WS(1−x)Sex Nanoparticles Decorated Three-Dimensional Graphene on Nickel Foam: A Robust and Highly Efficient Electrocatalyst for the Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Experimental Details
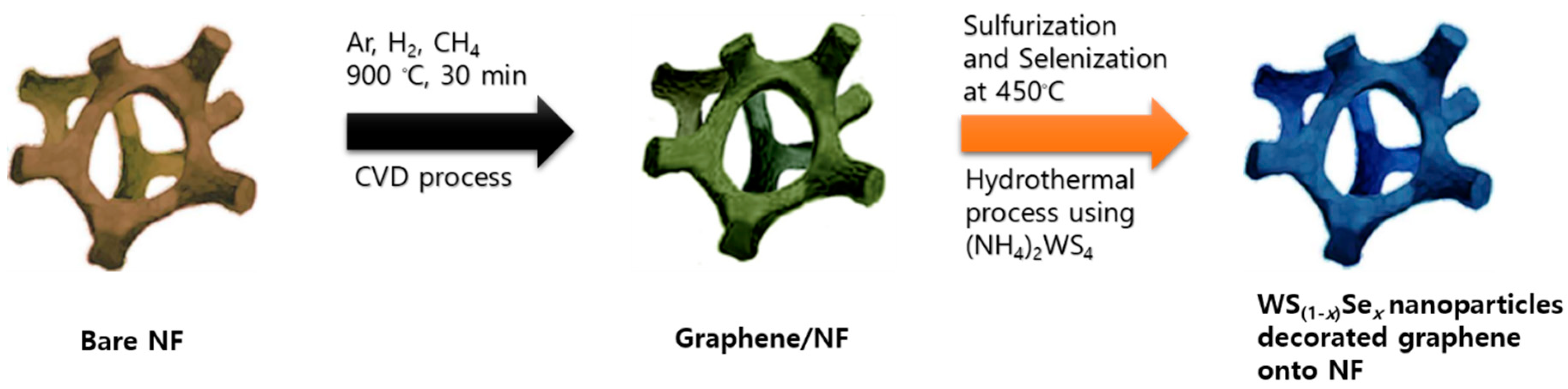
2.1. Synthesis of Graphene/NF, WS2/Graphene/NF, and WS(1−x)Sex/Graphene/NF
2.2. Electrochemical Measurements
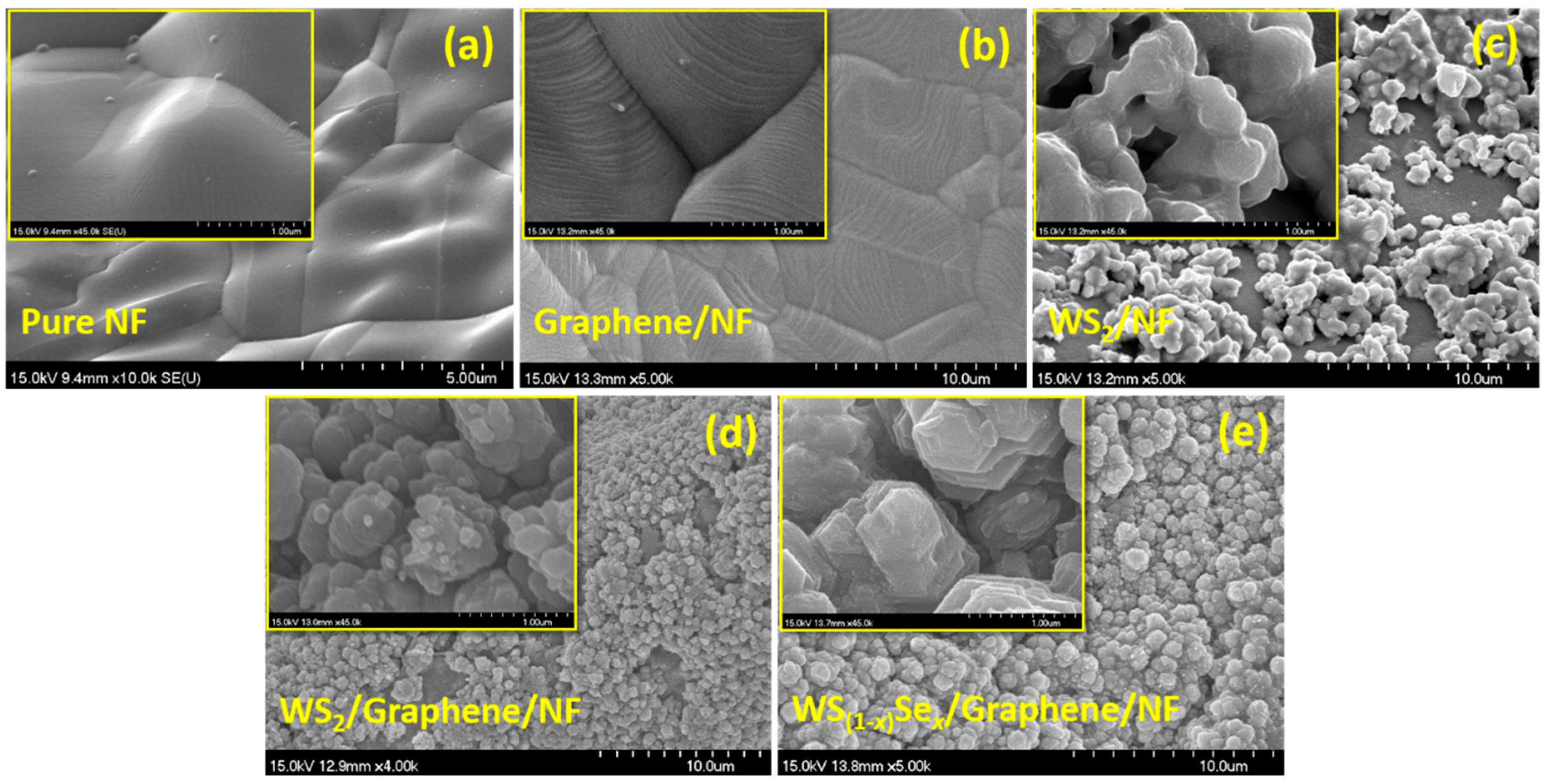
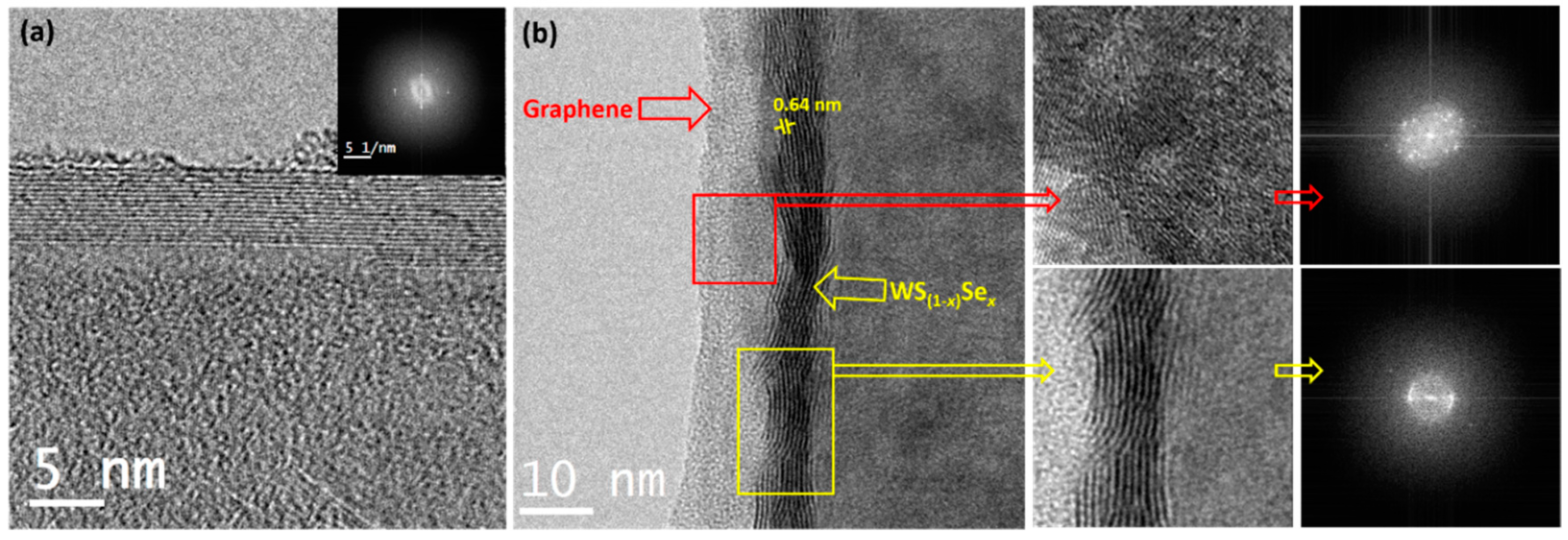
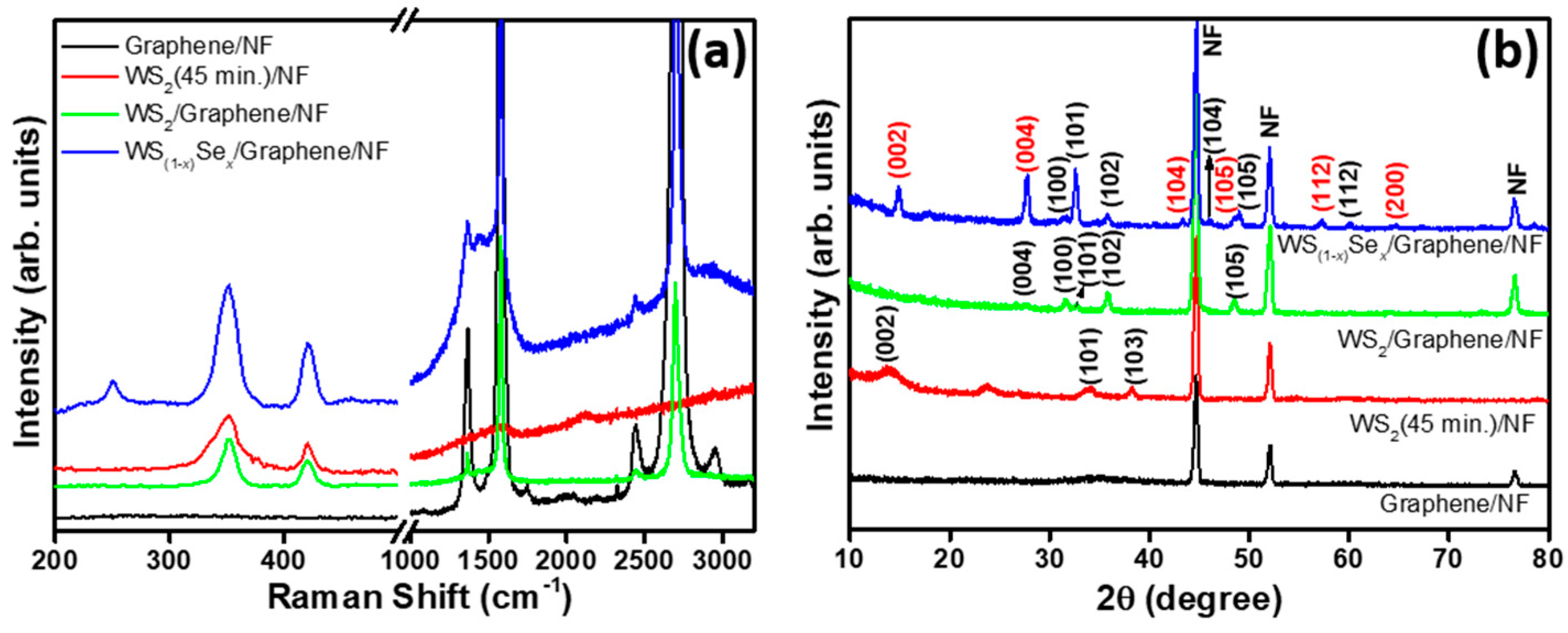
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Song, F.; Hu, X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 2016, 7, 12324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xiao, C.; Xie, S.; Liang, J.; Chen, X.; Tang, Y. Iron-Nickel Nitride Nanostructures in Situ Grown on Surface-Redox-Etching Nickel Foam: Efficient and Ultrasustainable Electrocatalysts for Overall Water Splitting. Chem. Mater. 2016, 28, 6934–6941. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, J.; Yao, T.; He, J.; Jiang, S.; Sun, Z.; Liu, Q.; Cheng, W.; Hu, F.; Jiang, Y. CoOOH nanosheets with high mass activity for water oxidation. Angew. Chem. Int. Ed. 2015, 54, 8722–8727. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Chang, S.L.; Hocking, R.K.; Bach, U.; Spiccia, L. Highly active nickel oxide water oxidation catalysts deposited from molecular complexes. Energy Environ. Sci. 2013, 6, 579–586. [Google Scholar] [CrossRef]
- Xu, K.; Ding, H.; Jia, K.; Lu, X.; Chen, P.; Zhou, T.; Cheng, H.; Liu, S.; Wu, C.; Xie, Y. Solution-Liquid-Solid Synthesis of Hexagonal Nickel Selenide Nanowire Arrays with a Nonmetal Catalyst. Angew. Chem. Int. Ed. 2016, 55, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Shifa, T.A.; Liu, K.; Wang, F.; Wang, Z.; Xu, P.; Wang, Q.; He, J. Selenium-Enriched Nickel Selenide Nanosheets as a Robust Electrocatalyst for Hydrogen Generation. Angew. Chem. 2016, 128, 7033–7038. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Akbar, K.; Adaikalam, K.; Lee, S.H.; Chun, S.-H.; Jung, J.; Kim, H.-S.; Park, H.J. Facile Synthesis of Molybdenum Diselenide Layers for High-Performance Hydrogen Evolution Electrocatalysts. ACS Omega 2018, 3, 5799–5807. [Google Scholar] [CrossRef]
- Xu, K.; Ding, H.; Lv, H.; Chen, P.; Lu, X.; Cheng, H.; Zhou, T.; Liu, S.; Wu, X.; Wu, C. Dual Electrical-Behavior Regulation on Electrocatalysts Realizing Enhanced Electrochemical Water Oxidation. Adv. Mater. 2016, 28, 3326–3332. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Leonard, B.M. Iron-doped molybdenum carbide catalyst with high activity and stability for the hydrogen evolution reaction. Chem. Mater. 2015, 27, 4281–4288. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, W.; Yang, L.; Lu, J.; Cheng, S.; Mai, W.; Tang, Z.; Li, L.; Chen, S. Ultrahigh-Performance Pseudocapacitor Electrodes Based on Transition Metal Phosphide Nanosheets Array via Phosphorization: A General and Effective Approach. Adv. Funct. Mater. 2015, 25, 7530–7538. [Google Scholar] [CrossRef]
- Stern, L.-A.; Feng, L.; Song, F.; Hu, X. Ni2P as a Janus catalyst for water splitting: The oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 2015, 8, 2347–2351. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Zhou, T.; Tong, Y.; Wu, J.; Cheng, H.; Lu, X.; Ding, H.; Wu, C.; Xie, Y. Strong-Coupled Cobalt Borate Nanosheets/Graphene Hybrid as Electrocatalyst for Water Oxidation Under Both Alkaline and Neutral Conditions. Angew. Chem. Int. Ed. 2016, 55, 2488–2492. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Tilley, T.D. Electrocatalytic water oxidation at neutral pH by a nanostructured Co(PO3)2 anode. Adv. Funct. Mater. 2013, 23, 227–233. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Huang, W.; Gong, Q.; Liu, C.; Liu, Z.; Li, Y.; Dai, H. Ultrathin WS2 nanoflakes as a high-performance electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 7860–7863. [Google Scholar] [CrossRef] [PubMed]
- Davodi, F.; Mühlhausen, E.; Tavakkoli, M.; Sainio, J.; Jiang, H.; Gökce, B.; Marzun, G.; Kallio, T. Catalyst Support Effect on the Activity and Durability of Magnetic Nanoparticles: Toward Design of Advanced Electrocatalyst for Full Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 31300–31311. [Google Scholar] [CrossRef] [PubMed]
- Davodi, F.; Tavakkoli, M.; Lahtinen, J.; Kallio, T. Straightforward synthesis of nitrogen-doped carbon nanotubes as highly active bifunctional electrocatalysts for full water splitting. J. Catal. 2017, 353, 19–27. [Google Scholar] [CrossRef]
- Luo, Z.; Martí-Sànchez, S.; Nafria, R.; Joshua, G.; de la Mata, M.; Guardia, P.; Flox, C.; Martínez-Boubeta, C.; Simeonidis, K.; Llorca, J. Fe3O4@NiFexOy Nanoparticles with Enhanced Electrocatalytic Properties for Oxygen Evolution in Carbonate Electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 29461–29469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yu, F.; Sun, J.; He, R.; Wang, Y.; Guo, C.F.; Wang, F.; Lan, Y.; Ren, Z.; Chen, S. Highly active and durable self-standing WS2/graphene hybrid catalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 9472–9476. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Sun, J.; Zhu, H.; Mishra, I.K.; Chen, S.; Ren, Z. Highly efficient hydrogen evolution from edge-oriented WS2(1−x)Se2x particles on three-dimensional porous NiSe2 foam. Nano Lett. 2016, 16, 7604–7609. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Akbar, K.; Hussain, S.; Yoo, G.; Jang, J.-Y.; Chun, S.-H.; Jung, J.; Park, H.J. Direct synthesis of thickness-tunable MoS2 quantum dot thin layers: Optical, structural and electrical properties and their application to hydrogen evolution. Nano Energy 2017, 35, 101–114. [Google Scholar] [CrossRef]
- Hussain, S.; Akbar, K.; Vikraman, D.; Liu, H.; Chun, S.-H.; Jung, J. WS2/CoSe2 heterostructure: A designed structure as catalysts for enhanced hydrogen evolution performance. J. Ind. Eng. Chem. 2018, 65, 167–174. [Google Scholar] [CrossRef]
- Hussain, S.; Akbar, K.; Vikraman, D.; Karuppasamy, K.; Kim, H.-S.; Chun, S.-H.; Jung, J. Synthesis of MoS2(1−x)Se2x and WS2(1−x)Se2x alloys for enhanced hydrogen evolution reaction performance. Inorg. Chem. Front. 2017, 4, 2068–2074. [Google Scholar] [CrossRef]
- Hussain, S.; Akbar, K.; Vikraman, D.; Choi, D.-C.; Kim, S.J.; An, K.-S.; Jung, S.; Jung, J. A highly sensitive enzymeless glucose sensor based on 3D graphene-Cu hybrid electrodes. New J. Chem. 2015, 39, 7481–7487. [Google Scholar] [CrossRef]
- Mattevi, C.; Kim, H.; Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Jung, Y.; Shen, J.; Sun, Y.; Cha, J.J. Chemically synthesized heterostructures of two-dimensional molybdenum/tungsten-based dichalcogenides with vertically aligned layers. ACS Nano 2014, 8, 9550–9557. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chen, G.; Joshi, P.; Tadigadapa, S.; Eklund, P. Raman scattering from high-frequency phonons in supported n-graphene layer films. Nano Lett. 2006, 6, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-G.; Park, J.; Lee, W.; Choi, T.; Jung, H.; Lee, C.W.; Hwang, S.-H.; Myoung, J.M.; Jung, J.-H.; Kim, S.-H.; et al. Layer-Controlled, Wafer-Scale, and Conformal Synthesis of Tungsten Disulfide Nanosheets Using Atomic Layer Deposition. ACS Nano 2013, 7, 11333–11340. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.A.; Chua, D.H.; Wee, A.T. One-step synthesis of few-layer WS2 by pulsed laser deposition. Sci. Rep. 2015, 5, 18116. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.V.; Patil, P.D.; Barman, P.K.; Kini, R.N.; Shaijumon, M.M. Controllable growth of few-layer spiral WS2. RSC Adv. 2016, 6, 376–382. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Tang, D.-M.; Zhao, W.; Xu, H.; Chu, L.; Bando, Y.; Golberg, D.; Eda, G. Halide-assisted atmospheric pressure growth of large WSe2 and WS2 monolayer crystals. Appl. Mater. Today 2015, 1, 60–66. [Google Scholar] [CrossRef]
- Ibrahem, M.A.; Lan, T.-W.; Huang, J.K.; Chen, Y.-Y.; Wei, K.-H.; Li, L.-J.; Chu, C.W. High quantity and quality few-layers transition metal disulfide nanosheets from wet-milling exfoliation. RSC Adv. 2013, 3, 13193–13202. [Google Scholar] [CrossRef]
- Rhyee, J.S.; Kwon, J.; Dak, P.; Kim, J.H.; Kim, S.M.; Park, J.; Hong, Y.K.; Song, W.G.; Omkaram, I.; Alam, M.A. High-Mobility Transistors Based on Large-Area and Highly Crystalline CVD-Grown MoSe2 Films on Insulating Substrates. Adv. Mater. 2016, 28, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, H.; Gu, H.; Fu, J.; Mo, S.; Wei, C.; Miao, Y.-E.; Liu, T. A CNT@MoSe2 hybrid catalyst for efficient and stable hydrogen evolution. Nanoscale 2015, 7, 18595–18602. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kong, D.; Johanes, P.; Cha, J.J.; Zheng, G.; Yan, K.; Liu, N.; Cui, Y. MoSe2 and WSe2 nanofilms with vertically aligned molecular layers on curved and rough surfaces. Nano Lett. 2013, 13, 3426–3433. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, X.; Hedhili, M.N.; Huang, K.-W.; Li, L.-J.; Zhang, W. Symmetrical synergy of hybrid CoS2-WS2 electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 15552–15558. [Google Scholar] [CrossRef]
- Yang, J.; Voiry, D.; Ahn, S.J.; Kang, D.; Kim, A.Y.; Chhowalla, M.; Shin, H.S. Two-Dimensional Hybrid Nanosheets of Tungsten Disulfide and Reduced Graphene Oxide as Catalysts for Enhanced Hydrogen Evolution. Angew. Chem. Int. Ed. 2013, 52, 13751–13754. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Hussain, S.; Akbar, K.; Truong, L.; Kathalingam, A.; Chun, S.-H.; Jung, J.; Park, H.J.; Kim, H.-S. Improved Hydrogen Evolution Reaction Performance using MoS2-WS2 Heterostructures by Physicochemical Process. ACS Sustain. Chem. Eng. 2018, 6, 8400–8409. [Google Scholar] [CrossRef]
- Yu, S.; Kim, J.; Yoon, K.R.; Jung, J.-W.; Oh, J.; Kim, I.-D. Rational design of efficient electrocatalysts for hydrogen evolution reaction: Single layers of WS2 nanoplates anchored to hollow nitrogen-doped carbon nanofibers. ACS Appl. Mater. Interfaces 2015, 7, 28116–28121. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jin, Z.; Chen, Y.; Gao, Z.; Yan, J.; Liu, H.; Wang, J.; Li, Y.; Liu, S.F. Graphdiyne-WS2 2D-Nanohybrid electrocatalysts for high-performance hydrogen evolution reaction. Carbon 2017. [Google Scholar] [CrossRef]
- Zheng, M.; Du, J.; Hou, B.; Xu, C.-L. Few-Layered Mo(1−x)WxS2 Hollow Nanospheres on Ni3S2 Nanorod Heterostructure as Robust Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 26066–26076. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, P.; Chen, Y.; Zheng, B.; Liu, J.; Zhou, J.; He, J.; Hao, X.; Zhang, W. Three-dimensional structure of WS2/graphene/Ni as a binder-free electrocatalytic electrode for highly effective and stable hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 7811–7819. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.; Li, N.; Xu, Z.; Fisher, A.; Wang, X. Vertically oriented MoS2 and WS2 nanosheets directly grown on carbon cloth as efficient and sTable 3-dimensional hydrogen-evolving cathodes. J. Mater. Chem. A 2015, 3, 131–135. [Google Scholar] [CrossRef]
- Shang, X.; Yan, K.-L.; Liu, Z.-Z.; Lu, S.-S.; Dong, B.; Chi, J.-Q.; Li, X.; Liu, Y.-R.; Chai, Y.-M.; Liu, C.-G. Oxidized carbon fiber supported vertical WS2 nanosheets arrays as efficient 3D nanostructure electrocatalyts for hydrogen evolution reaction. Appl. Surf. Sci. 2017, 402, 120–128. [Google Scholar] [CrossRef]
- Wen, Y.; Xia, Y.; Zhang, S. Tungsten disulphide nanorattle: A new type of high performance electrocatalyst for hydrogen evolution reaction. J. Power Sources 2016, 307, 593–598. [Google Scholar] [CrossRef]
- Shifa, T.A.; Wang, F.; Cheng, Z.; Zhan, X.; Wang, Z.; Liu, K.; Safdar, M.; Sun, L.; He, J. A vertical-oriented WS2 nanosheet sensitized by graphene: An advanced electrocatalyst for hydrogen evolution reaction. Nanoscale 2015, 7, 14760–14765. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, F.; Wang, Z.; Zhan, X.; Wang, Q.; Cheng, Z.; Safdar, M.; He, J. Component-Controllable WS2(1−x)Se2x Nanotubes for Efficient Hydrogen Evolution Reaction. Acs Nano 2014, 8, 8468–8476. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Yang, L.; Wang, W.; Han, A.; Huang, J.; Du, P.; Fan, Z.; Zhang, J.; Xiang, B. Synthesis and enhanced electrochemical catalytic performance of monolayer WS2(1−x)Se2x with a tunable band gap. Adv. Mater. 2015, 27, 4732–4738. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Cheng, L.; Liu, C.; Zhang, M.; Feng, Q.; Ye, H.; Zeng, M.; Xie, L.; Liu, Z.; Li, Y. Ultrathin MoS2(1−x)Se2x Alloy Nanoflakes For Electrocatalytic Hydrogen Evolution Reaction. ACS Catal. 2015, 5, 2213–2219. [Google Scholar] [CrossRef]
- Thomas, J.G.N. Kinetics of electrolytic hydrogen evolution and the adsorption of hydrogen by metals. Trans. Faraday Soc. 1961, 57, 1603–1611. [Google Scholar] [CrossRef]
- Merki, D.; Fierro, S.; Vrubel, H.; Hu, X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2011, 2, 1262–1267. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Wang, F.; Shifa, T.A.; Cheng, Z.; Wang, Z.; Xu, K.; Zhan, X.; Wang, Q.; Huang, Y. Enhanced Electrochemical H2 Evolution by Few-Layered Metallic WS2(1−x)Se2x Nanoribbons. Adv. Funct. Mater. 2015, 25, 6077–6083. [Google Scholar] [CrossRef]
- Xu, C.; Peng, S.; Tan, C.; Ang, H.; Tan, H.; Zhang, H.; Yan, Q. Ultrathin S-doped MoSe2 nanosheets for efficient hydrogen evolution. J. Mater. Chem. A 2014, 2, 5597–5601. [Google Scholar] [CrossRef]
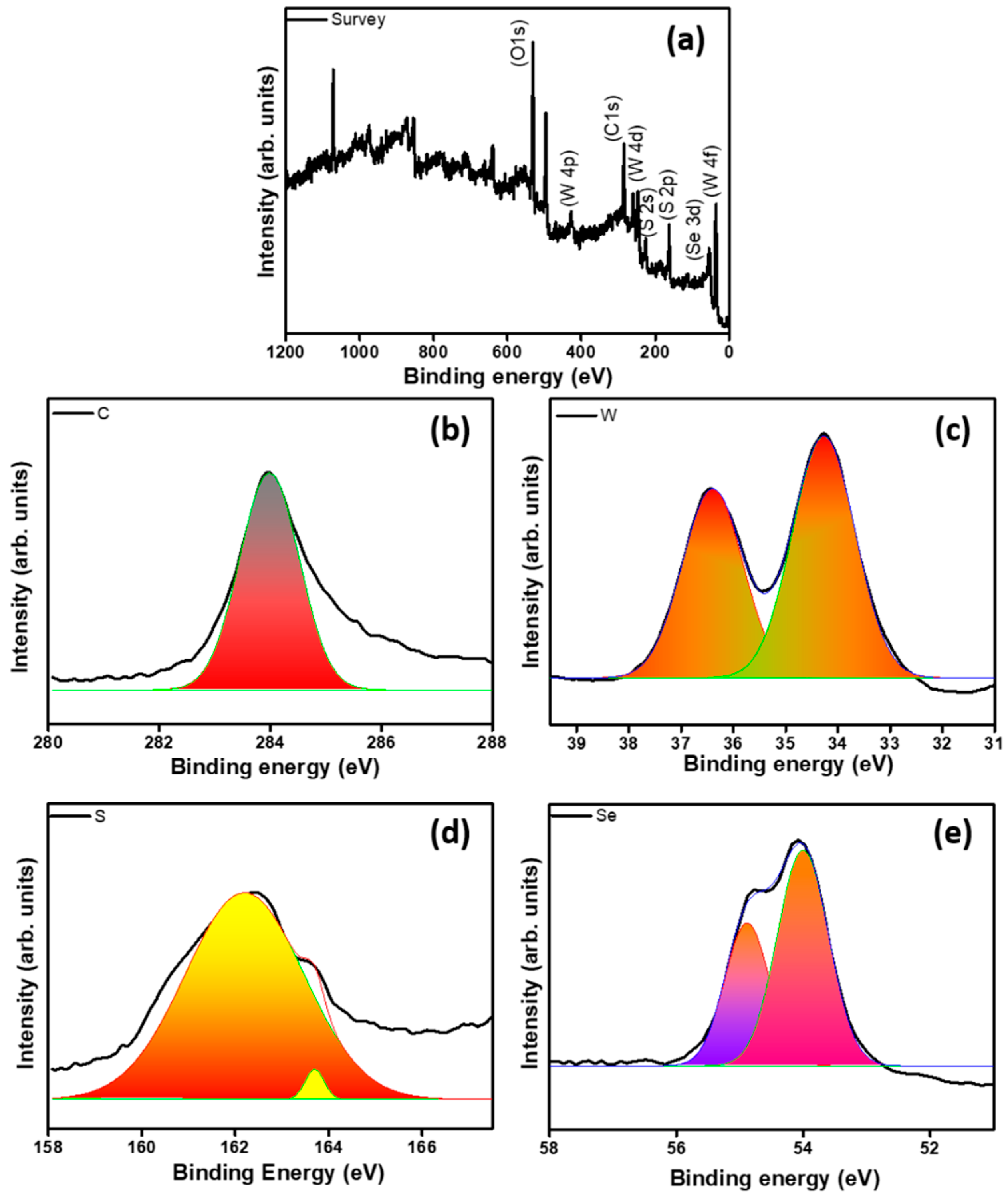
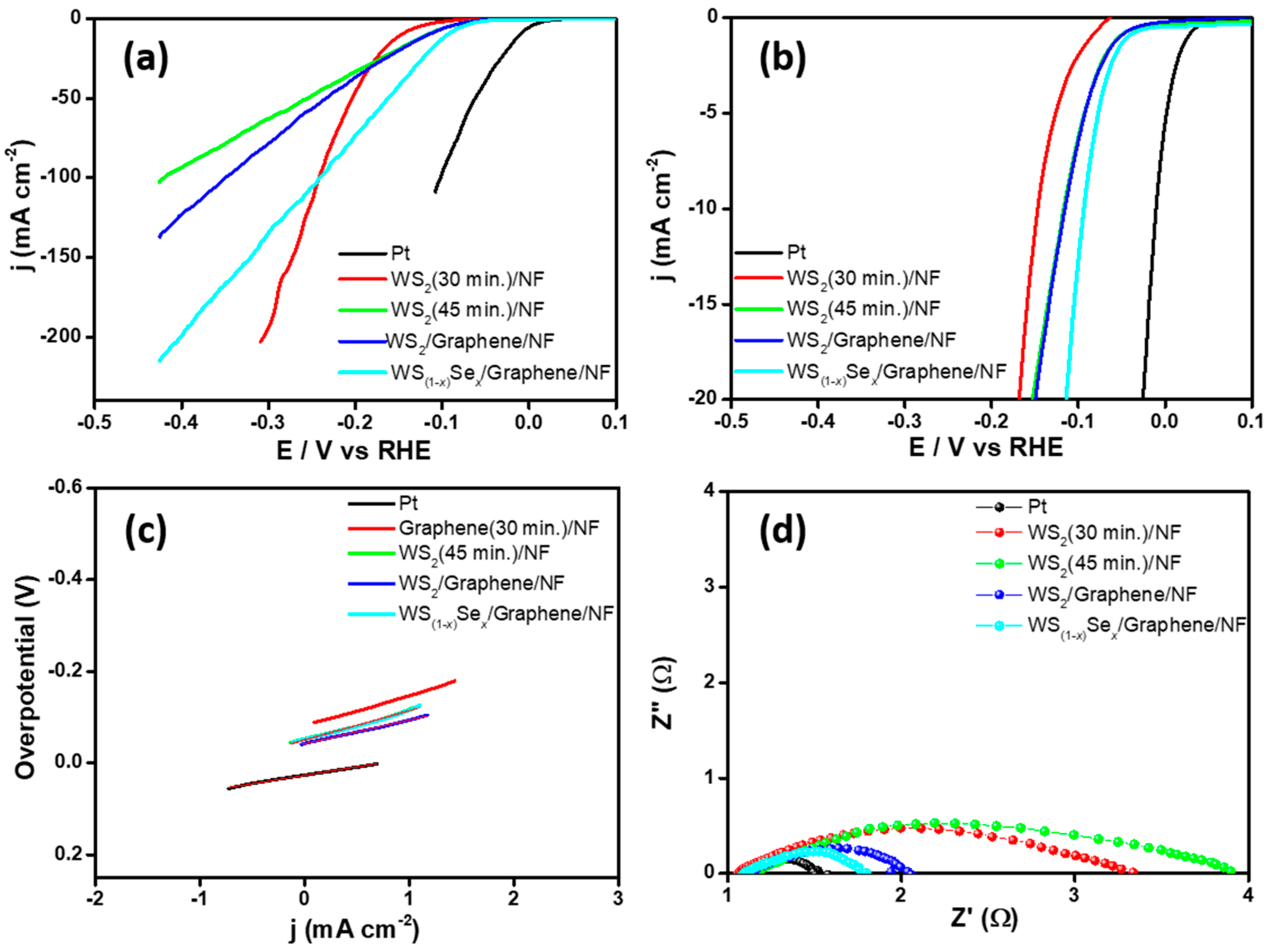

| Catalyst | Overpotential (mV vs. RHE) @10 mA cm−2 | Tafel Slope (mV dec−1) | Exchange Current Density (j0, mA cm−2) |
|---|---|---|---|
| Pt | −10 | 36 | 5.98 |
| WS2(45 min)/NF | −115 | 63 | 0.162 |
| WS2/graphene/NF | −114 | 62 | 0.165 |
| WS(1−x)Sex/graphene/NF | −93 | 51 | 0.274 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Akbar, K.; Vikraman, D.; Afzal, R.A.; Song, W.; An, K.-S.; Farooq, A.; Park, J.-Y.; Chun, S.-H.; Jung, J. WS(1−x)Sex Nanoparticles Decorated Three-Dimensional Graphene on Nickel Foam: A Robust and Highly Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Nanomaterials 2018, 8, 929. https://doi.org/10.3390/nano8110929
Hussain S, Akbar K, Vikraman D, Afzal RA, Song W, An K-S, Farooq A, Park J-Y, Chun S-H, Jung J. WS(1−x)Sex Nanoparticles Decorated Three-Dimensional Graphene on Nickel Foam: A Robust and Highly Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Nanomaterials. 2018; 8(11):929. https://doi.org/10.3390/nano8110929
Chicago/Turabian StyleHussain, Sajjad, Kamran Akbar, Dhanasekaran Vikraman, Rana Arslan Afzal, Wooseok Song, Ki-Seok An, Ayesha Farooq, Jun-Young Park, Seung-Hyun Chun, and Jongwan Jung. 2018. "WS(1−x)Sex Nanoparticles Decorated Three-Dimensional Graphene on Nickel Foam: A Robust and Highly Efficient Electrocatalyst for the Hydrogen Evolution Reaction" Nanomaterials 8, no. 11: 929. https://doi.org/10.3390/nano8110929
APA StyleHussain, S., Akbar, K., Vikraman, D., Afzal, R. A., Song, W., An, K.-S., Farooq, A., Park, J.-Y., Chun, S.-H., & Jung, J. (2018). WS(1−x)Sex Nanoparticles Decorated Three-Dimensional Graphene on Nickel Foam: A Robust and Highly Efficient Electrocatalyst for the Hydrogen Evolution Reaction. Nanomaterials, 8(11), 929. https://doi.org/10.3390/nano8110929








