Palygorskite Supported AuPd Alloy Nanoparticles as Efficient Nano-Catalysts for the Reduction of Nitroarenes and Dyes at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Pal-NH2
2.3. Preparation of Pal-NH2@AuPd, Pal-NH2@Au, Pal-NH2@Pd
2.4. Catalytic Reduction of Nitrobenzene
2.5. Preparation of Artificial Wastewater
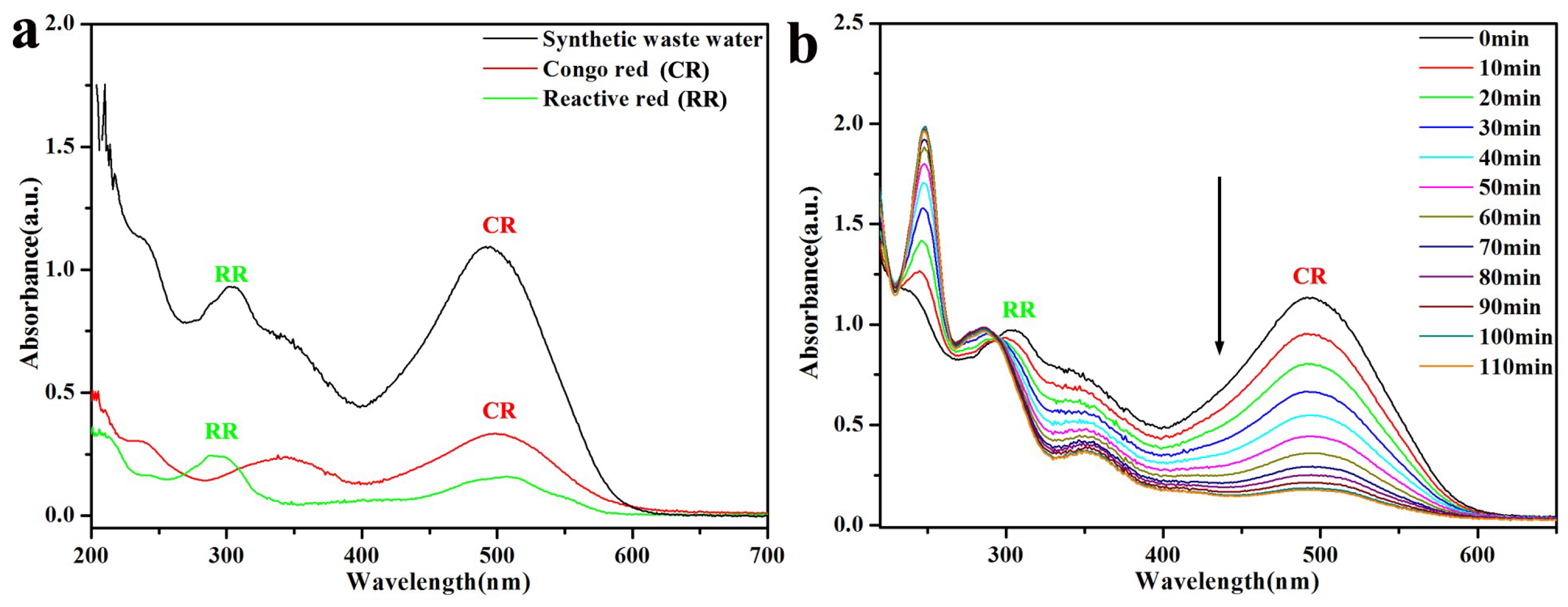
2.6. Treatment of Artificial Wastewater with Pal-NH2@Au48Pd52
2.7. Characterization
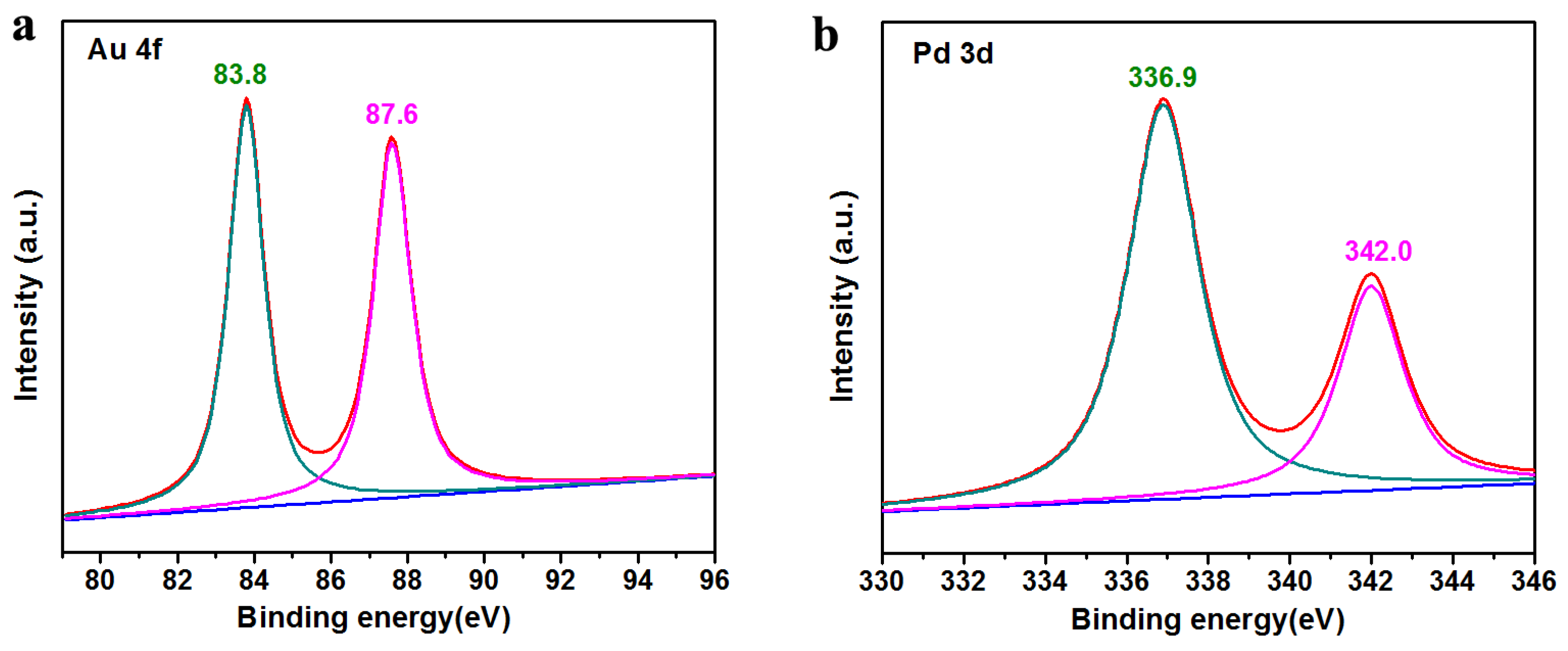
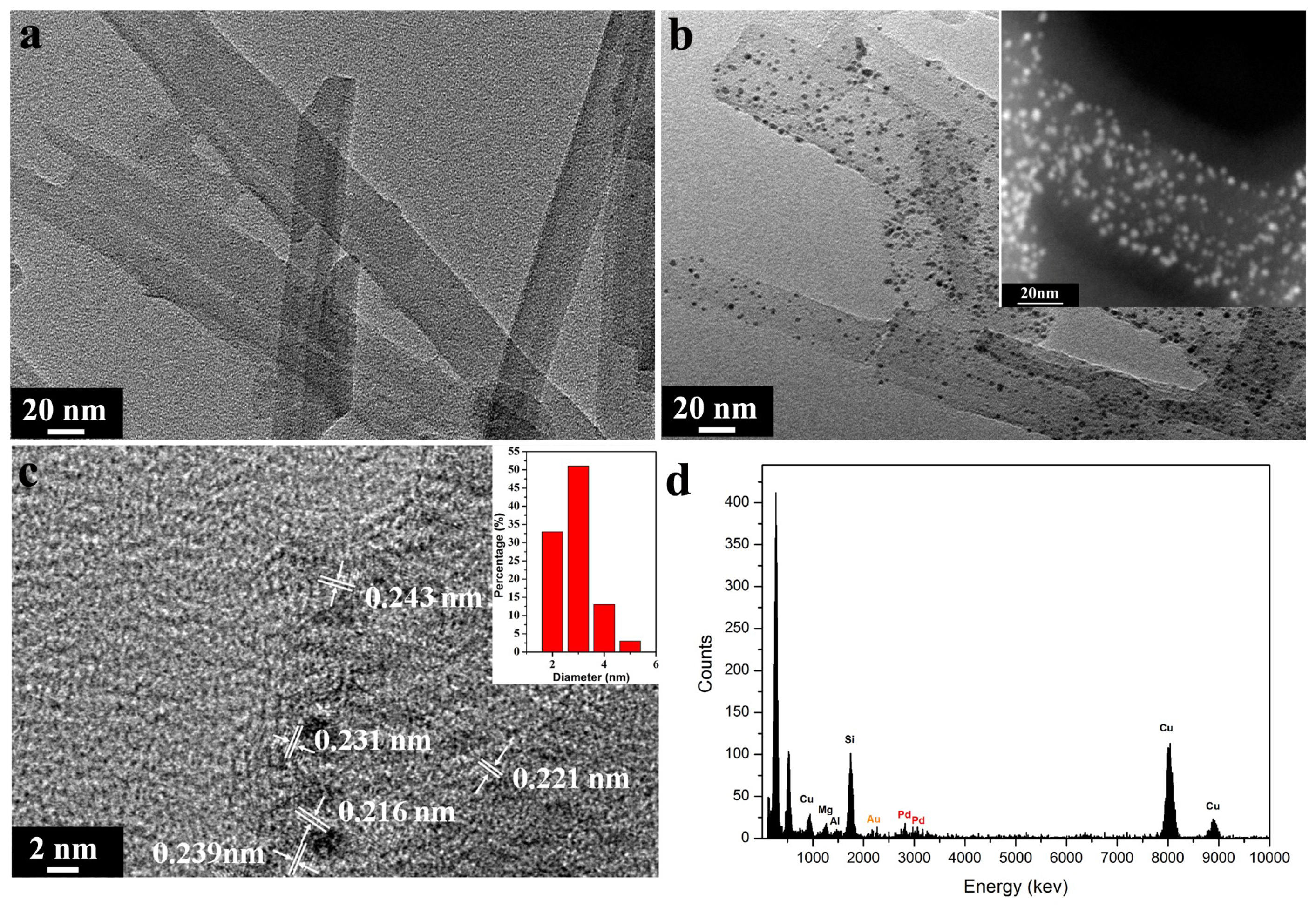
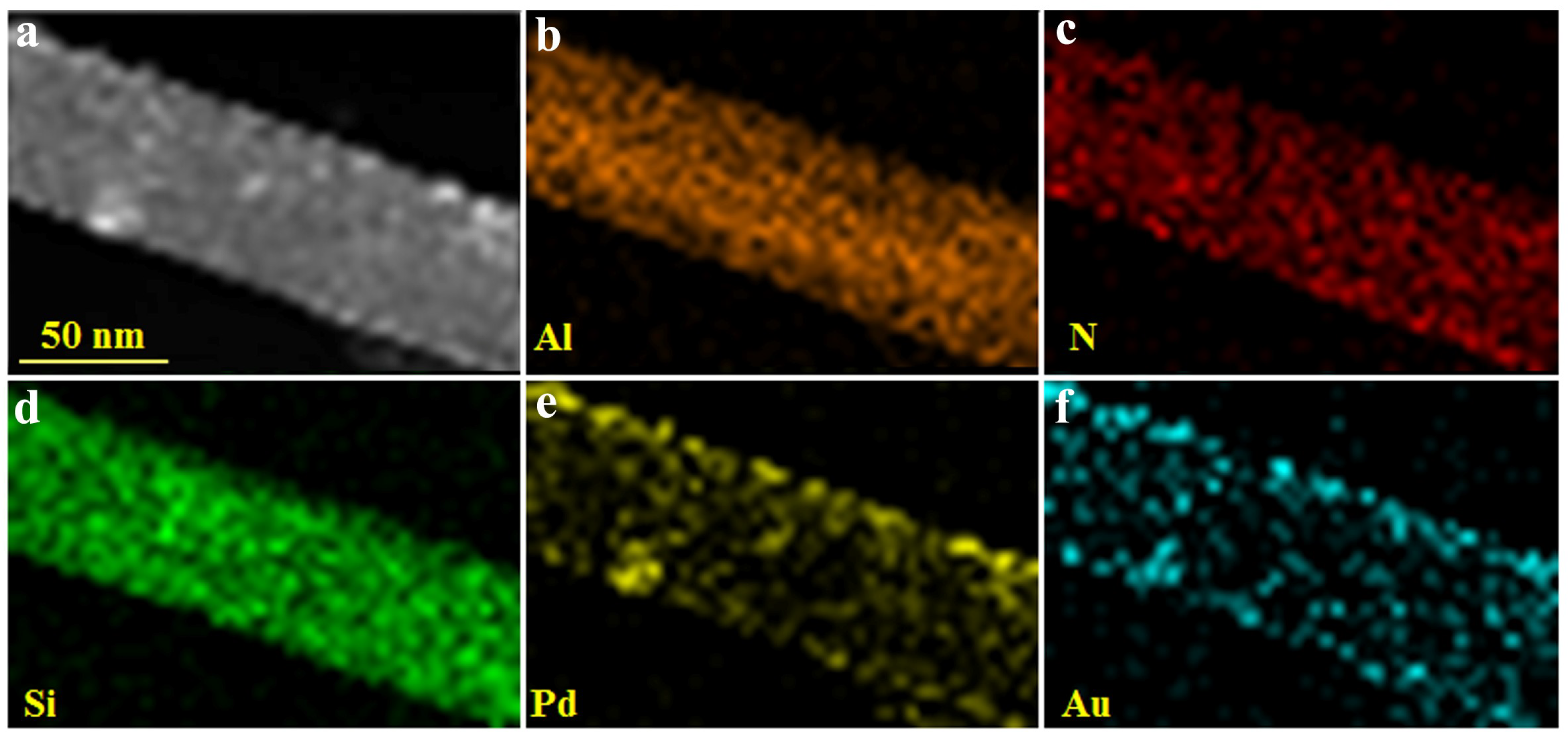
3. Results and Discussion
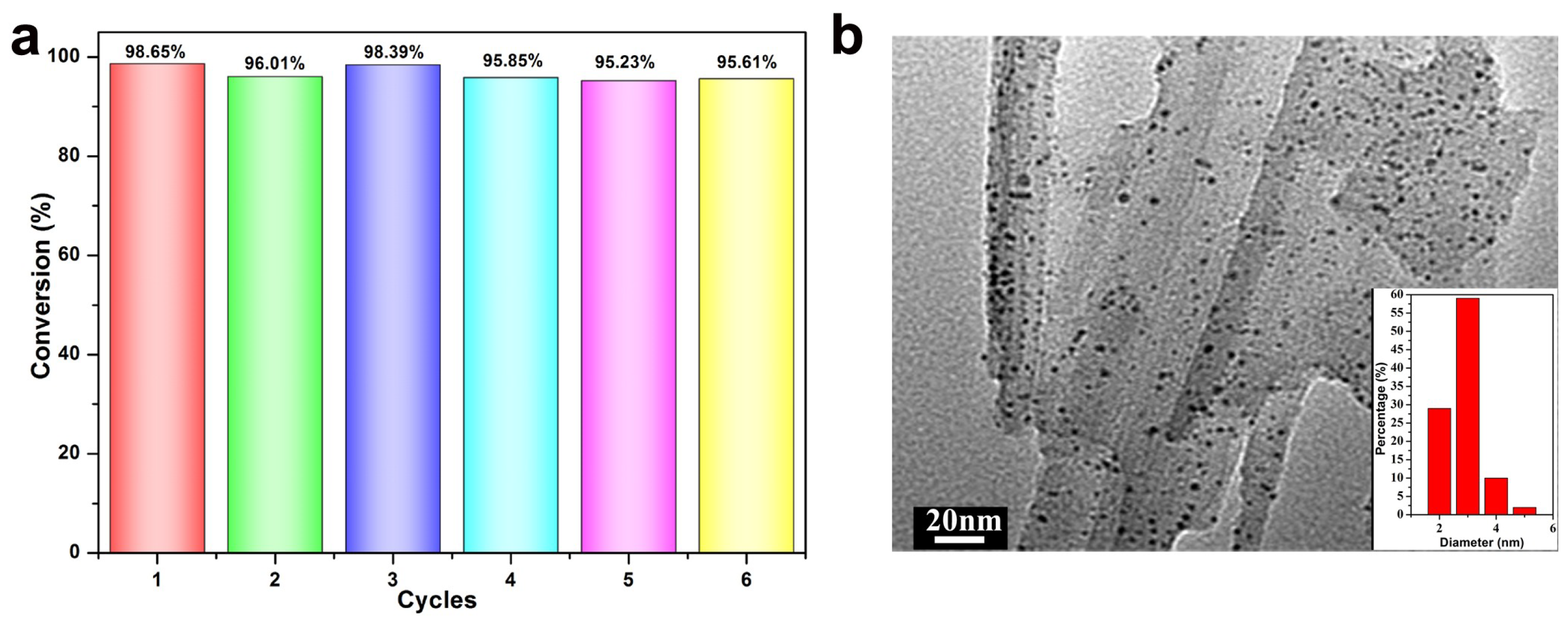
3.1. Structural and Morphology Characterization
3.2. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, A.J.; Cheng, H.Y.; Liang, B.; Ren, N.Q.; Cui, D.; Lin, N.; Kim, B.H.; Rabaey, K. Efficient reduction of nitrobenzene to aniline with a biocatalyzed cathode. Environ. Sci. Technol. 2011, 45, 10186–1019393. [Google Scholar] [CrossRef]
- Wei, W.; Sun, R.; Jin, Z.; Cui, J.; Wei, Z. Hydroxyapatite–gelatin nanocomposite as a novel adsorbent for nitrobenzene removal from aqueous solution. Appl. Surf. Sci. 2014, 292, 1020–1029. [Google Scholar] [CrossRef]
- Wu, Y.G.; Wen, M.; Wu, Q.S.; Fang, H. Ni/graphene nanostructure and its electron-enhanced catalytic action for hydrogenation reaction of nitrophenol. J. Phys. Chem. C 2014, 118, 6307–6313. [Google Scholar] [CrossRef]
- Andreou, D.; Iordanidou, D.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Reduction of nitroarenes into aryl amines and N-aryl hydroxylamines via activation of NaBH4 and ammonia-borane complexes by Ag/TiO2 catalyst. Nanomaterials 2016, 6, 54. [Google Scholar] [CrossRef]
- Noschese, A.; Buonerba, A.; Canton, P.; Milione, S.; Capacchione, C.; Grassi, A. Highly efficient and selective reduction of nitroarenes into anilines catalyzed by gold nanoparticles incarcerated in a nanoporous polymer matrix: Role of the polymeric support and insight into the reaction mechanism. J. Catal. 2016, 340, 30–40. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Zhao, F.; Lan, G.; Li, L.; Liu, Y.; Si, Y.; Jiang, Y.; Yang, B.; Yang, R. Palladium-modified functionalized cyclodextrin as an efficient and recyclable catalyst for reduction of nitroarenes. RSC. Adv. 2016, 6, 7950–7954. [Google Scholar] [CrossRef]
- Shukla, A.; Singha, R.K.; Sasaki, T.; Bal, R. Nanocrystalline Pt-CeO2 as an efficient catalyst for a room temperature selective reduction of nitroarenes. Green Chem. 2015, 17, 785–790. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Gallo, V.; Mastrorilli, P.; Romanazzi, G. A recyclable nanoparticle-supported rhodium catalyst for hydrogenation reactions. Molecules 2010, 15, 3311–3318. [Google Scholar] [CrossRef]
- Astruc, D. Palladium nanoparticles as efficient green homogeneous and heterogeneous carbon−carbon coupling precatalysts: A unifying view. Inorg. Chem. 2007, 46, 1884–1894. [Google Scholar] [CrossRef]
- Polavarapu, L.; Mourdikoudis, S.; Pastoriza-Santos, I.; Pérez-Juste, J. Nanocrystal engineering of noble metals and metal chalcogenides: controlling the morphology, composition and crystallinity. Cryst. Eng. Comm. 2015, 17, 3727–3762. [Google Scholar] [CrossRef]
- Filice, M.; Marciello, M.; Morales, M.P.; Palomo, J.M. Synthesis of heterogeneous enzyme–metal nanoparticle biohybrids in aqueous media and their applications in C–C bond formation and tandem catalysis. Chem. Commun. 2013, 49, 6876–6878. [Google Scholar] [CrossRef]
- Dutta, S.; Sarkar, S.; Ray, C.; Roy, A.; Sahoo, R.; Pal, T. Mesoporous gold and palladium nanoleaves from liquid–liquid interface: enhanced catalytic activity of the palladium analogue toward hydrazine-assisted room-temperature 4-nitrophenol reduction. ACS Appl. Mater. Interfaces 2014, 6, 9134–9143. [Google Scholar] [CrossRef]
- Wang, Z.M.; Xu, C.L.; Gao, G.Q.; Li, X. Facile synthesis of well-dispersed Pd-graphene nanohybrids and their catalytic properties in 4-nitrophenol reduction. RSC Adv. 2014, 4, 13644–13651. [Google Scholar] [CrossRef]
- Wang, C.Y.; Boucher, M.; Yang, M.; Saltsburg, H.; Flytzani-Stephanopoulos, M. ZnO-modified zirconia as gold catalyst support for the low-temperature methanol steam reforming reaction. Appl. Catal. B Environ. 2014, 154–155, 142–152. [Google Scholar] [CrossRef]
- Bychkov, V.Y.; Tyulenin, Y.P.; Gorenberg, A.Y.; Sokolov, S.; Korchak, V. Evolution of Pd catalyst structure and activity during catalytic oxidation of methane and ethane. Appl. Catal. A Gen. 2014, 485, 1–9. [Google Scholar] [CrossRef]
- Zheng, G.; Kaefer, K.; Mourdikoudis, S.; Polavarapu, L.; Vaz, B.; Cartmell, S.E.; Bouleghlimat, A.; Buurma, N.J.; Yate, L.; de Lera, Á.R.; et al. Palladium nanoparticle-loaded cellulose paper: A highly efficient, robust, and recyclable self-assembled composite catalytic system. J. Phys. Chem. Lett. 2015, 6, 230–238. [Google Scholar] [CrossRef]
- Le, X.; Dong, Z.; Li, X.; Zhang, W.; Le, M.; Ma, J. Fibrous nano-silica supported palladium nanoparticles: An efficient catalyst for the reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol under mild conditions. Catal. Commun. 2015, 59, 21–25. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Feng, C.; Gao, S.; Shang, N.; Wang, Z. Multifunctional Pd@MOF core–shell nanocomposite as highly active catalyst for p-nitrophenol reduction. Catal. Commun. 2015, 72, 29–32. [Google Scholar] [CrossRef]
- Notar, F.I.; Fontaine-Vive, F.; Antoniotti, S. Synergy in the catalytic activity of bimetallic nanoparticles and new synthetic methods for the preparation of fine chemicals. ChemCatChem 2014, 6, 2784–2791. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Chirea, M.; Zanaga, D.; Altantzis, T.; Mitrakas, M.; Bals, S.; Liz-Marzán, L.M.; Pérez-Juste, J.; Pastoriza-Santos, I. Governing the morphology of Pt–Au heteronanocrystals with improved electrocatalytic performance. Nanoscale 2015, 7, 8739–8747. [Google Scholar] [CrossRef]
- Hirasawa, S.; Watanabe, H.; Kizuka, T.; Nakagawa, Y.; Tomishige, K. Performance, structure and mechanism of Pd–Ag alloy catalyst for selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2013, 300, 205–216. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Liu, C. Perspective on CO oxidation over Pd-based catalysts. Catal. Sci. Technol. 2015, 5, 69–81. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, T.; Xu, J.; Xu, Z.; Li, H.; Liu, B.; Sun, J.; Cao, J.; Shen, X.; Li, X. Synthesis and characterization of PdRu alloy-coated palygorskite-based nanocomposites as a magnetically recyclable multifunctional catalyst for reduction of nitroarenes and azo dyes. Mater. Lett. 2017, 197, 24–27. [Google Scholar] [CrossRef]
- Hosseini, H.; Mahyari, M.; Bagheri, A.; Shaabani, A. Pd and PdCo alloy nanoparticles supported on polypropylenimine dendrimer-grafted graphene: A highly efficient anodic catalyst for direct formic acid fuel cells. J. Power Sources 2014, 247, 70–77. [Google Scholar] [CrossRef]
- Karatas, Y.; Bulut, A.; Yurderi, M.; Ertas, I.E.; Alal, O.; Gulcan, M. PdAu-MnOx nanoparticles supported on amine-functionalized SiO2 for the room temperature dehydrogenation of formic acid in the absence of additives. Appl. Catal. B Environ. 2016, 180, 586–595. [Google Scholar] [CrossRef]
- Su, B.; Shao, H.; Li, N.; Chen, X.; Cai, Z.; Chen, X. A sensitive bisphenol A voltammetric sensor relying on AuPd nanoparticles/graphene composites modified glassy carbon electrode. Talanta 2017, 166, 126–132. [Google Scholar] [CrossRef]
- Fageria, P.; Uppala, S.; Nazir, R.; Gangopadhyay, S.; Chang, C.H.; Basu, M.; Pande, S. Synthesis of Monometallic (Au and Pd) and Bimetallic (AuPd) Nanoparticles Using Carbon Nitride (C3N4) Quantum Dots via the Photochemical Route for Nitrophenol Reduction. Langmuir 2016, 32, 10054–10064. [Google Scholar] [CrossRef]
- Yang, X.; Pachfule, P.; Chen, Y.; Tsumori, N.; Xu, Q. Highly efficient hydrogen generation from formic acid using a reduced graphene oxide-supported AuPd nanoparticle catalyst. Chem. Commun. 2016, 52, 4171–4174. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, W.; Xie, A.; Wu, F.; Yao, C.; Li, X.; et al. Effect of MnO2 polymorphs structure on the selective catalytic reduction of NOx with NH3 over TiO2–Palygorskite. Chem. Eng. J. 2016, 286, 291–299. [Google Scholar] [CrossRef]
- Lu, Z.; Hao, Z.; Wang, J.; Chen, L. Efficient removal of europium from aqueous solutions using attapulgite-iron oxide magnetic composites. J. Ind. Eng. Chem. 2016, 34, 374–381. [Google Scholar] [CrossRef]
- Bulut, A.; Yurderi, M.; Karatas, Y.; Zahmakiran, M.; Kivrak, H.; Gulcan, M.; Kaya, M. Pd-MnOx nanoparticles dispersed on amine-grafted silica: highly efficient nano-catalyst for hydrogen production from additive-free dehydrogenation of formic acid under mild conditions. Appl. Catal. B Environ. 2015, 164, 324–333. [Google Scholar] [CrossRef]
- Sahoo, A.; Tripathy, S.K.; Dehury, N.; Patra, S. A porous trimetallic Au@Pd@Ru nanoparticle system: Synthesis, characterisation and efficient dye degradation and removal. J. Mater. Chem. A 2015, 3, 19376–19383. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, C.; Lu, J.; Liu, H.; Huang, L.; Chen, T.; Chen, D. Catalytic degradation of gaseous benzene by using TiO2/goethite immobilized on palygorskite: Preparation, characterization and mechanism. Solid. State Sci. 2015, 49, 1–9. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Hao, Q.; Duan, H. Nanoporous PdNi alloys as highly active and methanol-tolerant electrocatalysts towards oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 13542–13548. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Guo, D.Z.; Xing, Y.J.; Zhang, G.M. Fabrication of open-ended TiO2 nanotube arrays by anodizing a thermally evaporated Ti/Au bilayer film. Appl. Surf. Sci. 2011, 257, 4139–4143. [Google Scholar] [CrossRef]
- Qin, Y.H.; Li, Y.; Lv, R.L.; Wang, T.L.; Wang, W.G.; Wang, C.W. Pd-Au/C catalysts with different alloying degrees for ethanol oxidation in alkaline media. Electrochim. Acta 2014, 144, 50–55. [Google Scholar] [CrossRef]
- Han, C.; Wu, L.; Ge, L.; Li, Y.; Zhao, Z. AuPd bimetallic nanoparticles decorated graphitic carbon nitride for highly efficient reduction of water to H2 under visible light irradiation. Carbon 2015, 92, 31–34. [Google Scholar] [CrossRef]
- Xu, L.; Yao, F.; Luo, J.; Wan, C.; Ye, M.; Cui, P.; An, Y. Facile synthesis of amine-functionalized SBA-15-supported bimetallic Au–Pd nanoparticles as an efficient catalyst for hydrogen generation from formic acid. RSC Adv. 2017, 7, 4746–4752. [Google Scholar] [CrossRef]
- Li, S.J.; Ping, Y.; Yan, J.M.; Wang, H.L.; Wu, M.; Jiang, Q. Facile synthesis of AgAuPd/graphene with high performance for hydrogen generation from formic acid. J. Mater. Chem. A 2015, 3, 14535–14538. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yan, J.M.; Wang, H.L.; Ping, Y.; Jiang, Q. Au@Pd core-shell nanoclusters growing on nitrogen-doped mildly reduced graphene oxide with enhanced catalytic performance for hydrogen generation from formic acid. J. Mater. Chem. A 2013, 1, 12721–12725. [Google Scholar] [CrossRef]
- Wu, S.; Yang, F.; Wang, H.; Chen, R.; Sun, P.C.; Chen, T.H. Mg(II)-assisted low temperature reduction of alloyed AuPd/C: an efficient catalyst for hydrogen generation from formic acid at room temperature. Chem. Commun. 2015, 51, 10887–10890. [Google Scholar] [CrossRef]
- Zelekew, O.A.; Kuo, D.H. A two-oxide nanodiode system made of double-layered p-type Ag2O@n-type TiO2 for rapid reduction of 4-nitrophenol. Phys. Chem. Chem. Phys. 2016, 18, 4405–4414. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, S.; Shang, N.; Wang, C.; Wang, Z. Copper ferrite–graphene hybrid: A highly efficient magnetic catalyst for chemoselective reduction of nitroarenes. RSC. Adv. 2014, 4, 31328–31332. [Google Scholar] [CrossRef]
- Liu, L.; Chen, R.; Liu, W.; Wu, J.; Gao, D. Catalytic reduction of 4-nitrophenol over Ni-Pd nanodimers supported on nitrogen-doped reduced graphene oxide. J. Hazard. Mater. 2016, 320, 96–104. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Z.; Wang, Y.; Yang, C.; Li, Y. Facile fabrication of polystyrene microsphere supported gold-palladium alloy nanoparticles with superior catalytic performance for the reduction of 4-nitrophenol in water. Colloid. Surf. A 2017, 529, 417–424. [Google Scholar] [CrossRef]
- Srisombat, L.; Nonkumwong, J.; Suwannarat, K.; Kuntalue, B.; Ananta, S. Simple preparation Au/Pd Core/Shell nanoparticles for 4-Nitrophenol reduction. Colloid Surf. A 2016, 512, 17–25. [Google Scholar] [CrossRef]
- Zhao, R.; Gong, M.; Zhu, H.; Chen, Y.; Tang, Y.; Lu, T. Seed-assisted synthesis of Pd@Au core–shell nanotetrapods and their optical and catalytic properties. Nanoscale 2014, 6, 9273–9278. [Google Scholar] [CrossRef]
- Jiang, F.; Li, R.; Cai, J.; Xu, W.; Cao, A.; Chen, D.; et al. Ultrasmall Pd/Au bimetallic nanocrystals embedded in hydrogen-bonded supramolecular structures: Facile synthesis and catalytic activities in the reduction of 4-nitrophenol. J. Mater. Chem. A 2015, 3, 19433–19438. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Z.; Chen, X.; Oyama, M. AuPd bimetallic nanoparticles decorated on graphene nanosheets: their green synthesis, growth mechanism and high catalytic ability in 4-nitrophenol reduction. J. Mater. Chem. A 2014, 2, 5668–5674. [Google Scholar] [CrossRef]


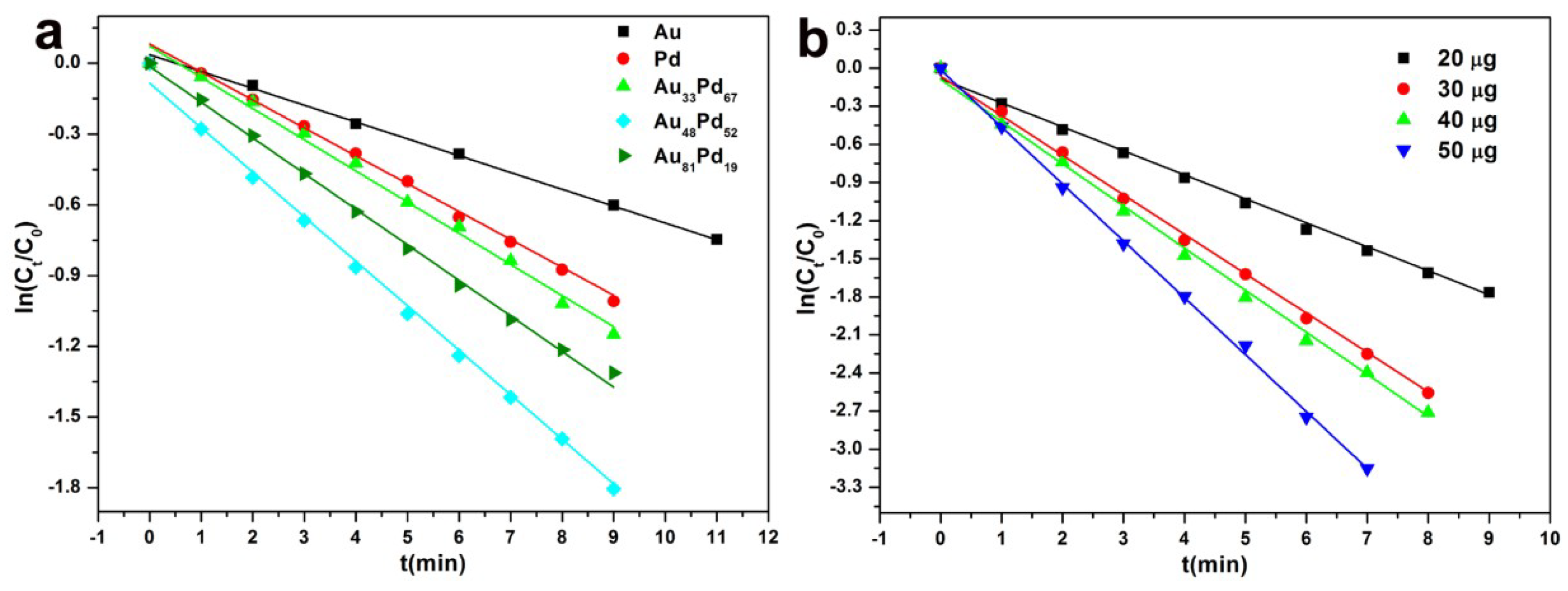

| Catalysts | Au (wt%) | Pd (wt%) | K (Min−1) | TOF (Min−1) |
|---|---|---|---|---|
| Au | 3.94 | 0 | 0.021 | 4.25 |
| Pd | 0 | 2.12 | 0.071 | 7.08 |
| Au33Pd67 | 1.32 | 1.41 | 0.132 | 12.39 |
| Au48Pd52 | 1.81 | 1.10 | 0.194 | 16.51 |
| Au81Pd19 | 3.19 | 0.40 | 0.102 | 10.11 |
| Catalysts | kapp (min−1) | (nAu + nPd)/n4-NP | Turnover Number (TON) | K (min−1) | TOF (min−1) | Reference |
|---|---|---|---|---|---|---|
| Pal-NH2@Au48Pd52 | 0.194 | 0.0047 | 212.77 | 45.116 | 11.82 | Here |
| Au1Pd4 core/shell | 0.39 | 15.00 | 0.037 | 0.078 | 3 | [43] |
| Pd@Au core-shell nanotetrapods | 0.139 | 0.380 | 2.63 | 0.366 | 0.035 | [44] |
| Melamine cyanurate-Pd/Au | 0.280 | 0.0364 | 27.41 | 7.692 | 0.036 | [45] |
| Au-on-Pd heteronanostructure | 0.867 | 0.16 | 33.33 | 5.419 | 9.52 | [46] |
| Compound | Time/min | Conversion/% | Amount of Catalyst/μg | TOF (min−1) |
|---|---|---|---|---|
| p-Nitroaniline | 20 | 99 | 20 | 9.88 |
| m-Nitroaniline | 10 | 99 | 20 | 19.77 |
| o-Nitroaniline | 5 | 99 | 20 | 39.54 |
| 2,4-Nitroaniline | 19 | 99 | 20 | 10.40 |
| m-Nitrotoluene | 74 | 79 | 20 | 2.11 |
| o-Nitrotoluene | 86 | 85 | 20 | 1.95 |
| 2,4-Dinitrotoluene | 97 | 89 | 20 | 1.81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Guo, S.; Jia, L.; Zhang, W. Palygorskite Supported AuPd Alloy Nanoparticles as Efficient Nano-Catalysts for the Reduction of Nitroarenes and Dyes at Room Temperature. Nanomaterials 2018, 8, 1000. https://doi.org/10.3390/nano8121000
Xu J, Guo S, Jia L, Zhang W. Palygorskite Supported AuPd Alloy Nanoparticles as Efficient Nano-Catalysts for the Reduction of Nitroarenes and Dyes at Room Temperature. Nanomaterials. 2018; 8(12):1000. https://doi.org/10.3390/nano8121000
Chicago/Turabian StyleXu, Jun, Shengli Guo, Lei Jia, and Wensheng Zhang. 2018. "Palygorskite Supported AuPd Alloy Nanoparticles as Efficient Nano-Catalysts for the Reduction of Nitroarenes and Dyes at Room Temperature" Nanomaterials 8, no. 12: 1000. https://doi.org/10.3390/nano8121000
APA StyleXu, J., Guo, S., Jia, L., & Zhang, W. (2018). Palygorskite Supported AuPd Alloy Nanoparticles as Efficient Nano-Catalysts for the Reduction of Nitroarenes and Dyes at Room Temperature. Nanomaterials, 8(12), 1000. https://doi.org/10.3390/nano8121000




