Three-Dimensional Hierarchical Reticular Nanostructure of Fulfora candelaria Wing Decorated by Ag Nanoislands as Practical SERS-Active Substrates
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Materials and Instruments
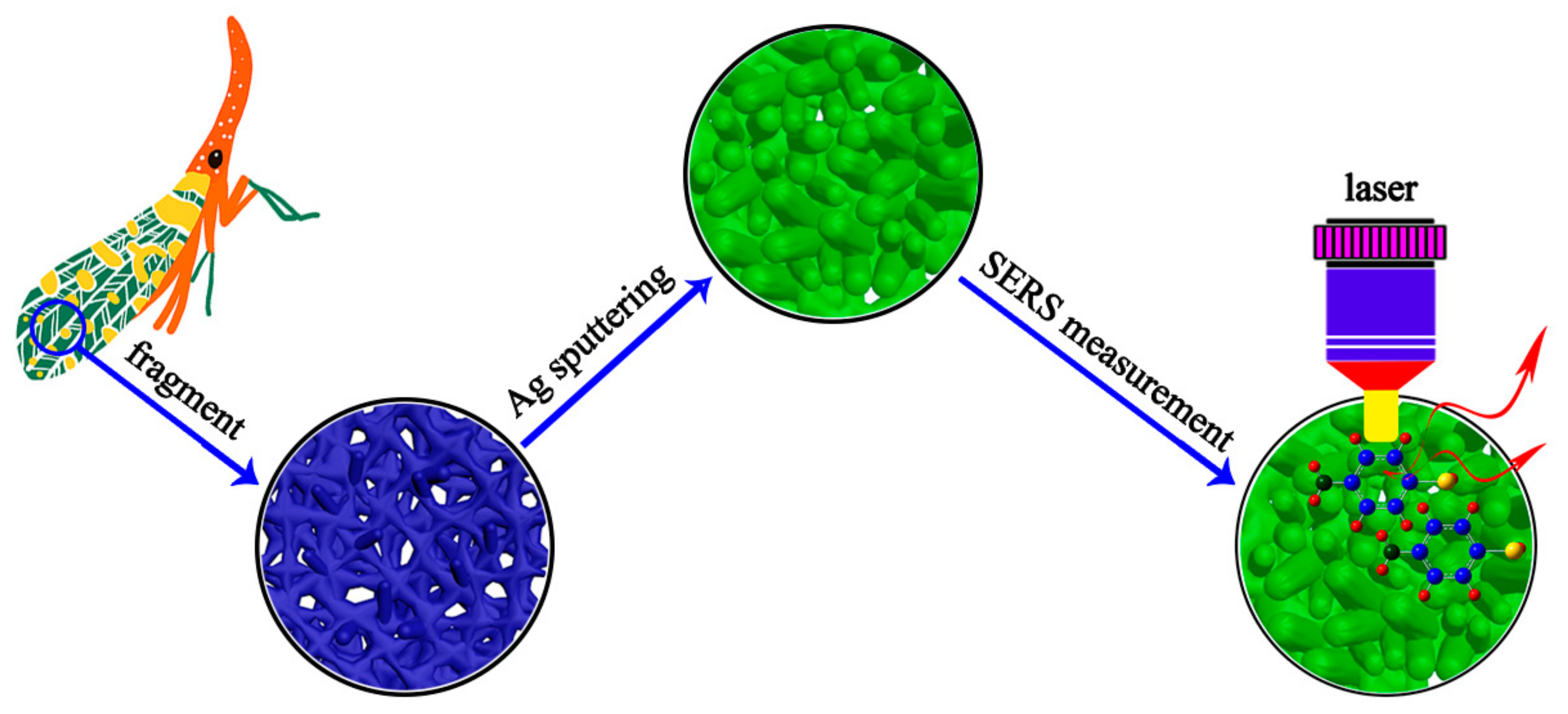
2.2. Fabrication of SERS-Active Substrates
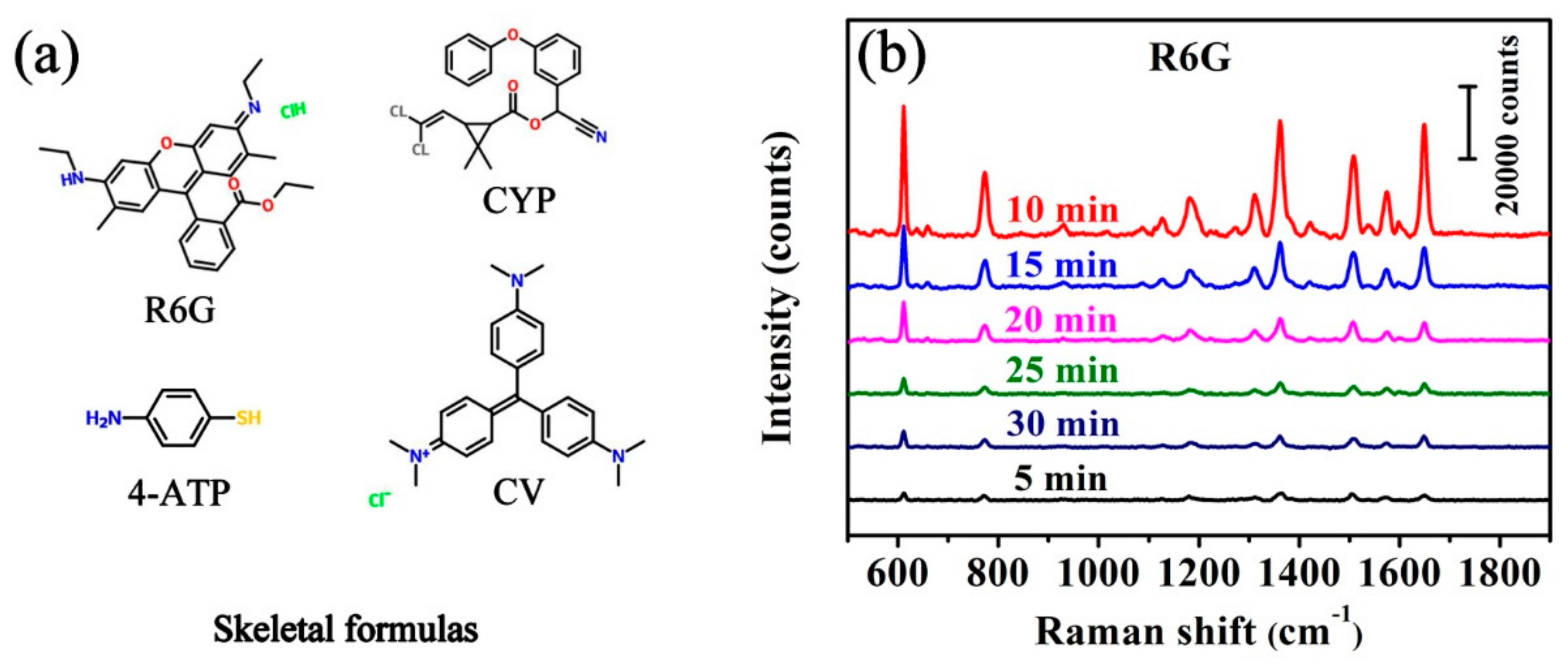
2.3. SERS Measurements
3. Results and Discussion
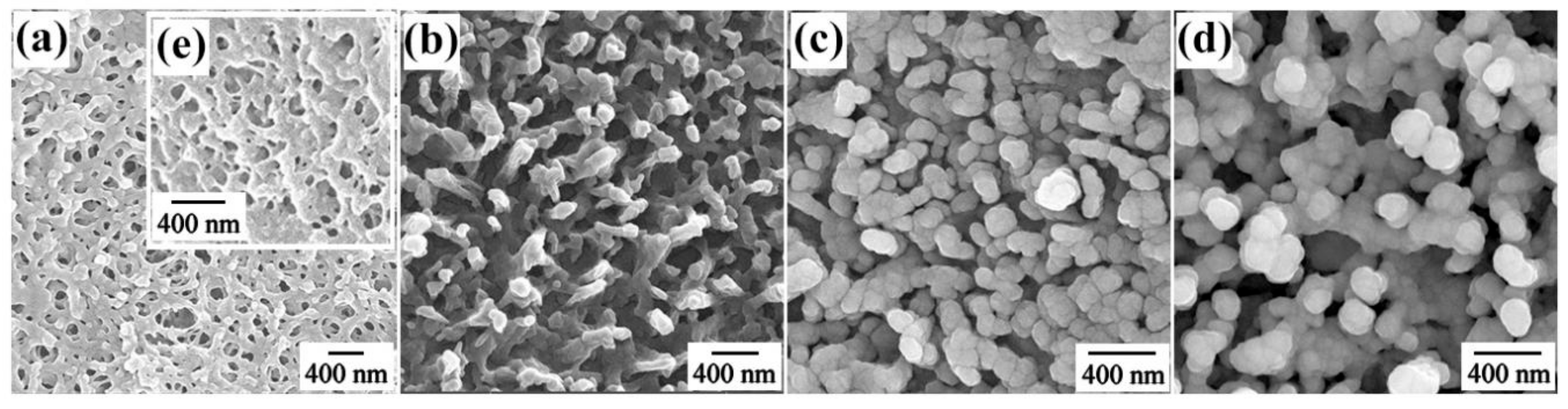
3.1. Characterization and Three-Dimensional Finite-Difference Time-Domain Simulation
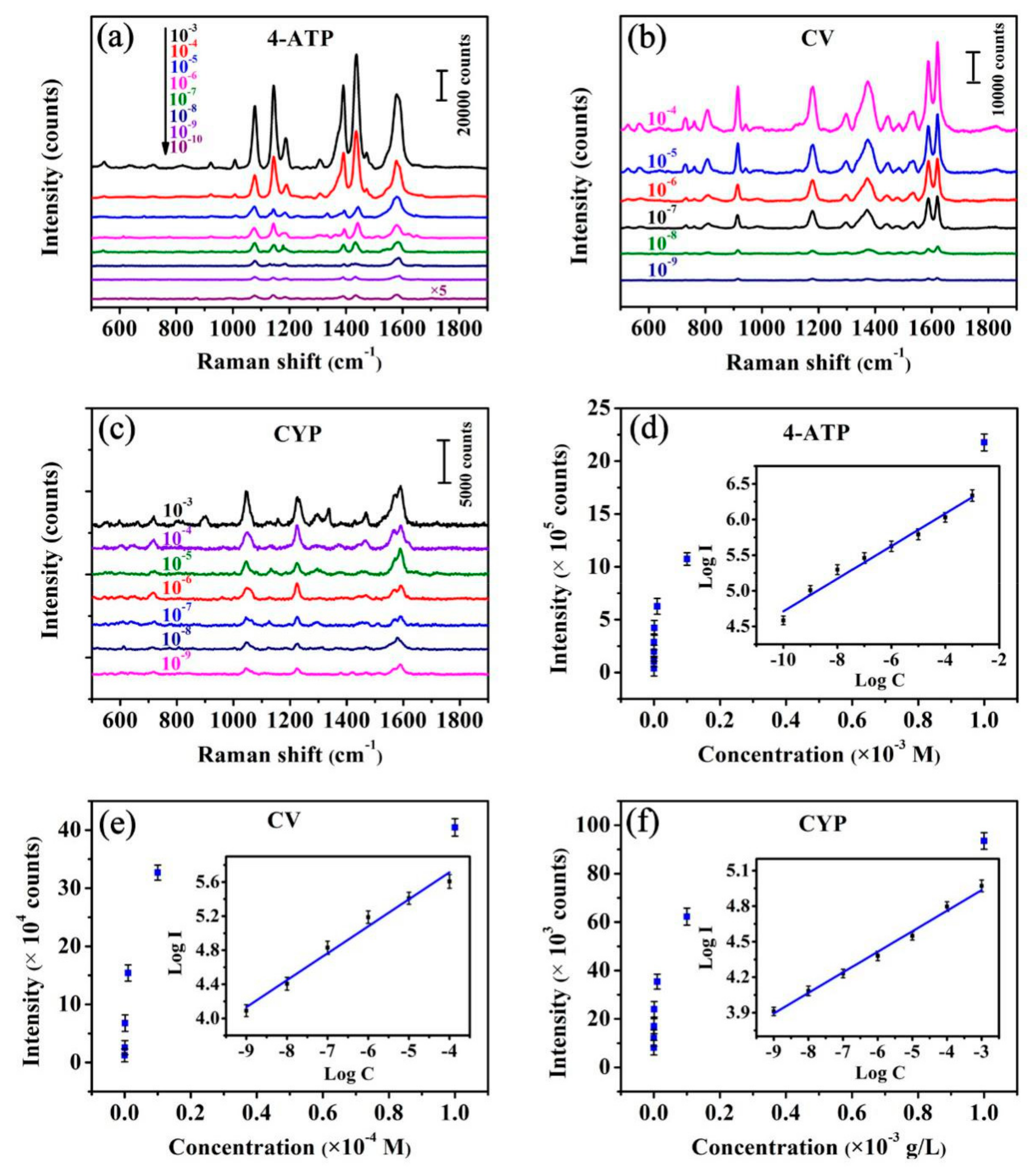
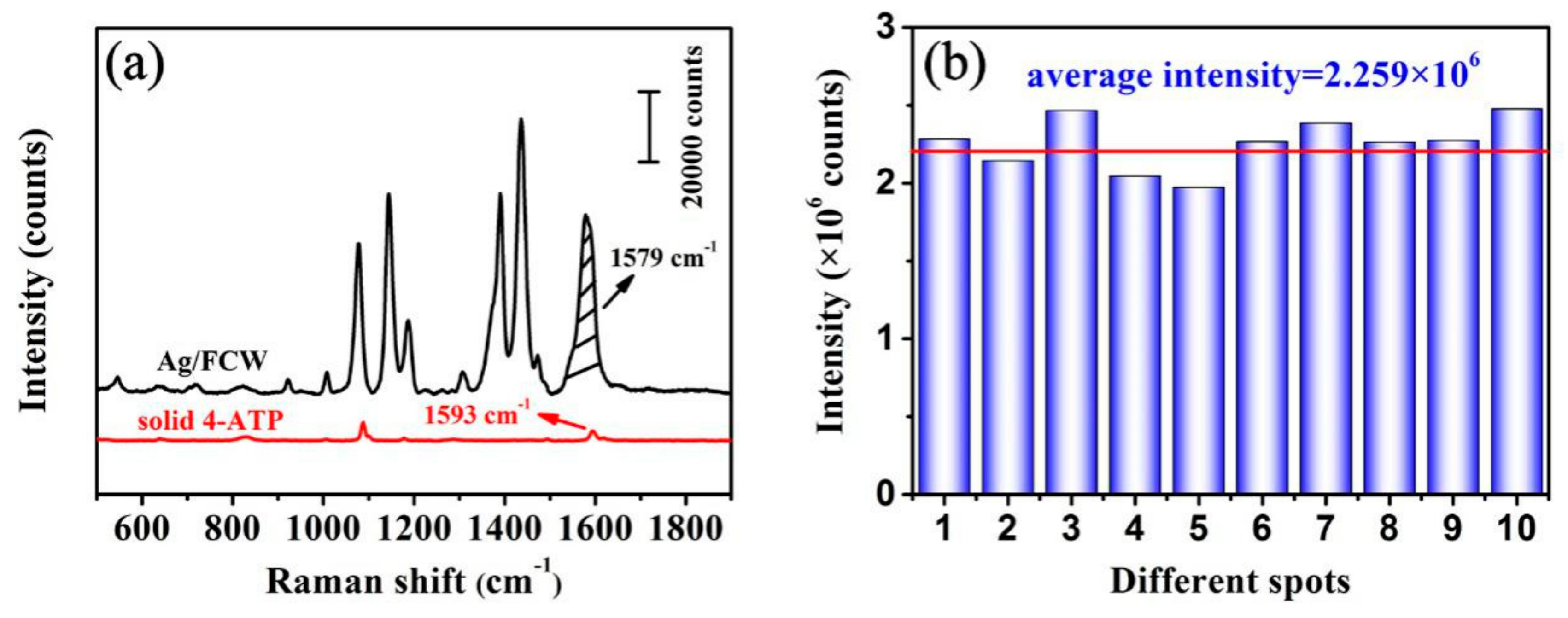
3.2. SERS Performances
3.3. EF Calculation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, H.Y.; Liu, Y.; Huang, Y.; Zhao, Y. A high sensitive fiber SERS probe based on silver nanorod arrays. Opt. Express 2007, 15, 12230–12239. [Google Scholar] [CrossRef] [PubMed]
- Fisk, H.; Westley, C.; Turner, N.J.; Goodacre, R. Achieving optimal SERS through enhanced experimental design. J. Raman Spectrosc. 2016, 47, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Q.; Fu, Z.W.; Qin, D. Transformation of Ag nanocubes into Ag-Au hollow nanostructures with enriched Ag contents to improve SERS activity and chemical stability. ACS Appl. Mater. Inter. 2014, 6, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Graham, D. ChemInform abstract: Molecularly-mediated assemblies of plasmonic nanoparticles for surface-enhanced Raman spectroscopy applications. Chem. Soc. Rev. 2012, 41, 7085–7107. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Jiang, L.; Hu, J.; Wang, A.; Li, X.; Lu, Y. Optical field enhancement in Au nanoparticle-decorated nanorod arrays prepared by femtosecond laser and their tunable surface-enhanced Raman scattering applications. ACS Appl. Mater. Inter. 2017, 10, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Blackie, E.J.; Ru, E.C.L.; Etchegoin, P.G. Single-molecule surface-enhanced Raman spectroscopy of nonresonant molecules. J. Am. Chem. Soc. 2009, 131, 14466–14472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Liu, S.S.; Gao, W.T.; Li, W.; Zhang, Y.J. Surface-enhanced Raman spectroscopy based on ordered nanocap arrays. Superlatt. Microstruct. 2012, 52, 750–758. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, M.L.; Shen, L.; Sun, X.; Shi, G.C.; Ma, W.L.; Yan, X.Y. High-performance flexible surface-enhanced Raman scattering substrates fabricated by depositing Ag nanoislands on the dragonfly wing. Appl. Surf. Sci. 2018, 436, 391–397. [Google Scholar] [CrossRef]
- Seniutinas, G.; Gervinskas, G.; Verma, R.; Gupta, B.D.; Lapierre, F.; Stoddart, P.R.; Clark, F.; McArthur, S.L.; Juodkazis, S. Versatile SERS sensing based on black silicon. Opt. Express 2015, 23, 6763–6772. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.N.; Lim, H.; Lee, H.N.; Park, Y.M.; Yoo, B.; Kim, H. Large-area and cost-effective fabrication of Ag-coated polymeric nanopillar array for surface-enhanced Raman spectroscopy. Appl. Surf. Sci. 2018, 446, 114–121. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, G.; Huang, Q.; Yang, Y.; Zhu, C.; Tang, C. Improved SERS performance from Au nanopillar arrays by abridging the pillar tip spacing by Ag sputtering. Adv. Mater. 2010, 22, 4136–4139. [Google Scholar] [CrossRef] [PubMed]
- Men, D.; Wu, Y.; Wang, C.; Xiang, J.; Yang, G.; Wan, C.; Zhang, H. Wafer-scale hierarchical nanopillar arrays based on Au masks and reactive ion etching for effective 3D SERS substrate. Materials 2018, 11, 239. [Google Scholar] [CrossRef]
- Tanwar, S.; Haldar, K.K.; Sen, T. DNA origami directed Au nanostar dimers for single molecule surface enhanced Raman scattering. J. Am. Chem. Soc. 2017, 139, 17639–17648. [Google Scholar] [CrossRef] [PubMed]
- Fales, A.M.; Yuan, H.; Vo-Dinh, T. Silica-coated gold nanostars for combined surface-enhanced Raman scattering (SERS) detection and singlet-oxygen generation: A potential nanoplatform for theranostics. Langmuir 2011, 27, 12186–12190. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Zhang, M.; Jiang, X.; Wang, Y.; Lv, J.; He, G.; Sun, Z. Three-dimensional hierarchical anatase@rutile TiO2 nanotree array films decorated by silver nanoparticles as ultrasensitive recyclable surface-enhanced Raman scattering substrates. J. Alloys Compd. 2017, 725, 1166–1174. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, C.; Sarkar, S.; Pradhan, M.; Negishi, Y.; Pal, T. Silver nanoparticle decorated reduced graphene oxide (rGO) nanosheet: a platform for SERS based low-level detection of uranyl ion. ACS Appl. Mater. Inter. 2013, 5, 8724–8732. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, C.X.; Liu, L.; Zhao, Y.M.; Xu, H.J. Recyclable three-dimensional Ag nanoparticle-decorated TiO2, nanorod arrays for surface-enhanced Raman scattering. Biosens. Bioelectron. 2015, 64, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Meng, J.; Wang, D.; Tang, Q.; Chen, T.; Rong, S.; Liu, J.; Wu, Y. Biomimetic synthesis of hierarchical 3D Ag butterfly wing scales arrays/graphene composites as ultrasensitive SERS substrates for efficient trace chemical detection. J. Mater. Chem. C 2018, 6, 1933–1943. [Google Scholar] [CrossRef]
- Shao, F.; Lu, Z.; Liu, C.; Han, H.; Chen, K.; Li, W.; He, Q.; Peng, H.; Chen, J. Hierarchical nanogaps within bioscaffold arrays as a high-performance SERS substrate for animal virus biosensing. ACS Appl. Mater. Inter. 2014, 6, 6281–6289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wang, M.L.; Sun, X.; Shi, G.C.; Zhang, J.Z.; Ma, W.L.; Ren, L.J. Grating-like SERS substrate with tunable gaps based on nanorough Ag nanoislands/moth wing scale arrays for quantitative detection of cypermethrin. Opt. Express 2018, 26, 22168–22181. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, C.; Wang, M.; Pan, X.; Zhang, C.; Liu, M.; Xu, S.; Jiang, S.; Man, B. Adsorbable and self-supported 3D AgNPs/G@Ni foam as cut-and-paste highly-sensitive SERS substrates for rapid in situ detection of residuum. Opt. Express 2017, 25, 16437–16451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Lai, C.; Zhou, H.; Zhu, Y. Silver-decorated aligned CNT arrays as SERS substrates by high temperature annealing. Opt. Express 2014, 22, 21157–21166. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, A.; Bukowska, J.; Janik-Czachor, M.; Grochala, W.; Szummer, A.; Dolata, M. Characterization of the copper surface optimized for use as a substrate for surface-enhanced Raman scattering. Vib. Spectrosc. 1998, 16, 21–29. [Google Scholar] [CrossRef]
- García-Vidal, F.J.; Pendry, J.B. Collective Theory of Surface Enhanced Raman Scattering. Phys. Rev. Lett. 1996, 77, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Dong, S.; Wang, E. Surface enhanced Raman scattering of p-aminothiophenol self-assembled monolayers in sandwich structure fabricated on glass. J. Chem. Phys. 2006, 124, 74709. [Google Scholar] [CrossRef] [PubMed]
- Limnonthakul, P.; Limwichean, S.; Eiamchai, P.; Horprathum, M.; Supatti, A.; Nuntawong, N.; Patthanasetakul, V.; Chindaudom, P. Vertically aligned Ag nanorod arrays for trace cypermethrin detection. Adv. Mater. Res. 2014, 979, 259–262. [Google Scholar] [CrossRef]
- Lv, M.Y.; Teng, H.Y.; Chen, Z.Y.; Zhao, Y.M.; Zhang, X.; Liu, L.; Wu, Z.L.; Liu, L.M.; Xu, H.J. Low-cost Au nanoparticle-decorated cicada wing as sensitive and recyclable substrates for surface enhanced Raman scattering Sensor. Sens. Actuators B Chem. 2015, 209, 820–827. [Google Scholar] [CrossRef]
- Wu, D.Y.; Liu, X.M.; Huang, Y.F.; Ren, B.; Xu, X.; Tian, Z.Q. Surface Catalytic Coupling Reaction of p-Mercaptoaniline Linking to Silver Nanostructures Responsible for Abnormal SERS Enhancement: A DFT Study. J. Phys. Chem. C 2009, 113, 18212–18222. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhu, H.P.; Liu, G.K.; Wu, D.Y.; Ren, B.; Tian, Z.Q. When the signal is not from the original molecule to be detected: Chemical transformation of para-Aminothiophenol on Ag during the SERS Measurement. J. Am. Chem. Soc. 2010, 132, 9244–9246. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shen, M.; Liu, S.; Li, F.; Sun, D.; Wang, T. A simple technique for direct growth of Au into a nanoporous alumina layer on conductive glass as a reusable SERS substrate. Appl. Surf. Sci. 2017, 406, 285–293. [Google Scholar] [CrossRef]
- Wei, G.; Wang, L.; Liu, Z.; Song, Y.; Sun, L.; Yang, T.; Li, Z. DNA-network-templated self-assembly of silver nanoparticles and their application in surface-enhanced Raman scattering. J. Phys. Chem. B 2005, 109, 23941–23947. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Li, C.; Qi, L. Facile fabrication of two-dimensionally ordered macroporous silver thin films and their application in molecular sensing. Adv. Funct. Mater. 2010, 20, 3774–3783. [Google Scholar] [CrossRef]
- Gole, A.; Sainkar, S.; Sastry, M. Electrostatically controlled organization of carboxylic acid derivatized colloidal silver particles on amine-terminated self-assembled monolayers. Chem. Mater. 2000, 12, 1234–1239. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wang, Y.; Yan, X.; Sun, X.; Shi, G.; Zhang, K.; Ren, L.; Ma, W. Three-Dimensional Hierarchical Reticular Nanostructure of Fulfora candelaria Wing Decorated by Ag Nanoislands as Practical SERS-Active Substrates. Nanomaterials 2018, 8, 905. https://doi.org/10.3390/nano8110905
Wang M, Wang Y, Yan X, Sun X, Shi G, Zhang K, Ren L, Ma W. Three-Dimensional Hierarchical Reticular Nanostructure of Fulfora candelaria Wing Decorated by Ag Nanoislands as Practical SERS-Active Substrates. Nanomaterials. 2018; 8(11):905. https://doi.org/10.3390/nano8110905
Chicago/Turabian StyleWang, Mingli, Yuhong Wang, Xiaoya Yan, Xin Sun, Guochao Shi, Keqin Zhang, Lijian Ren, and Wanli Ma. 2018. "Three-Dimensional Hierarchical Reticular Nanostructure of Fulfora candelaria Wing Decorated by Ag Nanoislands as Practical SERS-Active Substrates" Nanomaterials 8, no. 11: 905. https://doi.org/10.3390/nano8110905
APA StyleWang, M., Wang, Y., Yan, X., Sun, X., Shi, G., Zhang, K., Ren, L., & Ma, W. (2018). Three-Dimensional Hierarchical Reticular Nanostructure of Fulfora candelaria Wing Decorated by Ag Nanoislands as Practical SERS-Active Substrates. Nanomaterials, 8(11), 905. https://doi.org/10.3390/nano8110905





