Peculiarities of Synthesis and Properties of Lignin–Silica Nanocomposites Prepared by Sol-Gel Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Analysis
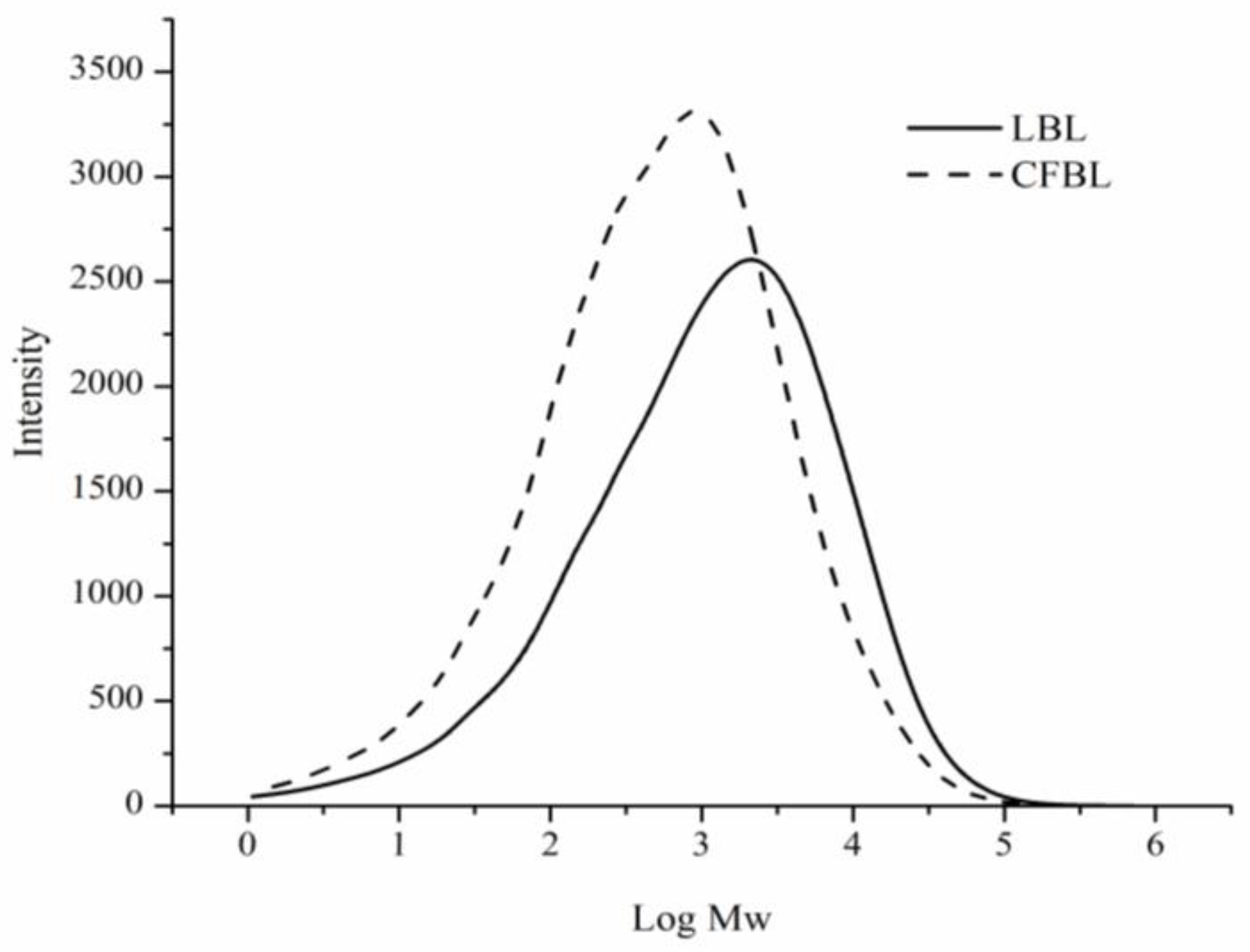
2.3.1. Size-Exclusion Chromatography (SEC)
2.3.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
31P-NMR
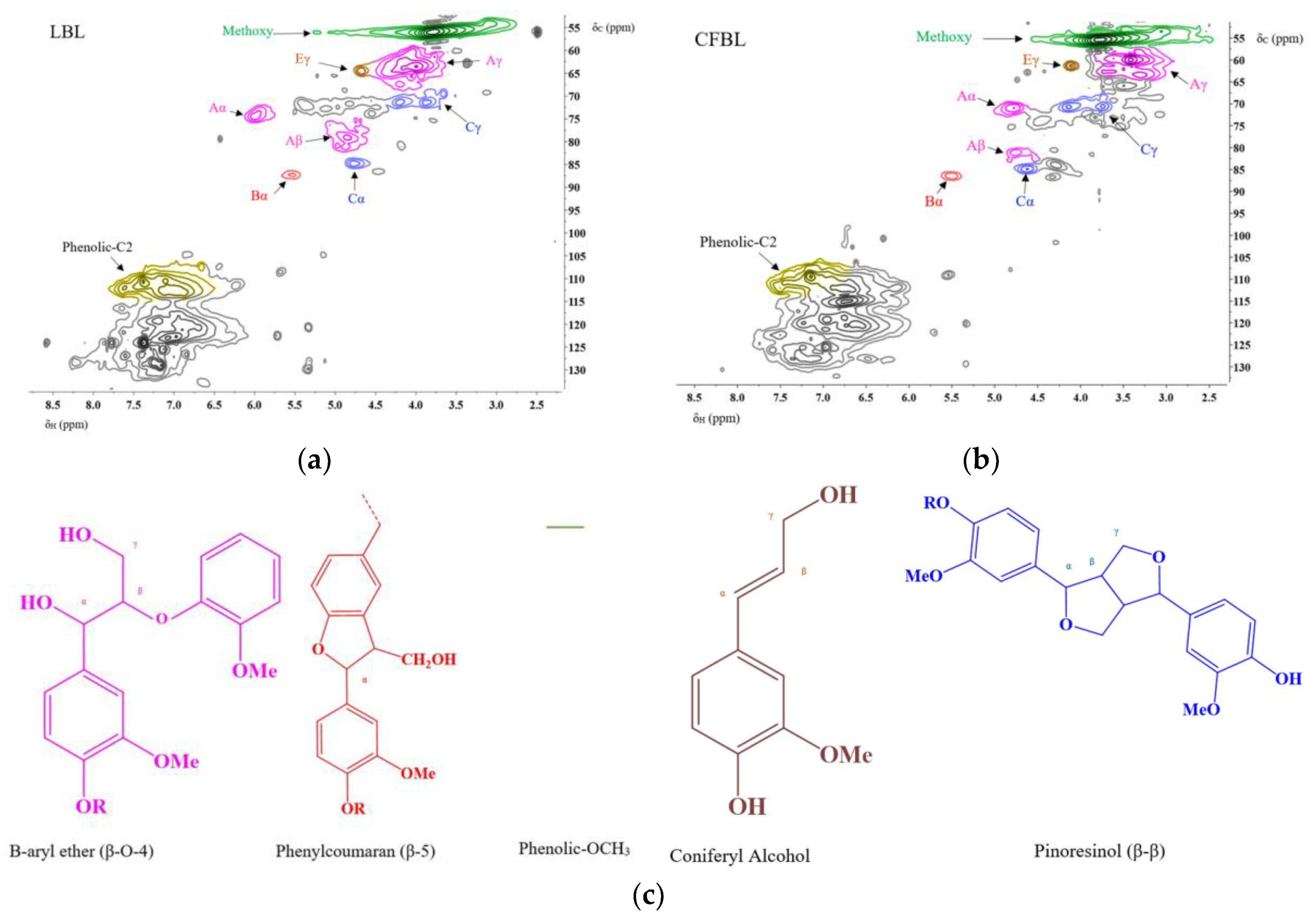
2D-Heteronuclear Single Quantum Coherence (2D-HSQC) NMR
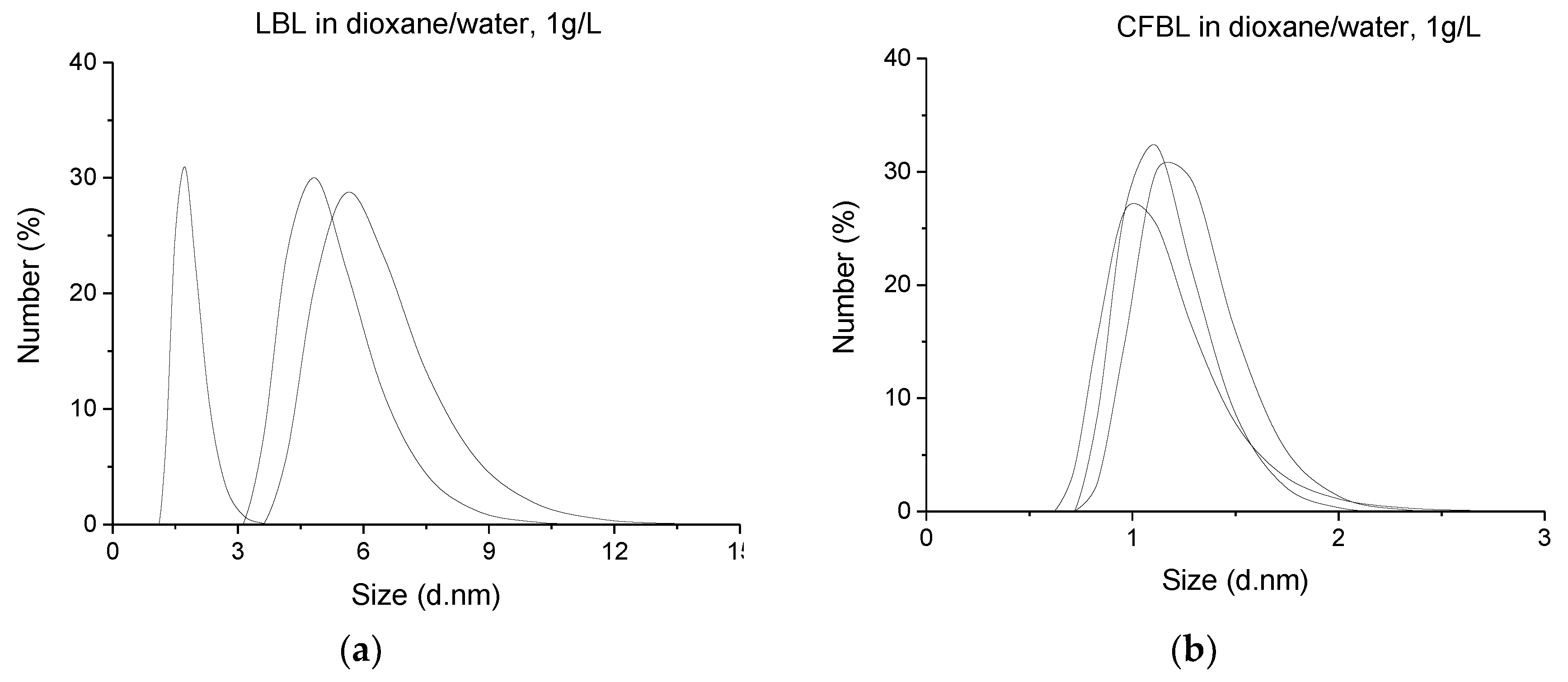
Dynamic Light Scattering (DLS)
FTIR Spectroscopy
X-Ray Photoelectron Spectroscopy (XPS)
Thermal Analysis
Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Characterization of the Technical Lignins
3.2. Dynamic Light Scattering
3.3. Design and Synthesis of Sorbent
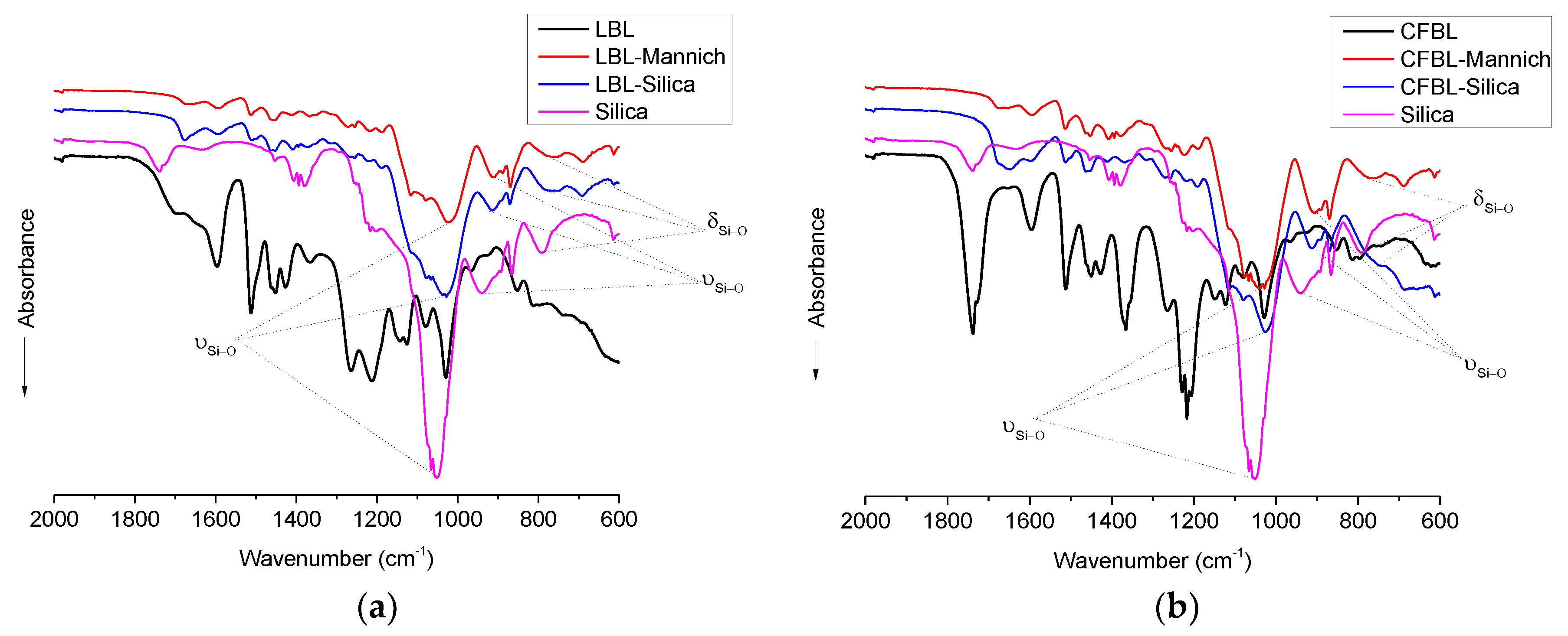
3.4. FTIR Analysis
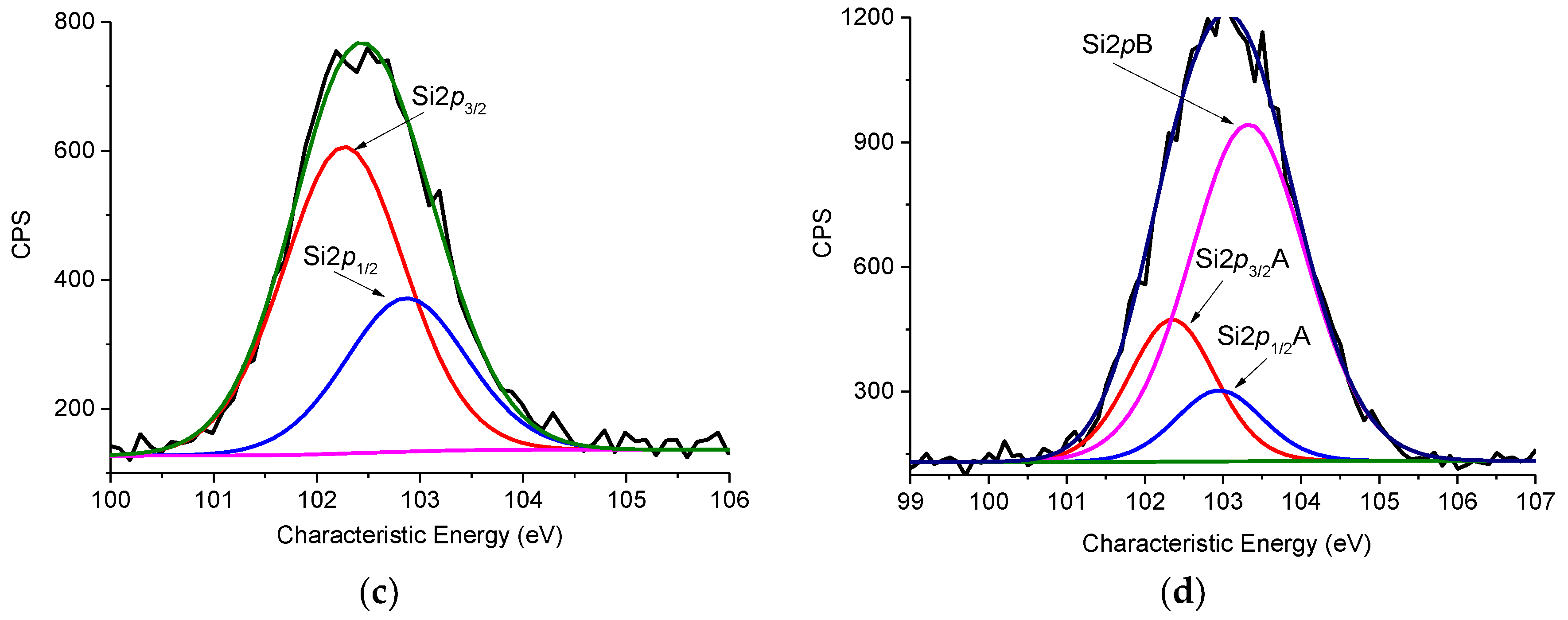
3.5. XPS Analysis
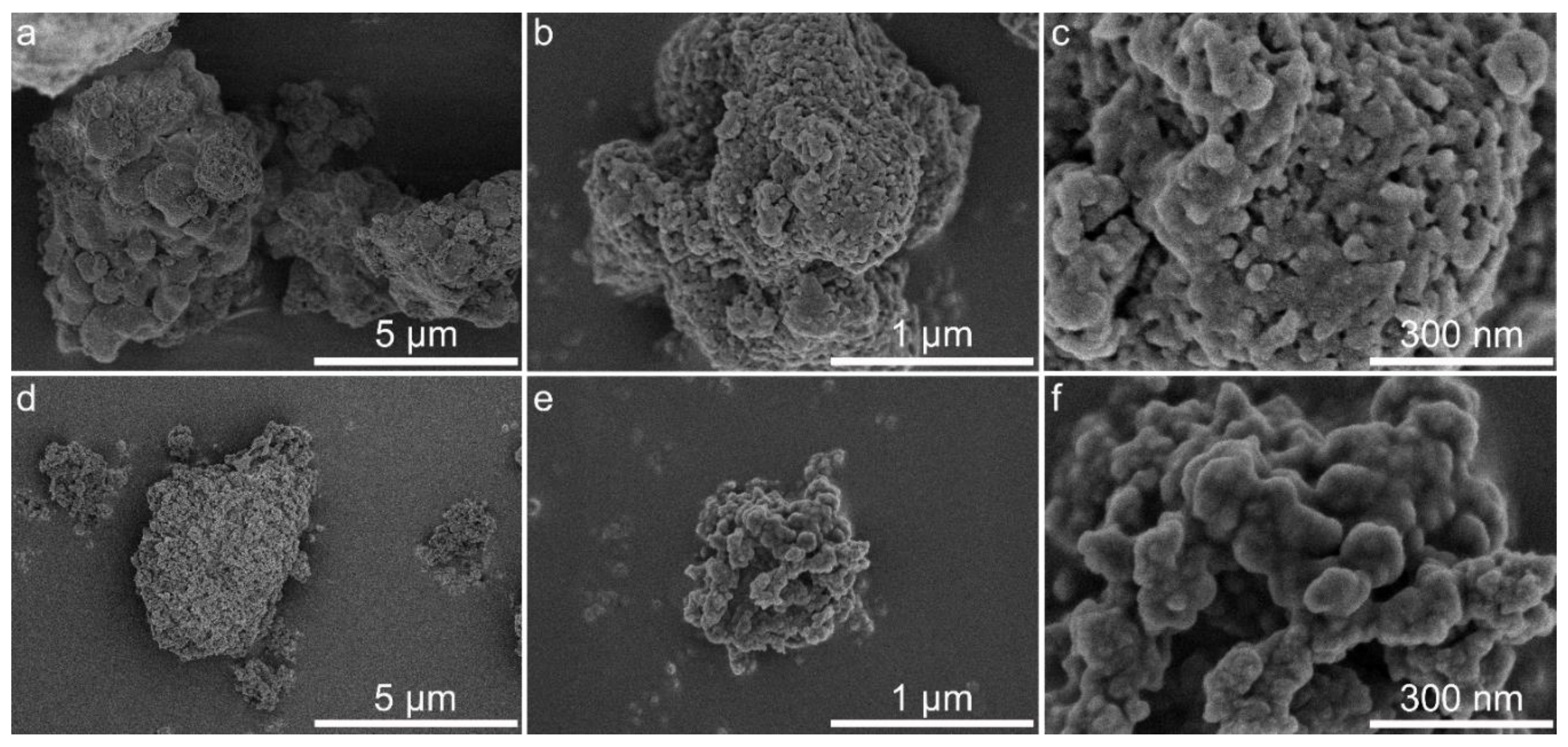
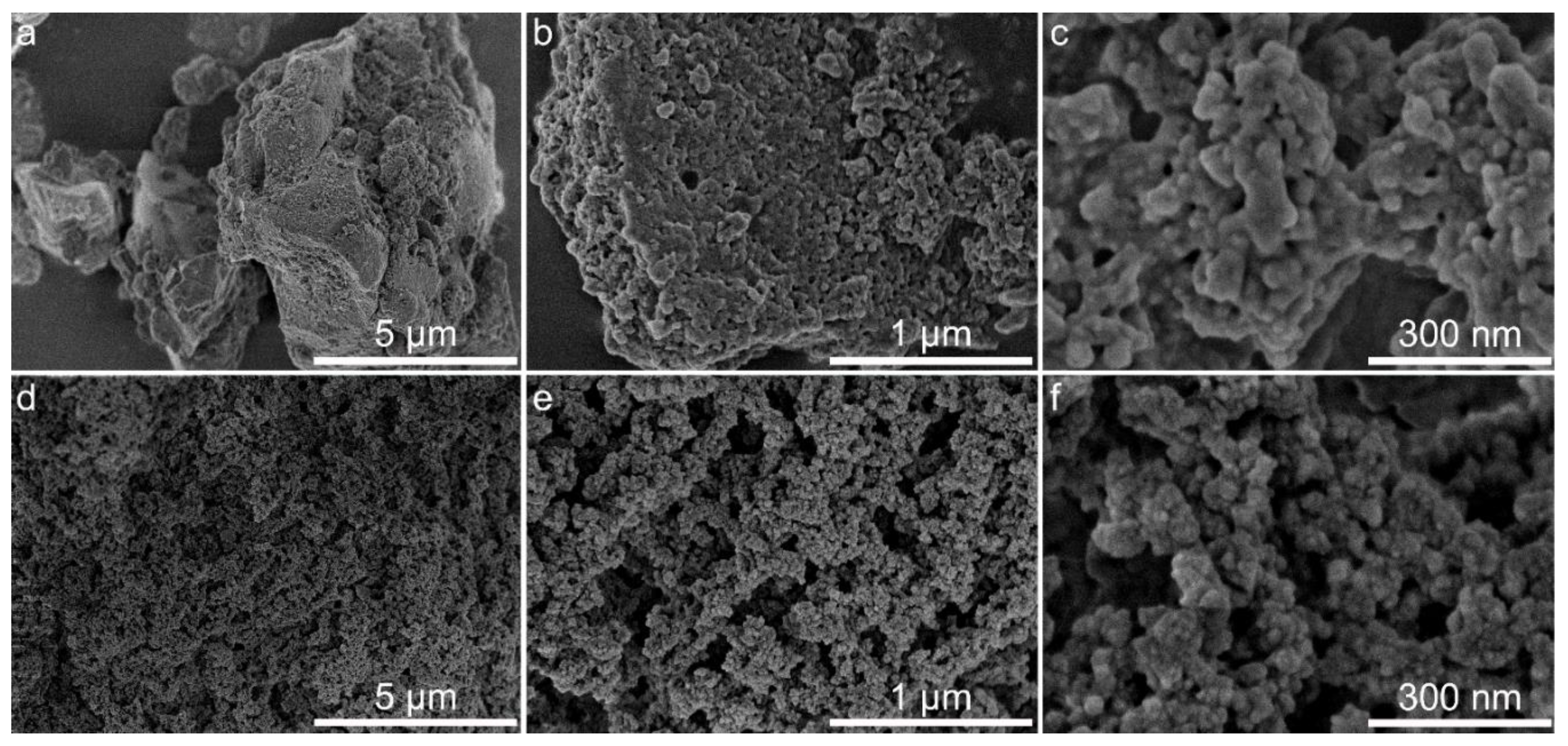
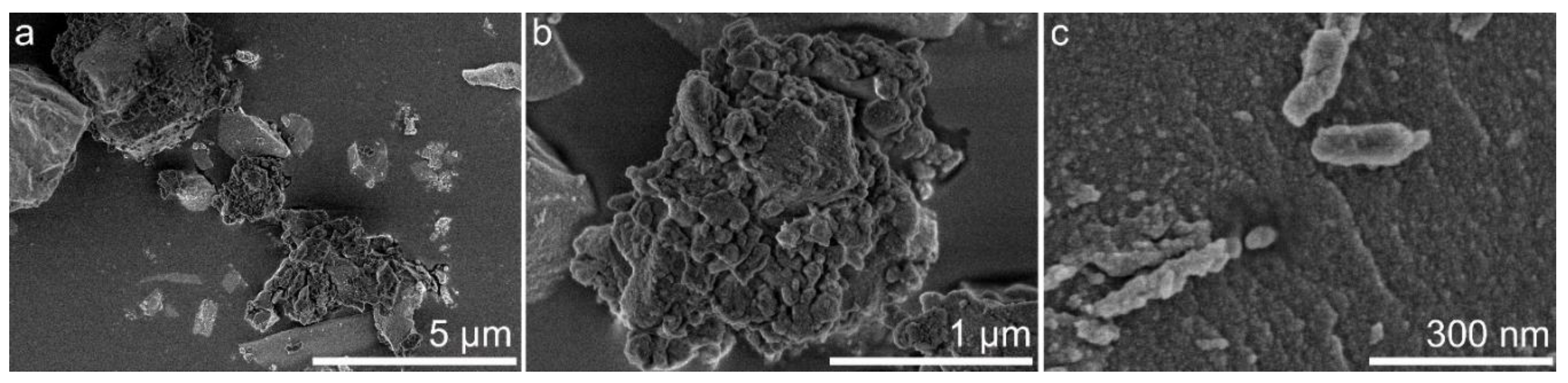
3.6. Morphology and Textural Characteristics
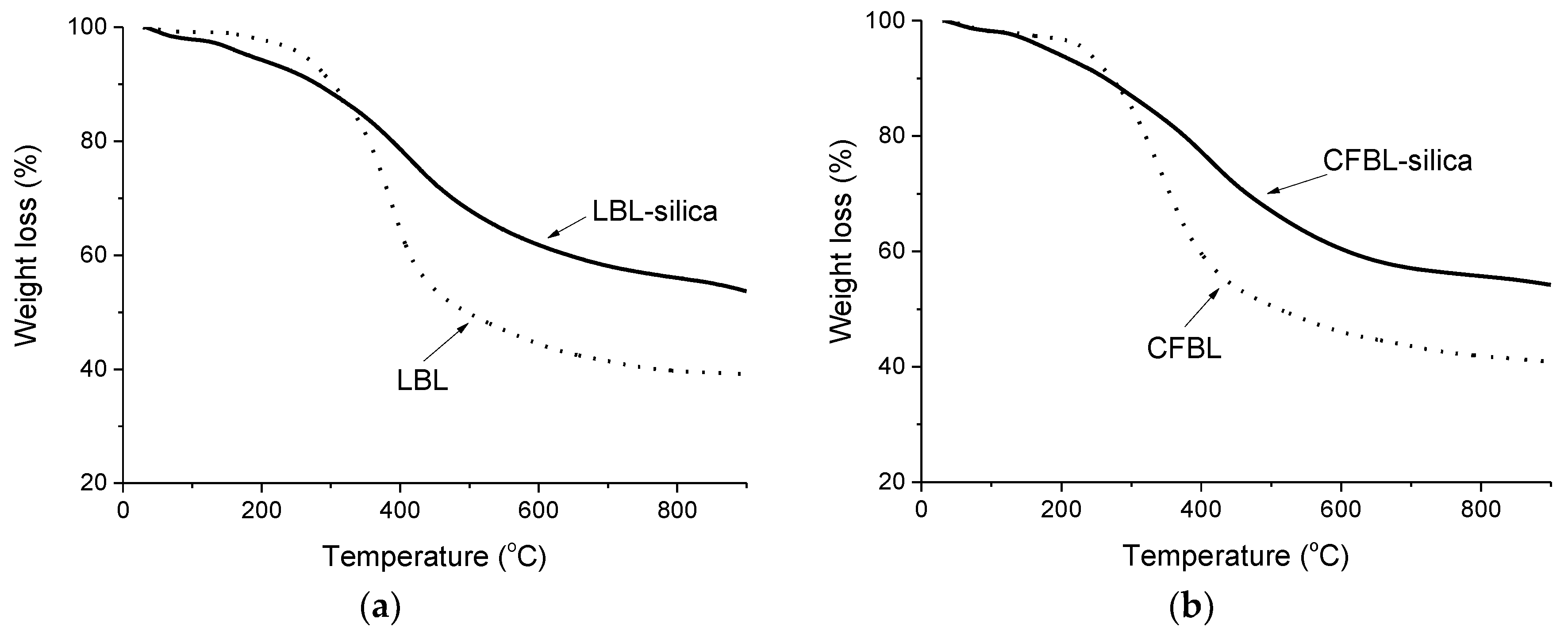
3.7. Thermal Analysis
3.8. Application as Sorbzents in Water Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hu, T.Q. Chemical Modification, Properties, and Usage of Lignin; Springer: New York, NY, USA, 2002. [Google Scholar]
- Gellerstedt, G.; Lindfors, E.-L. Structural changes in lignin during kraft pulping. Holzforschung 1984, 38, 151–158. [Google Scholar] [CrossRef]
- Robert, D.R.; Bardet, M.; Gellerstedt, G.; Lindfors, E.L. Structural changes in lignin during kraft cooking part 3. On the structure of dissolved lignins. J. Wood Chem. Technol. 1984, 4, 239–263. [Google Scholar] [CrossRef]
- Gordobil, O.; Moriana, R.; Zhang, L.; Labidi, J.; Sevastyanova, O. Assesment of technical lignins for uses in biofuels and biomaterials: Structure-related properties, proximate analysis and chemical modification. Ind. Crop. Prod. 2016, 83, 155–165. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Tomani, P. The lignoboost process. Cell. Chem. Technol. 2010, 44, 53. [Google Scholar]
- Aminzadeh, S.; Lauberts, M.; Dobele, G.; Ponomarenko, J.; Mattsson, T.; Lindström, M.E.; Sevastyanova, O. Membrane filtration of kraft lignin: Structural charactristics and antioxidant activity of the low-molecular-weight fraction. Ind. Crop. Prod. 2018, 112, 200–209. [Google Scholar] [CrossRef]
- Sevastyanova, O.; Helander, M.; Chowdhury, S.; Lange, H.; Wedin, H.; Zhang, L.; Ek, M.; Kadla, J.F.; Crestini, C.; Lindström, M.E. Tailoring the molecular and thermo–mechanical properties of kraft lignin by ultrafiltration. J. Appl. Polym. Sci. 2014, 131, 40799. [Google Scholar] [CrossRef]
- Jablonskis, A.; Arshanitsa, A.; Arnautov, A.; Telysheva, G.; Evtuguin, D. Evaluation of Ligno Boost™ softwood kraft lignin epoxidation as an approach for its application in cured epoxy resins. Ind. Crop. Prod. 2018, 112, 225–235. [Google Scholar] [CrossRef]
- Lagerquist, L.; Pranovich, A.; Smeds, A.; von Schoultz, S.; Vähäsalo, L.; Rahkila, J.; Kilpeläinen, I.; Tamminen, T.; Willför, S.; Eklund, P. Structural characterization of birch lignin isolated from a pressurized hot water extraction and mild alkali pulped biorefinery process. Ind. Crop. Prod. 2018, 111, 306–316. [Google Scholar] [CrossRef]
- Liu, C.; Si, C.; Wang, G.; Jia, H.; Ma, L. A novel and efficient process for lignin fractionation in biomass-derived glycerol-ethanol solvent system. Ind. Crop. Prod. 2018, 111, 201–211. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; González, I.; Tarrés, Q.; Pèlach, M.À.; Alcalà, M.; Mutjé, P. The key role of lignin in the production of low-cost lignocellulosic nanofibres for papermaking applications. Ind. Crop. Prod. 2016, 86, 295–300. [Google Scholar] [CrossRef]
- Jääskeläinen, A.S.; Liitiä, T.; Mikkelson, A.; Tamminen, T. Aqueous organic solvent fractionation as means to improve lignin homogeneity and purity. Ind. Crop. Prod. 2017, 103, 51–58. [Google Scholar] [CrossRef]
- García, A.; Spigno, G.; Labidi, J. Antioxidant and biocide behaviour of lignin fractions from apple tree pruning residues. Ind. Crop. Prod. 2017, 104, 242–252. [Google Scholar] [CrossRef]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood kraft lignin for value-added applications: Fractionation and structural characterization. Ind. Crop. Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- Lauberts, M.; Sevastyanova, O.; Ponomarenko, J.; Dizhbite, T.; Dobele, G.; Volperts, A.; Lauberte, L.; Telysheva, G. Fractionation of technical lignin with ionic liquids as a method for improving purity and antioxidant activity. Ind. Crop. Prod. 2017, 95, 512–520. [Google Scholar] [CrossRef]
- Podkościelna, B.; Goliszek, M.; Sevastyanova, O. New approach in the application of lignin for the synthesis of hybrid materials. Pure Appl. Chem. 2017, 89, 161–171. [Google Scholar] [CrossRef]
- Podkościelna, B.; Sobiesiak, M.; Zhao, Y.; Gawdzik, B.; Sevastyanova, O. Preparation of lignin-containing porous microspheres through the copolymerization of lignin acrylate derivatives with styrene and divinylbenzene. Holzforschung 2015, 69, 769–776. [Google Scholar] [CrossRef]
- Ye, W.; Li, X.; Luo, J.; Wang, X.; Sun, R. Lignin as a green reductant and morphology directing agent in the fabrication of 3D graphene-based composites for high-performance supercapacitors. Ind. Crop. Prod. 2017, 109, 410–419. [Google Scholar] [CrossRef]
- Gómez-Fernández, S.; Ugarte, L.; Calvo-Correas, T.; Peña-Rodríguez, C.; Corcuera, M.A.; Eceiza, A. Properties of flexible polyurethane foams containing isocyanate functionalized kraft lignin. Ind. Crop. Prod. 2017, 100, 51–64. [Google Scholar] [CrossRef]
- Wahlström, R.; Kalliola, A.; Heikkinen, J.; Kyllönen, H.; Tamminen, T. Lignin cationization with glycidyltrimethylammonium chloride aiming at water purification applications. Ind. Crop. Prod. 2017, 104, 188–194. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Hu, H.; Huang, Z.; Yang, M.; Chen, D.; Huang, K.; Huang, A.; Qin, X.; Feng, Z. Effect of mechanical activation on structure changes and reactivity in further chemical modification of lignin. Int. J. Biol. Macromol. 2016, 91, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Yang, S.; Fang, W.; Yuan, T.-Q.; Argyropoulos, D.S.; Sun, R.-C. Structure-property relationships for technical lignins for the production of lignin-phenol-formaldehyde resins. Ind. Crop. Prod. 2017, 108, 316–326. [Google Scholar] [CrossRef]
- Myglovets, M.; Poddubnaya, O.; Sevastyanova, O.; Lindström, M.E.; Gawdzik, B.; Sobiesiak, M.; Tsyba, M.; Sapsay, V.; Klymchuk, D.; Puziy, A. Preparation of carbon adsorbents from lignosulfonate by phosphoric acid activation for the adsorption of metal ions. Carbon 2014, 80, 771–783. [Google Scholar] [CrossRef]
- Kadla, J.; Kubo, S.; Venditti, R.; Gilbert, R.; Compere, A.; Griffith, W. Lignin-based carbon fibers for composite fiber applications. Carbon 2002, 40, 2913–2920. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. The formation of strong intermolecular interactions in immiscible blends of poly (vinyl alcohol)(PVA) and lignin. Biomacromolecules 2003, 4, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Nowacka, M.; Milczarek, G.; Jesionowski, T. Physicochemical and electrokinetic properties of silica/lignin biocomposites. Carbohydr. Polym. 2013, 94, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, L.; Madrawska, M.; Jesionowski, T. Preparation and characterisation of hydrated silica/lignin biocomposites. Physicochem. Probl. Miner. Process. 2012, 48, 463–473. [Google Scholar]
- Klapiszewski, Ł.; Nowacka, M.; Szwarc-Rzepka, K.; Jesionowski, T. Advanced biocomposites based on silica and lignin precursors. Physicochem. Probl. Miner. Process. 2013. [Google Scholar] [CrossRef]
- Nowacka, M.; Klapiszewski, Ł.; Norman, M.; Jesionowski, T. Dispersive evaluation and surface chemistry of advanced, multifunctional silica/lignin hybrid biomaterials. Open Chem. 2013, 11, 1860–1873. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Bartczak, P.; Wysokowski, M.; Jankowska, M.; Kabat, K.; Jesionowski, T. Silica conjugated with kraft lignin and its use as a novel ‘green’ sorbent for hazardous metal ions removal. Chem. Eng. J. 2015, 260, 684–693. [Google Scholar] [CrossRef]
- Qin, L.; Ge, Y.; Deng, B.; Li, Z. Poly (ethylene imine) anchored lignin composite for heavy metals capturing in water. J. Taiwan Inst. Chem. E 2017, 71, 84–90. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Bula, K.; Sobczak, M.; Jesionowski, T. Influence of processing conditions on the thermal stability and mechanical properties of PP/silica-lignin composites. Int. J. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Strzemiecka, B.; Klapiszewski, Ł.; Jamrozik, A.; Szalaty, T.J.; Matykiewicz, D.; Sterzyński, T.; Voelkel, A.; Jesionowski, T. Physicochemical Characterization of Functional Lignin-Silica Hybrid Fillers for Potential Application in Abrasive Tools. Materials 2016, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Yang, D.; Zhong, R.; Li, Y.; Zhou, H.; Qiu, X. Preparation of lignin-based silica composite submicron particles from alkali lignin and sodium silicate in aqueous solution using a direct precipitation method. Ind. Crop. Prod. 2015, 74, 285–292. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, L.; Guo, Y.; Fu, Y.; Shao, Q.; Wu, T.; Guo, S.; Sun, K.; Guo, X.; Wujcik, E.K. Porous lignin based poly (acrylic acid)/organo-montmorillonite nanocomposites: Swelling behaviors and rapid removal of Pb (II) ions. Polymer 2017, 128, 12–23. [Google Scholar] [CrossRef]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Mishra, Y.K.; Thakur, V.K. Progress in lignin hydrogels and nanocomposites for water purification: Future perspectives. Vacuum 2017, 146, 342–355. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Yuan, T.; Sun, R. A lignosulfonate-modified graphene hydrogel with ultrahigh adsorption capacity for Pb (ii) removal. J. Mater. Chem. A 2016, 4, 11888–11896. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Budnyak, T.; Hubicki, Z.; Tertykh, V. Sol-Gel Derived Organic–Inorganic Hybrid Ceramic Materials for Heavy Metal Removal. In Sol-Gel Based Nanoceramic Materials: Preparation, Properties and Applications; Springer: New York, NY, USA, 2017; pp. 253–274. [Google Scholar]
- Qu, Y.; Tian, Y.; Zou, B.; Zhang, J.; Zheng, Y.; Wang, L.; Li, Y.; Rong, C.; Wang, Z. A novel mesoporous lignin/silica hybrid from rice husk produced by a sol-gel method. Bioresour. Technol. 2010, 101, 8402–8405. [Google Scholar] [CrossRef] [PubMed]
- Telysheva, G.; Dizhbite, T.; Jashina, L.; Andersone, A.; Volperts, A.; Ponomarenko, J.; Mironova-Ulmane, N. Synthesis of lignin-based inorganic/organic hybrid materials favorable for detoxification of ecosystem components. BioResources 2009, 4, 1276–1284. [Google Scholar]
- Telysheva, G.; Dizhbite, T.; Evtuguin, D.; Mironova-Ulmane, N.; Lebedeva, G.; Andersone, A.; Bikovens, O.; Chirkova, J.; Belkova, L. Design of siliceous lignins-novel organic/inorganic hybrid sorbent materials. Scr. Mater. 2009, 60, 687–690. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Aminzadeh, S.; Pylypchuk, I.V.; Sternik, D.; Tertykh, V.A.; Lindström, M.E.; Sevastyanova, O. Methylene Blue dye sorption by hybrid materials from technical lignins. J. Environ. Chem. Eng. 2018, 6, 4997–5007. [Google Scholar] [CrossRef]
- Argyropoulos, D.S. Quantitative Phosphorus-31 NMR Analysis of Lignins, a New Tool for the Lignin Chemist. J. Wood Chem. Technol. 1994, 14, 45–63. [Google Scholar] [CrossRef]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer: Berlin, Germany, 1992. [Google Scholar]
- Capanema, E.A.; Balakshin, M.Y.; Kadla, J.F. A comprehensive approach for quantitative lignin characterization by NMR spectroscopy. J. Agric. Food Chem. 2004, 52, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, A.N.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ralph, J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org. Biomol. Chem. 2010, 8, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gellerstedt, G. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. Magn. Reson. Chem. 2007, 45, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Biorefin. 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Vainio, U.; Maximova, N.; Hortling, B.; Laine, J.; Stenius, P.; Simola, L.K.; Gravitis, J.; Serimaa, R. Morphology of dry lignins and size and shape of dissolved kraft lignin particles by X-ray scattering. Langmuir 2004, 20, 9736–9744. [Google Scholar] [CrossRef] [PubMed]
- Tertykh, V.; Yanishpolskii, V.; Panova, O.Y. Covalent attachment of some phenol derivatives to the silica surface by use of single-stage aminomethylation. J. Therm. Anal. Calorim. 2000, 62, 545–549. [Google Scholar] [CrossRef]
- Yanovska, E.; Ryabchenko, K.; Tertykh, V.; Kichkiruk, O.Y. Complexing properties of silica gel-polyaniline composites with grefted heterocyclic azo reagents. Chem. Phys. Technol. Surf. 2012, 3, 439–447. [Google Scholar]
- Budnyak, T.M.; Pylypchuk, I.V.; Tertykh, V.A.; Yanovska, E.S.; Kolodynska, D. Synthesis and adsorption properties of chitosan-silica nanocomposite prepared by sol-gel method. Nanoscale Res. Lett. 2015, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Budnyak, T.; Yanovska, E.; Kołodyńska, D.; Sternik, D.; Pylypchuk, I.V.; Ischenko, M.; Tertykh, V. Preparation and properties of organomineral adsorbent obtained by sol-gel technology. J. Therm. Anal. Calorim. 2016, 125, 1335–1351. [Google Scholar] [CrossRef]
- Wang, M.; Sjöholm, E.; Li, J. Fast and reliable quantification of lignin reactivity via reaction with dimethylamine and formaldehyde (Mannich reaction). Holzforschung 2017, 71, 27–34. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Lindström, M.E. Modification of industrial softwood kraft lignin using Mannich reaction with and without phenolation pretreatment. Ind. Crop. Prod. 2014, 52, 729–735. [Google Scholar] [CrossRef]
- Jamois, D.; Tessier, M.; Maréchal, E. Preparation of amphiphilic polyisobutylenes-b-polyethylenamines by mannich reaction. II. Study of mannich reaction on model systems. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1941–1958. [Google Scholar] [CrossRef]
- Budnyak, T.; Yanovska, E.; Kichkiruk, O.Y.; Sternik, D.; Tertykh, V. Natural Minerals Coated by Biopolymer Chitosan: Synthesis, Physicochemical, and Adsorption Properties. Nanoscale Res. Lett. 2016, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Podkościelna, B.; Gordobil, O.; Riazanova, A.V.; Dobele, G.; Labidi, J.; Lindström, M.E.; Gun’ko, V.M.; Sevastyanova, O. Novel Porous Materials Obtained from Technical Lignins and Their Methacrylate Derivatives Copolymerized with Styrene and Divinylbenzene. Chem. Sel. 2017, 2, 2257–2264. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Bartczak, P.; Jesionowski, T. Enhanced removal of hazardous dye form aqueous solutions and real textile wastewater using bifunctional chitin/lignin biosorbent. Int. J. Boil. Macromol. 2017, 99, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Ryoritani, M.; Saka, S. Different pyrolytic cleavage mechanisms of β-ether bond depending on the side-chain structure of lignin dimers. J. Anal. Appl. Pyrolysis 2008, 81, 88–94. [Google Scholar] [CrossRef]
- Xu, C.; Ferdosian, F. Degradation of Lignin by Pyrolysis. In Conversion of Lignin into Bio-Based Chemicals and Materials; Springer: New York, NY, USA, 2017; pp. 13–33. [Google Scholar]
- Sarkanen, K.V.; Ludwig, C.H. Lignins: Occurrence, Formation, Structure and Reactions; Wiley-Interscience: Hoboken, NJ, USA, 1971. [Google Scholar]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353. [Google Scholar]

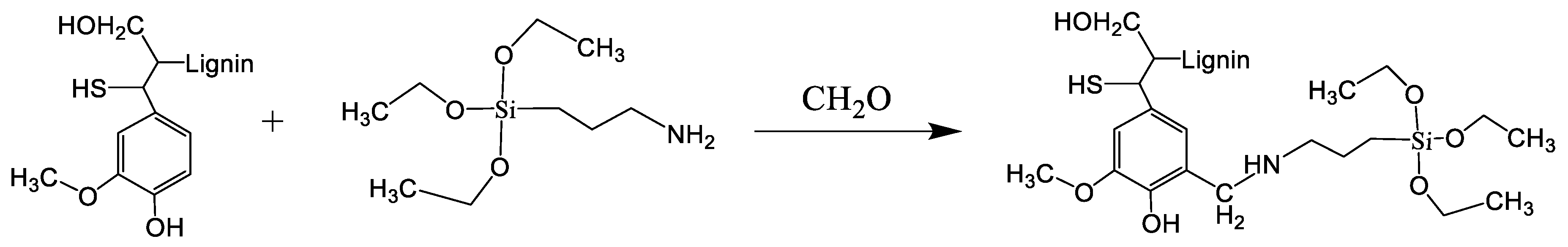



| Sample | Molecular Weight | Content of Functional Groups, mmol·g−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mw, Da | Mn, Da | PDI | Phenolic OH | Aliphatic OH | Total OH | COOH | |||
| Condensed | Non-Condensed | Total | |||||||
| LBL | 1300 | 5600 | 4.2 | 1.88 | 2.11 | 3.99 | 1.73 | 5.72 | 0.41 |
| CFBL | 880 | 3000 | 3.5 | 2.11 | 3.36 | 5.47 | 1.81 | 7.28 | 0.28 |
| Sample | Atom | Position | Concentration, at.% | Assignment |
|---|---|---|---|---|
| CFBL–silica | N1sA | 399.4 | 54.4 | amine, amide, cyanides |
| N1sB | 400.7 | 29.7 | imide | |
| N1sC | 402.0 | 15.8 | quaternary nitrogen | |
| Si2p3/2A | 102.3 | 32.1 | silicon/siloxane (SiOEt)3 from TEOS and 3 aminopropyltriethoxysilane (APTES) (unhydrolyzed ethoxy groups) | |
| Si2pB | 103.3 | 67.9 | SiO2 | |
| CFBL–Mannich | N1sA | 399.5 | 47.8 | amine, amide, cyanides |
| N1sB | 400.7 | 32.8 | imide | |
| N1sC | 402.1 | 19.4 | quaternary nitrogen | |
| Si2p3/2 | 102.3 | 100 | silicon/siloxane, (SiOEt)3 |
| M. | Tmax, °C (DTG) | ∆m, % (TG) | ∆mtotal, % (TG) | Process |
|---|---|---|---|---|
| LBL | 51 | <0.4 | 61 | Moisture evaporation |
| 184 | Water evaporation due to self-condensation reactions (up to 400 °C) [66] | |||
| 293 | The β–β and C–C linkages between the lignin monomeric units cleave at 275–350 °C, while the recombination of the formed radicals leads to guaiacyl and syringyl compounds [67]; aryl-ether bonds cleavage [68] | |||
| 390 | Conversion of phenols into pyrocatechols [65], the conversion of short substituents of the benzene rings [66] | |||
| ≥400 | Rearrangement of backbone, carbonization | |||
| CFBL | 62 | <0.4 | 59 | Moisture evaporation |
| 150 | Water evaporation due to self-condensation reactions (up to 400 °C) [66] | |||
| 256 | The β–β and C–C linkages between the lignin monomeric units cleave at 275–350 °C, while the recombination of the formed radicals leads to guaiacyl and syringyl compounds [67]; aryl-ether bonds cleavage [68] | |||
| 345 | Conversion of phenols into pyrocatechols [65]; conversion of short substituents of the benzene rings [66] | |||
| ≥400 | Rearrangement of backbone, carbonization | |||
| LBL–M | 149 | 13.4 | 52.4 | Water evaporation, EtO elimination |
| 338 | 18.6 | Condensation and elimination of the hydroxyl groups; decomposition of the aminopropyl radical; conversion of phenols into pyrocatechols (demethylation of the dimethothoxy groups) [65] | ||
| 464 | 16.0 | Conversion of short substituents of the benzene rings [66], rearrangement of backbone, carbonization | ||
| CFBL–M | 139 | 12.5 | 48.7 | Water evaporation, EtO elimination |
| 333 | 15.7 | Condensation and elimination of the hydroxyl groups; decomposition of the aminopropyl radical; conversion of phenols into pyrocatechols (demethylation of the dimethothoxy-groups) [65] | ||
| 422 | 18.9 | Conversion of short substituents of the benzene rings [66], rearrangement of backbone, carbonization | ||
| LBL–Silica | 55 | 0.7 | 45 | Moisture evaporation |
| 172 | 4.2 | Water evaporation, EtO elimination | ||
| 290 | 12.0 | Onset of lignin decomposition | ||
| 416 | 25 | Conversion of short substituents of the benzene rings [66], rearrangement of backbone, carbonization, hydroxyl radical elimination from silica | ||
| CFBL–Silica | 50 | 0.5 | 46 | Moisture evaporation |
| 159 | 12 | Water evaporation, EtO elimination | ||
| 290 | Onset of lignin decomposition | |||
| 414 | Conversion of short substituents of the benzene rings [66], rearrangement of backbone, carbonization, hydroxyl radical elimination from silica | |||
| Silica/W | 78 | 9.3 | 15.6 | Physically adsorbed water evaporation |
| 260 | 15.4 | Condensation of the silica hydroxyl groups | ||
| Silica/D:W | 73 | 5.8 | 17.1 | Dioxan evaporation |
| 213 | 13.1 | Condensation of the silica hydroxyl groups |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budnyak, T.M.; Aminzadeh, S.; Pylypchuk, I.V.; Riazanova, A.V.; Tertykh, V.A.; Lindström, M.E.; Sevastyanova, O. Peculiarities of Synthesis and Properties of Lignin–Silica Nanocomposites Prepared by Sol-Gel Method. Nanomaterials 2018, 8, 950. https://doi.org/10.3390/nano8110950
Budnyak TM, Aminzadeh S, Pylypchuk IV, Riazanova AV, Tertykh VA, Lindström ME, Sevastyanova O. Peculiarities of Synthesis and Properties of Lignin–Silica Nanocomposites Prepared by Sol-Gel Method. Nanomaterials. 2018; 8(11):950. https://doi.org/10.3390/nano8110950
Chicago/Turabian StyleBudnyak, Tetyana M., Selda Aminzadeh, Ievgen V. Pylypchuk, Anastasia V. Riazanova, Valentin A. Tertykh, Mikael E. Lindström, and Olena Sevastyanova. 2018. "Peculiarities of Synthesis and Properties of Lignin–Silica Nanocomposites Prepared by Sol-Gel Method" Nanomaterials 8, no. 11: 950. https://doi.org/10.3390/nano8110950
APA StyleBudnyak, T. M., Aminzadeh, S., Pylypchuk, I. V., Riazanova, A. V., Tertykh, V. A., Lindström, M. E., & Sevastyanova, O. (2018). Peculiarities of Synthesis and Properties of Lignin–Silica Nanocomposites Prepared by Sol-Gel Method. Nanomaterials, 8(11), 950. https://doi.org/10.3390/nano8110950








