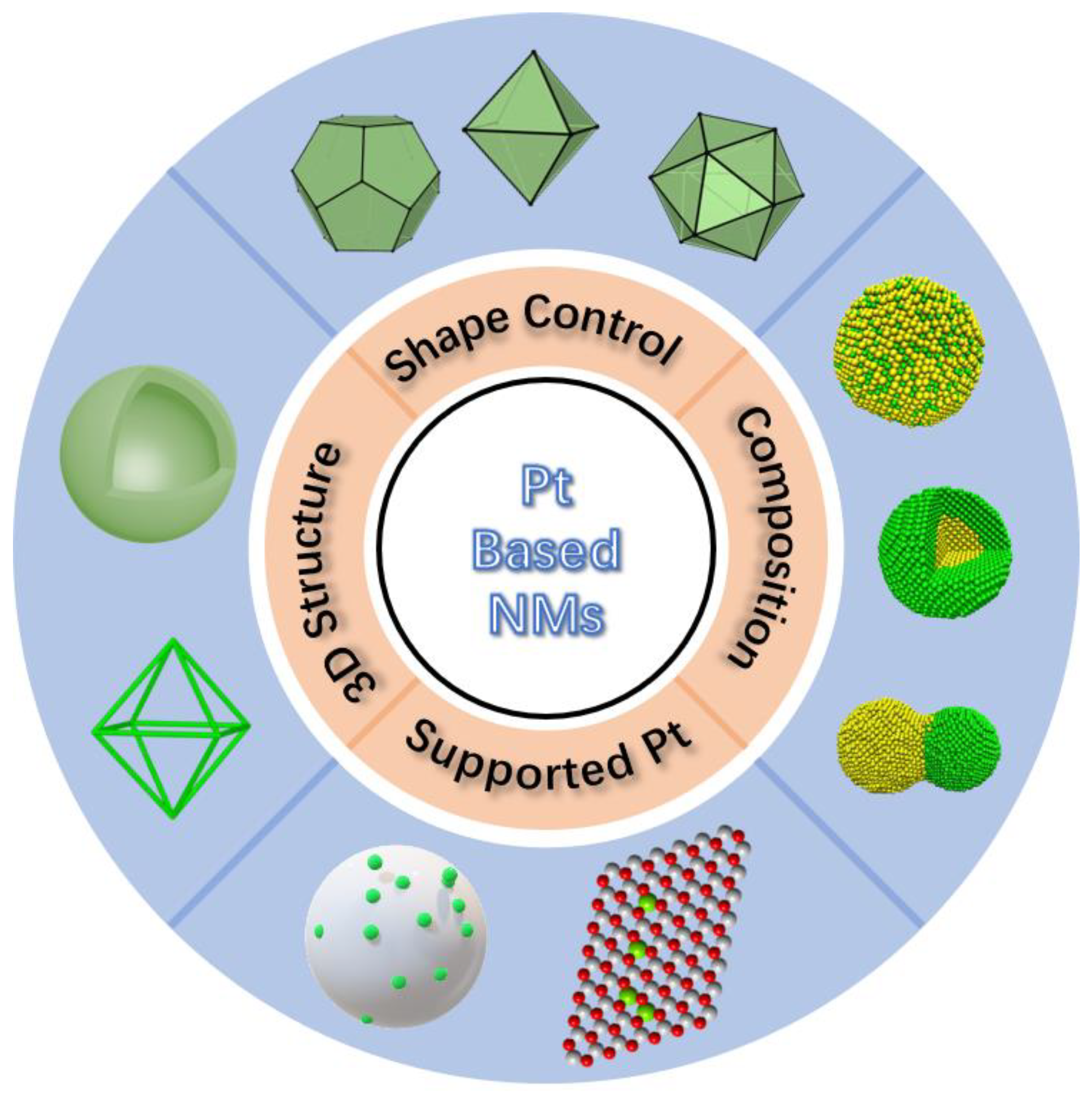
Nanostructure Optimization of Platinum-Based Nanomaterials for Catalytic Applications
Abstract
1. Introduction
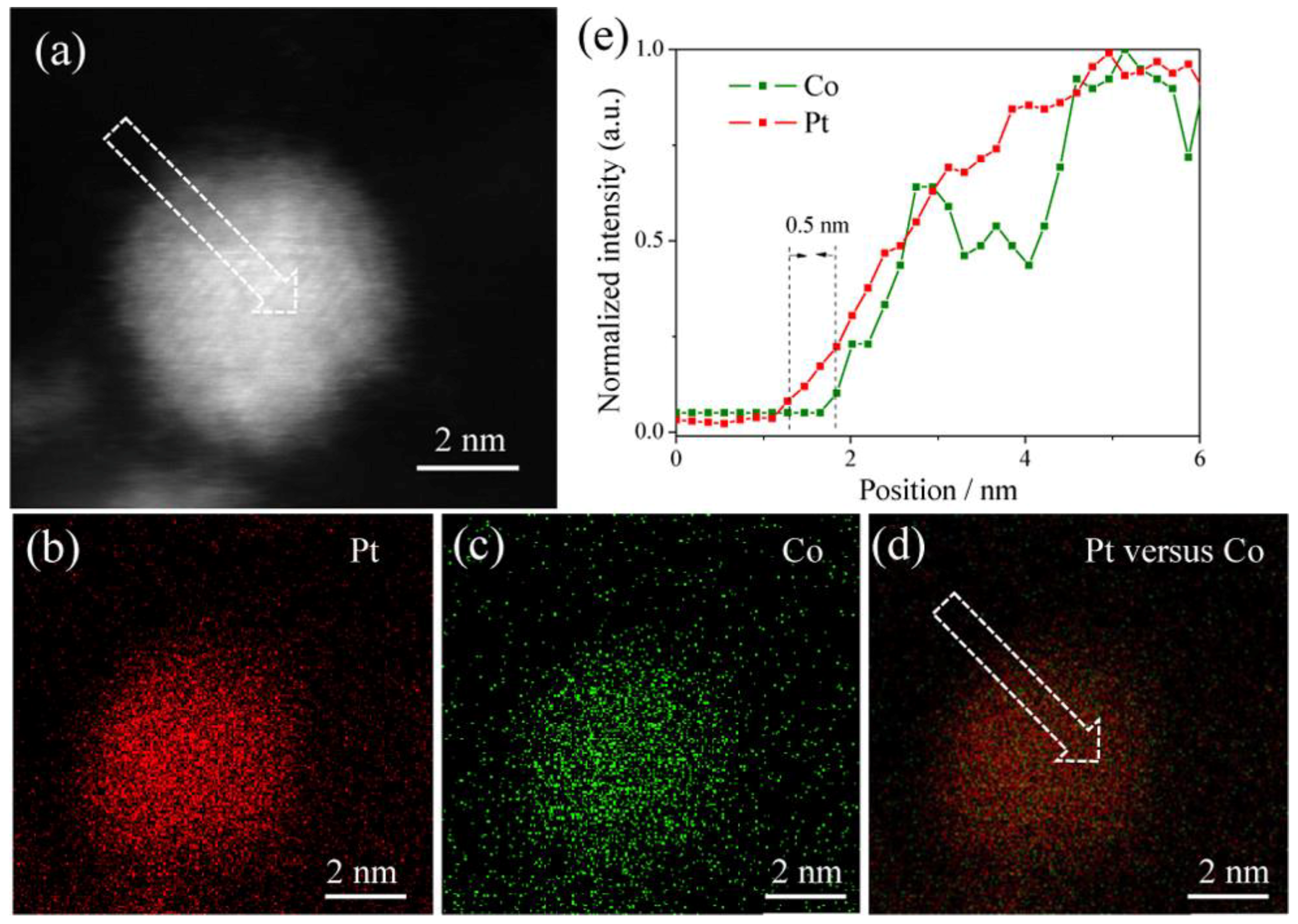
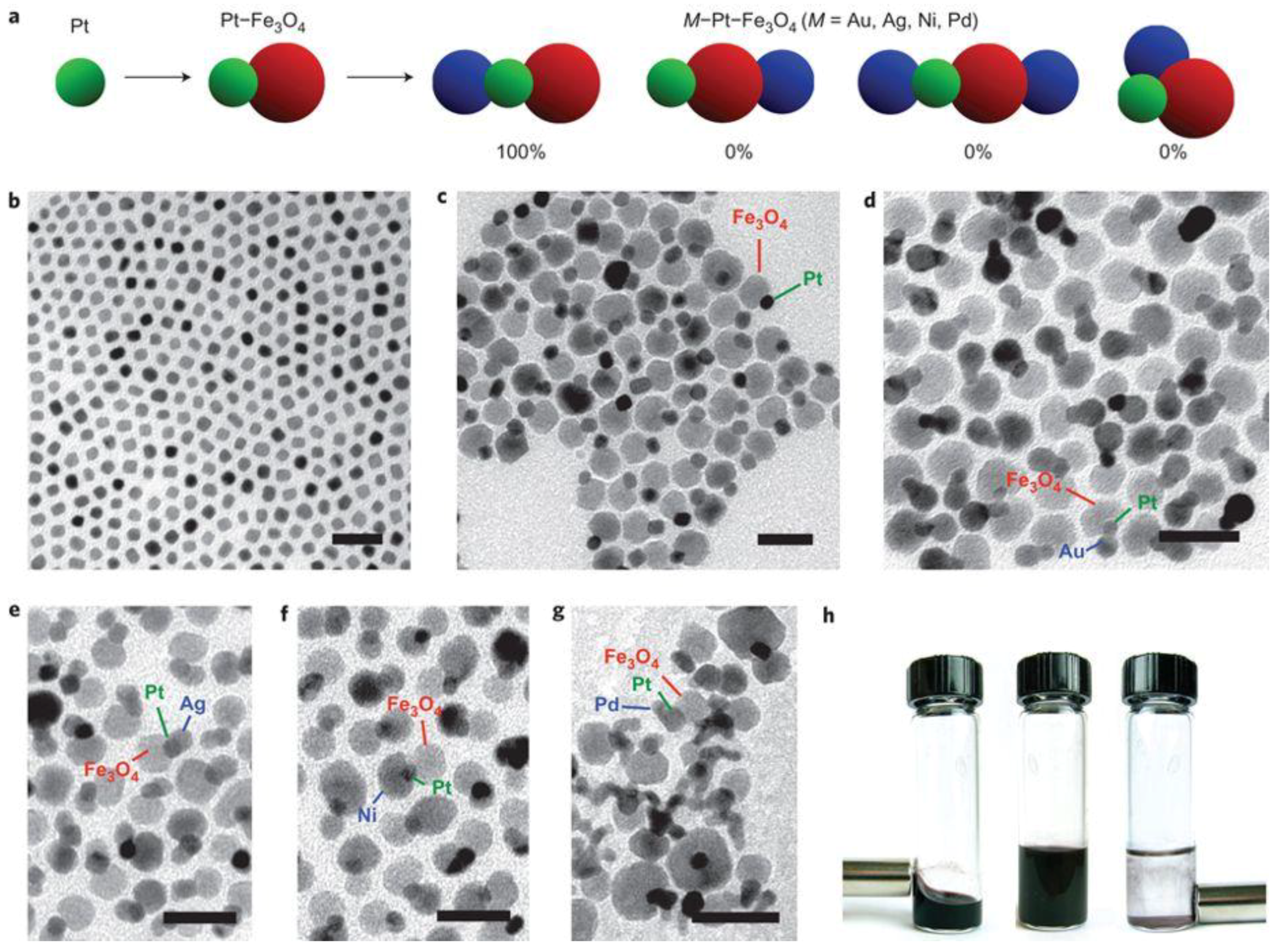
2. Composition Regulation
3. Shape Control
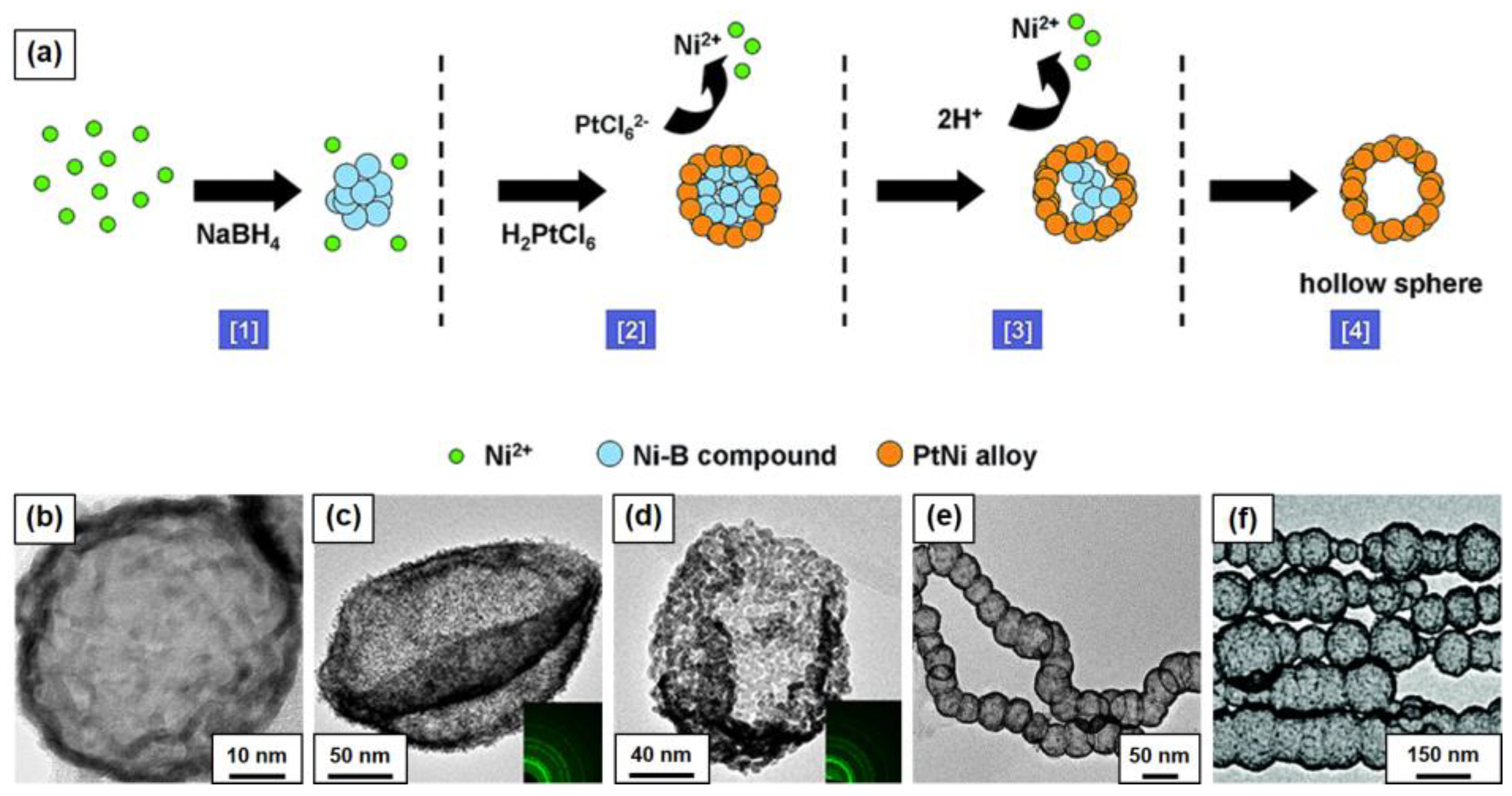
4. Construction of 3D Structures
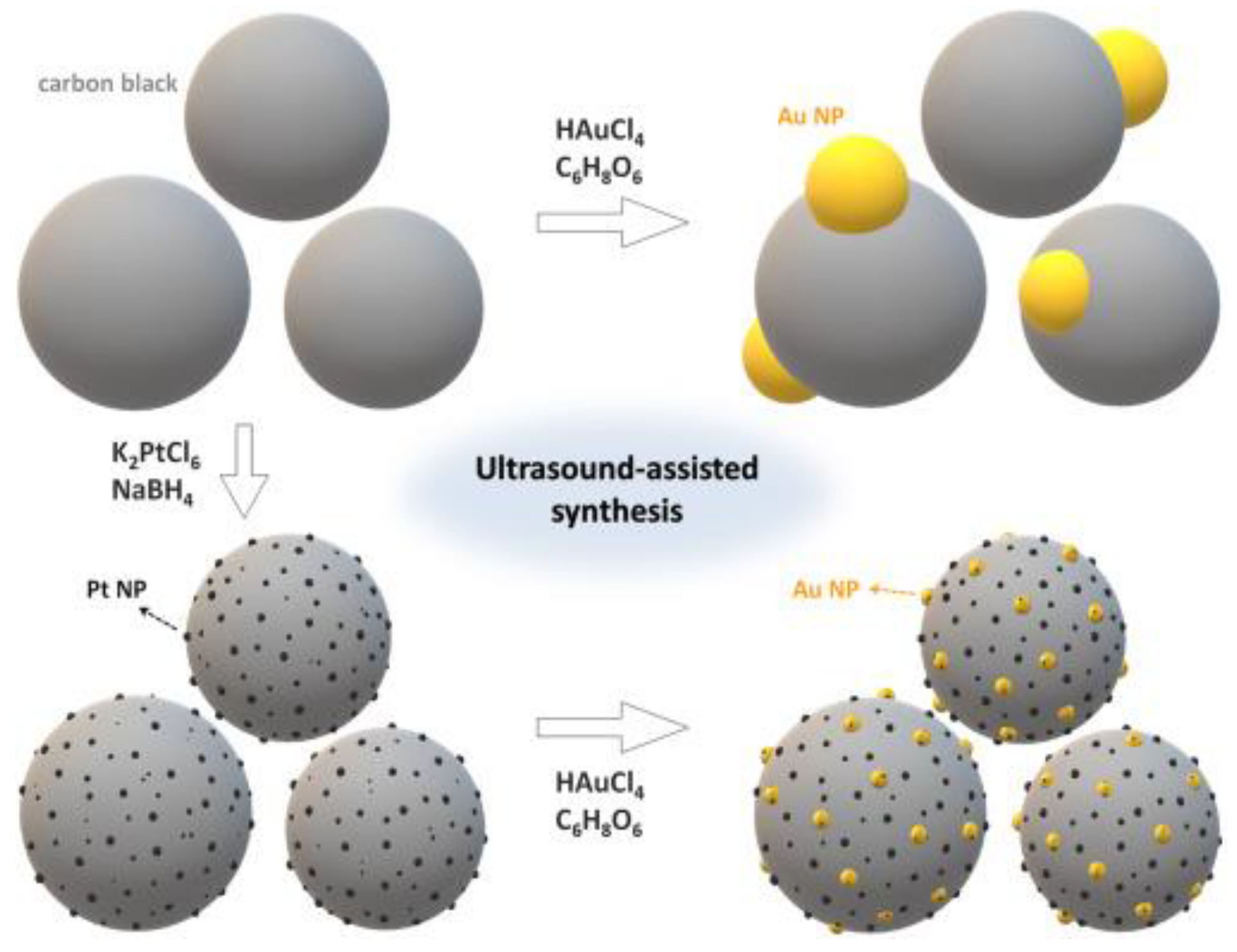
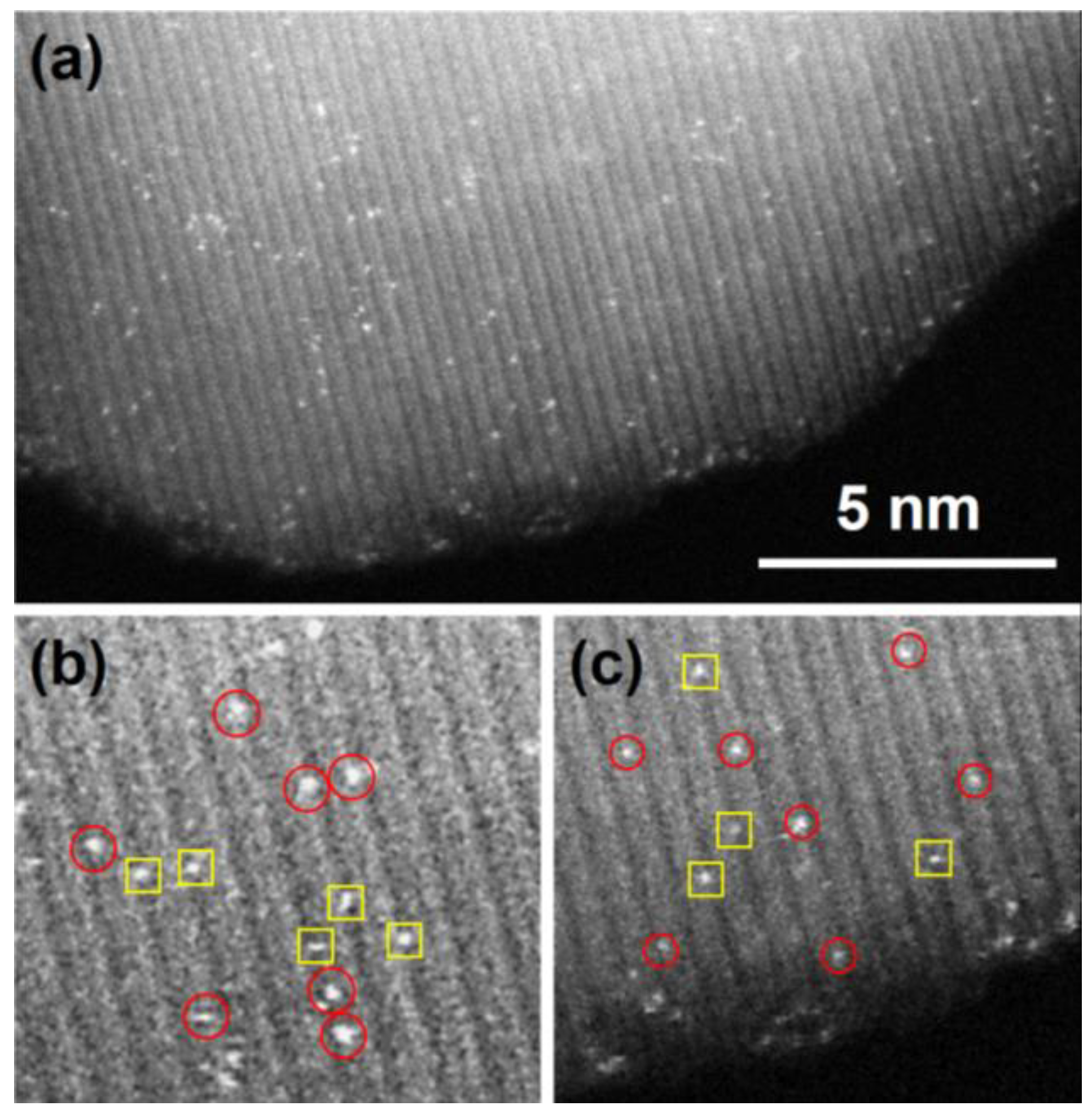
5. Supported Pt-Based NMs
6. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Chen, A.; Holt-Hindle, P. Platinum-based nanostructured materials: Synthesis, properties, and applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, B. Recent advances in porous Pt-based nanostructures: Synthesis and electrochemical applications. Chem. Soc. Rev. 2014, 43, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Badwal, S.P.S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, J.; Li, Y.; Datye, A.K.; Wang, Y. Bimetallic catalysts for hydrogen generation. Chem. Soc. Rev. 2012, 41, 7994–8008. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.; Hannan, M.A.; Mohamed, A.; Majlan, E.H.; Daud, W.R.W. A review on energy management system for fuel cell hybrid electric vehicle: Issues and challenges. Renew. Sustain. Energy Rev. 2015, 52, 802–814. [Google Scholar] [CrossRef]
- Elmer, T.; Worall, M.; Wu, S.Y.; Riffat, S.B. Fuel cell technology for domestic built environment applications: State of-the-art review. Renew. Sustain. Energy Rev. 2015, 42, 913–931. [Google Scholar] [CrossRef]
- Wolf, O.; Dasog, M.; Yang, Z.; Balberg, I.; Veinot, J.G.; Millo, O. Doping and quantum confinement effects in single Si nanocrystals observed by scanning tunneling spectroscopy. Nano Lett. 2013, 13, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Sichert, J.A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.; Milowska, K.Z.; Garcia Cortadella, R.; Nickel, B.; Cardenas-Daw, C.; Stolarczyk, J.K.; et al. Quantum size effect in organometal halide perovskite nanoplatelets. Nano Lett. 2015, 15, 6521–6527. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 2013, 46, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, H.C.; Liu, J.; Huang, C.Z.; Xia, Y. Use of reduction rate as a quantitative knob for controlling the twin structure and shape of palladium nanocrystals. Nano Lett. 2015, 15, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Tan, H.B.; Lin, J.J.; Luo, X.L.; Wang, S.P.; You, J.; Kang, Y.H.; Bando, Y.; Yamauchi, Y.; Kim, J. Emerging Pt-based electrocatalysts with highly open nanoarchitectures for boosting oxygen reduction reaction. Nano Today 2018, 21, 91–105. [Google Scholar] [CrossRef]
- Lin, R.; Cai, X.; Zeng, H.; Yu, Z. Stability of high-performance Pt-based catalysts for oxygen reduction reactions. Adv. Mater. 2018, 30, e1705332. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Shen, M.; Zhou, S.; Lee, C.T.; Zhao, M.; Lyu, Z.H.; Hood, Z.D.; Vara, M.; Gilroy, K.D.; Wang, K.; et al. Synthesis of Pt nanocrystals with different shapes using the same protocol to optimize their catalytic activity toward oxygen reduction. Mater. Today 2018, 21, 834–844. [Google Scholar] [CrossRef]
- Porter, N.S.; Wu, H.; Quan, Z.; Fang, J. Shape-control and electrocatalytic activity-enhancement of Pt-based bimetallic nanocrystals. Acc. Chem. Res. 2013, 46, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Kloke, A.; von Stetten, F.; Zengerle, R.; Kerzenmacher, S. Strategies for the fabrication of porous platinum electrodes. Adv. Mater. 2011, 23, 4976–5008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.W.; Yang, H.L.; Wang, Y.X.; Dou, S.X.; Liu, H.K. A comprehensive review on controlling surface composition of Pt-based bimetallic electrocatalysts. Adv. Energy Mater. 2018, 8, 1703597. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lim, B.; Lee, E.P.; Xia, Y.N. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Jackson, C.; Smith, G.T.; Inwood, D.W.; Leach, A.S.; Whalley, P.S.; Callisti, M.; Polcar, T.; Russell, A.E.; Levecque, P.; Kramer, D. Electronic metal-support interaction enhanced oxygen reduction activity and stability of boron carbide supported platinum. Nat. Commun. 2017, 8, 15802. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, H.; Gao, W.; Xue, W.; Liu, Z.; Huang, J.; Pan, X.; Huang, Y. Surface-engineered PtNi-O nanostructure with record-high performance for electrocatalytic hydrogen evolution reaction. J. Am. Chem. Soc. 2018, 140, 9046–9050. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, X.; Luo, M.; Chen, X.; Wang, L.; Li, Y.; Li, M.; Qin, Y.; Li, C.; Xu, N.; et al. Ultrathin PtPd-based nanorings with abundant step atoms enhance oxygen catalysis. Adv. Mater. 2018, 30, e1802136. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; You, Y.; Zhang, S.; Cai, W.; Xu, M.; Xie, L.; Wu, R.; Graham, G.W.; Pan, X. In situ atomic-scale observation of oxygen-driven core-shell formation in Pt3Co nanoparticles. Nat. Commun. 2017, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, G.; Cui, X.; Chen, B.; Zhu, Y.; Gong, Y.; Saleem, F.; Xi, S.; Du, Y.; Borgna, A.; et al. Crystal phase and architecture engineering of lotus-thalamus-shaped Pt-Ni anisotropic superstructures for highly efficient electrochemical hydrogen evolution. Adv. Mater. 2018, 30, e1801741. [Google Scholar] [CrossRef] [PubMed]
- Rizo, R.; Aran-Ais, R.M.; Padgett, E.; Muller, D.A.; Lazaro, M.J.; Solla-Gullon, J.; Feliu, J.M.; Pastor, E.; Abruna, H.D. Pt-richcore/Sn-richsubsurface/Ptskin nanocubes as highly active and stable electrocatalysts for the ethanol oxidation reaction. J. Am. Chem. Soc. 2018, 140, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yang, J.; Wen, M.; Wu, Q. Nanoalloy materials for chemical catalysis. Adv. Mater. 2018, 30, e1705698. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.M.; Dmitrieva, O.; Farle, M.; Dumpich, G.; Ye, H.Q.; Poppa, H.; Kilaas, R.; Kisielowski, C. Layer resolved structural relaxation at the surface of magnetic FePt icosahedral nanoparticles. Phys. Rev. Lett. 2008, 100, 017205. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.M.; Dmitrieva, O.; Farle, M.; Dumpich, G.; Acet, M.; Mejia-Rosales, S.; Perez-Tijerina, E.; Yacaman, M.J.; Kisielowski, C. FePt icosahedra with magnetic cores and catalytic shells. J. Phys. Chem. C 2009, 113, 4395–4400. [Google Scholar] [CrossRef]
- Buck, M.R.; Bondi, J.F.; Schaak, R.E. A total-synthesis framework for the construction of high-order colloidal hybrid nanoparticles. Nat. Chem. 2011, 4, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Wang, Y.; Fang, J. High-index faceted noble metal nanocrystals. Acc. Chem. Res. 2013, 46, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Gocyla, M.; Kuehl, S.; Shviro, M.; Heyen, H.; Selve, S.; Dunin-Borkowski, R.E.; Heggen, M.; Strasser, P. Shape stability of octahedral PtNi nanocatalysts for electrochemical oxygen reduction reaction studied by in situ transmission electron microscopy. ACS Nano 2018, 12, 5306–5311. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yang, P.; Markovic, N.M.; Stamenkovic, V.R. Shaping electrocatalysis through tailored nanomaterials. Nano Today 2016, 11, 587–600. [Google Scholar] [CrossRef]
- Du, H.Y.; Luo, S.P.; Wang, K.; Tang, M.; Sriphathoorat, R.; Jin, Y.S.; Shen, P.K. High-quality and deeply excavated Pt3Co nanocubes as efficient catalysts for liquid fuel electrooxidation. Chem. Mater. 2017, 29, 9613–9617. [Google Scholar] [CrossRef]
- Xia, T.; Liu, J.; Wang, S.; Wang, C.; Sun, Y.; Gu, L.; Wang, R. Enhanced catalytic activities of NiPt truncated octahedral nanoparticles toward ethylene glycol oxidation and oxygen reduction in alkaline electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 10841–10849. [Google Scholar] [CrossRef] [PubMed]
- Shan, A.X.; Chen, C.P.; Zhang, W.; Cheng, D.J.; Shen, X.; Yu, R.C.; Wang, R.M. Giant enhancement and anomalous temperature dependence of magnetism in monodispersed NiPt2 nanoparticles. Nano Res. 2017, 10, 3238–3247. [Google Scholar] [CrossRef]
- Xia, T.Y.; Liu, J.L.; Wang, S.G.; Wang, C.; Sun, Y.; Wang, R.M. Nanomagnetic CoPt truncated octahedrons: Facile synthesis, superior electrocatalytic activity and stability for methanol oxidation. Sci. China-Mater. 2017, 60, 57–67. [Google Scholar] [CrossRef]
- Beermann, V.; Gocyla, M.; Kuhl, S.; Padgett, E.; Schmies, H.; Goerlin, M.; Erini, N.; Shviro, M.; Heggen, M.; Dunin-Borkowski, R.E.; et al. Tuning the electrocatalytic oxygen reduction reaction activity and stability of shape-controlled Pt-Ni nanoparticles by thermal annealing–elucidating the surface atomic structural and compositional changes. J. Am. Chem. Soc. 2017, 139, 16536–16547. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Shen, P.K. Concave platinum-copper octopod nanoframes bounded with multiple high-index facets for efficient electrooxidation catalysis. ACS Nano 2017, 11, 11946–11953. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Li, S.; Wang, R.; Song, Y. Surface and interface engineering of FePt/C nanocatalysts for electro-catalytic methanol oxidation: Enhanced activity and durability. Nanoscale 2017, 9, 4066–4075. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.F.; Chong, Y.; Fang, G.; Jiang, X.M.; Pan, Y.; Chen, C.Y.; Yin, J.J.; Ge, C.C. Synthesis of Pt hollow nanodendrites with enhanced peroxidase-like activity against bacterial infections: Implication for wound healing. Adv. Funct. Mater. 2018, 28, 1801484. [Google Scholar] [CrossRef]
- Park, J.; Kwon, T.; Kim, J.; Jin, H.; Kim, H.Y.; Kim, B.; Joo, S.H.; Lee, K. Hollow nanoparticles as emerging electrocatalysts for renewable energy conversion reactions. Chem. Soc. Rev. 2018, 47, 8173–8202. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.P.; Yuan, X.L.; Hu, H.C.; Liu, Y.L.; Zheng, H.W.; Yang, D.; Chen, L.; Cao, M.H.; Xu, Y.; Min, Y.L.; et al. Solvothermal synthesis of alloyed PtNi colloidal nanocrystal clusters (CNCs) with enhanced catalytic activity for methanol oxidation. Adv. Funct. Mater. 2018, 28, 1704774. [Google Scholar] [CrossRef]
- Kim, J.; Park, W.H.; Doh, W.H.; Lee, S.W.; Noh, M.C.; Gallet, J.J.; Bournel, F.; Kondoh, H.; Mase, K.; Jung, Y.; et al. Adsorbate-driven reactive interfacial Pt-NiO1-x nanostructure formation on the Pt3Ni(111) alloy surface. Sci. Adv. 2018, 4, eaat3151. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, X.D.; Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Chin. J. Catal. 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Zhang, B.W.; Zhang, Z.C.; Liao, H.G.; Gong, Y.; Gu, L.; Qu, X.M.; You, L.X.; Liu, S.; Huang, L.; Tian, X.C.; et al. Tuning Pt-skin to Ni-rich surface of Pt3Ni catalysts supported on porous carbon for enhanced oxygen reduction reaction and formic electro-oxidation. Nano Energy 2016, 19, 198–209. [Google Scholar] [CrossRef]
- Moliner, M.; Gabay, J.E.; Kliewer, C.E.; Carr, R.T.; Guzman, J.; Casty, G.L.; Serna, P.; Corma, A. Reversible Transformation of Pt Nanoparticles into Single Atoms inside High-Silica Chabazite Zeolite. J. Am. Chem. Soc. 2016, 138, 15743–15750. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Lee, S.; Zugic, B.; Huang, J.; Allard, L.F.; Flytzani-Stephanopoulos, M. A common single-site Pt(II)-O(OH)x- species stabilized by sodium on “active” and “inert” supports catalyzes the water-gas shift reaction. J. Am. Chem. Soc. 2015, 137, 3470–3473. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Choi, M. Physicochemical stabilization of Pt against sintering for a dehydrogenation catalyst with high activity, selectivity, and durability. ACS Catal. 2016, 6, 2819–2826. [Google Scholar] [CrossRef]
- Zheng, B.; Wu, S.; Yang, X.; Jia, M.; Zhang, W.; Liu, G. Room temperature CO oxidation over Pt/MgFe2O4: A stable inverse spinel oxide support for preparing highly efficient Pt catalyst. ACS Appl. Mater. Interfaces 2016, 8, 26683–26689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.W.; Sheng, T.; Wang, Y.X.; Qu, X.M.; Zhang, J.M.; Zhang, Z.C.; Liao, H.G.; Zhu, F.C.; Dou, S.X.; Jiang, Y.X.; et al. Platinum–cobalt bimetallic nanoparticles with Pt skin for electro-oxidation of ethanol. ACS Catal. 2016, 7, 892–895. [Google Scholar] [CrossRef]
- Ferrin, P.; Mavrikakis, M. Structure sensitivity of methanol electrooxidation on transition metals. J. Am. Chem. Soc. 2009, 131, 14381–14389. [Google Scholar] [CrossRef] [PubMed]
- Frelink, T.; Visscher, W.; van Veen, J.A.R. On the role of Ru and Sn as promotors of methanol electro-oxidation over Pt. Surf. Sci. 1995, 335, 353–360. [Google Scholar] [CrossRef]
- Hammer, B.; Morikawa, Y.; Norskov, J.K. CO chemisorption at metal surfaces and overlayers. Phys. Rev. Lett. 1996, 76, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.; Hammer, B.; Stoltze, P.; Skriver, H.L.; Nørskov, J.K. Surface electronic structure and reactivity of transition and noble metals. J. Mol. Catal. A-Chem 1997, 115, 421–429. [Google Scholar] [CrossRef]
- Kua, J.; Goddard, W.A. Oxidation of methanol on 2nd and 3rd row group VIII transition metals (Pt, Ir, Os, Pd, Rh, and Ru): Application to direct methanol fuel cells. J. Am. Chem. Soc. 1999, 121, 10928–10941. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.; Ferrin, P.; Tritsaris, G.A.; Nilekar, A.U.; Koh, S.; Bae, S.E.; Brankovic, S.R.; Strasser, P.; Mavrikakis, M. Bifunctional anode catalysts for direct methanol fuel cells. Energy Environ. Sci. 2012, 5, 8335–8342. [Google Scholar] [CrossRef]
- Liu, P.; Norskov, J.K. Ligand and ensemble effects in adsorption on alloy surfaces. Phys. Chem. Chem. Phys. 2001, 3, 3814–3818. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, J.; Jeon, M.; Ridwan, M.; Park, H.S.; Choi, S.H.; Nam, S.W.; Han, J.; Lim, T.H.; Ham, H.C.; et al. Experimental and computational studies of formic acid dehydrogenation over PdAu: Influence of ensemble and ligand effects on catalysis. J. Mater. Chem. A 2016, 4, 14141–14147. [Google Scholar] [CrossRef]
- Grabow, L.; Xu, Y.; Mavrikakis, M. Lattice strain effects on CO oxidation on Pt(111). Phys. Chem. Chem. Phys. 2006, 8, 3369–3374. [Google Scholar] [CrossRef] [PubMed]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kwon, K.; Rhee, C.K.; Han, J.; Lim, T.H. Modification of Pt nanoelectrodes dispersed on carbon support using irreversible adsorption of Bi to enhance formic acid oxidation. Electrochim. Acta 2008, 53, 7744–7750. [Google Scholar] [CrossRef]
- Choi, S.I.; Lee, S.U.; Kim, W.Y.; Choi, R.; Hong, K.; Nam, K.M.; Han, S.W.; Park, J.T. Composition-controlled PtCo alloy nanocubes with tuned electrocatalytic activity for oxygen reduction. ACS Appl. Mater. Interfaces 2012, 4, 6228–6234. [Google Scholar] [CrossRef] [PubMed]
- Erini, N.; Loukrakpam, R.; Petkov, V.; Baranova, E.A.; Yang, R.; Teschner, D.; Huang, Y.; Brankovic, S.R.; Strasser, P. Ethanol electro-oxidation on ternary platinum–rhodium–tin nanocatalysts: Insights in the atomic 3D structure of the active catalytic phase. ACS Catal. 2014, 4, 1859–1867. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, C.; Qin, R.; Shao, Z.; An, D.; Zhang, W.; Wang, Y. Uniform Pd–Pt alloy nanoparticles supported on graphite nanoplatelets with high electrocatalytic activity towards methanol oxidation. J. Mater. Chem. A 2015, 3, 5204–5211. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; Xu, Z.; Carlton, C.E.; Kim, J.; Han, B.; Lee, S.W.; Bonnet, N.; Marzari, N.; Allard, L.F.; Gasteiger, H.A.; et al. Surface composition tuning of Au-Pt bimetallic nanoparticles for enhanced carbon monoxide and methanol electro-oxidation. J. Am. Chem. Soc. 2013, 135, 7985–7991. [Google Scholar] [CrossRef] [PubMed]
- Kuttiyiel, K.A.; Sasaki, K.; Choi, Y.; Su, D.; Liu, P.; Adzic, R.R. Nitride stabilized PtNi core-shell nanocatalyst for high oxygen reduction activity. Nano Lett. 2012, 12, 6266–6271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hao, Y.; Su, D.; Doan-Nguyen, V.V.; Wu, Y.; Li, J.; Sun, S.; Murray, C.B. Monodisperse core/shell Ni/FePt nanoparticles and their conversion to Ni/Pt to catalyze oxygen reduction. J. Am. Chem. Soc. 2014, 136, 15921–15924. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Choi, S.I.; Lu, N.; Roling, L.T.; Herron, J.A.; Zhang, L.; Park, J.; Wang, J.; Kim, M.J.; Xie, Z.; et al. Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Lett. 2014, 14, 3570–3576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hsieh, Y.C.; Volkov, V.; Su, D.; An, W.; Si, R.; Zhu, Y.; Liu, P.; Wang, J.X.; Adzic, R.R. High performance Pt monolayer catalysts produced via core-catalyzed coating in ethanol. ACS Catal. 2014, 4, 738–742. [Google Scholar] [CrossRef]
- Sasaki, K.; Naohara, H.; Choi, Y.; Cai, Y.; Chen, W.F.; Liu, P.; Adzic, R.R. Highly stable Pt monolayer on PdAu nanoparticle electrocatalysts for the oxygen reduction reaction. Nat. Commun. 2012, 3, 1115. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Core-shell compositional fine structures of dealloyed PtxNi1-x nanoparticles and their impact on oxygen reduction catalysis. Nano Lett. 2012, 12, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Yu, R.; Luo, J.; Cheng, Z.; Zhu, J. Lattice strain distributions in individual dealloyed Pt-Fe catalyst nanoparticles. J. Phys. Chem. Lett. 2012, 3, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Frenkel, A.I.; Su, D.; Teng, X. Enhanced electrokinetics of C–C bond splitting during ethanol oxidation by using a Pt/Rh/Sn catalyst with a partially oxidized Pt and Rh core and a SnO2 shell. ChemCatChem 2016, 8, 2876–2880. [Google Scholar] [CrossRef]
- Sang, W.; Zheng, T.; Wang, Y.; Li, X.; Zhao, X.; Zeng, J.; Hou, J.G. One-step synthesis of hybrid nanocrystals with rational tuning of the morphology. Nano Lett. 2014, 14, 6666–6671. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Qi, K.; Zhang, L.; Zhang, H.; Yu, S.; Cui, X. Engineering Pt/Pd interfacial electronic structures for highly efficient hydrogen evolution and alcohol oxidation. ACS Appl. Mater. Interfaces 2017, 9, 18008–18014. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, Y.W.; Tao, F.F. Shape control of bimetallic nanocatalysts through well-designed colloidal chemistry approaches. Chem. Soc. Rev. 2012, 41, 8050–8065. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Cozzoli, P.D. Colloidal heterostructured nanocrystals: Synthesis and growth mechanisms. Nano Today 2010, 5, 449–493. [Google Scholar] [CrossRef]
- Kang, Y.; Li, M.; Cai, Y.; Cargnello, M.; Diaz, R.E.; Gordon, T.R.; Wieder, N.L.; Adzic, R.R.; Gorte, R.J.; Stach, E.A.; et al. Heterogeneous catalysts need not be so “heterogeneous”: Monodisperse Pt nanocrystals by combining shape-controlled synthesis and purification by colloidal recrystallization. J. Am. Chem. Soc. 2013, 135, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qi, L.; You, H.; Gross, A.; Li, J.; Yang, H. Icosahedral platinum alloy nanocrystals with enhanced electrocatalytic activities. J. Am. Chem. Soc. 2012, 134, 11880–11883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, J.; Yang, H. Highly uniform platinum icosahedra made by hot injection-assisted GRAILS method. Nano Lett. 2013, 13, 2870–2874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Fang, J.; Zou, S. Synthesis and oxygen reduction activity of shape-controlled Pt3Ni nanopolyhedra. Nano Lett. 2010, 10, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Martens, B.; Luo, Z.; Xu, D.; Wang, Y.; Zou, S.; Fang, J. Pt–Cu nanoctahedra: Synthesis and comparative study with nanocubes on their electrochemical catalytic performance. Chem. Sci. 2012, 3, 3302–3306. [Google Scholar] [CrossRef]
- Wang, C.; Lin, C.; Zhang, L.; Quan, Z.; Sun, K.; Zhao, B.; Wang, F.; Porter, N.; Wang, Y.; Fang, J. Pt3Co concave nanocubes: Synthesis, formation understanding, and enhanced catalytic activity toward hydrogenation of styrene. Chem.-Eur. J. 2014, 20, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Pyo, J.B.; Ye, X.; Gordon, T.R.; Murray, C.B. Synthesis, shape control, and methanol electro-oxidation properties of Pt-Zn alloy and Pt3Zn intermetallic nanocrystals. ACS Nano 2012, 6, 5642–5647. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Guo, S.; Zhang, X.; Shen, X.; Su, D.; Lu, G.; Zhu, X.; Yao, J.; Guo, J.; Huang, X. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat. Commun. 2016, 7, 11850. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.X.; Min, X.Q.; Zhu, W.; Wu, H.S.; Zhang, Y.W.; Yan, C.H. Multiply twinned Pt-Pd nanoicosahedrons as highly active electrocatalysts for methanol oxidation. Chem. Commun. 2012, 48, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Bian, T.; Choi, S.I.; Jiang, Y.; Jin, C.; Fu, M.; Zhang, H.; Yang, D. Kinetically controlled synthesis of Pt-Cu alloy concave nanocubes with high-index facets for methanol electro-oxidation. Chem. Commun. 2014, 50, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.X.; Min, X.Q.; Zhang, Y.W.; Yan, C.H. Shape-selective synthesis and facet-dependent enhanced electrocatalytic activity and durability of monodisperse sub-10 nm Pt-Pd tetrahedrons and cubes. J. Am. Chem. Soc. 2011, 133, 3816–3819. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, S.B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Direct observations of oxygen-induced platinum nanoparticle ripening studied by in situ TEM. J. Am. Chem. Soc. 2010, 132, 7968–7975. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, M.S.; Goodman, D.W. Sintering of Au particles supported on TiO2(110) during CO oxidation. J. Phys. Chem. C 2008, 113, 254–260. [Google Scholar] [CrossRef]
- Hu, J.; Chen, M.; Fang, X.; Wu, L. Fabrication and application of inorganic hollow spheres. Chem. Soc. Rev. 2011, 40, 5472–5491. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.P.; Zhang, H.M.; Hu, J.S.; Guo, Y.G.; Wan, L.J.; Bai, C.L. Pt hollow nanospheres: Facile synthesis and enhanced electrocatalysts. Angew. Chem. Int. Ed. 2004, 43, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Becknell, N.; Yu, Y.; Kim, D.; Chen, C.; Kornienko, N.; Somorjai, G.A.; Yang, P. Anisotropic phase segregation and migration of Pt in nanocrystals en route to nanoframe catalysts. Nat. Mater. 2016, 15, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Roling, L.T.; Wang, X.; Vara, M.; Chi, M.; Liu, J.; Choi, S.I.; Park, J.; Herron, J.A.; Xie, Z.; et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 2015, 349, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Kang, S.W.; Choi, B.S.; Kim, D.; Lee, S.B.; Han, S.W. Controlled synthesis of Pd-Pt alloy hollow nanostructures with enhanced catalytic activities for oxygen reduction. ACS Nano 2012, 6, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.; Baik, H.; Choi, D.S.; Cheon, J.Y.; Kim, B.; Kim, H.; Kwon, S.J.; Joo, S.H.; Jung, Y.; Lee, K. Skeletal octahedral nanoframe with Cartesian coordinates via geometrically precise nanoscale phase segregation in a Pt@Ni core-shell nanocrystal. ACS Nano 2015, 9, 2856–2867. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, J.; Lu, X. Tailoring galvanic replacement reaction for the preparation of Pt/Ag bimetallic hollow nanostructures with controlled number of voids. ACS Nano 2012, 6, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, W.; Sun, Q.; Wang, S.; Sun, K.; Schwank, J.; Wang, R. In situ tracing of atom migration in Pt/NiPt hollow spheres during catalysis of CO oxidation. Chem. Commun. 2014, 50, 1804–1807. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, W.; Wang, R.M. Double-layered NiPt nanobowls with ultrathin shell synthesized in water at room temperature. CrystEngComm 2012, 14, 5151–5154. [Google Scholar] [CrossRef]
- Sun, Q.; Ren, Z.; Wang, R.M.; Wang, N.; Cao, X. Platinum catalyzed growth of NiPt hollow spheres with an ultrathin shell. J. Mater. Chem. 2011, 21, 1925–1930. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.G.; Wang, R.M. Well-aligned CoPt hollow nanochains synthesized in water at room temperature. J. Phys. Chem. C 2012, 116, 5352–5357. [Google Scholar] [CrossRef]
- Shan, A.X.; Chen, Z.C.; Li, B.Q.; Chen, C.P.; Wang, R.M. Monodispersed, ultrathin NiPt hollow nanospheres with tunable diameter and composition via a green chemical synthesis. J. Mater. Chem. A 2015, 3, 1031–1036. [Google Scholar] [CrossRef]
- Fan, H.; Cheng, M.; Wang, Z.; Wang, R. Layer-controlled Pt-Ni porous nanobowls with enhanced electrocatalytic performance. Nano Res. 2016, 10, 187–198. [Google Scholar] [CrossRef]
- Liu, J.; Xia, T.; Wang, S.; Yang, G.; Dong, B.; Wang, C.; Ma, Q.; Sun, Y.; Wang, R. Oriented-assembly of hollow FePt nanochains with tunable catalytic and magnetic properties. Nanoscale 2016, 8, 11432–11440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.; Sun, Y.; Wang, R. Magnetic field modulated SERS enhancement of CoPt hollow nanoparticles with sizes below 10 nm. Nanoscale 2018, 10, 12650–12656. [Google Scholar] [CrossRef] [PubMed]
- Shan, A.X.; Cheng, M.; Fan, H.S.; Chen, Z.C.; Wang, R.M.; Chen, C.P. NiPt hollow nanocatalyst: Green synthesis, size control and electrocatalysis. Prog. Nat. Sci.-Mater. 2014, 24, 175–178. [Google Scholar] [CrossRef]
- Shen, X.; Sun, Q.; Zhu, J.; Yao, Y.; Liu, J.; Jin, C.Q.; Yu, R.C.; Wang, R.M. Structural stability and Raman scattering of CoPt and NiPt hollow nanospheres under high pressure. Prog. Nat. Sci.-Mater. 2013, 23, 382–387. [Google Scholar] [CrossRef]
- Dubau, L.; Asset, T.; Chattot, R.; Bonnaud, C.; Vanpeene, V.; Nelayah, J.; Maillard, F. Tuning the performance and the stability of porous hollow PtNi/C nanostructures for the oxygen reduction reaction. ACS Catal. 2015, 5, 5333–5341. [Google Scholar] [CrossRef]
- Shviro, M.; Polani, S.; Zitoun, D. Hollow octahedral and cuboctahedral nanocrystals of ternary Pt-Ni-Au alloys. Nanoscale 2015, 7, 13521–13529. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Niu, Z.; Xie, C.; Gao, M.; Lai, M.; Li, M.; Yang, P. Effects of catalyst processing on the activity and stability of Pt-Ni nanoframe electrocatalysts. ACS Nano 2018, 12, 8697–8705. [Google Scholar] [CrossRef] [PubMed]
- Becknell, N.; Son, Y.; Kim, D.; Li, D.; Yu, Y.; Niu, Z.; Lei, T.; Sneed, B.T.; More, K.L.; Markovic, N.M.; et al. Control of architecture in rhombic dodecahedral Pt-Ni nanoframe electrocatalysts. J. Am. Chem. Soc. 2017, 139, 11678–11681. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, D.; Zhou, G.; Yu, R.; Chen, C.; Li, Y. Sophisticated construction of Au islands on Pt-Ni: An ideal trimetallic nanoframe catalyst. J. Am. Chem. Soc. 2014, 136, 11594–11597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Ye, S.Y. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC–Part I. Physico-chemical and electronic interaction between Pt and carbon support, and activity enhancement of Pt/C catalyst. J. Power Sources 2007, 172, 133–144. [Google Scholar] [CrossRef]
- Li, B.; Fan, H.; Cheng, M.; Song, Y.; Li, F.; Wang, X.; Wang, R. Porous Pt–NiOx nanostructures with ultrasmall building blocks and enhanced electrocatalytic activity for the ethanol oxidation reaction. RSC Adv. 2018, 8, 698–705. [Google Scholar] [CrossRef]
- Fan, H.S.; Cheng, M.; Wang, L.; Song, Y.J.; Cui, Y.M.; Wang, R.M. Extraordinary electrocatalytic performance for formic acid oxidation by the synergistic effect of Pt and Au on carbon black. Nano Energy 2018, 48, 1–9. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, M.; Zhao, X.; Li, K.; Liu, C.; Xing, W. Nitrogen-doped carbon-graphene composites enhance the electrocatalytic performance of the supported Pt catalysts for methanol oxidation. Chem. Commun. 2014, 50, 12201–12203. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y.; Li, S.; Jiang, S.P. Self-assembled platinum nanoparticles on sulfonic acid-grafted graphene as effective electrocatalysts for methanol oxidation in direct methanol fuel cells. Sci. Rep. 2016, 6, 21530. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Z.; Long, R.; Xiao, K.; Xi, J. Synthesis of ultrafine Pt nanoparticles stabilized by pristine graphene nanosheets for electro-oxidation of methanol. ACS Appl. Mater. Interfaces 2014, 6, 15162–15170. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; He, G.; Shen, P.K.; Luo, Z.; Xie, J.; Chen, M. MoC–graphite composite as a Pt electrocatalyst support for highly active methanol oxidation and oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4014–4022. [Google Scholar] [CrossRef]
- Qiu, Z.; Huang, H.; Du, J.; Tao, X.; Xia, Y.; Feng, T.; Gan, Y.; Zhang, W. Biotemplated synthesis of bark-structured TiC nanowires as Pt catalyst supports with enhanced electrocatalytic activity and durability for methanol oxidation. J. Mater. Chem. A 2014, 2, 8003–8008. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, M.L.; Yu, Y.D.; Zhu, Y.A.; Sui, Z.J.; Zhou, X.G.; Holmen, A.; Chen, D. Size-dependent reaction mechanism and kinetics for propane dehydrogenation over Pt catalysts. ACS Catal. 2015, 5, 6310–6319. [Google Scholar] [CrossRef]
- Wei, S.; Li, A.; Liu, J.C.; Li, Z.; Chen, W.; Gong, Y.; Zhang, Q.; Cheong, W.C.; Wang, Y.; Zheng, L.; et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 2018, 13, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.J.; Hensley, A.J.R.; Marcinkowski, M.D.; Zhang, R.Q.; Lucci, F.R.; Coughlin, B.; Schilling, A.C.; McEwen, J.S.; Sykes, E.C.H. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 2018, 1, 192–198. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Pereira Hernandez, X.I.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.I.P.; DeLaRiva, A.; Wang, M.; Engelhard, M.H.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wang, R.; Liu, J. Stability investigation of a high number density Pt1/Fe2O3 single-atom catalyst under different gas environments by HAADF-STEM. Nanotechnology 2018, 29, 204002. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, S.; Du, Z.; Fan, H.; Wang, R. Nanostructure Optimization of Platinum-Based Nanomaterials for Catalytic Applications. Nanomaterials 2018, 8, 949. https://doi.org/10.3390/nano8110949
Duan S, Du Z, Fan H, Wang R. Nanostructure Optimization of Platinum-Based Nanomaterials for Catalytic Applications. Nanomaterials. 2018; 8(11):949. https://doi.org/10.3390/nano8110949
Chicago/Turabian StyleDuan, Sibin, Zhe Du, Hongsheng Fan, and Rongming Wang. 2018. "Nanostructure Optimization of Platinum-Based Nanomaterials for Catalytic Applications" Nanomaterials 8, no. 11: 949. https://doi.org/10.3390/nano8110949
APA StyleDuan, S., Du, Z., Fan, H., & Wang, R. (2018). Nanostructure Optimization of Platinum-Based Nanomaterials for Catalytic Applications. Nanomaterials, 8(11), 949. https://doi.org/10.3390/nano8110949






