A Surface Mediated Supramolecular Chiral Phenomenon for Recognition of l- and d-Cysteine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Situ Synthesis of l-/d-/dl-Cys Capped Ag NPs
2.3. Synthesis of SC-Stabilized Ag NPs
2.4. Synthesis of l-Cys Capped Ag NPs by Ligand Exchange Reaction
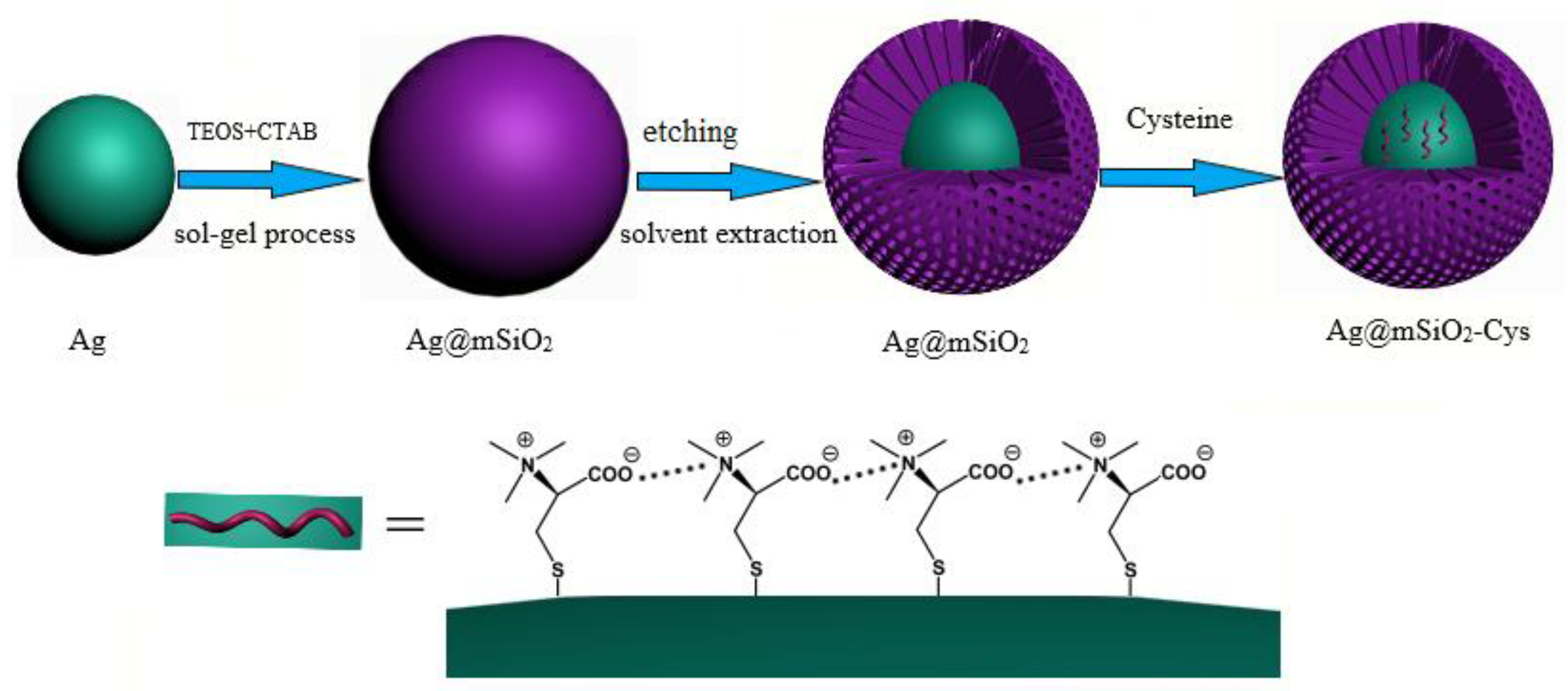
2.5. Synthesis of Mesoporous Silica Coated Ag Particle (Ag@mSiO2)
2.6. Modification of Ag@mSiO2 with Cys
2.7. Characterization
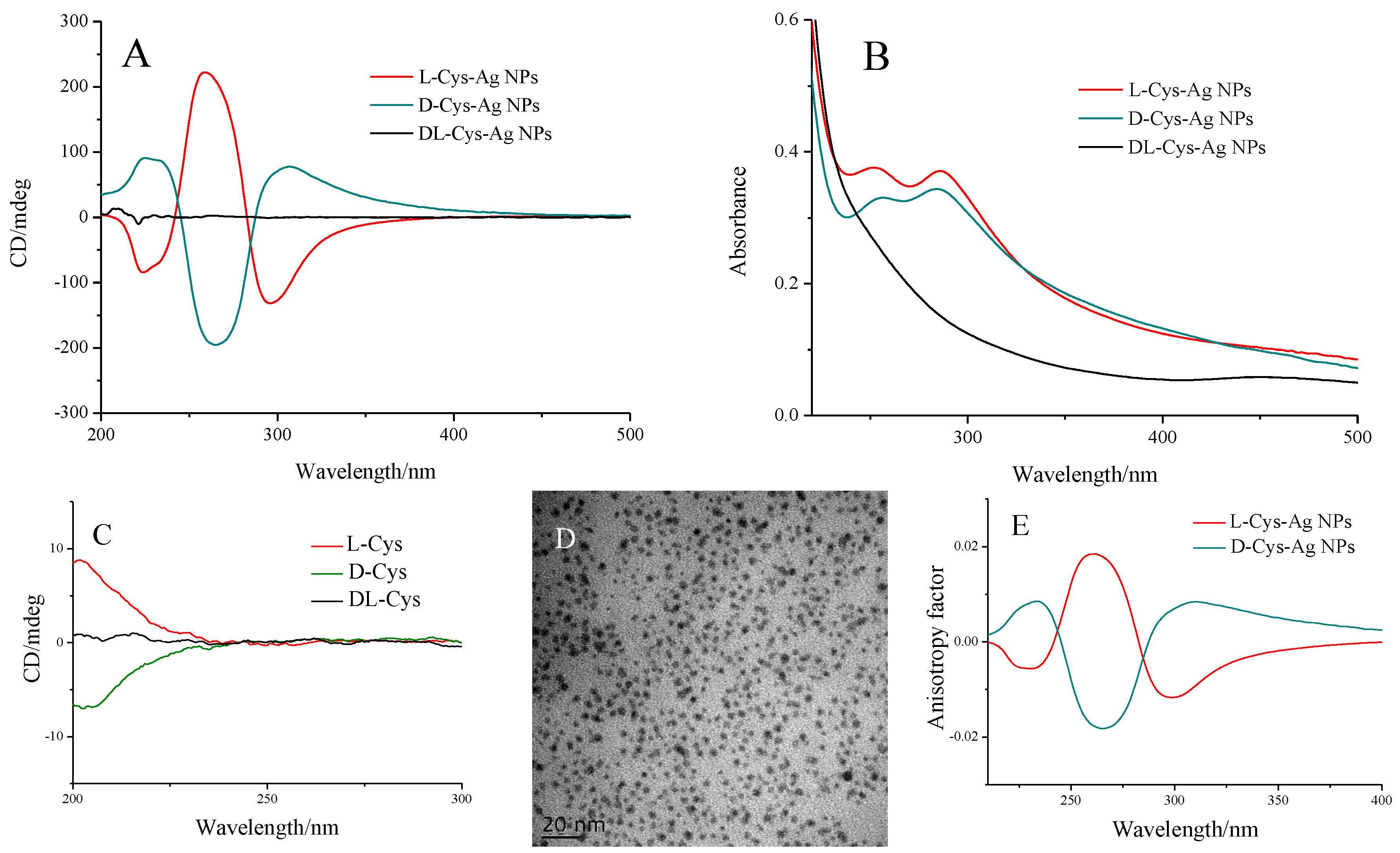
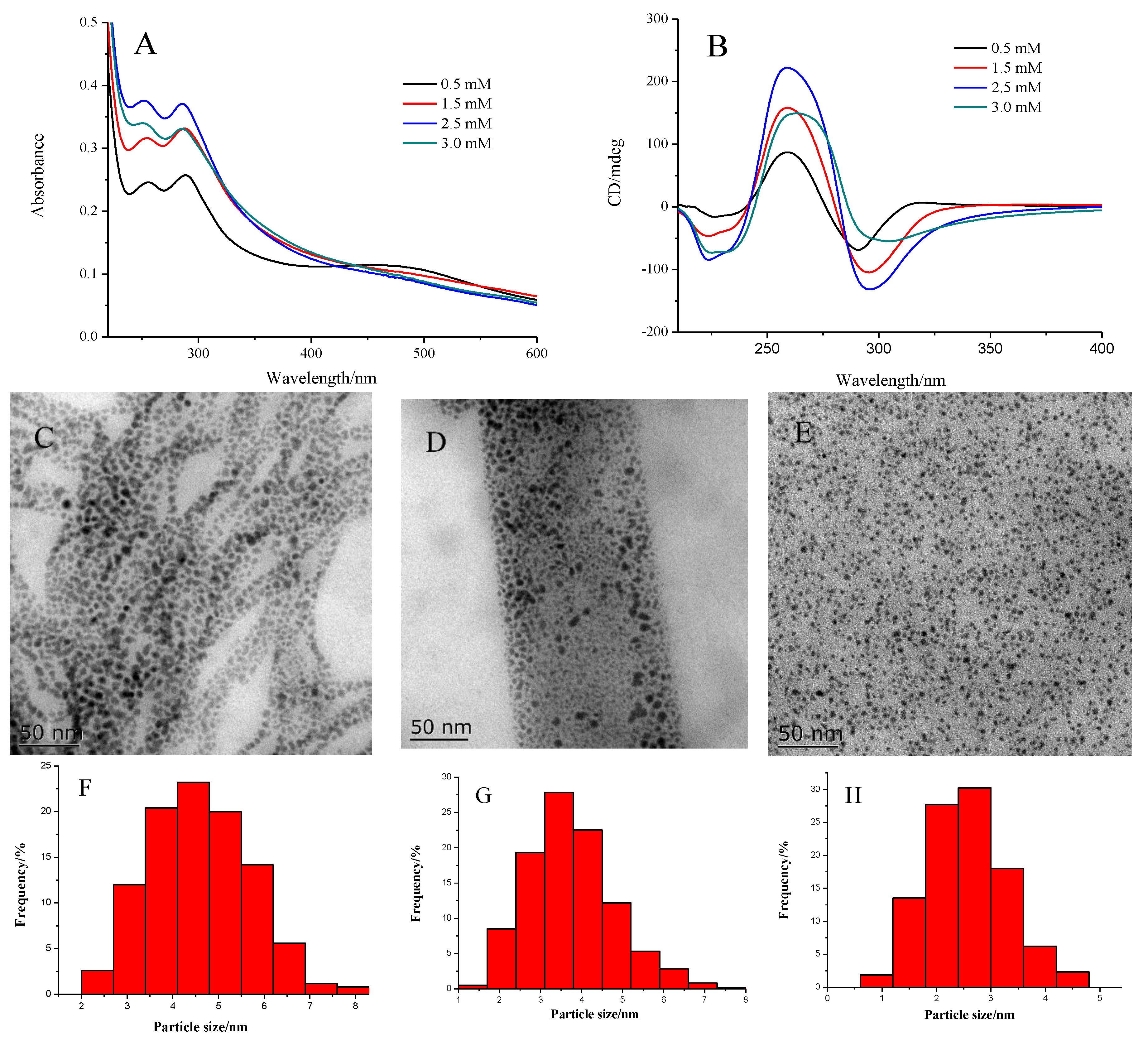
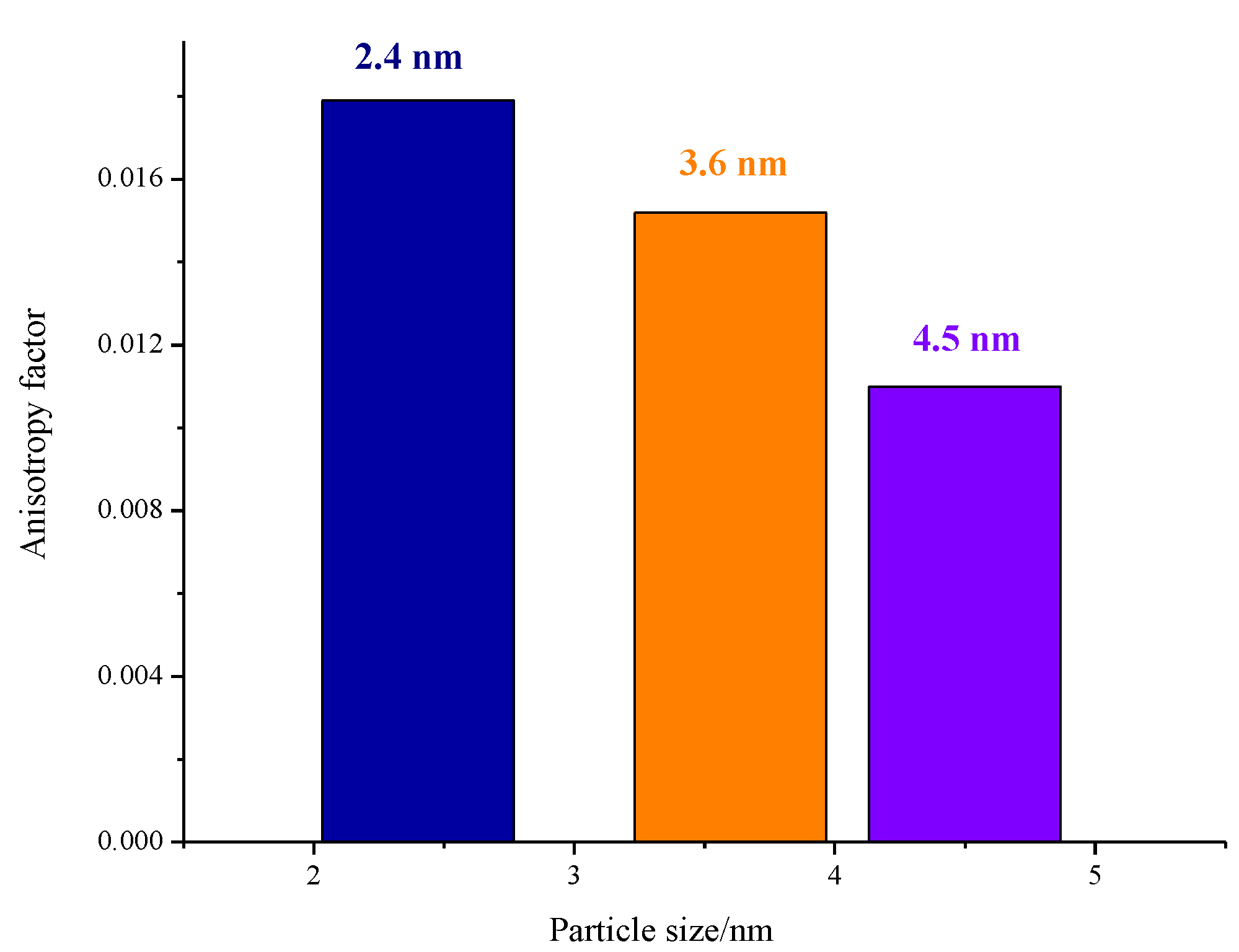
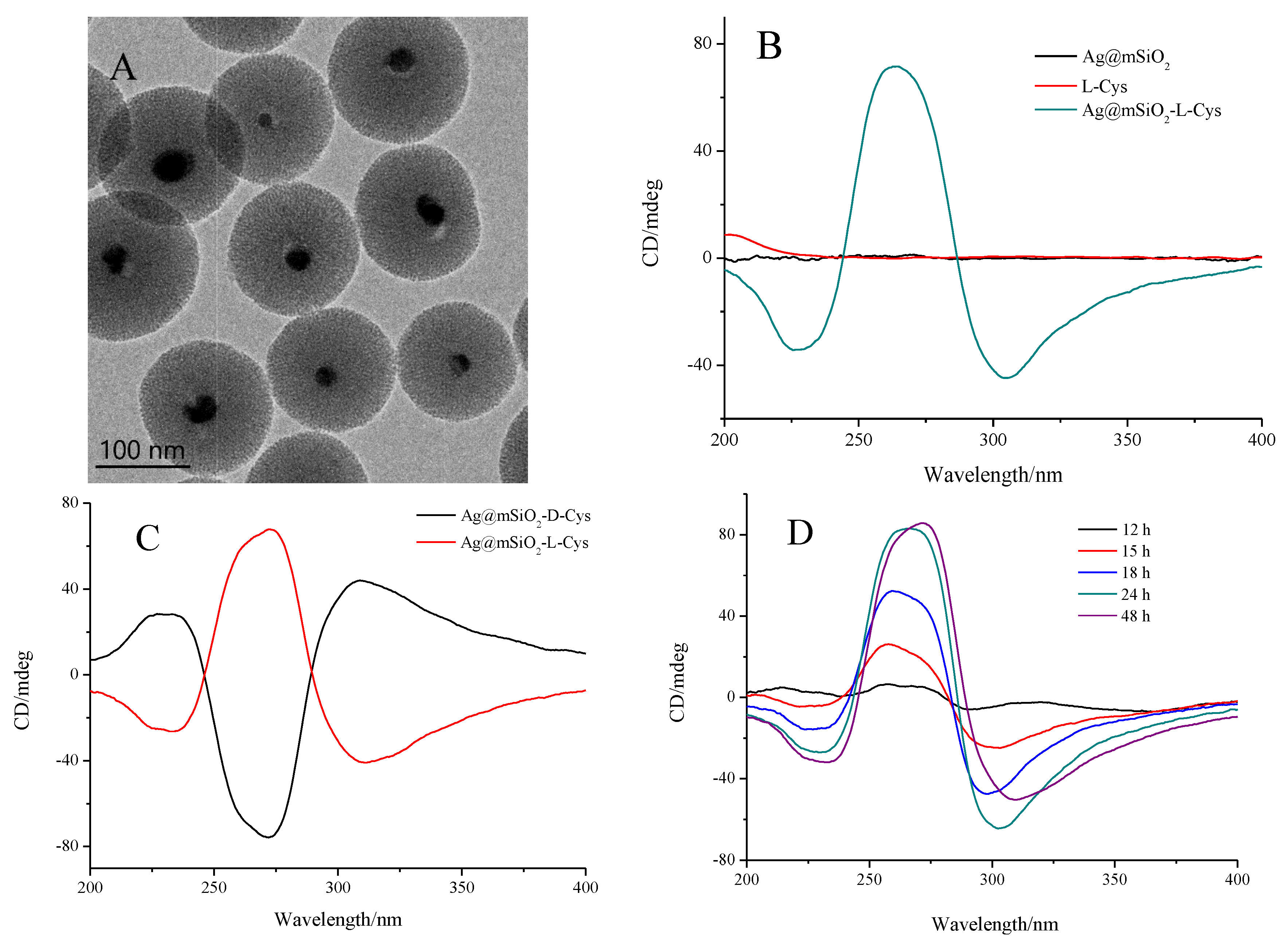
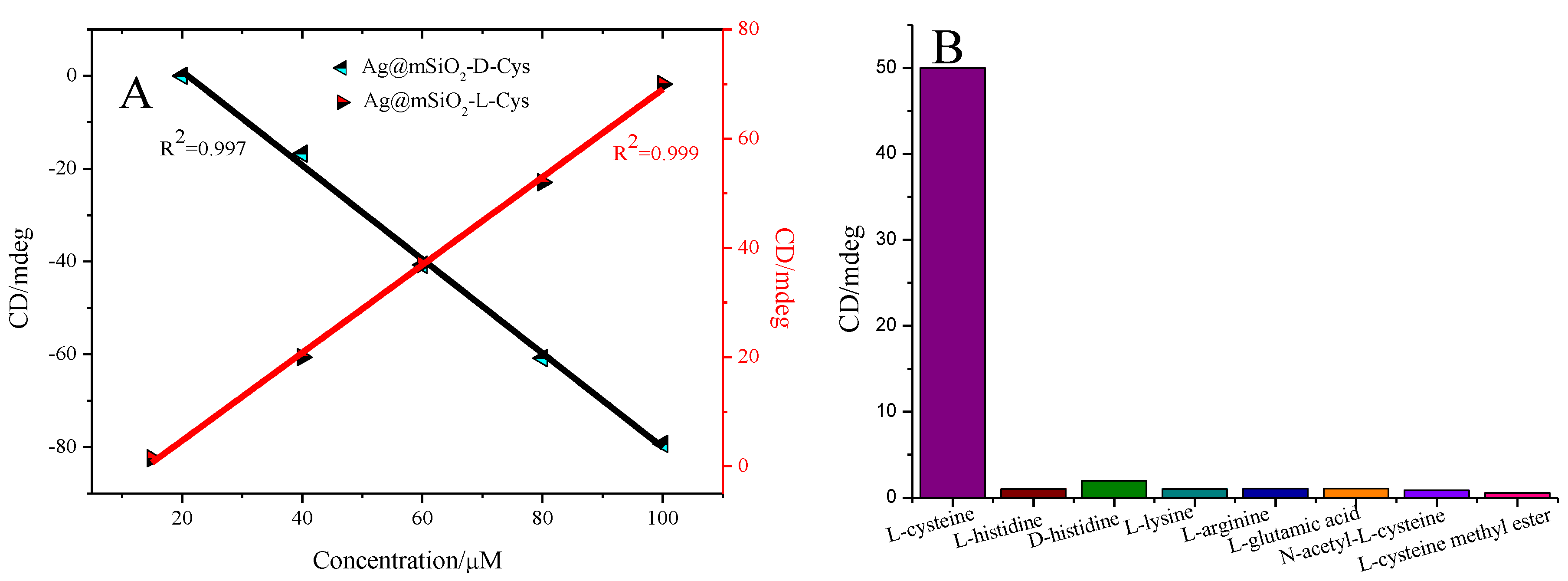
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fra, A.; Yoboue, E.D.; Sitia, R. Cysteines as redox molecular switches and targets of disease. Front. Mol. Neurosci. 2017, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Soutourina, J.; Blanquet, S.; Plateau, P. Role of d-cysteine desulfhydrase in the adaptation of Escherichia coli to d-cysteine. J. Biol. Chem. 2001, 276, 40864–40872. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zheng, Q.; Li, H. Colorimetric detection and separation of chiral tyrosine based on N-acetyl-l-cysteine modified gold nanoparticles. J. Mater. Chem. 2012, 22, 6546–6548. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, B.C. Colorimetric chiral recognition of enantiomers using the nucleotide-capped silver nanoparticles. Anal. Chem. 2011, 83, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Kim, S.; Min, S.H. Gold nanoparticle-based colorimetric chiral discrimination of histidine: Application to determining the enantiomeric excess of histidine. Anal. Methods 2013, 6, 73–76. [Google Scholar] [CrossRef]
- Zor, E.; Bekar, N. Lab-in-a-syringe using gold nanoparticles for rapid colorimetric chiral discrimination of enantiomers. Biosens. Bioelectron. 2017, 91, 211–216. [Google Scholar] [CrossRef]
- Song, L.; Wang, S.F.; Kotov, N.A.; Xia, Y.S. Nonexclusive fluorescent sensing for L/D enantiomers enabled by dynamic nanoparticle-nanorod assemblies. Anal. Chem. 2012, 84, 7330–7335. [Google Scholar] [CrossRef]
- Gao, F.; Ma, S.Y.; Xiao, X.C.; Hu, Y.; Zhao, D.; He, Z.K. Sensing tyrosine enantiomers by using chiral CdSe/CdS quantum dots capped with N-acetyl-l-cysteine. Talanta 2017, 163, 102–110. [Google Scholar] [CrossRef]
- Liu, T.; Su, Y.Y.; Song, H.J.; Lv, Y. Microwave-assisted green synthesis of ultrasmall fluorescent water-soluble silver nanoclusters and its application in chiral recognition of amino acids. Analyst 2013, 138, 6558–6564. [Google Scholar] [CrossRef]
- Han, X.Y.; Zhen, Z.H.; Zeng, J.Z.; Fan, Q.X.; Fang, Z.Q.; Shi, G.Y.; Zhang, M. Inorganic–organic hybrid tongue-mimic for time-resolved luminescent noninvasive pattern and chiral recognition of thiols in biofluids toward healthcare monitoring. ACS Appl. Mater. Interfaces 2018, 10, 31725–31734. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.J.; Guo, Y.J.; Li, J.Z.; Yuan, M.K.; Long, T.F.; Liu, Z.D. Optically active ultrafine Au–Ag alloy nanoparticles used for colorimetric chiral recognition and circular dichroism sensing of enantiomers. Anal. Chem. 2017, 89, 9781–9787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.W.; Wang, Y.; Zhang, X.M. Homochiral MOF as circular dichroism sensor for enantioselective recognition on nature and chirality of unmodified amino acids. ACS Appl. Mater. Interfaces 2017, 9, 20991–20999. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Shi, L.; Gao, X.Q.; Guo, J.; Hou, K.; Zheng, Y.L.; Tang, Z.Y. Ultra-stable silica-coated chiral Au-nanorod assemblies: Core–shell nanostructures with enhanced chiroptical properties. Nano Res. 2016, 9, 451–457. [Google Scholar] [CrossRef]
- Hou, S.; Wen, T.; Zhang, H.; Liu, W.Q.; Hu, X.N.; Wang, R.Y.; Hu, Z.J.; Wu, X.C. Fabrication of chiral plasmonic oligomers using cysteine-modified gold nanorods as monomers. Nano Res. 2014, 7, 1699–1705. [Google Scholar] [CrossRef]
- Randazzo, R.; Mauro, A.D.; D’Urso, A.; Messina, G.C.; Compagnini, G.; Villari, V.; Micali, N.; Purrello, R.; Fragalà, M.E. Hierarchical effect behind the supramolecular chirality of silver(I)−cysteine coordination polymers. J. Phys. Chem. B 2015, 119, 4898–4904. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Haynie, B.E.; Tohgha, U.; Pap, L.; Elliott, K.W.; Leonard, B.M.; Dzyuba, S.V.; Varga, K.; Kubelka, J.; Balaz, M. Chirality inversion of CdSe and CdS quantum dots without changing the stereochemistry of the capping ligand. ACS Nano 2016, 10, 3809–3815. [Google Scholar] [CrossRef]
- Zhu, F.; Li, X.Y.; Li, Y.C.; Yan, M.; Liu, S.Q. Enantioselective circular dichroism sensing of cysteine and glutathione with gold nanorods. Anal. Chem. 2015, 87, 357–361. [Google Scholar] [CrossRef]
- Zhang, H.; Govorov, A.O. Giant Circular dichroism of a molecule in a region of strong plasmon resonances between two neighboring gold nanocrystals. Phys. Rev. B 2012, 87, 138–143. [Google Scholar] [CrossRef]
- Govorov, A.O. Plasmon-induced circular dichroism of a chiral molecule in the vicinity of metal nanocrystals. Application to various geometries. J. Phys. Chem. C 2011, 115, 7914–7923. [Google Scholar] [CrossRef]
- Govorov, A.O.; Fan, Z.; Hernandez, P.; Slocik, J.M.; Naik, R.R. Theory of circular dichroism of nanomaterials comprising chiral molecules and nanocrystals: Plasmon enhancement, dipole Interactions, and dielectric Effects. Nano Lett. 2010, 10, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Layani, M.E.; Moshe, A.B.; Varenik, M.; Regev, O.; Zhang, H.; Govorov, A.O.; Markovich, G. Chiroptical activity in silver cholate nanostructures induced by the formation of nanoparticle assemblies. J. Phys. Chem. C 2013, 117, 22240–22244. [Google Scholar] [CrossRef]
- Guerrero-Martíneza, A.; Alonso-Gómezb, J.L.; Auguiéa, B.; Cidb, M.M.; Liz-Marzána, L.M. From individual to collective chirality in metal nanoparticles. Nano Today 2011, 6, 381–400. [Google Scholar] [CrossRef]
- Ge′rard, V.A.; Gun’ko, Y.K.; Defrancq, E.; Govorov, A.O. Plasmon-induced CD response of oligonucleotide-conjugated metal Nanoparticles. Chem. Commun. 2011, 47, 7383–7385. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Wang, P.; Liu, Y.N.; Zhao, W.J.; Zhai, D.W.; Hong, X.H.; Ji, Y.L.; Wu, X.C.; Wang, F. Experimental observation of giant chiroptical amplification of small chiral molecules by gold nanosphere clusters. J. Phys. Chem. C 2014, 118, 9690–9695. [Google Scholar] [CrossRef]
- Xu, L.G.; Xu, Z.; Ma, W.; Liu, L.Q.; Wang, L.B.; Kuang, H.; Xu, C.L. Highly selective recognition and ultrasensitive quantification of enantiomer. J. Mater. Chem. B 2013, 1, 4478–4483. [Google Scholar] [CrossRef]
- Wang, J.; Fei, K.X.; Yang, X.; Zhang, S.S.; Peng, Y.X. Synthesis and plasmonic chiroptical studies of sodium deoxycholate modified silver nanoparticles. Materials 2018, 11, 1291. [Google Scholar] [CrossRef]
- Sanz-Ortiz, M.N.; Sentosun, K.; Bals, S.; Liz-Marzan, L.M. Templated growth of Surface Enhanced Raman Scattering-active branched gold nanoparticles within radial mesoporous silica shells. ACS Nano 2015, 9, 10489–10497. [Google Scholar] [CrossRef]
- Teng, Z.G.; Su, X.D.; Zheng, Y.Y.; Sun, J.; Chen, G.T.; Tian, C.C.; Wang, J.D.; Li, H.; Zhao, Y.N.; Lu, G.M. Mesoporous silica hollow spheres with ordered radial mesochannels by a spontaneous self-transformation approach. Chem. Mater. 2013, 25, 98–105. [Google Scholar] [CrossRef]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, J.D.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; et al. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: Core and monolayer properties as a function of core size. Langmuir 1998, 14, 17–30. [Google Scholar] [CrossRef]
- Wang, R.; Ji, X.; Huang, Z.; Xue, Y.; Wang, D.; Yang, W. Citrate-regulated surface morphology of SiO2@Au particles to control the surface plasmonic properties. Phys. Chem. C 2016, 120, 377–385. [Google Scholar] [CrossRef]
- Yao, H.; Miki, K.; Nishida, N.; Sasaki, A.; Kimura, K. Large optical activity of gold nanocluster enantiomers induced by a pair of optically active penicillamines. J. Am. Chem. Soc. 2005, 127, 15536–15543. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yao, H.; Ueda, T.; Sasaki, A.; Kimura, K. Synthesis and chiroptical study of D/L-penicillamine-capped silver nanoclusters. Chem. Mater. 2007, 19, 2831–2841. [Google Scholar] [CrossRef]
- Koktan, J.; Sedlácková, H.; Osante, I.; Cativiela, C.; Díaz, D.D.; Rezanka, P. Chiral supramolecular nanoparticles: The study of chiral surface modification of silver nanoparticles by cysteine and its derivatives. Colloids Surf. A 2015, 470, 142–148. [Google Scholar] [CrossRef]
- Lu, F.; Tian, Y.; Liu, M.; Su, D.; Zhang, H.; Govorov, A.O.; Gang, O. Discrete nanocubes as plasmonic reporters of molecular chirality. Nano Lett. 2013, 13, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Slocik, J.M.; Govorov, A.O.; Naik, R.R. Plasmonic circular dichroism of peptide-functionalized gold nanoparticles. Nano Lett. 2011, 11, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, J.; Liu, M.H. Reversible plasmonic circular dichroism via hybrid supramolecular gelation of achiral gold nanorods. ACS Nano 2016, 10, 11179–11186. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Solovyov, A.; Katz, A. Postsynthetic modification of gold nanoparticles with calix[4]arene enantiomers: Origin of chiral surface plasmon resonance. Langmuir 2009, 25, 153–158. [Google Scholar] [CrossRef]
- Maoz, B.M.; van der Weegen, R.; Fan, Z.Y.; Govorov, A.O.; Ellestad, G.; Berova, N.; Meijer, E.W.; Markovich, G. Plasmonic chiroptical response of silver nanoparticles interacting with chiral supramolecular assemblies. J. Am. Chem. Soc. 2012, 134, 17807–17813. [Google Scholar] [CrossRef]
- Molotsky, T.; Tamarin, T.; Moshe, A.B.; Markovich, G.; Kotlyar, A.B. Synthesis of chiral silver clusters on a DNA template. J. Phys. Chem. C 2010, 114, 15951–15954. [Google Scholar] [CrossRef]
- Rezanka, P.; Záruba, K.; Král, V. Supramolecular chirality of cysteine modified silver nanoparticles. Colloids Surf. A 2011, 374, 77–83. [Google Scholar] [CrossRef]
- Liu, H.L.; Ye, Y.J.; Chen, J.; Lin, D.Y.; Jiang, Z.; Liu, Z.J.; Sun, B.; Yang, L.B.; Liu, J.H. In situ photoreduced silver nanoparticles on cysteine: An insight into the origin of chirality. Chem. Eur. J. 2012, 18, 8037–8041. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.Y.; Zhang, W.; Zhang, Y.L.; He, J.J.; Dai, J.Y.; Yeung, C.T.; Law, G.L.; Lei, D.Y. Interband absorption enhanced optical activity in discrete Au@Ag core–shell nanocuboids: Probing extended helical conformation of chemisorbed cysteine molecules. Angew. Chem. Int. Ed. 2017, 56, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Fajín, J.L.; Gomes, J.R.; Cordeiro, M.N. DFT study of the adsorption of d-(l-)cysteine on flat and chiral stepped gold surfaces. Langmuir 2013, 29, 8856–8864. [Google Scholar] [CrossRef] [PubMed]
- Im, S.H.; Lee, Y.T.; Wiley, B.; Xia, Y. Large-scale synthesis of silver nanocubes: The role of HCl in promoting cube perfection and monodispersity. Angew. Chem. Int. Ed. 2005, 44, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Herricks, T.; Sun, Y.; Xia, Y. Polyol synthesis of silver nanoparticles: Use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons. Nano Lett. 2004, 4, 1733–1739. [Google Scholar] [CrossRef]
- Long, R.; Zhou, S.; Wiley, B.J.; Xiong, Y.J. Oxidative etching for controlled synthesis of metal nanocrystals: Atomic addition and subtraction. Chem. Soc. Rev. 2014, 43, 6288–6310. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, S.-S.; Xu, X.; Fei, K.-X.; Peng, Y.-X. A Surface Mediated Supramolecular Chiral Phenomenon for Recognition of l- and d-Cysteine. Nanomaterials 2018, 8, 1027. https://doi.org/10.3390/nano8121027
Wang J, Zhang S-S, Xu X, Fei K-X, Peng Y-X. A Surface Mediated Supramolecular Chiral Phenomenon for Recognition of l- and d-Cysteine. Nanomaterials. 2018; 8(12):1027. https://doi.org/10.3390/nano8121027
Chicago/Turabian StyleWang, Jing, Shuai-Shuai Zhang, Xu Xu, Kai-Xuan Fei, and Yin-Xian Peng. 2018. "A Surface Mediated Supramolecular Chiral Phenomenon for Recognition of l- and d-Cysteine" Nanomaterials 8, no. 12: 1027. https://doi.org/10.3390/nano8121027
APA StyleWang, J., Zhang, S.-S., Xu, X., Fei, K.-X., & Peng, Y.-X. (2018). A Surface Mediated Supramolecular Chiral Phenomenon for Recognition of l- and d-Cysteine. Nanomaterials, 8(12), 1027. https://doi.org/10.3390/nano8121027




