Effect of Aminosilane Coupling Agents with Different Chain Lengths on Thermo-Mechanical Properties of Cross-Linked Epoxy Resin
Abstract
1. Introduction
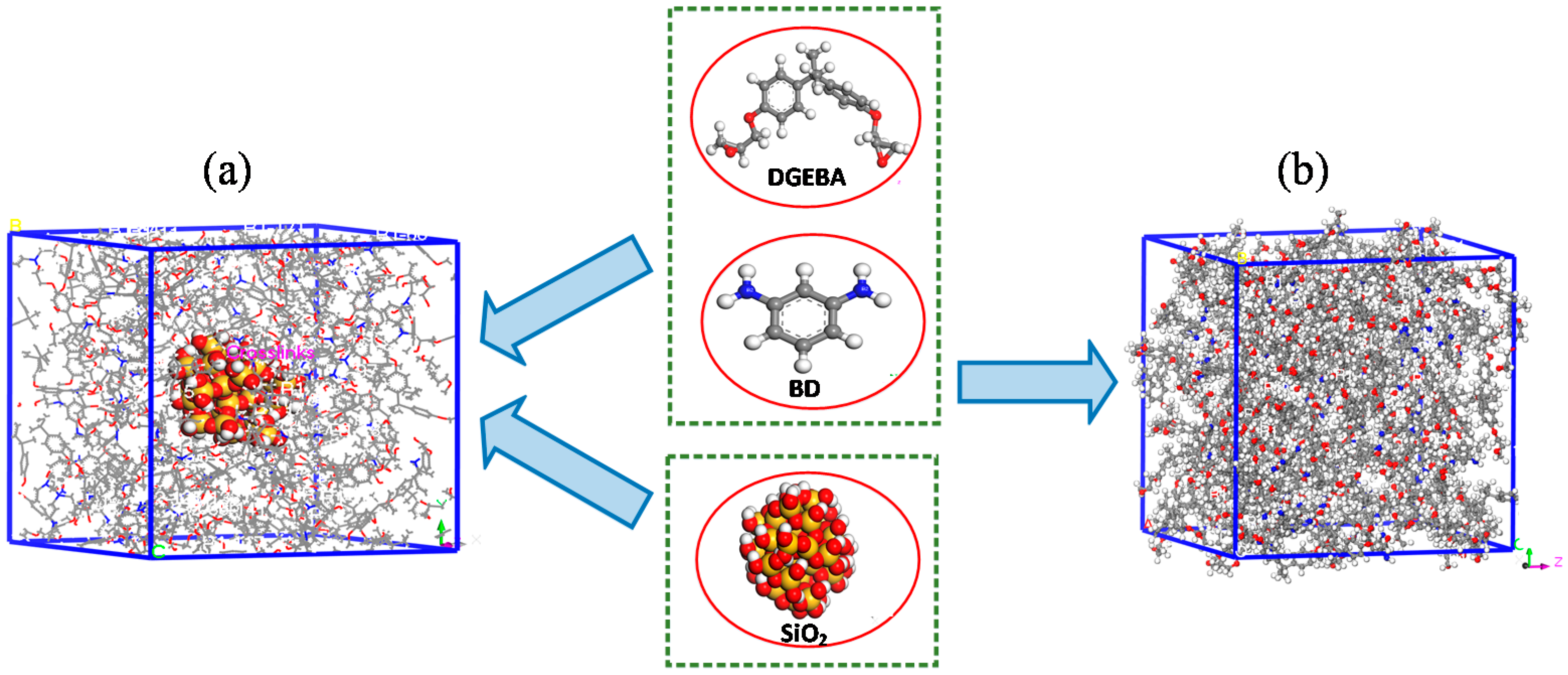
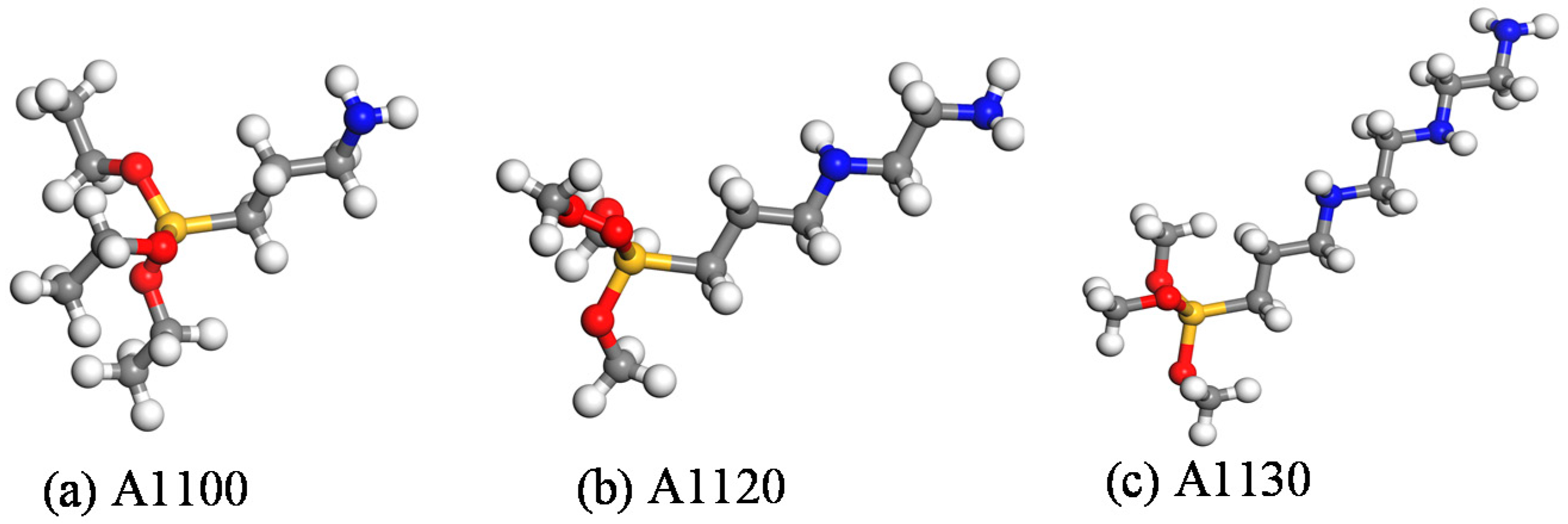
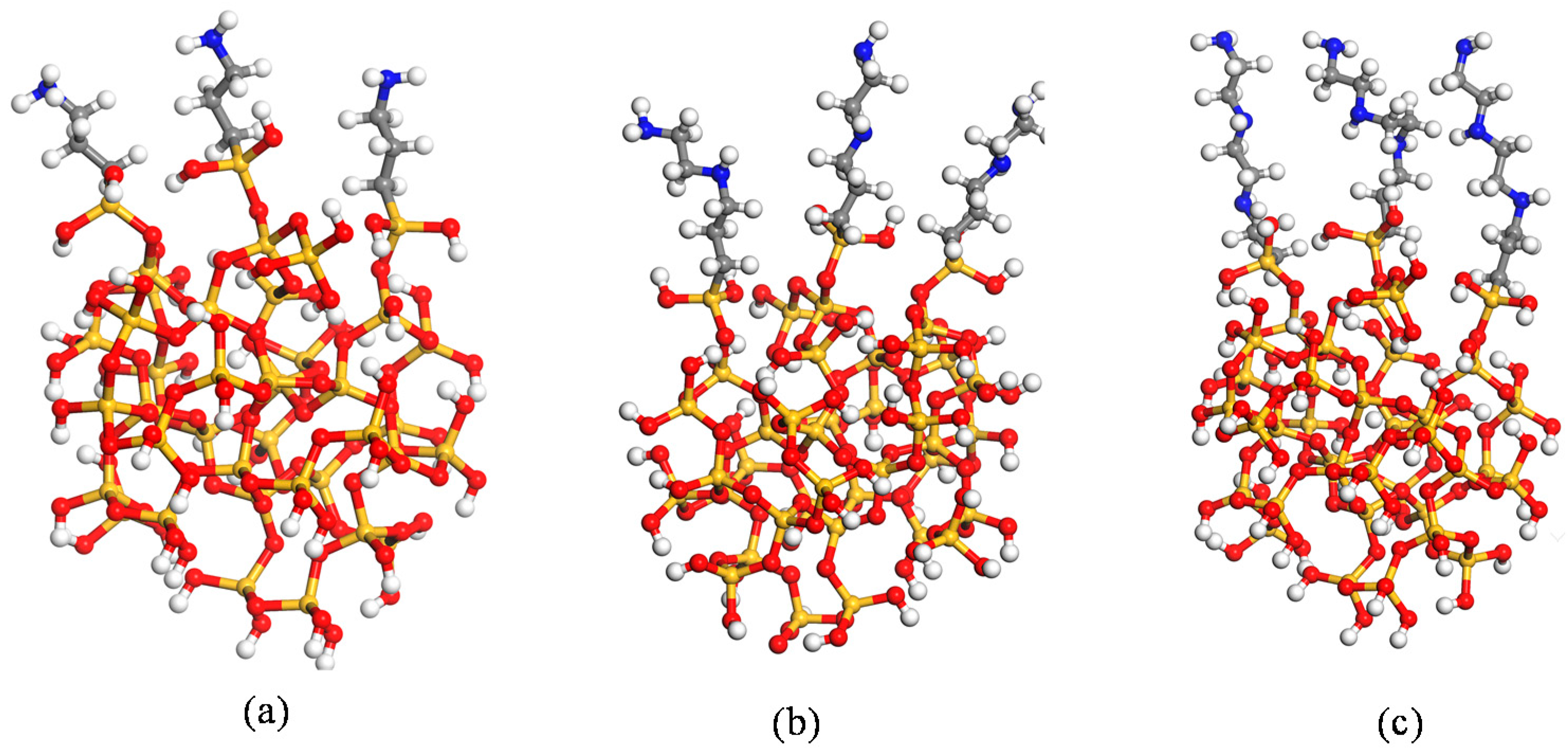
2. Materials and Methods
3. Results
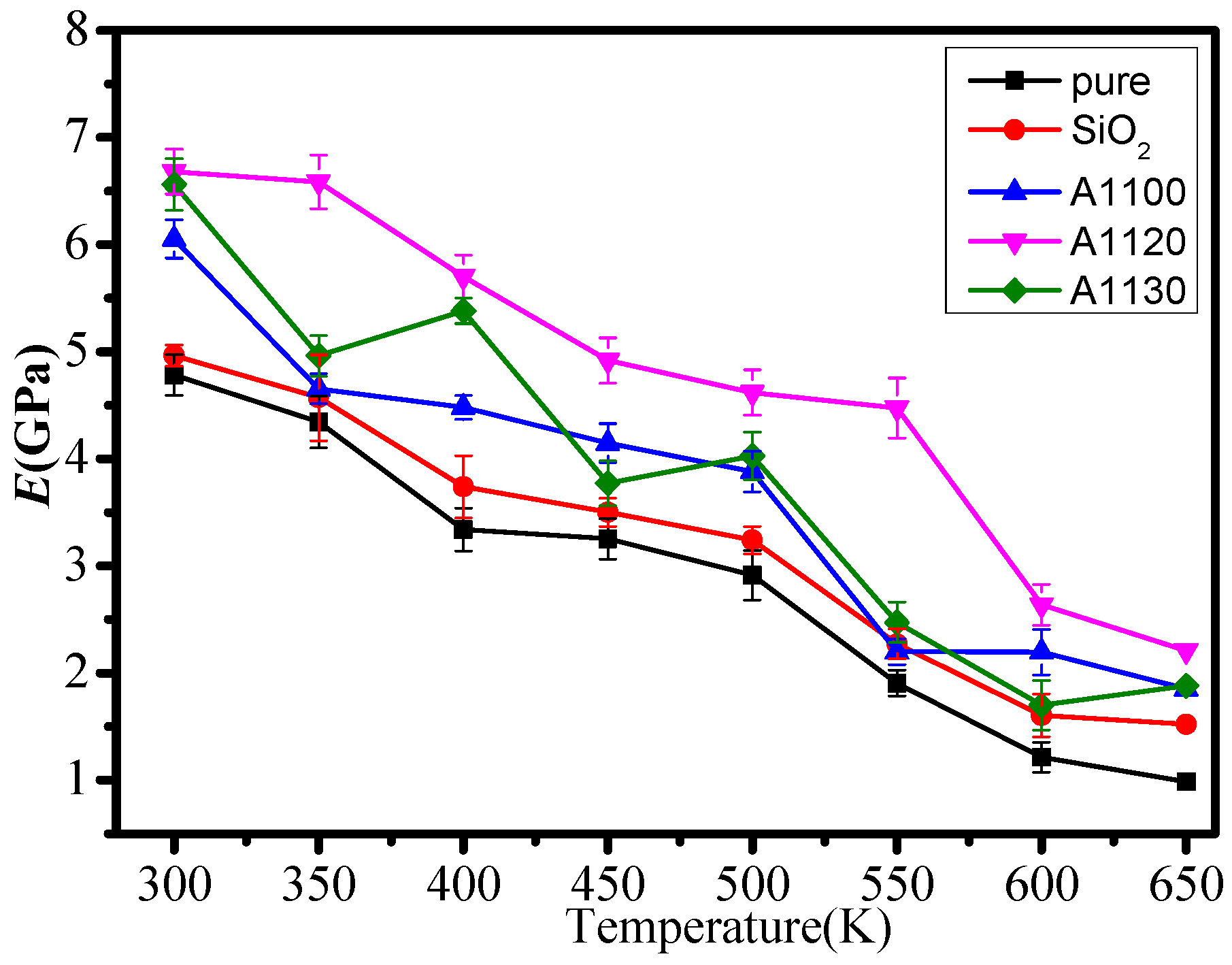
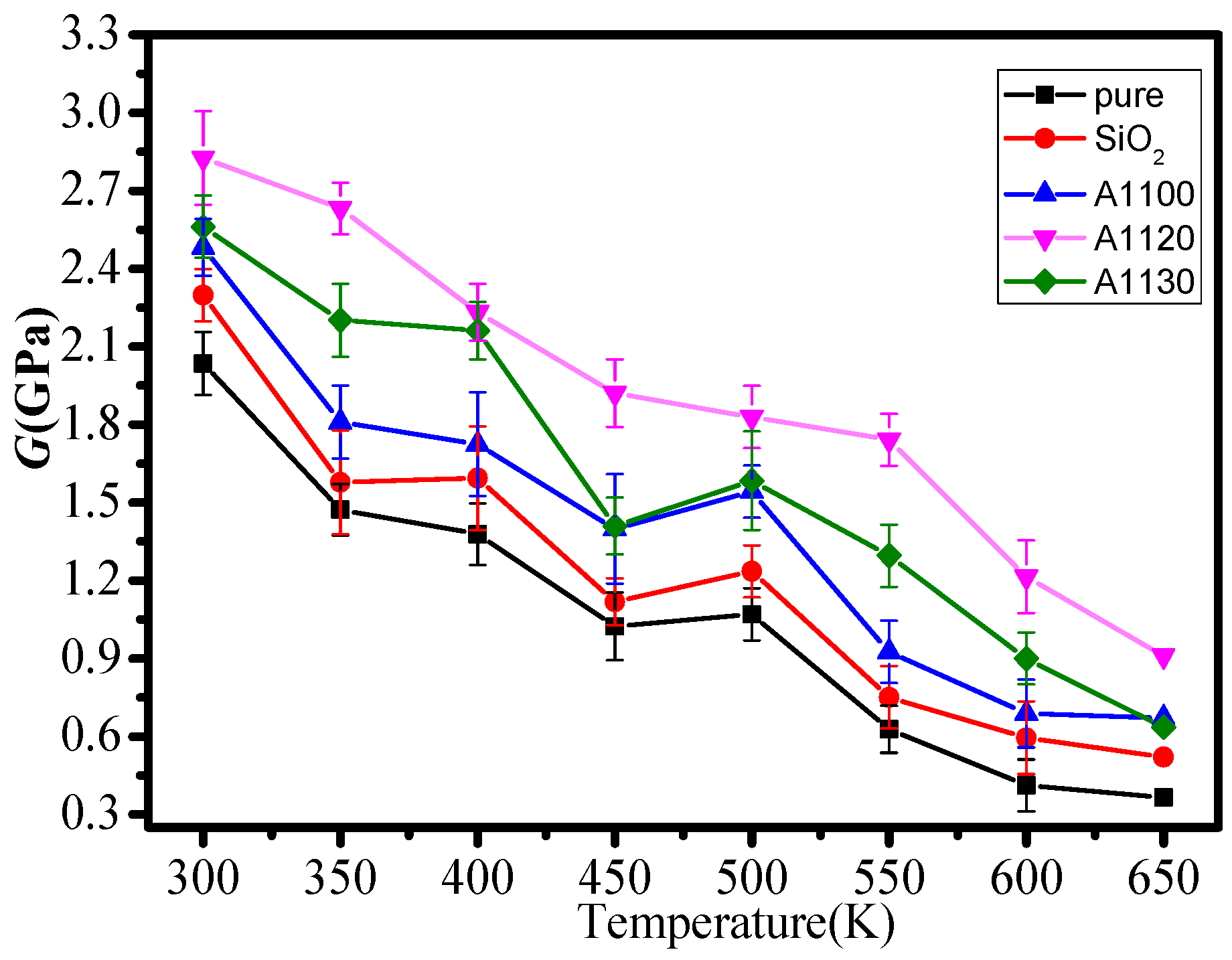
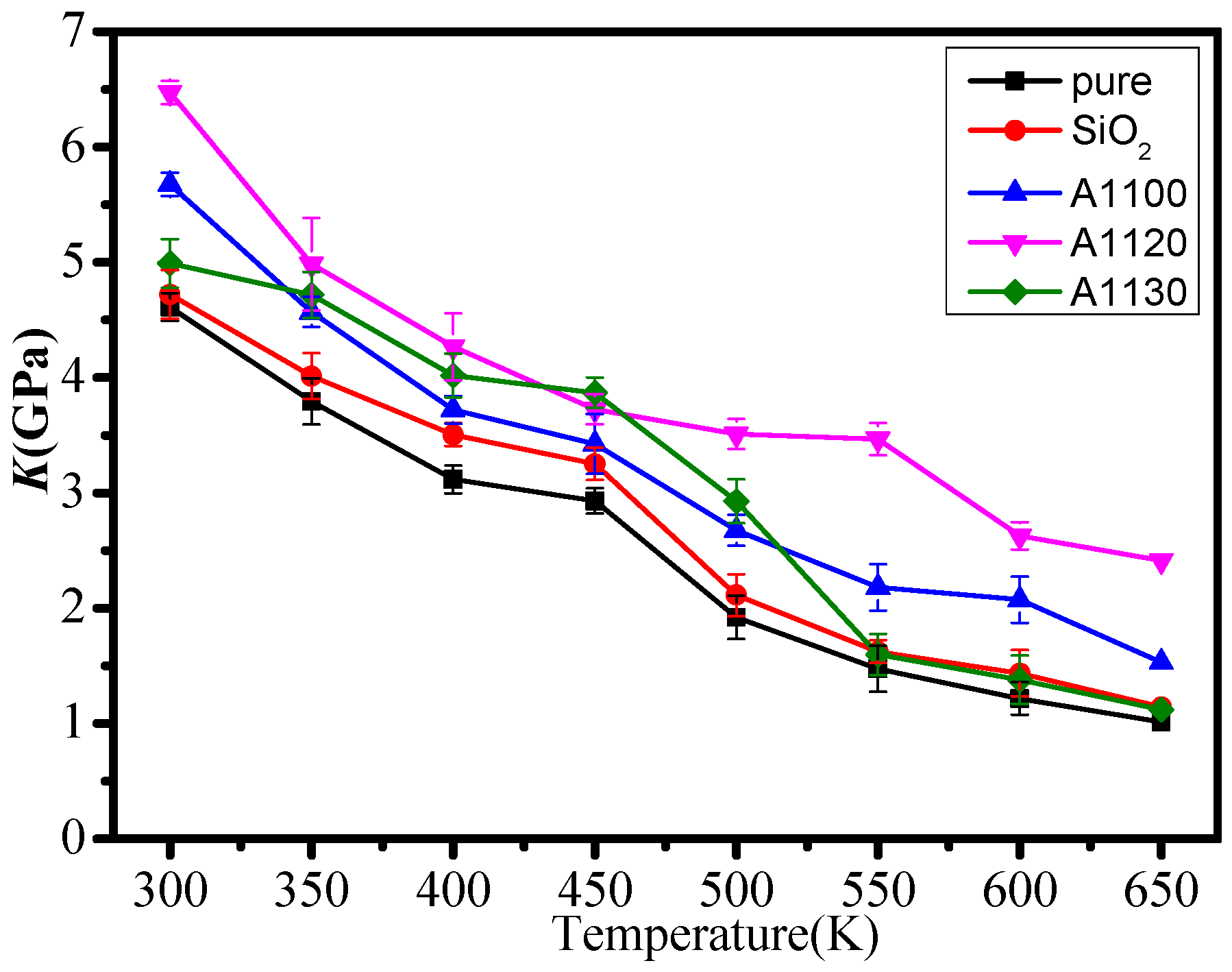
3.1. Mechanical Properties
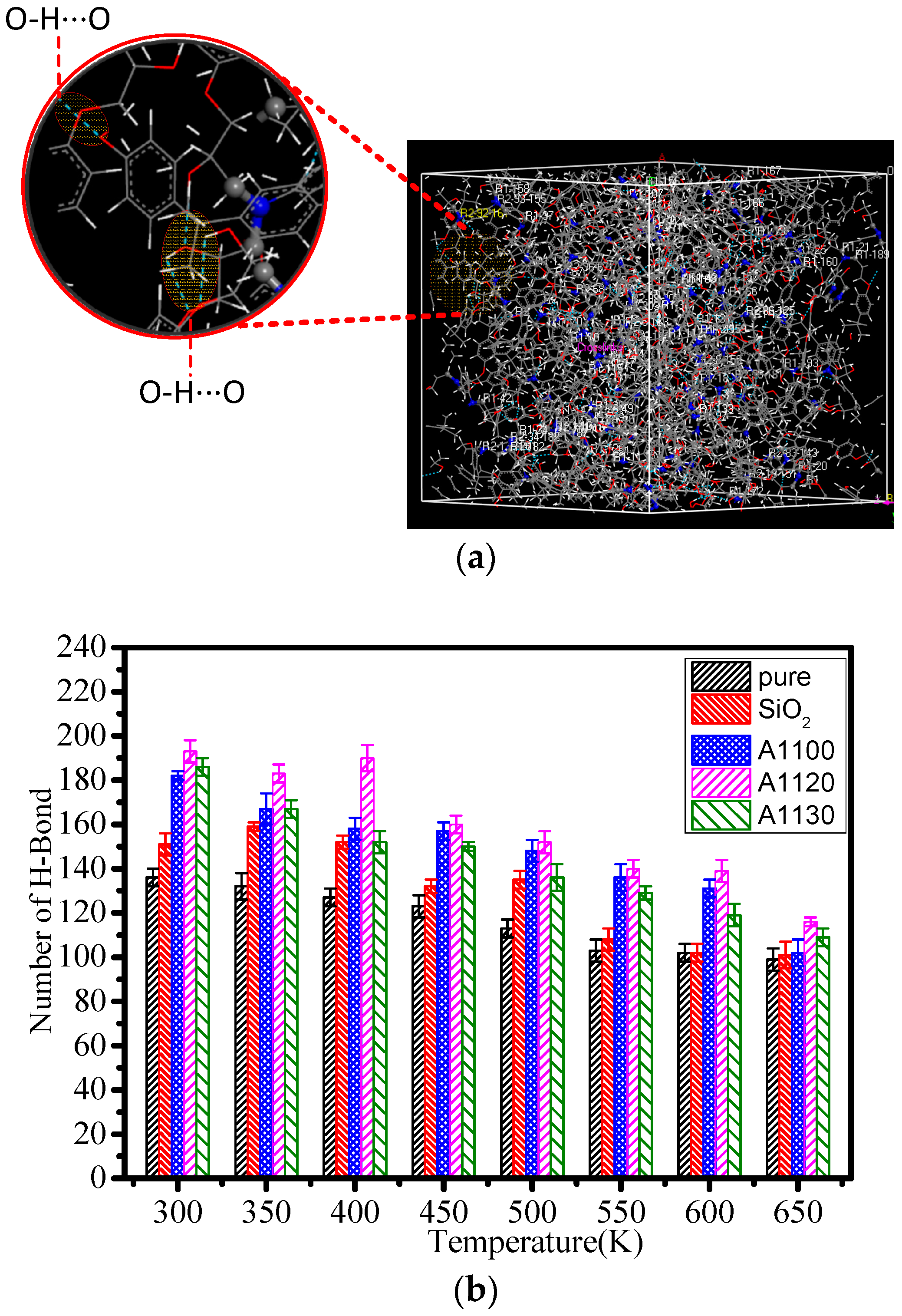
3.2. Hydrogen Bond Calculation
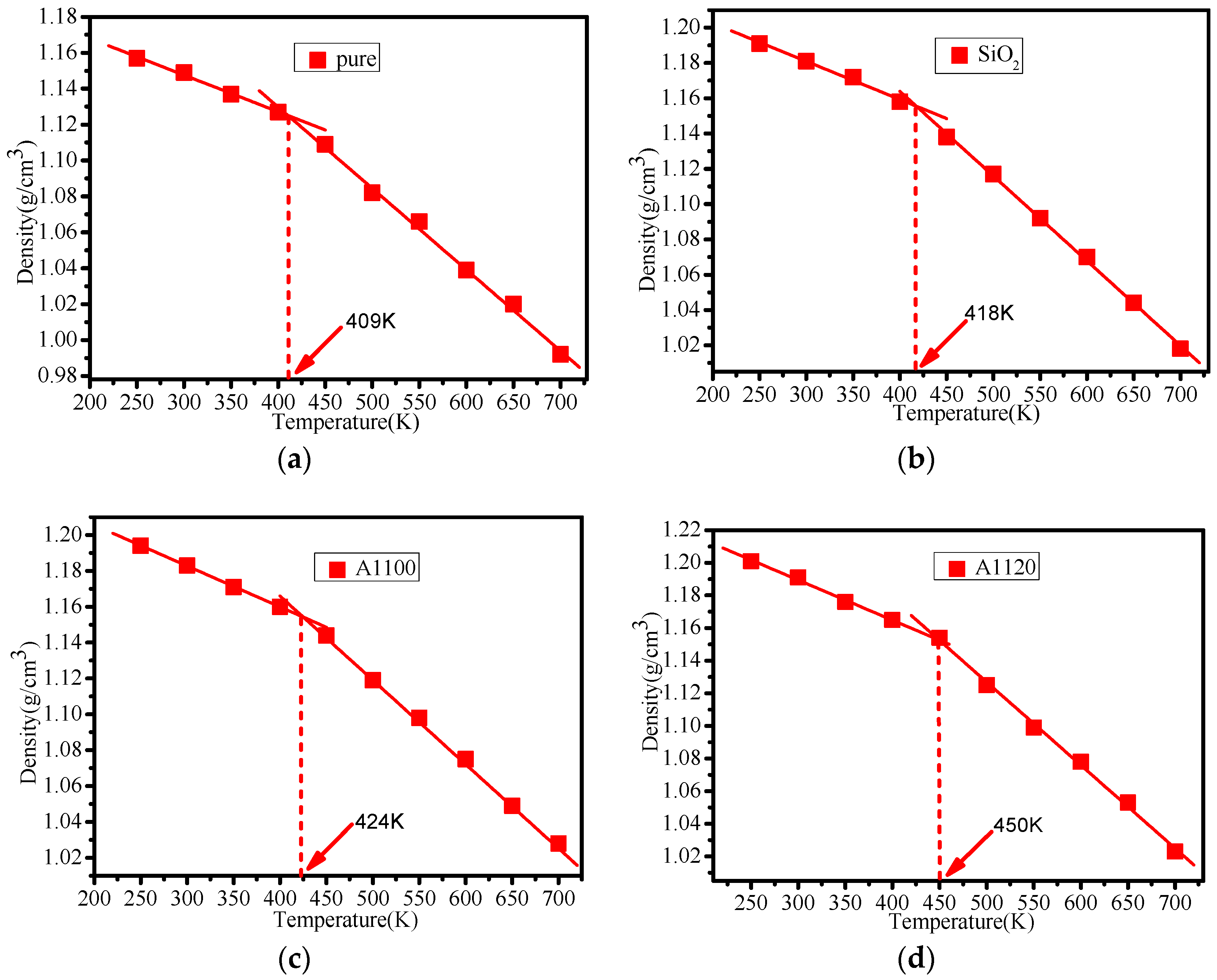
3.3. Glass Transition Temperature Analysis
3.4. Free Volume Calculation
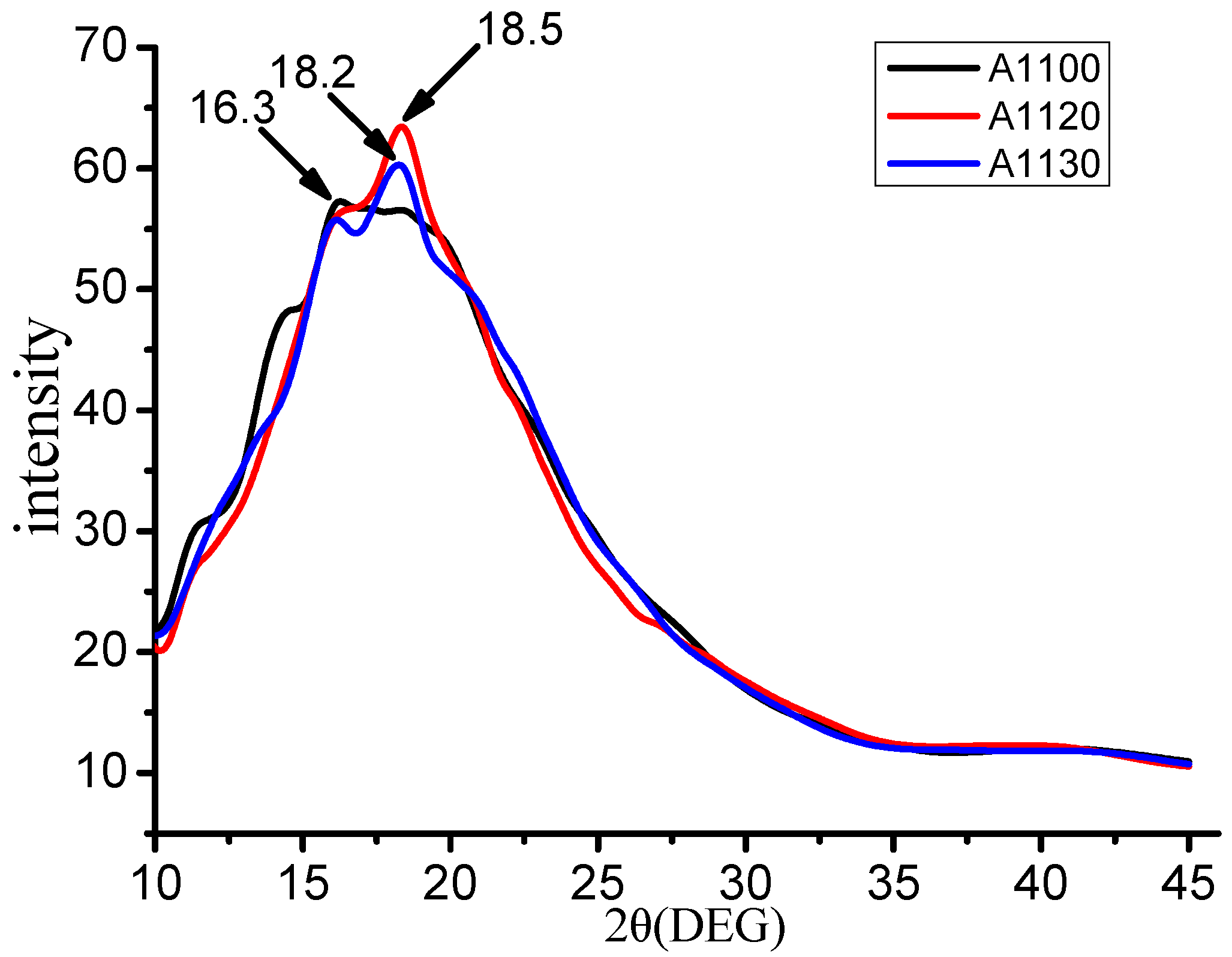
3.5. Polymers Chain Spacing Calculation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Wang, S.; Wang, Q.; Xing, M. Enhancement of fracture properties of polymer composites reinforced by carbon nanotubes: A molecular dynamics study. Carbon 2018, 129, 504–509. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Li, L.; Guo, Z.H.; Peng, Z.R.; Liu, J.Y. Effects of silane coupling agent treatment on properties of SiO2/epoxy nanocomposites. Insul. Mater. 2017, 50, 1–8. [Google Scholar]
- Yang, Q.; Lan, F.T.; He, Z.W.; Liu, H.; Chen, X. Molecular simulation study on effects of nano-titanium. Insul. Mater. 2015, 48, 40–43. [Google Scholar]
- Bray, D.J.; Dittanet, P.; Guild, F.J.; Kinloch, A.J.; Masania, K.; Pearson, R.A.; Taylor, A.C. The modelling of the toughening of epoxy polymers via silica nanoparticles: The effects of volume fraction and particle size. Polymer 2013, 54, 7022–7032. [Google Scholar] [CrossRef]
- Tang, L.C.; Zhang, H.; Sprenger, S.; Ye, L.; Zhang, Z. Fracture mechanisms of epoxy-based ternary composites filled with rigid-soft particles. Compos. Sci. Technol. 2012, 72, 558–565. [Google Scholar] [CrossRef]
- Blackman, B.R.K.; Kinloch, A.J.; Lee, J.S.; Taylor, A.C.; Agarwal, R.; Schueneman, G.; Sprenger, S. The fracture and fatigue behaviour of nano-modified epoxy polymers. J. Mater. Sci. 2007, 42, 7049–7051. [Google Scholar] [CrossRef]
- He, Z.; Yu, J. Preparation and Thermal Property of Liquid Crystal Epoxy Grafted Alumina/Epoxy Resin Composite. Insul. Mater. 2015, 48, 15–20. [Google Scholar]
- Chang, K.C.; Lin, C.Y.; Lin, H.F.; Chiou, S.C.; Huang, W.C.; Yeh, J.M.; Yang, J.C. Thermally and mechanically enhanced epoxy resin-silica hybrid materials containing primary amine-modified silica nanoparticles. J. Appl. Polym. Sci. 2010, 108, 1629–1635. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Wu, Y. Computational thermomechanical properties of silica-epoxy nanocomposites by molecular dynamic simulation. Polymers 2017, 9, 430. [Google Scholar] [CrossRef]
- Chang, K.C.; Lin, H.F.; Lin, C.Y.; Kuo, T.H.; Huang, H.H.; Hsu, S.C.; Yeh, J.M.; Yang, J.C.; Yu, Y.H. Effect of amino-modified silica nanoparticles on the corrosion protection properties of epoxy resin-silica hybrid materials. J. Nanosci. Nanotechnol. 2008, 8, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiang, L.; Chen, S.; Li, C.; Sun, S.; Hu, S. Effect of Interfacial Bonding on Interphase Properties in SiO2/Epoxy Nanocomposite: A Molecular Dynamics Simulation Study. ACS Appl. Mater. Int. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Scamardella, A.M.; Romeo, V.; Lavorgna, M.; Barra, G.; Amendola, E. Epoxy composites based on amino-silylated MMT: The role of interfaces and clay morphology. J. Appl. Polym. Sci. 2012, 124, 616–628. [Google Scholar] [CrossRef]
- Piscitelli, F.; Posocco, P.; Toth, R.; Fermeglia, M.; Pricl, S.; Mensitieri, G.; Lavorgna, M. Sodium montmorillonite silylation: Unexpected effect of the aminosilane chain length. J. Colloid Interface Sci. 2010, 351, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Arab, B. The effect of cross linking density on the mechanical properties and structure of the epoxy polymers: Molecular dynamics simulation. J. Mol. Model. 2013, 19, 3719–3731. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Ab initio calculations and force field development for computer simulation of polysilanes. Macromolecules 1995, 28, 701–712. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, X.; Ma, J.; Song, L.; Yang, Y.; Yang, Q. Interface of polyimide–silica grafted with different silane coupling agents: Molecular dynamic simulation. J. Appl. Polym. Sci. 2017, 135, 45725. [Google Scholar] [CrossRef]
- Nose, S. Constant Temperature Molecular Dynamics Methods. Prog. Theor. Phys. Suppl. 1991, 103, 1–46. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Gunsteren, W.F.V.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Jeyranpour, F.; Alahyarizadeh, G.; Arab, B. Comparative investigation of thermal and mechanical properties of cross-linked epoxy polymers with different curing agents by molecular dynamics simulation. J. Mol. Model. 2015, 62, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Choi, J.; Yang, S.; Yu, S.; Cho, M. Influence of crosslink density on the interfacial characteristics ofepoxy nanocomposites. Polymer 2015, 60, 186–197. [Google Scholar] [CrossRef]
- Yu, S.; Yang, S.; Cho, M. Multi-scale modeling of cross-linked epoxy nanocomposites. Polymer 2009, 50, 945–952. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, Y.; Geng, C.; Zheng, T.; Yang, X.; Du, B. Preparation and Anti-wet Flashover Performance of Epoxy Resin/Nano-SiO2 Composite Super-hydrophobic Surface. Insul. Mater. 2017, 50, 14–19. [Google Scholar]
- Jeyranpour, F.; Alahyarizadeh, G.; Minuchehr, H. The thermo-mechanical properties estimation of fullerene-reinforced resin epoxy composites by molecular dynamics simulation—A comparative study. Polymer 2016, 88, 9–18. [Google Scholar] [CrossRef]
- Chelli, R.; Procacci, P.; Cardini, G.; Califano, S. Glycerol condensed phases Part II. A molecular dynamics study of the conformational structure and hydrogen bonding. Phys. Chem. Chem. Phys. 1999, 1, 879–885. [Google Scholar] [CrossRef]
- Chelli, R.; Procacci, P.; Cardini, G.; Della Valle, R.G.; Califano, S. Glycerol condensed phases Part I. A molecular dynamics study. Phys. Chem. Chem. Phys. 1999, 1, 871–877. [Google Scholar] [CrossRef]
- Chen, C.; Li, W.Z. Molecular Dynamics Simulation of Hydrogen Bonding Characteristics in Aqueous Glycerol Solutions. Acta Phys.-Chim. Sin. 2009, 25, 507–512. [Google Scholar] [CrossRef]
- Callam, C.S.; Singer, S.J.; Lowary, T.L.; Hadad, C.M. Computational analysis of the potential energy surfaces of glycerol in the gas and aqueous phases: Effects of level of theory, basis set, and solvation on strongly intramolecularly hydrogen-bonded systems. J. Am. Chem. Soc. 2001, 123, 11743–11754. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Joarder, R.N. Intermolecular ordering of hydrogen-bonded liquid glycerol. Phys. Lett. A 1996, 222, 195–198. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.A.; Blackburn, R.S. IR study on hydrogen bonding in epoxy resin–silica nanocomposites. Prog. Nat. Sci.-Mater. 2008, 18, 801–805. [Google Scholar] [CrossRef]
- Wu, C.; Xu, W. Atomistic molecular simulations of structure and dynamics of crosslinked epoxy resin. Polymer 2007, 48, 5802–5812. [Google Scholar] [CrossRef]
- Yang, S.; Qu, J. Computing thermomechanical properties of crosslinked epoxy by molecular dynamic simulations. Polymer 2012, 53, 4806–4817. [Google Scholar] [CrossRef]
- Li, C.; Strachan, A. Molecular dynamics predictions of thermal and mechanical properties of thermoset polymer EPON862/DETDA. Polymer 2011, 52, 2920–2928. [Google Scholar] [CrossRef]
- Fan, H.B.; Yuen, M.M.F. Material properties of the cross-linked epoxy resin compound predicted by molecular dynamics simulation. Polymer 2007, 48, 2174–2178. [Google Scholar] [CrossRef]
- Fox, T.G.; Loshaek, S. Influence of molecular weight and degree of crosslinking on the specific volume and glass temperature of polymers. J. Polym. Sci. Polym. Chem. 1955, 15, 371–390. [Google Scholar] [CrossRef]
- Fried, J.R.; Sadat-Akhavi, M.; Mark, J.E. Molecular simulation of gas permeability: Poly(2,6-dimethyl-1,4-phenylene oxide). J. Membr. Sci. 1998, 149, 115–126. [Google Scholar] [CrossRef]
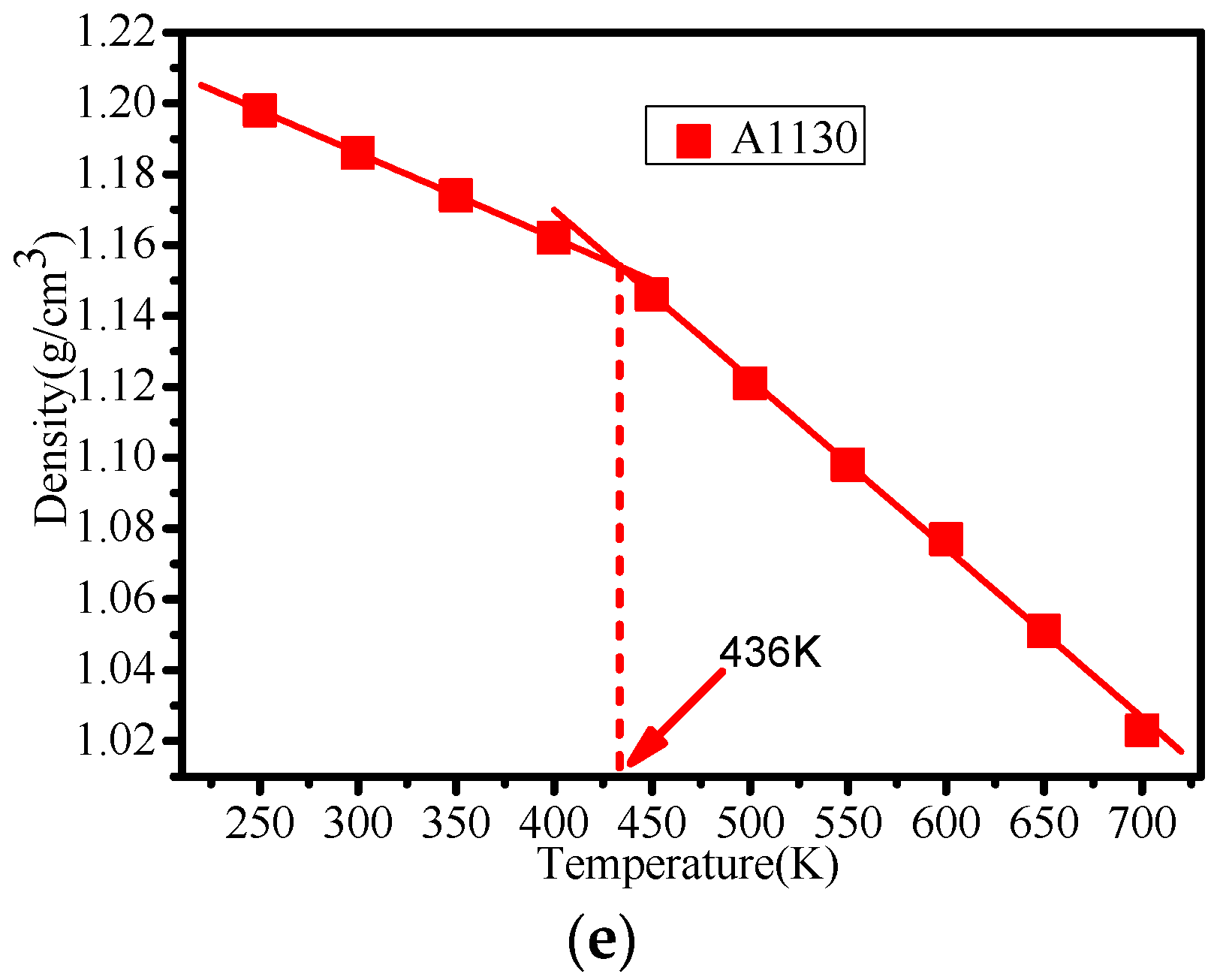
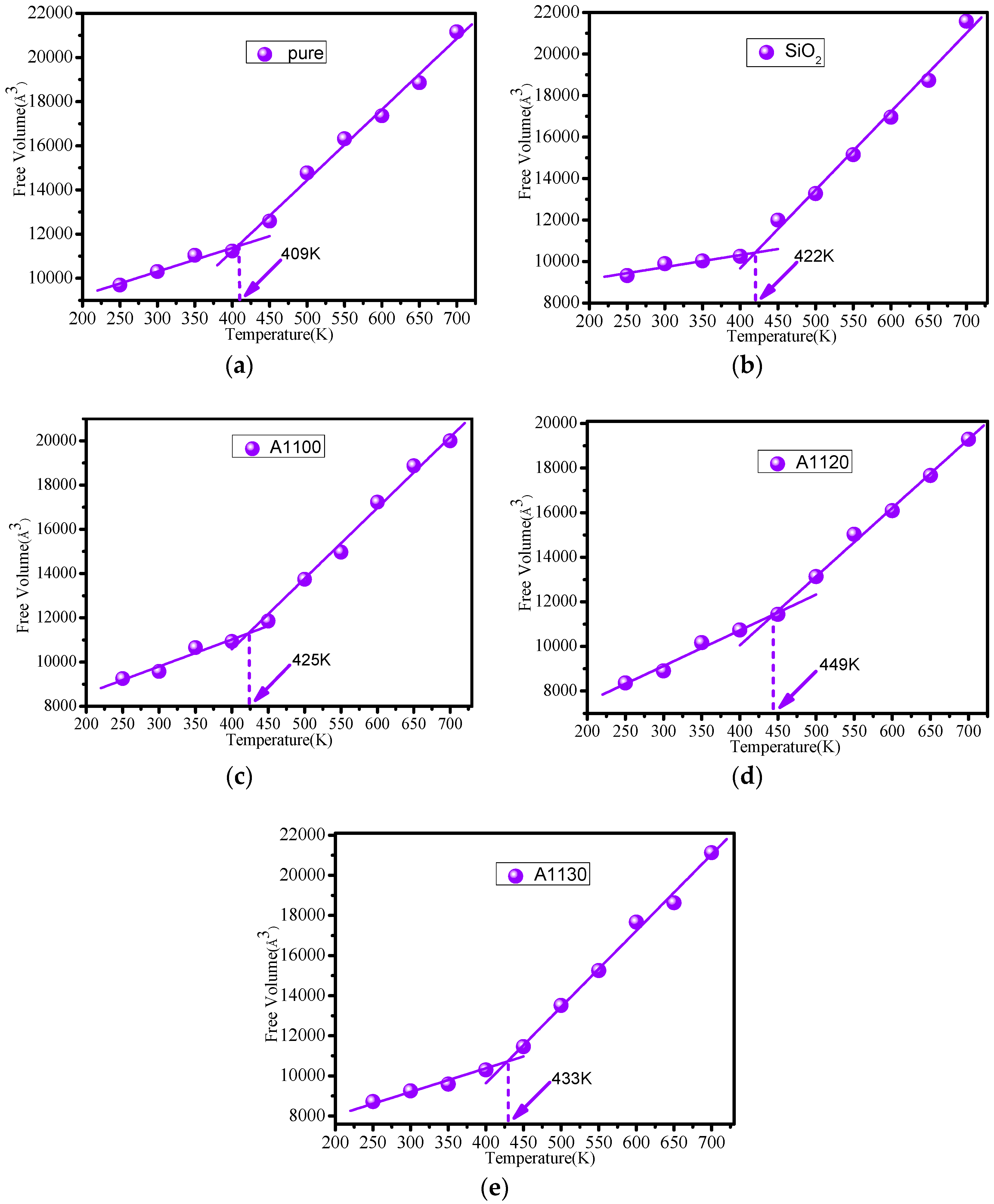
| Temperature (K) | 250 | 300 | 350 | 400 | 450 | 500 | 550 | 600 | 650 |
|---|---|---|---|---|---|---|---|---|---|
| pure | 0.151 | 0.159 | 0.168 | 0.170 | 0.187 | 0.212 | 0.231 | 0.246 | 0.290 |
| SiO2 | 0.148 | 0.156 | 0.157 | 0.176 | 0.183 | 0.198 | 0.203 | 0.233 | 0.260 |
| A1100 | 0.148 | 0.151 | 0.166 | 0.173 | 0.178 | 0.196 | 0.217 | 0.246 | 0.263 |
| A1120 | 0.135 | 0.142 | 0.160 | 0.167 | 0.182 | 0.197 | 0.220 | 0.240 | 0.251 |
| A1130 | 0.141 | 0.148 | 0.149 | 0.168 | 0.184 | 0.200 | 0.220 | 0.249 | 0.259 |
| System | 2θ (°) | d-Spacing (Å) |
|---|---|---|
| A1100 | 16.3 | 5.43 |
| A1120 | 18.5 | 4.79 |
| A1130 | 18.2 | 4.87 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Tang, C.; Hu, D.; Gui, Y. Effect of Aminosilane Coupling Agents with Different Chain Lengths on Thermo-Mechanical Properties of Cross-Linked Epoxy Resin. Nanomaterials 2018, 8, 951. https://doi.org/10.3390/nano8110951
Tang Y, Tang C, Hu D, Gui Y. Effect of Aminosilane Coupling Agents with Different Chain Lengths on Thermo-Mechanical Properties of Cross-Linked Epoxy Resin. Nanomaterials. 2018; 8(11):951. https://doi.org/10.3390/nano8110951
Chicago/Turabian StyleTang, Yujing, Chao Tang, Dong Hu, and Yingang Gui. 2018. "Effect of Aminosilane Coupling Agents with Different Chain Lengths on Thermo-Mechanical Properties of Cross-Linked Epoxy Resin" Nanomaterials 8, no. 11: 951. https://doi.org/10.3390/nano8110951
APA StyleTang, Y., Tang, C., Hu, D., & Gui, Y. (2018). Effect of Aminosilane Coupling Agents with Different Chain Lengths on Thermo-Mechanical Properties of Cross-Linked Epoxy Resin. Nanomaterials, 8(11), 951. https://doi.org/10.3390/nano8110951






