Preparation of Hydrogen Peroxide Sensitive Nanofilms by a Layer-by-Layer Technique
Abstract
1. Introduction
2. Materials and Methods
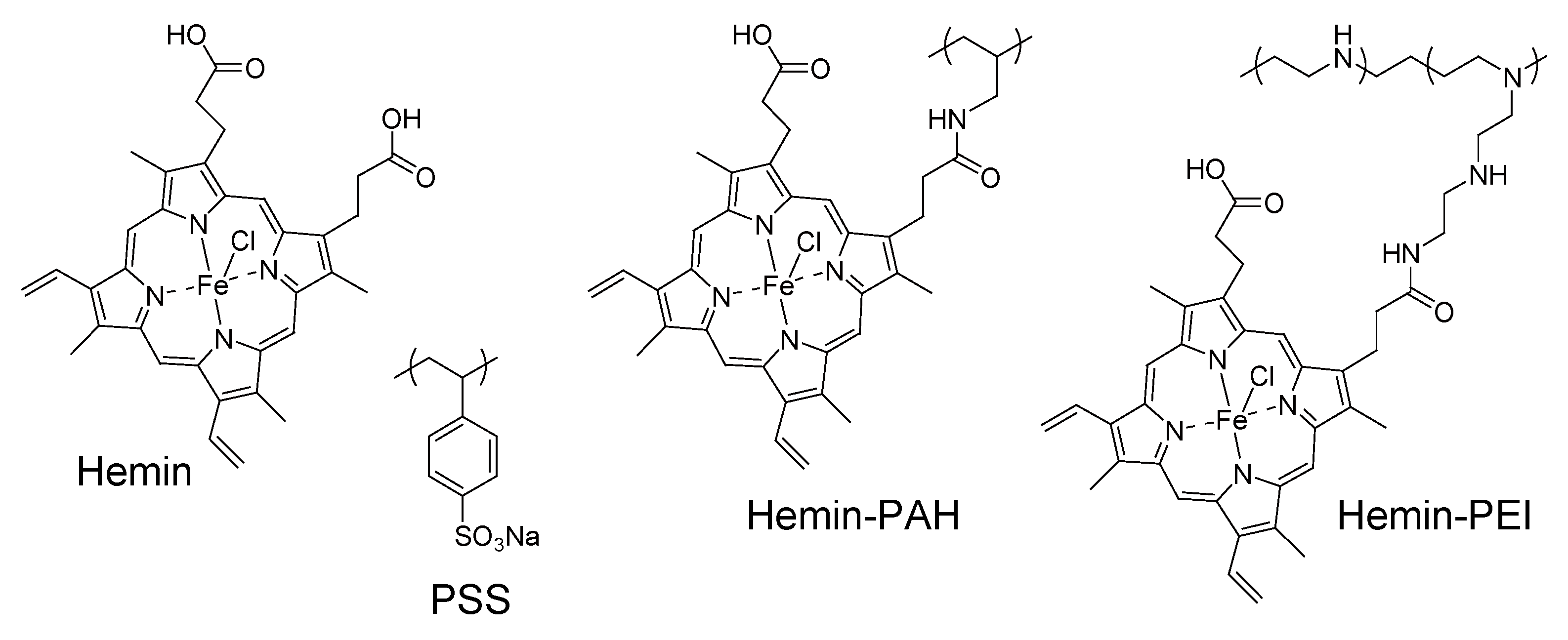
2.1. Materials
2.2. Apparatus
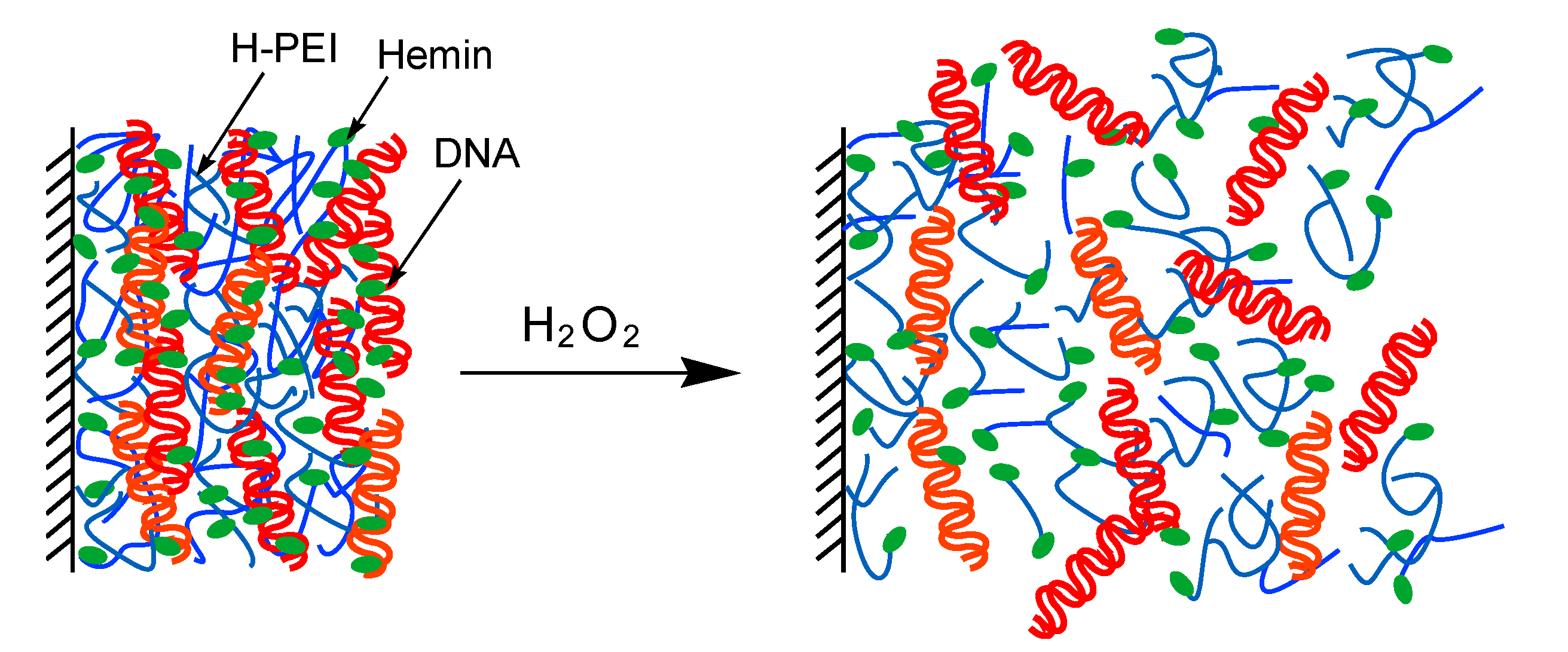
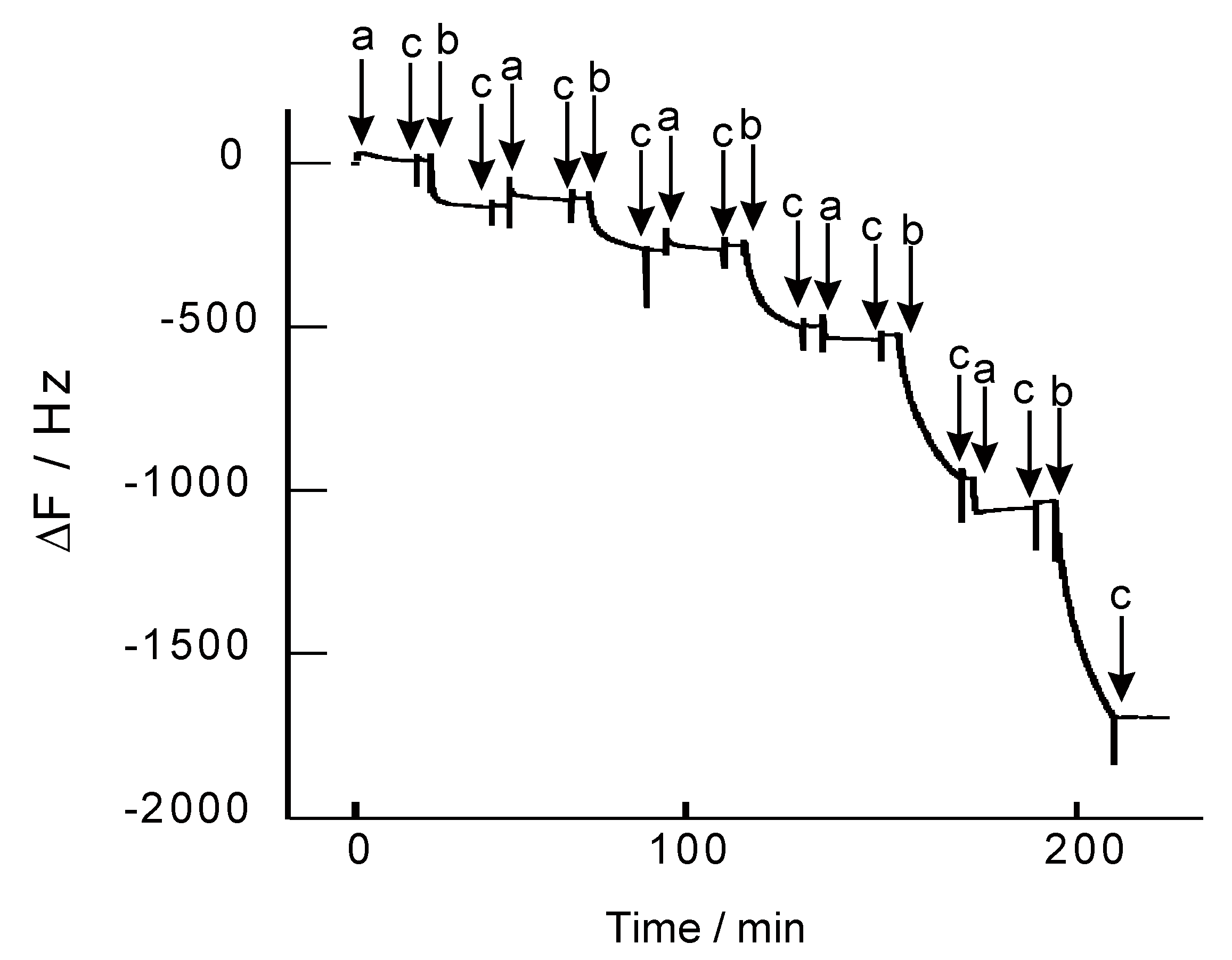
2.3. Preparation of Nanofilms
2.4. Decomposition of Nanofilms
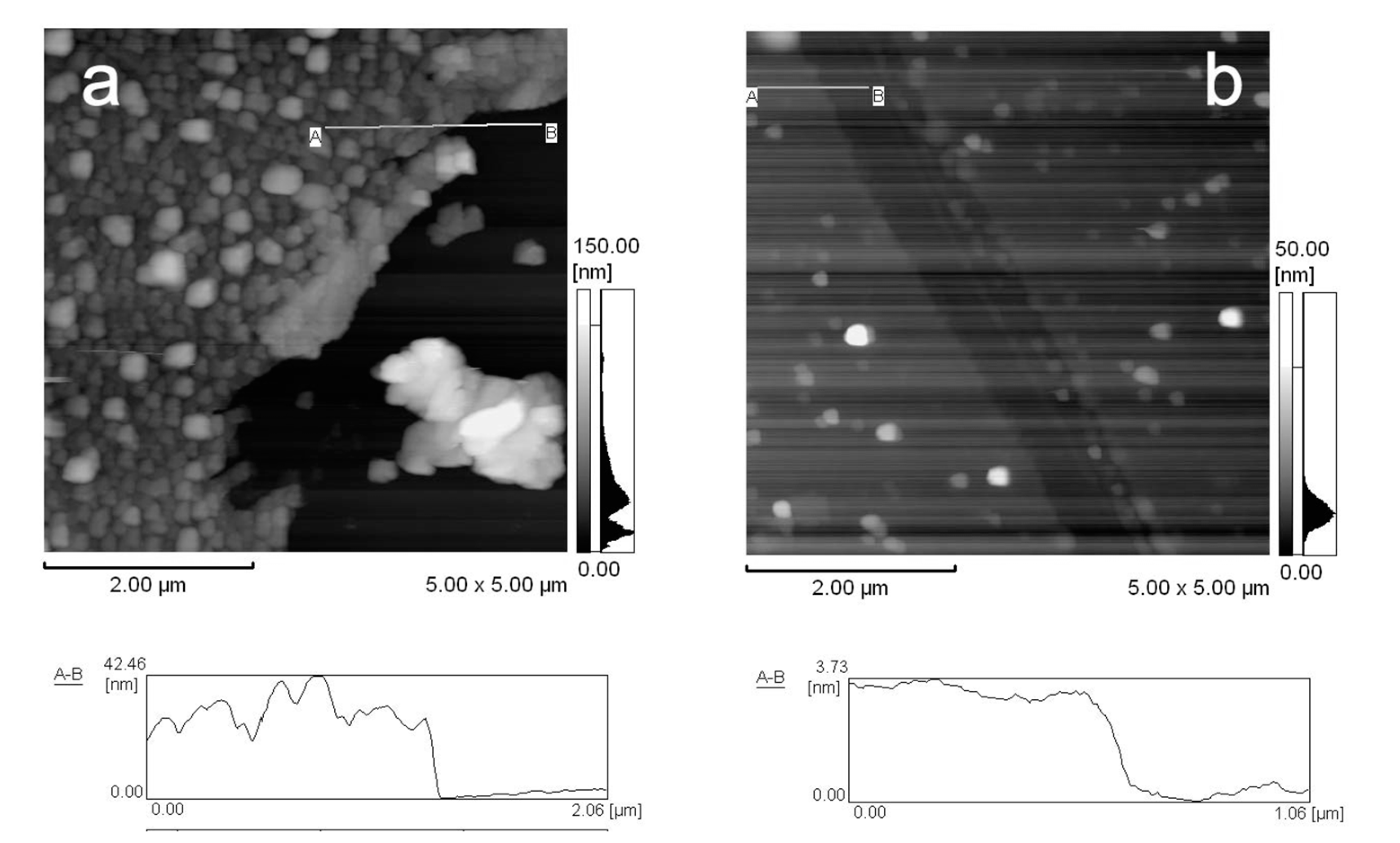
2.5. Observation of Nanofilms with AFM
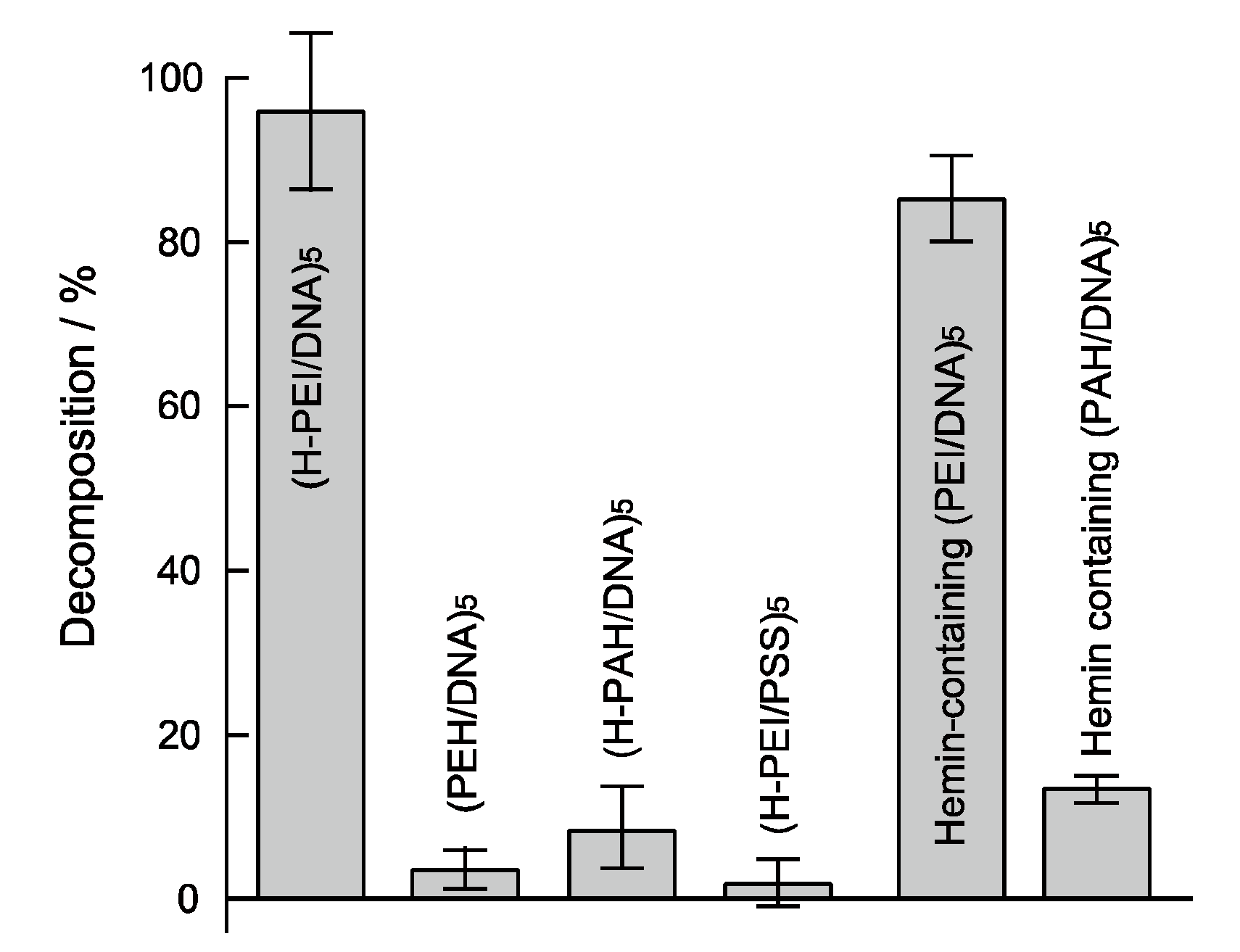
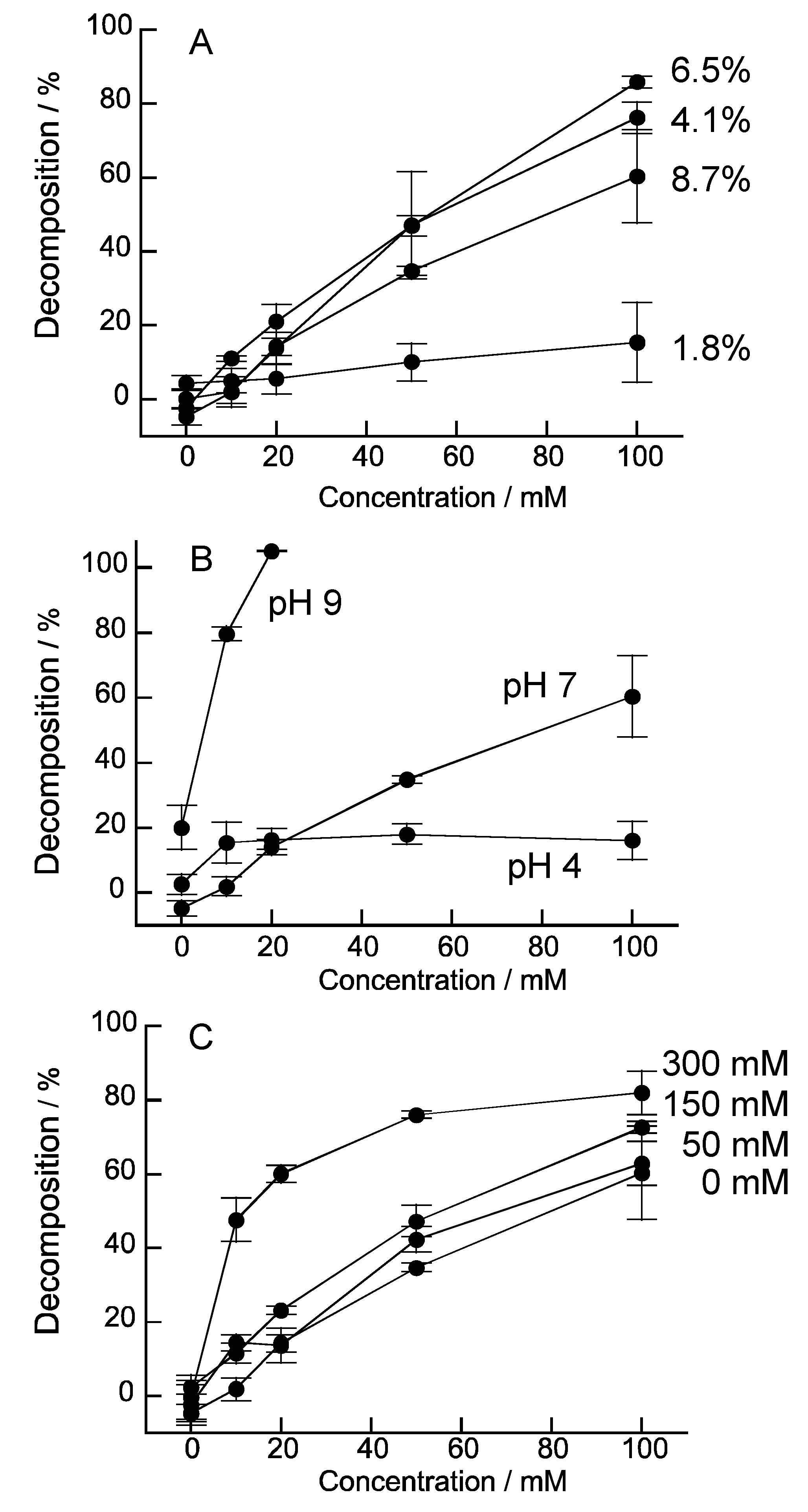
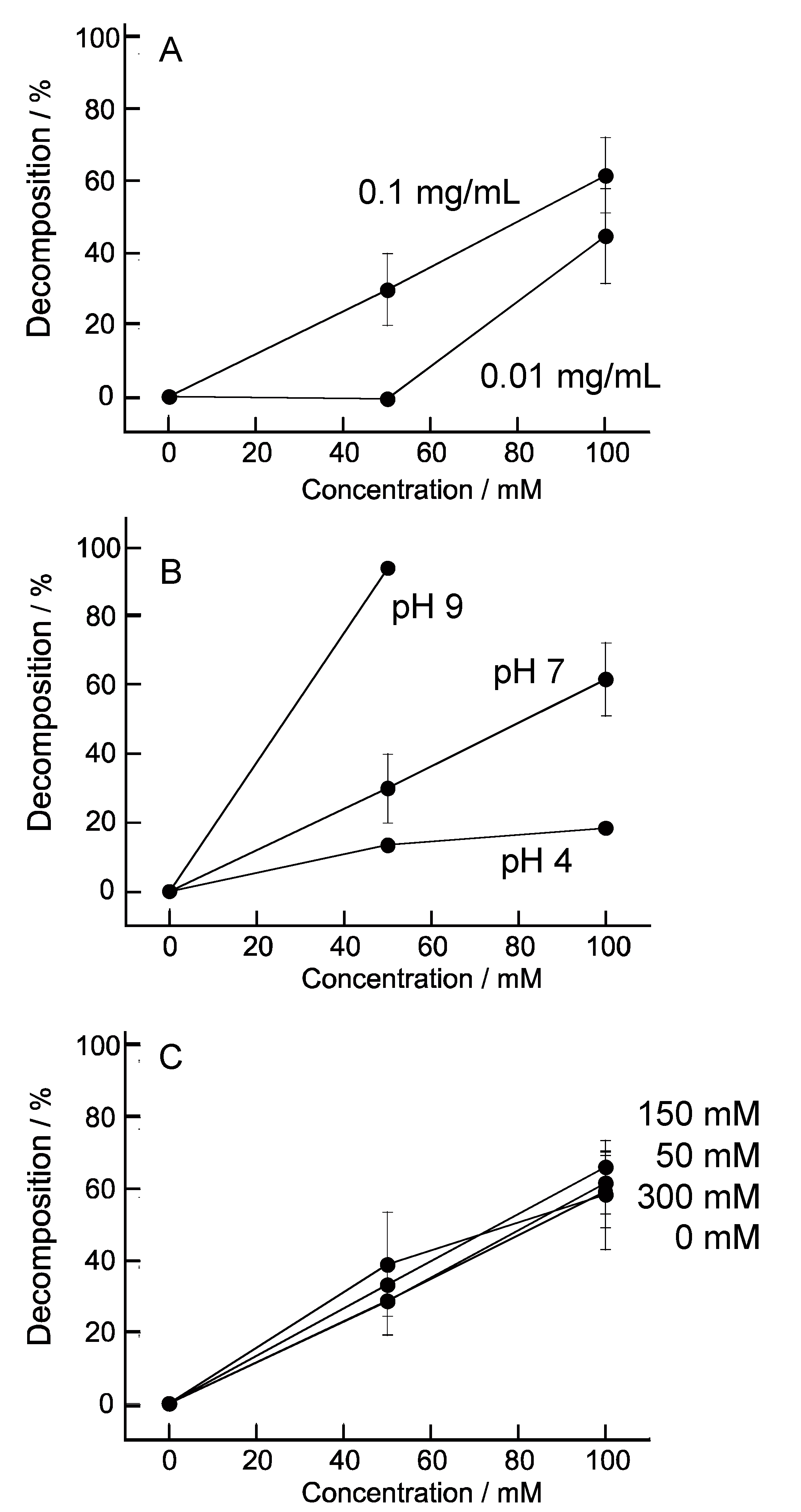
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- He, T.; Jańczewski, D.; Guo, S.; Man, S.M.; Jiang, S.; Tan, W.S. Stable pH responsive layer-by-layer assemblies of partially hydrolysed poly(2-ethyl-2-oxazoline) and poly(acrylic acid) for effective prevention of protein, cell and bacteria surface attachment. Colloids Surf. B Biointerfaces 2018, 161, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, H.; Casdorff, K.; Muff, L.F.; Ammann, R.; Burgert, I.; Michen, B. Layer-by-layer deposition on a heterogeneous surface: Effect of sorption kinetics on the growth of polyelectrolyte multilayers. J. Colloid Interface Sci. 2017, 500, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xu, J.J.; Chen, H.Y. Electrochemical biosensors based on layer-by-layer assemblies. Electroanalysis 2006, 18, 1737–1748. [Google Scholar] [CrossRef]
- Hoshi, T.; Anzai, J.; Osa, T. Controlled deposition of glucose oxidase on platinum electrode based on an avidin/biotin system for the regulation of output current of glucose sensors. Anal. Chem. 1995, 67, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wilson, J.T.; Chaikof, E.L. Construction of pegylated multilayer architectures via (strept)avidin/biotin interactions. Mater. Sci. Eng. C 2007, 27, 402–408. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Becker, A.L.; Johnston, A.P.R.; Wark, K.L.; Turatti, F.; Caruso, F. A general approach for DNA encapsulation in degradable polymer microcapsules. ACS Nano 2007, 1, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Patel, A.A.; Sukhorukov, G.B.; Lvov, Y.M. Nanoassembly of biodegradable microcapsules for DNA encasing. J. Am. Chem. Soc. 2004, 126, 3374–3375. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Matsusaki, M.; Kida, T.; Akashi, M. Enzyme-responsive release of encapsulated proteins from biodegradable hollow capsules. Biomacromolecules 2006, 7, 2715–2718. [Google Scholar] [CrossRef] [PubMed]
- Chinnayelka, S.; McShane, M.J. Glucose-sensitive nanoassemblies comprising affinity-binding complexes trapped in fuzzy microshells. J. Fluoresc. 2004, 14, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.; Tieke, B. Layer-by-layer assembled membranes containing hexacyclen-hexaacetic acid and polyethyleneimine N-acetic acid and their ion selective permeation behavior. J. Membr. Sci. 2009, 341, 261–267. [Google Scholar] [CrossRef]
- Huang, Z.; Li, M.; Li, N.; Tang, X.; Ouyang, Z. Antibacterial properties enhancement of layer-by-layer self-assembled nanofiltration membranes. J. Nanosci. Nanotechnol. 2018, 18, 4524–4533. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yoshida, K.; Takahashi, S.; Anzai, J. pH- and sugar-sensitive layer-by-layer films and microcapsules for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Hong, J. Layer-by-layer assembly of multilayer films for controlled drug release. Arch. Pharm. Res. 2014, 37, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.M.; Serpe, M.J.; Lyon, L.A. Thermally modulated insulin release from microgel thin films. Biomacromolecules 2004, 5, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Mercato, L.L.D.; Ferraro, M.M.; Baldassarre, F.; Mancarella, S.; Greco, V.; Rinaldi, R.; Leporatti, S. Biological applications of LbL multilayer capsules: From drug delivery to sensing. Adv. Colloid Interface Sci. 2014, 207, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Song, X.; Gao, C. Layer-by-layer assembly of microcapsules and their biomedical applications. Chem. Soc. Rev. 2012, 41, 6103–6124. [Google Scholar] [CrossRef] [PubMed]
- Biesheuvel, P.M.; Mauser, T.; Sukhorukov, G.B.; Möhwald, H. Micromechanical theory for pH-dependent polyelectrolyte multilayer capsule swelling. Macromolecules 2006, 39, 8480–8486. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Sukhishvili, S.A. Amphoteric hydrogel capsules: Multiple encapsulation and release routes. Macromolecules 2006, 39, 6191–6199. [Google Scholar] [CrossRef]
- Levy, T.; De’jugnat, C.; Sukhorukov, G.B. Polymer microcapsules with carbohydrate-sensitive properties. Adv. Funct. Mater. 2008, 18, 1586–1594. [Google Scholar] [CrossRef]
- Geest, B.G.D.; Jonas, A.M.; Demeester, J.; Smedt, S.C.D. Glucose-responsive polyelectrolyte capsules. Langmuir 2006, 22, 5070–5074. [Google Scholar] [CrossRef] [PubMed]
- Chinnayelka, S.; McShane, M.J. Microcapsule biosensors using competitive binding resonance energy transfer assays based on apoenzymes. Anal. Chem. 2005, 77, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Prevot, M.; Déjugnat, C.; Mçhwald, H.; Sukhorukov, G.B. Behavior of temperature-sensitive PNIPAM confined in polyelectrolyte capsules. Chem. Phys. Chem. 2006, 7, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Selin, V.; Ankner, J.F.; Sukhishvili, S.A. Diffusional response of layer-by-layer assembled polyelectrolyte chains to salt annealing. Macromolecules 2015, 48, 3983–3990. [Google Scholar] [CrossRef]
- Schmidt, D.J.; Moskowitz, J.S.; Hammond, P.T. Electrically triggered release of a small molecule drug from a polyelectrolyte multilayer coating. Chem. Mater. 2010, 22, 6416–6425. [Google Scholar] [CrossRef] [PubMed]
- Travascio, P.; Li, Y.S.D. DNA-enhanced peroxidase activity of a DNA-aptamer-hemin complex. Chem. Biol. 1998, 5, 505–517. [Google Scholar] [CrossRef]
- Yao, Y.; Mao, Y.; Huang, Q.; Wang, L.; Huang, Z.; Lu, W.; Chen, W. Enhanced decomposition of dyes by hemin-ACF with significant improvement in pH tolerance and stability. J. Hazard. Mater. 2014, 264, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yao, Y.; Xie, R.; Dai, D.; Lu, W.; Chen, W.; Zang, L. Enhanced generation of reactive oxygen species for efficient pollutant elimination catalyzed by hemin based on persistent free radicals. Appl. Catal. B Environ. 2016, 183, 291–297. [Google Scholar] [CrossRef]
- Breen, A.P.; Murphy, J.A. Reactions of oxyl radicals with DNA. Free Radic. Biol. Med. 1995, 18, 1033–1077. [Google Scholar] [CrossRef]
- Hertzberg, R.P.; Dervan, P.B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): Reaction conditions and product analyses. Biochemistry 1984, 23, 3934–3945. [Google Scholar] [CrossRef] [PubMed]
- Sigman, D.S. Nuclease activity of 1,10-phenanthroline-copper ion. Acc. Chem. Res. 1986, 19, 180–186. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S.; Murata, M.; Tsukitome, H.; Saito, I. Site-specific oxidation at GG and GGG sequences in double-stranded DNA by benzoyl peroxide as a tumor promoter. Biochemistry 1999, 51, 16733–16739. [Google Scholar] [CrossRef]
- Drechsel, D.A.; Patel, M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic. Biol. Med. 2008, 44, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- De Gracia, L.C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef] [PubMed]
- Rehor, A.; Hubbell, J.A.; Tirelli, N. Oxidation-sensitive polymeric nanoparticles. Langmuir 2005, 21, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Broaders, K.E.; Grandhe, S.; Fréchet, J.M. A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. J. Am. Chem. Soc. 2011, 133, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Awaji, K.; Shimizu, S.; Iwasaki, M.; Oide, Y.; Ito, M.; Dairaku, T.; Ono, T.; Kashiwagi, Y.; Sato, K. Preparation of microparticles capable of glucose-induced insulin release under physiological conditions. Polymers 2018, 10, 1164. [Google Scholar] [CrossRef]
- Inoue, H.; Anzai, J. Stimuli-sensitive thin films prepared by a layer-by-layer deposition of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Langmuir 2005, 21, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.F.; Goldsmith, J.I.; Szép, S.; Gibbs, E.J. A spectroscopic and thermodynamic study of porphyrin/DNA supramolecular assemblies. Biophys. J. 1998, 75, 1024–1031. [Google Scholar] [CrossRef]
- Suenaga, H.; Nango, M.; Sugiyama, T.; Iwasaki, T.; Takeuti, Y.; Shinkai, S. DNA cleavage by a polyethylenimine-appended porphyrin. Chem. Lett. 2000, 29, 306–307. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J. H2O2-induced decomposition of layer-by-layer films consisting of phenylboronic acid-bearing poly(allylamine) and poly(vinyl alcohol). Langmuir 2014, 30, 9247–9250. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Awaji, K.; Ito, M.; Anzai, J. Preparation of H2O2-induced poly(amidoamine) dendrimer release nanofilms. Colloid Polym. Sci. 2017, 295, 877–882. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J. Glucose-induced decomposition of layer-by-layer films composed of phenylboronic acid-bearing poly(allylamine) and poly(vinyl alcohol) under physiological conditions. J. Mater. Chem. B 2015, 3, 7796–7802. [Google Scholar] [CrossRef]
- Sato, K.; Shimizu, S.; Awaji, K.; Hitomi, O.; Anzai, J. Lactate-induced decomposition of layer-by-layer films composed of phenylboronic acid-modified poly(allylamine) and poly(vinyl alcohol) under extracellular tumor conditions. J. Colloid Interface Sci. 2018, 510, 302–307. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Ono, T.; Dairaku, T.; Kashiwagi, Y.; Sato, K. Preparation of Hydrogen Peroxide Sensitive Nanofilms by a Layer-by-Layer Technique. Nanomaterials 2018, 8, 941. https://doi.org/10.3390/nano8110941
Yoshida K, Ono T, Dairaku T, Kashiwagi Y, Sato K. Preparation of Hydrogen Peroxide Sensitive Nanofilms by a Layer-by-Layer Technique. Nanomaterials. 2018; 8(11):941. https://doi.org/10.3390/nano8110941
Chicago/Turabian StyleYoshida, Kentaro, Tetsuya Ono, Takenori Dairaku, Yoshitomo Kashiwagi, and Katsuhiko Sato. 2018. "Preparation of Hydrogen Peroxide Sensitive Nanofilms by a Layer-by-Layer Technique" Nanomaterials 8, no. 11: 941. https://doi.org/10.3390/nano8110941
APA StyleYoshida, K., Ono, T., Dairaku, T., Kashiwagi, Y., & Sato, K. (2018). Preparation of Hydrogen Peroxide Sensitive Nanofilms by a Layer-by-Layer Technique. Nanomaterials, 8(11), 941. https://doi.org/10.3390/nano8110941





