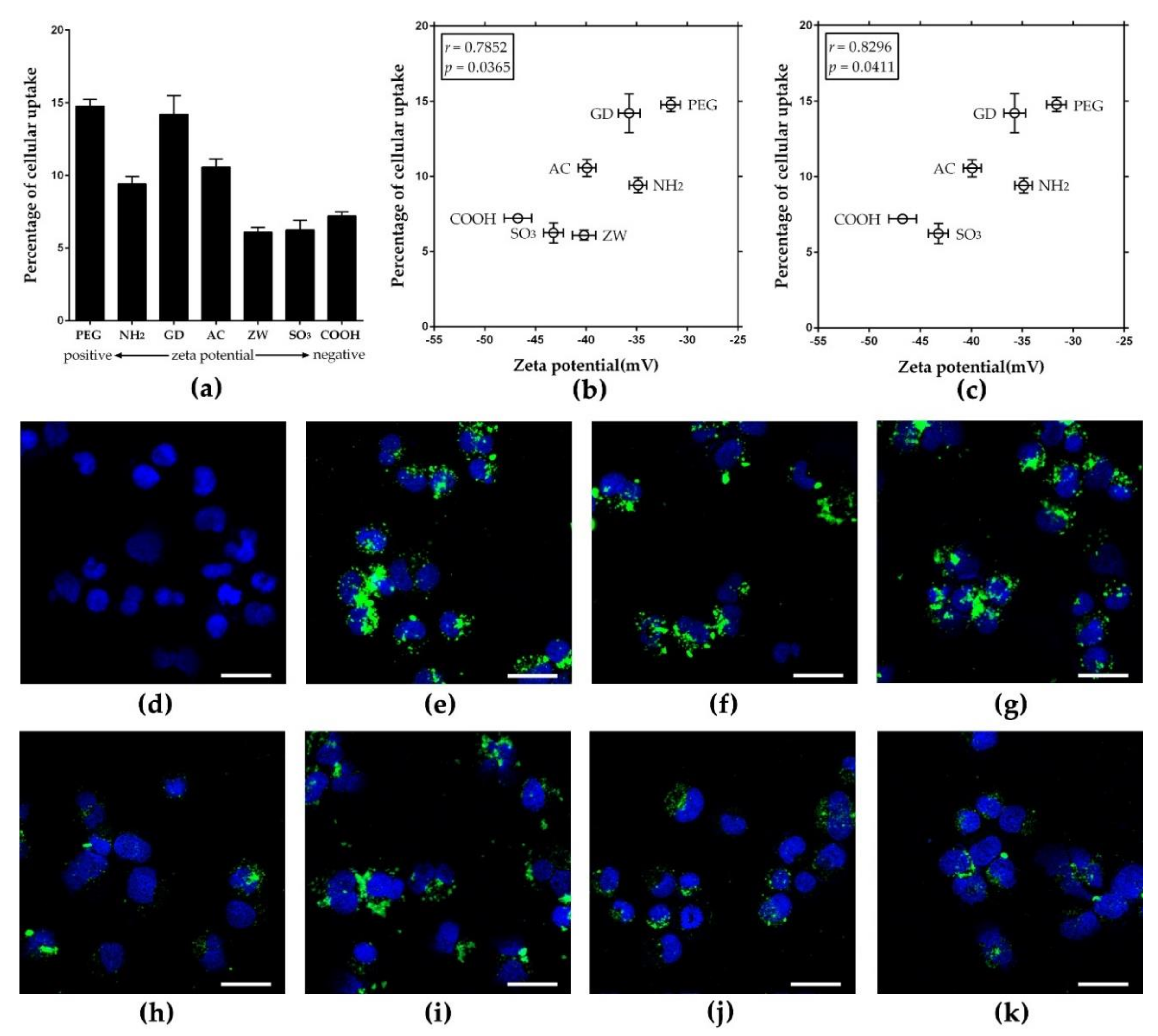
3.4. Zeta Potential-Dependent Cellular Uptake of Nanoparticles in Non-Phagocytic A549 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Papavlassopoulos, H.; Mishra, Y.K.; Kaps, S.; Paulowicz, I.; Abdelaziz, R.; Elbahri, M.; Maser, E.; Adelung, R.; Rohl, C. Toxicity of functional nano-micro zinc oxide tetrapods: Impact of cell culture conditions, cellular age and material properties. PLoS ONE 2014, 9, e84983. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Cho, W.S.; Thielbeer, F.; Duffin, R.; Johansson, E.M.; Megson, I.L.; Macnee, W.; Bradley, M.; Donaldson, K. Surface functionalization affects the zeta potential, coronal stability and membranolytic activity of polymeric nanoparticles. Nanotoxicology 2014, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chankeshwara, S.V.; Thielbeer, F.; Jeong, J.; Donaldson, K.; Bradley, M.; Cho, W.S. Surface charge determines the lung inflammogenicity: A study with polystyrene nanoparticles. Nanotoxicology 2016, 10, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Thielbeer, F.; Jeong, J.; Han, Y.; Chankeshwara, S.V.; Bradley, M.; Cho, W.S. Dual contribution of surface charge and protein-binding affinity to the cytotoxicity of polystyrene nanoparticles in nonphagocytic A549 cells and phagocytic THP-1 cells. J. Toxicol. Environ. Health A 2016, 79, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, T.S.; Bae, J.Y.; Jung, A.Y.; Lee, S.M.; Seok, J.H.; Roh, H.S.; Song, C.W.; Choi, M.J.; Jeong, J.; et al. Organ-specific distribution of gold nanoparticles by their surface functionalization. J. Appl. Toxicol. 2015, 35, 573–580. [Google Scholar] [CrossRef]
- Donaldson, K.; Schinwald, A.; Murphy, F.; Cho, W.S.; Duffin, R.; Tran, L.; Poland, C. The biologically effective dose in inhalation nanotoxicology. Acc. Chem. Res. 2013, 46, 723–732. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Thielbeer, F.; Bradley, M.; Megson, I.L.; MacNee, W.; Poland, C.A.; Tran, C.L.; Donaldson, K. Zeta potential and solubility to toxic ions as mechanisms of lung inflammation caused by metal/metal oxide nanoparticles. Toxicol. Sci. 2012, 126, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Wang, J.L.; Iqbal, S.; Li, H.J.; Cao, Z.T.; Wang, Y.C.; Du, J.Z.; Wang, J. The effect of surface charge on oral absorption of polymeric nanoparticles. Biomater. Sci. 2018, 6, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Nakanishi, T.; Shanmugam, S.; Takahama, S.; Zhang, H. Effect of surface charge of magnetite nanoparticles on their internalization into breast cancer and umbilical vein endothelial cells. Colloids Surf. B Biointerfaces 2009, 71, 325–330. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Bradley, M.; Megson, I.L.; MacNee, W.; Lee, J.K.; Jeong, J.; Donaldson, K. Predictive value of in vitro assays depends on the mechanism of toxicity of metal oxide nanoparticles. Part. Fibre Toxicol. 2013, 10, 55. [Google Scholar] [CrossRef]
- Thielbeer, F.; Chankeshwara, S.V.; Bradley, M. Polymerizable fluorescein derivatives: Synthesis of fluorescent particles and their cellular uptake. Biomacromolecules 2011, 12, 4386–4391. [Google Scholar] [CrossRef] [PubMed]
- Thielbeer, F.; Donaldson, K.; Bradley, M. Zeta potential mediated reaction monitoring on nano and microparticles. Bioconjug Chem. 2011, 22, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Shoshi, A.; Schotter, J.; Schroeder, P.; Milnera, M.; Ertl, P.; Charwat, V.; Purtscher, M.; Heer, R.; Eggeling, M.; Reiss, G.; et al. Magnetoresistive-based real-time cell phagocytosis monitoring. Biosens. Bioelectron. 2012, 36, 116–122. [Google Scholar] [CrossRef]
- Santos, T.D.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Quantitative assessment of the comparative nanoparticle-uptake efficiency of a range of cell lines. Small 2011, 7, 3341–3349. [Google Scholar] [CrossRef]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delacher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailander, V.; Landfester, K.; et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef]
- De Castro, C.E.; Ribeiro, C.A.S.; Alavarse, A.C.; Albuquerque, L.J.C.; da Silva, M.C.C.; Jager, E.; Surman, F.; Schmidt, V.; Giacomelli, C.; Giacomelli, F.C. Nanoparticle-Cell Interactions: Surface Chemistry Effects on the Cellular Uptake of Biocompatible Block Copolymer Assemblies. Langmuir 2018, 34, 2180–2188. [Google Scholar] [CrossRef]
- Ding, H.M.; Ma, Y.Q. Design strategy of surface decoration for efficient delivery of nanoparticles by computer simulation. Sci. Rep. 2016, 6, 26783. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Zhao, D.; Li, L.; Zhou, J. Understanding the Cellular Uptake of pH-Responsive Zwitterionic Gold Nanoparticles: A Computer Simulation Study. Langmuir 2017, 33, 14480–14489. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Park, J.; Bichelberger, M.A.; Kantner, K.; Hartmann, R.; Maffre, P.; Said, A.H.; Feliu, N.; Lee, J.; Lee, D.; et al. Zwitterionic surface coating of quantum dots reduces protein adsorption and cellular uptake. Nanoscale 2016, 8, 17794–17800. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Popp, L.; Yang, J.; Kumar, A.; Gangoli, V.S.; Segatori, L. The autophagic response to polystyrene nanoparticles is mediated by transcription factor EB and depends on surface charge. J. Nanobiotechnol. 2015, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Ershov, D.; Gucht, J.; Alink, G.M.; Rietjens, I.M.; Zuilhof, H.; Marcelis, A.T. Surface charge-specific cytotoxicity and cellular uptake of tri-block copolymer nanoparticles. Nanotoxicology 2013, 7, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Chen, W.; Hung, Y.; Mou, C.Y. Molecular Elucidation of Biological Response to Mesoporous Silica Nanoparticles in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 22235–22251. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Wang, X.; Ji, Z.; Chang, C.H.; Zhang, H.; Wang, M.; Liao, Y.P.; Lin, S.; Meng, H.; Li, R.; Sun, B.; et al. Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small 2014, 10, 385–398. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Y.; Sullivan, N.; Chen, Y. Modeling the primary size effects of citrate-coated silver nanoparticles on their ion release kinetics. Environ. Sci. Technol. 2011, 45, 4422–4428. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Ceridono, M.; Urban, P.; Bogni, A.; Ponti, J.; Gioria, S.; Kinsner-Ovaskainen, A. The agglomeration state of nanoparticles can influence the mechanism of their cellular internalisation. J. Nanobiotechnol. 2017, 15, 48. [Google Scholar] [CrossRef]
- Muller, K.H.; Motskin, M.; Philpott, A.J.; Routh, A.F.; Shanahan, C.M.; Duer, M.J.; Skepper, J.N. The effect of particle agglomeration on the formation of a surface-connected compartment induced by hydroxyapatite nanoparticles in human monocyte-derived macrophages. Biomaterials 2014, 35, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhang, Y.; Xia, J.; Ma, M.; He, S.; Nie, F.; Gu, N. Effect of surface charge and agglomerate degree of magnetic iron oxide nanoparticles on KB cellular uptake in vitro. Colloids Surf. B Biointerfaces 2009, 73, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.; Sandberg, W.J.; Wegierek-Ciuk, A.; Lisowska, H.; Refsnes, M.; Sartowska, B.; Schwarze, P.E.; Meczynska-Wielgosz, S.; Wojewodzka, M.; Kruszewski, M. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol. Lett. 2012, 208, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476–11521. [Google Scholar] [CrossRef] [PubMed]
| PLNPs | Zeta Potential (mV) | Hydrodynamic Size (nm) | Polydispersity Index (PDI) | Endotoxin (EU/mL) | ||
|---|---|---|---|---|---|---|
| In DW | In RPMI-1640 | In DMEM | ||||
| NH2F-PLNP | 42.1 ± 1.0 | −33.0 ± 1.3 | −34.9 ± 0.9 | 324.2 ± 4.6 | 0.3 ± 0.0 | Not detected |
| GDF-PLNP | 39.7 ± 3.6 | −29.8 ± 0.9 | −35.8 ± 1.1 | 429.6 ± 9.6 | 0.3 ± 0.0 | |
| PEGF-PLNP | 27.3 ± 1.9 | −27.2 ± 0.6 | −31.7 ± 1.0 | 280.6 ± 1.4 | 0.1 ± 0.0 | |
| ZWF-PLNP | 24.2 ± 1.5 | −30.2 ± 1.3 | −40.2 ± 1.2 | 369.1 ± 2.6 | 0.3 ± 0.0 | |
| ACF-PLNP | −17.8 ± 1.1 | −32.1 ± 1.3 | −39.9 ± 0.9 | 301.1 ± 3.5 | 0.2 ± 0.0 | |
| COOHF-PLNP | −33.4 ± 0.8 | −41.9 ± 1.5 | −46.8 ± 1.4 | 254.4 ± 0.9 | 0.0 ± 0.0 | |
| SO3F-PLNP | −40.9 ± 0.6 | −35.9 ± 1.1 | −43.3 ± 1.0 | 258.4 ± 1.4 | 0.0 ± 0.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Clavadetscher, J.; Lee, D.-K.; Chankeshwara, S.V.; Bradley, M.; Cho, W.-S. Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles. Nanomaterials 2018, 8, 1028. https://doi.org/10.3390/nano8121028
Jeon S, Clavadetscher J, Lee D-K, Chankeshwara SV, Bradley M, Cho W-S. Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles. Nanomaterials. 2018; 8(12):1028. https://doi.org/10.3390/nano8121028
Chicago/Turabian StyleJeon, Soyeon, Jessica Clavadetscher, Dong-Keun Lee, Sunay V. Chankeshwara, Mark Bradley, and Wan-Seob Cho. 2018. "Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles" Nanomaterials 8, no. 12: 1028. https://doi.org/10.3390/nano8121028
APA StyleJeon, S., Clavadetscher, J., Lee, D.-K., Chankeshwara, S. V., Bradley, M., & Cho, W.-S. (2018). Surface Charge-Dependent Cellular Uptake of Polystyrene Nanoparticles. Nanomaterials, 8(12), 1028. https://doi.org/10.3390/nano8121028