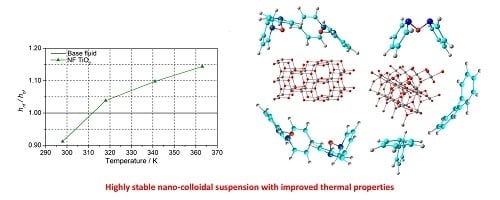
A Solvothermal Synthesis of TiO2 Nanoparticles in a Non-Polar Medium to Prepare Highly Stable Nanofluids with Improved Thermal Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
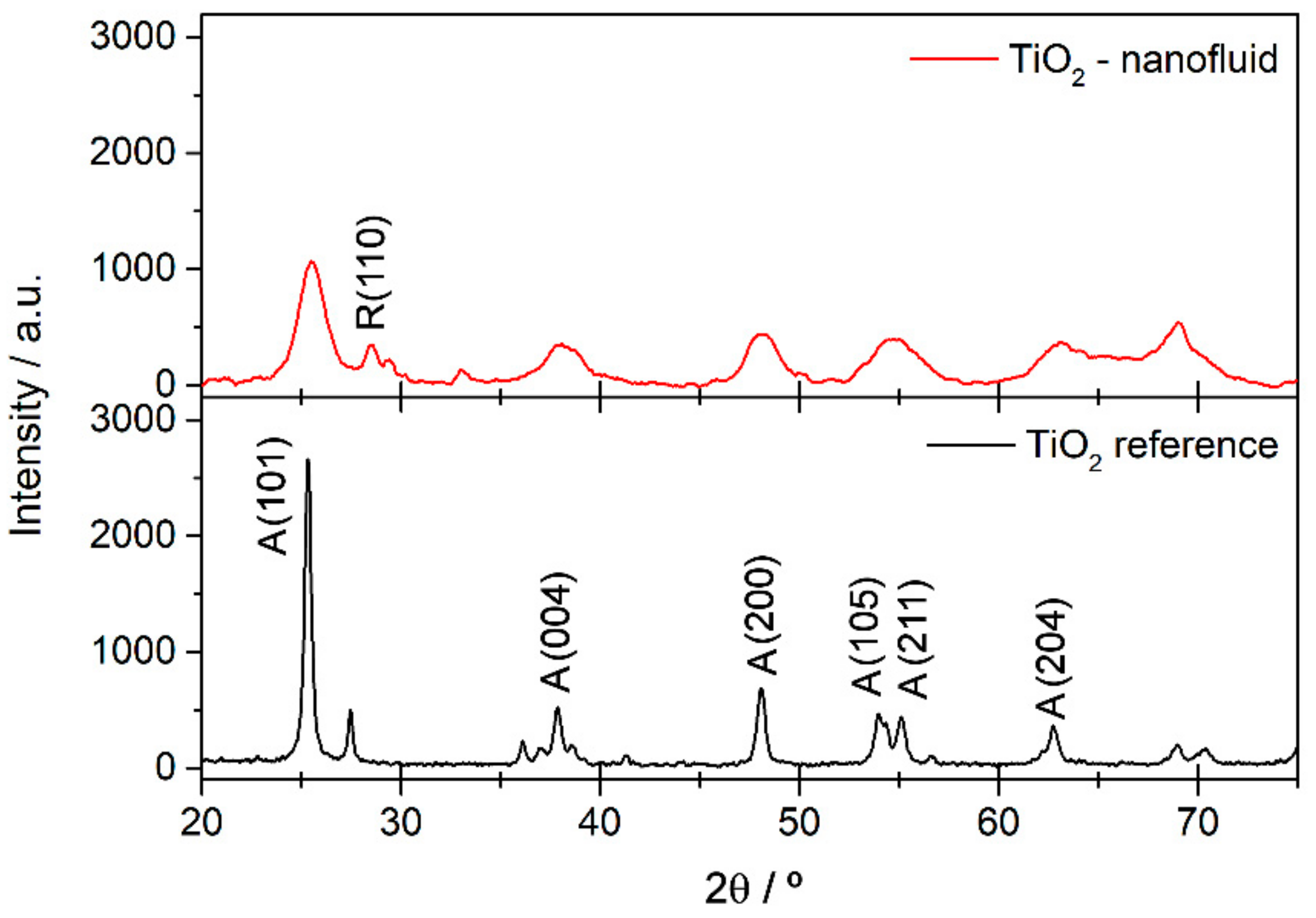
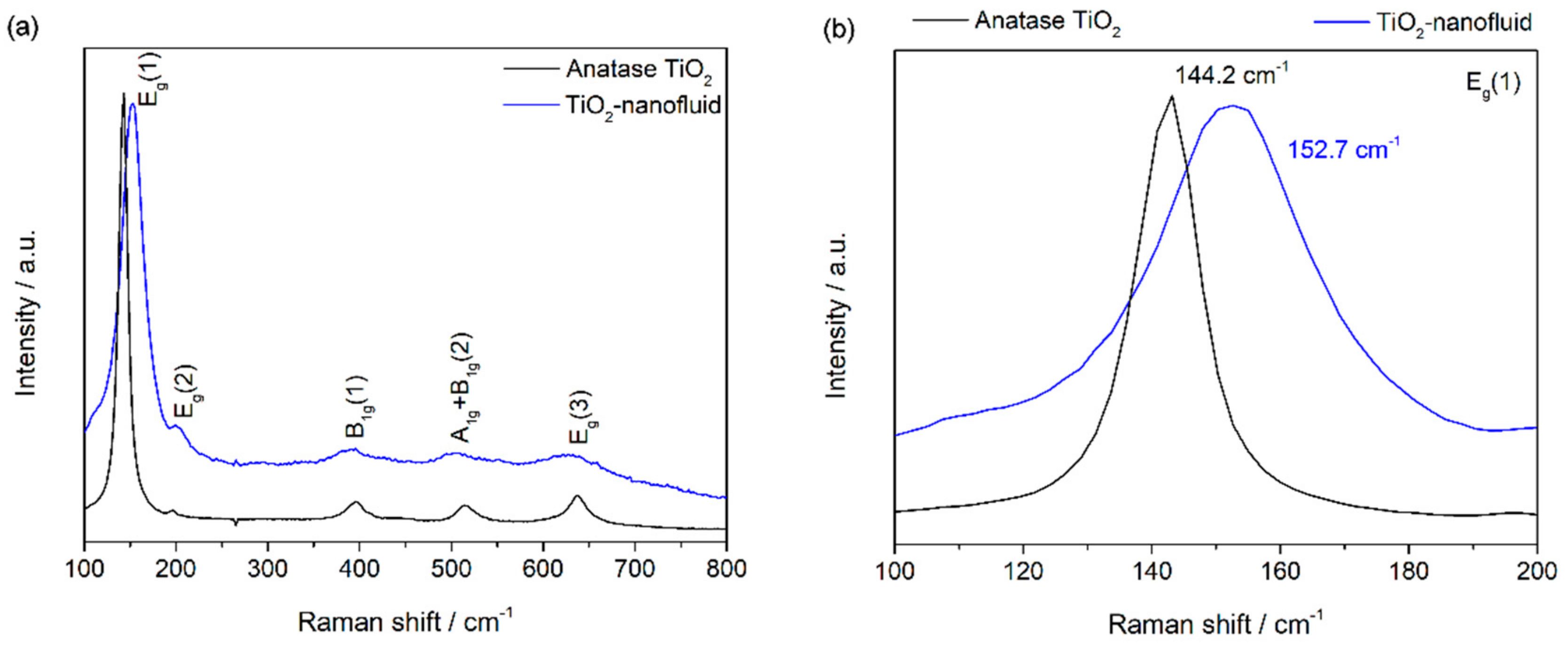
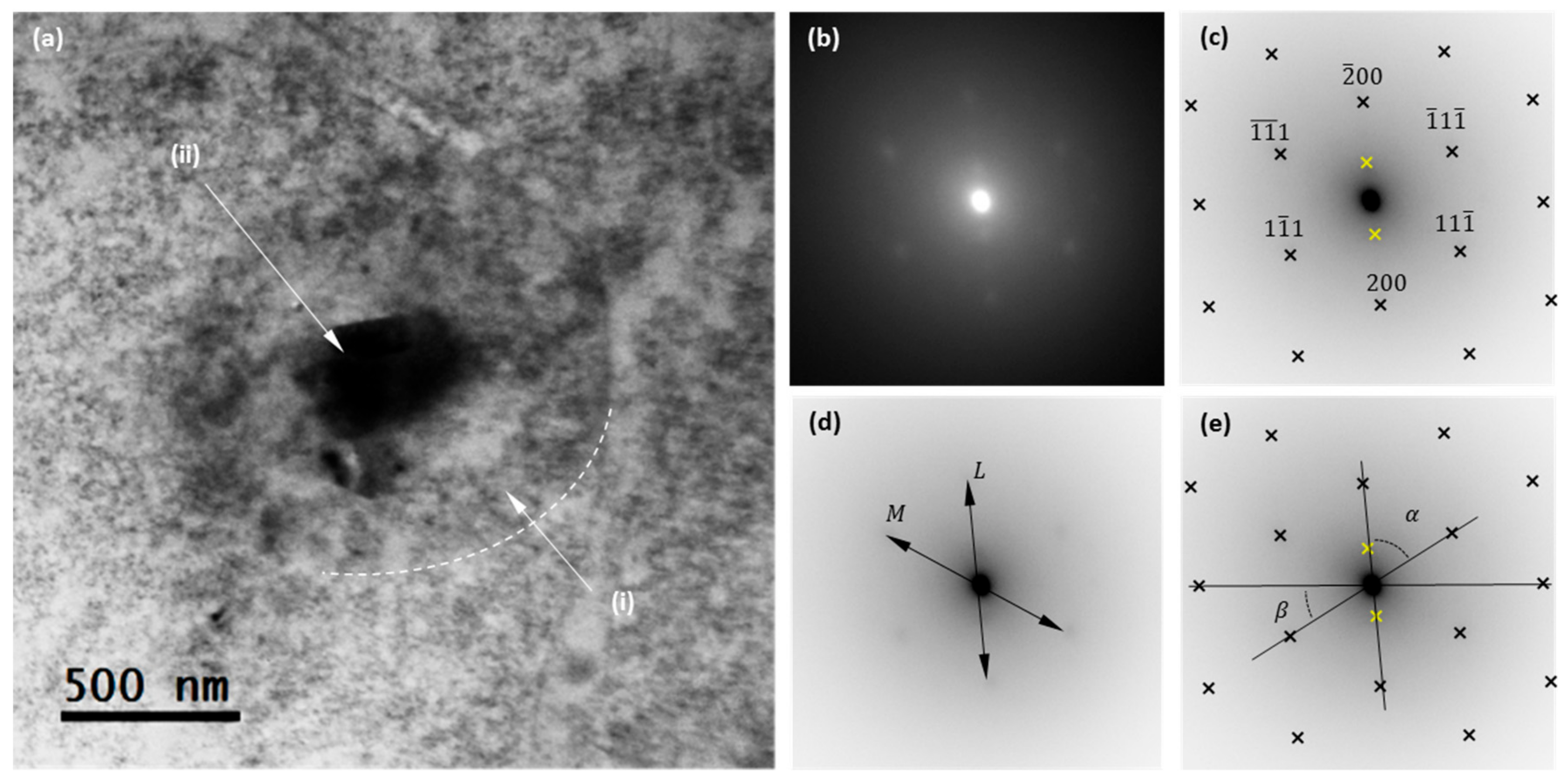
3.1. Material Characterization
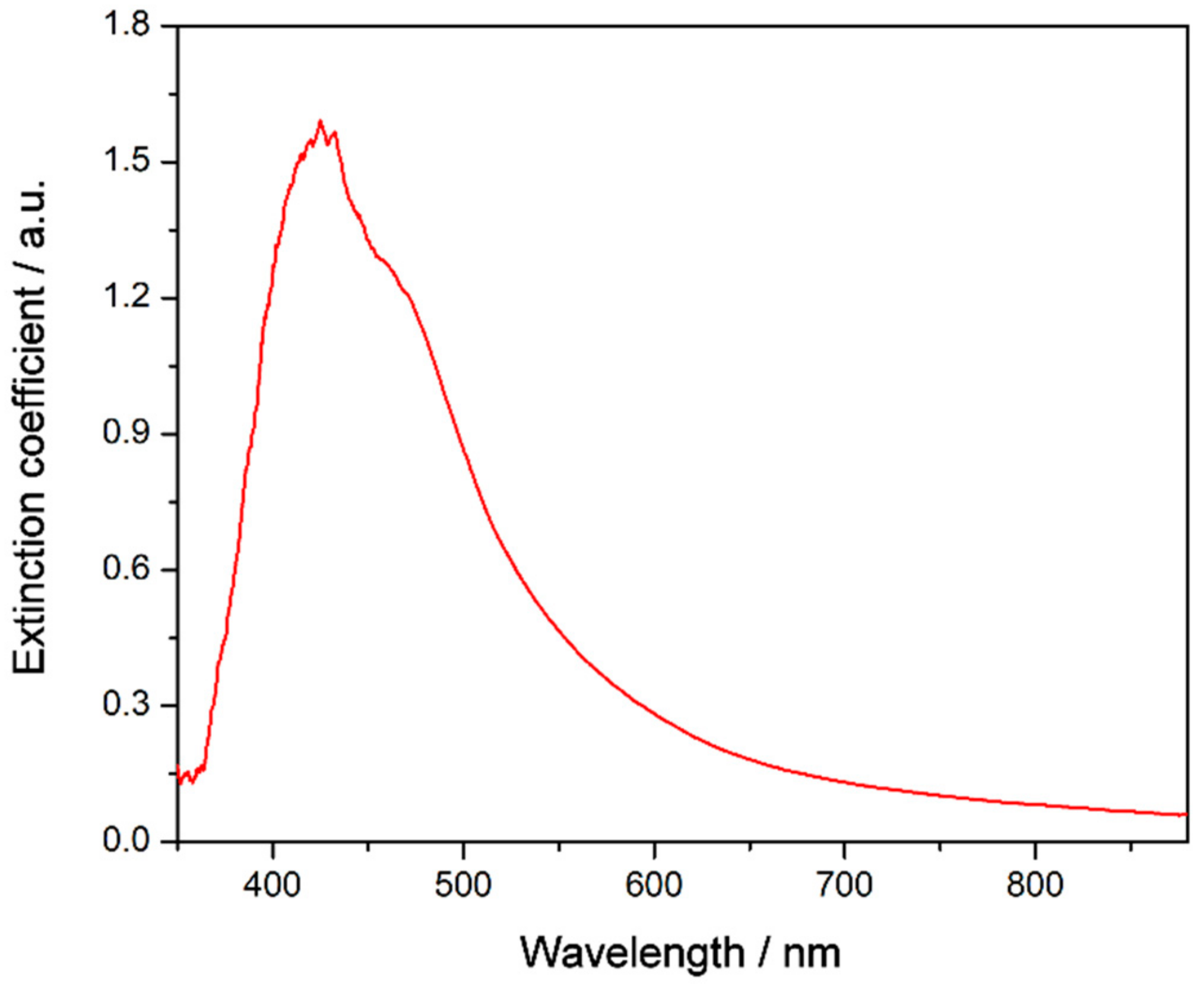
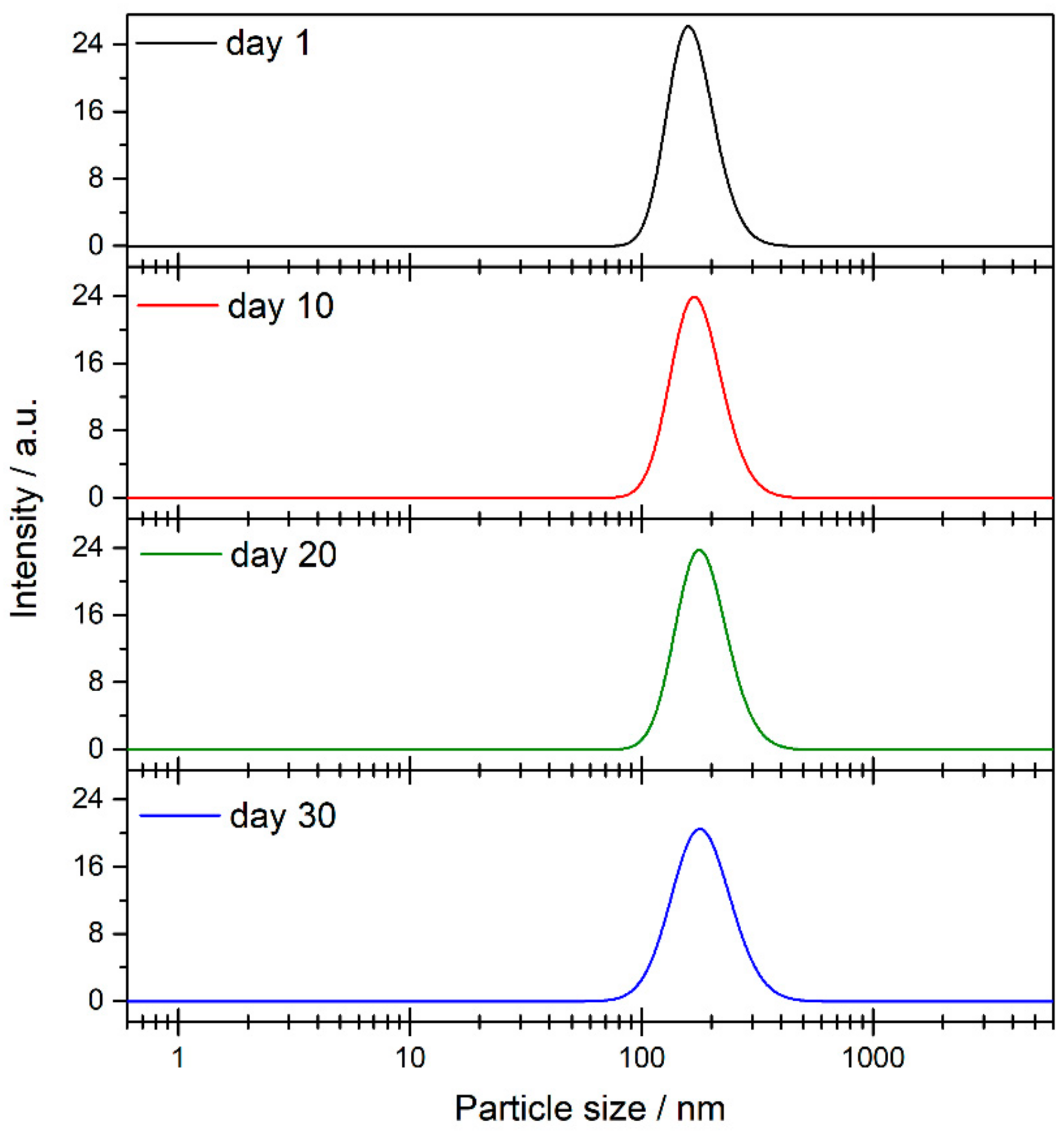
3.2. Nanofluid Stability
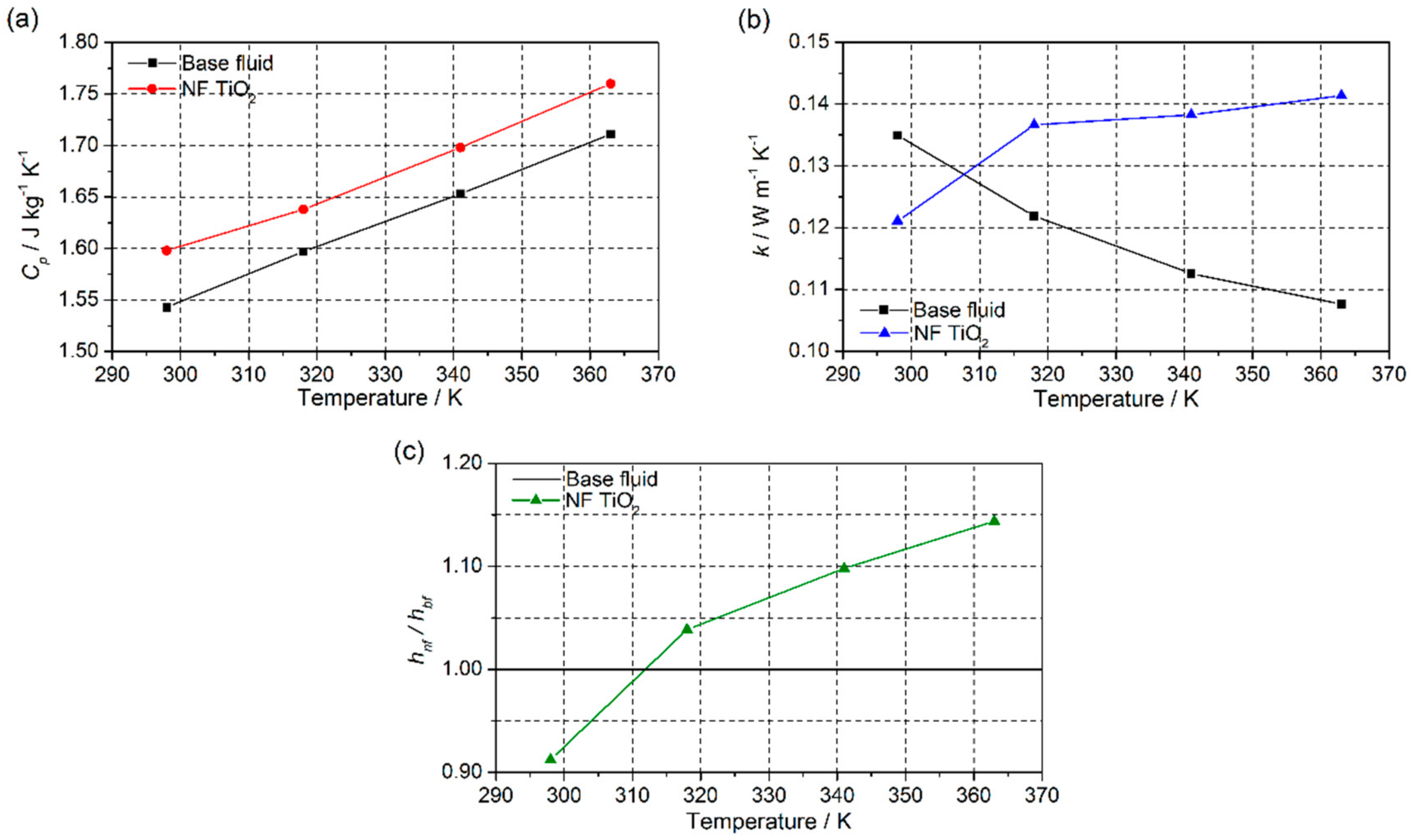
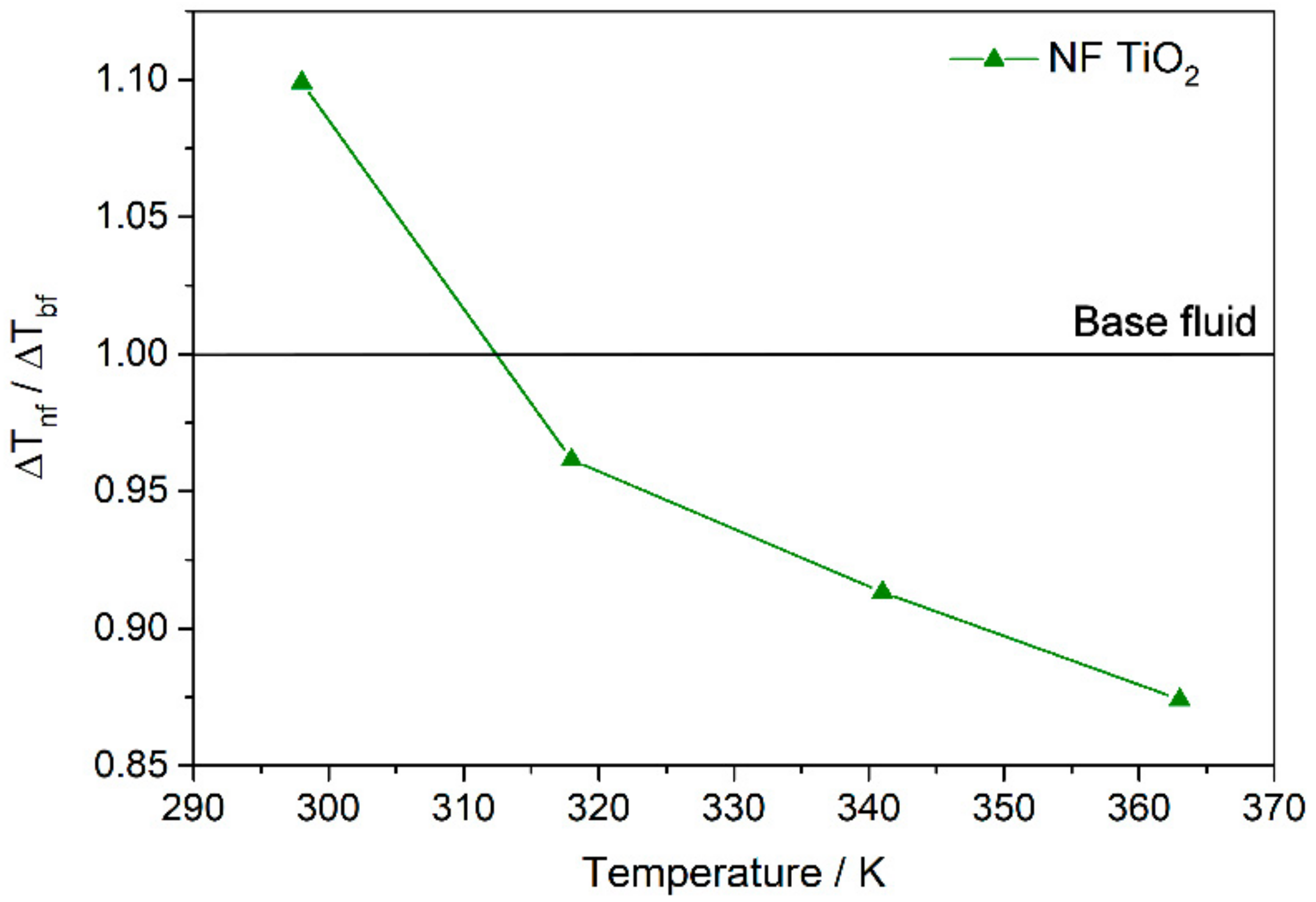
3.3. Nanofluid Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| a, b, c | Lattice constants (Å) |
| B.E. | Binding Energy (eV) |
| CP | Isobaric specific heat (J kg−1 K−1) |
| h | Convective heat transfer coefficient (W m−2 K−1) |
| k | Thermal conductivity (W m−1 K−1) |
| Heat flux (W m−2) | |
| Tm,o | Mean temperature of the fluid at the pipe outlet (K) |
| Ts | Temperature on the surface of the pipe (K) |
| α | Thermal diffusivity (m2 s−1) |
| ϕ | Volume fraction (vol.%) |
| μ | Dynamic viscosity (Pa s) |
| ρ | Density (kg m−3) |
| Subscripts | |
| bf | Base fluid |
| nf | Nanofluid |
| np | Nanoparticle |
References
- Choi, S.U.S. Enhancing thermal conductivity of fluids with nanoparticles. ASME-Publ.-Fed 1995, 231, 99–106. [Google Scholar]
- Colangelo, G.; Favale, E.; Milanese, M.; de Risi, A.; Laforgia, D. Cooling of electronic devices: Nanofluids contribution. Appl. Therm. Eng. 2017, 127, 421–435. [Google Scholar] [CrossRef]
- Asadi, M.; Asadi, A.; Aberoumand, S. An experimental and theoretical investigation on the effects of adding hybrid nanoparticles on heat transfer efficiency and pumping power of an oil-based nanofluid as a coolant fluid. Int. J. Refrig 2018, 89, 83–92. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Miglieta, P.; Milanese, M.; de Risi, A. Thermal conductivity, viscosity and stability of Al3O3-diathermic oil nanofluids for solar energy systems. Energy 2016, 95, 124–136. [Google Scholar] [CrossRef]
- Águila, V.B.; Vasco, D.A.; Galvez, P.P.; Zapata, P.A. Effect of temperature and CuO-nanoparticle concentration on the thermal conductivity and viscosity of an organic phase-change material. Int. J. Heat Mass Transf. 2018, 120, 1009–1019. [Google Scholar] [CrossRef]
- Yang, L.; Du, K. A comprehensive review on heat transfer characteristics of TiO2 nanofluids. Int. J. Heat Mass Transf. 2017, 108, 11–31. [Google Scholar] [CrossRef]
- Alawi, O.A.; Sidik, N.A.C.; Xian, H.W.; Kean, T.H.; Kazi, S.N. Thermal conductivity and viscosity models of metallic oxides nanofluids. Int. J. Heat Mass Transf. 2018, 116, 1314–1325. [Google Scholar] [CrossRef]
- Fuskele, V.; Sarviya, R.M. Recent developments in nanoparticles synthesis, preparation and stability of nanofluids. Mater. Today Proc. 2017, 4, 4049–4060. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.-K.; Lee, J.-K.; Jeong, Y.-M.; Cheong, S.-I.; Ahn, Y.-C.; Kim, S.H. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012, 17. [Google Scholar] [CrossRef]
- Sánchez-Coronilla, A.; Navas, J.; Aguilar, T.; Martín, E.I.; Gallardo, J.J.; Gómez-Villarejo, M.R.; Carrillo-Berdugo, M.I.; Alcántara, R.; Fernández-Lorenzo, C.; Martín-Calleja, J. The role of surfactants in the stability of NiO nanofluids: An experimental and DFT study. ChemPhysChem 2017, 18, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Yasinskiy, A.; Navas, J.; Aguilar, T.; Alcántara, R.; Gallardo, J.J.; Sánchez-Coronilla, A.; Martín, E.I.; De Los Santos, D.; Fernández-Lorenzo, C. Dramatically enhanced thermal properties for TiO2-based nanofluids for being used as heat transfer fluids in concentrating solar power plants. Renew. Energy 2018, 119, 809–819. [Google Scholar] [CrossRef]
- Sadeghi, R.; Etemad, S.G.; Keshavarzi, E.; Haghshenasfard, M. Investigation of alumina nanofluid stability by UV-vis spectrum. Microfluid. Nanofluid. 2015, 18, 1023–1030. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Tie, P. The effect of surfactants on heat transfer feature of nanofluids. Exp. Therm Fluid Sci. 2013, 46, 259–262. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Ali, H.M.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation techniques of TiO2 nanofluids and challenges: A review. Appl. Sci. 2018, 8, 587. [Google Scholar]
- Arshd, W.; Ali, H.M. Experimental investigations of heat transfer and pressure drop in a straight minichannel heat sink using TiO2 nanofluids. Int. J. Heat Mass Transf. 2017, 110, 248–256. [Google Scholar] [CrossRef]
- Paul, G.; Sarkar, S.; Pal, T.; Das, P.K.; Manna, I. Concentration and size dependence of nano-silver dispersed water based nanofluids. J. Colloid Interface Sci. 2012, 371, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-J.; Kim, C.K.; Lee, M.K.; Rhee, C.K.; Kim, S.; Kim, C. Thermal conductivity enhancement of ZnO nanofluid using a one-step physical method. Thermochim. Acta 2012, 542, 24–27. [Google Scholar] [CrossRef]
- Chang, H.; Jwo, C.S.; Lo, C.H.; Tsung, T.T.; Kao, M.J.; Lin, H.M. Rheology of CuO nanoparticle suspension prepared by ASNSS. Adv. Mater. Sci. 2005, 10, 128–132. [Google Scholar]
- Lee, S.; Choi, S.U.S.; Li, S.; Eastman, J.A. Measuring Thermal Conductivity of Fluids Containing Oxide Nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Niederberger, M.; Bartl, M.H.; Stucky, G.D. Benzyl alcohol and transition metal chlorides as a versatile reaction system for the nonaqueous and low-Temperature synthesis of crystalline nano-objects with controlled dimensionality. J. Am. Chem. Soc. 2002, 124, 13642–13643. [Google Scholar] [CrossRef] [PubMed]
- Pinna, N.; Garnweitner, G.; Antonietti, M.; Niederberg, M. Non- Aqueous Synthesis of High-Purity Metal Oxide Nanopowders Using an Ether Elimination Process. Adv. Mater. 2004, 16, 23–24. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gajbhiye, N.S.; Date, S.K. Ferromagnetism of Mn-doped TiO2 nanorods synthesized by hydrothermal method. J. Alloys Compd. 2011, 509, 4274. [Google Scholar] [CrossRef]
- Li Bassi, A.; Cattaneo, D.; Russo, V.; Bottani, C.E.; Barborini, E.; Mazza, T.; Piseri, P.; Milani, P.; Ernst, F.O.; Wegner, K.; et al. Raman spectroscopy characterization of titania nanoparticles produced by flame pyrolysis: The influence of size and stoichiometry. J. Appl. Phys. 2005, 98, 074305. [Google Scholar] [CrossRef]
- Alcántara, R.; Navas, J.; Fernández-Lorenzo, C.; Martín, J.; Guillén, E.; Anta, J.A. Synthesis and Raman spectroscopy study of TiO2 nanoparticles. Phys. Status Solidi C 2011, 8, 1970–1973. [Google Scholar] [CrossRef]
- Sun, L.; Haidry, A.A.; Fatima, Q.; Li, Z.; Yao, Z. Improving the humidity sensing below 30% RH of TiO2 with GO modification. Mater. Res. Bull. 2018, 99, 124–131. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Singh, M.K.; Mathpal, M.C.; Mishra, S.K.; Agarwal, A. Study of structural transformation in TiO2 nanoparticles and its optical properties. J. Alloys Compd. 2013, 549, 114–120. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.; Méndez Medrano, M.G.; Remita, H.; Escobar-Barrios, V. Photocatalytic properties of BiOCl-TiO2 composites for phenol photodegradation. J. Environ. Chem. Eng. 2018, 6, 1601–1612. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Huang, J.; Fei, J.; Cao, L.; Li, C. In situ synthesis of mesoporous C-doped TiO2 single crystal with oxygen vacancy and its enhanced sunlight photocatalytic properties. Dyes Pigm. 2017, 144, 203–211. [Google Scholar] [CrossRef]
- Chenakin, S.; Kruse, N. Combining XPS and ToF-SIMS for assessing the CO oxidation activity of Au/TiO2 catalysts. J. Catal. 2018, 358, 224–236. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Song, W.; Li, M.; Zeng, D.; Xie, C. Catalytic oxidation of formaldehyde on surface of HTiO2/HCTiO2 without light illumination at room temperature. Appl. Catal. B 2014, 147, 490–498. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Martín, E.I.; Teruel, M.; Gallardo, J.J.; Aguilar, T.; Gómez-Villarejo, R.; Alcántara, R.; Fernández-Lorenzo, C.; Piñero, J.C.; et al. On the enhancement of heat transfer fluid for concentrating solar power using Cu and Ni nanofluids: An experimental and molecular dynamics study. Nano Energy 2016, 27, 213–224. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.K.; Lee, C.H.; Jung, Y.M.; Cheong, S.I.; Lee, C.G.; Ku, B.C.; Jang, S.P. Stability and thermal conductivity characteristics of nanofluids. Thermochim. Acta 2007, 455, 70–74. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.S.; Yu, W.; Pradeep, T. Synthesis of Nanofluids. In Nanofluids; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Chapter 2; pp. 39–100. [Google Scholar]
- Das, S.K.; Choi, S.U.S.; Yu, W.; Pradeep, T. Convection in Nanofluids. In Nanofluids; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Chapter 5; pp. 209–296. [Google Scholar]
- Agarwal, D.K.; Vaidyanathan, A.; Sunil Kumar, S. Synthesis and characterization of kerosene-alumina nanofluids. Appl. Therm. Eng. 2013, 60, 275–284. [Google Scholar] [CrossRef]
- Hadadian, M.; Samiee, S.; Ahmadzadeh, H.; Goharshadi, E.K. Nanofluids for heat transfer enhancement—A review. Phys. Chem. Res. 2013, 1, 1–33. [Google Scholar]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Rheological behaviour of nanofluids: A review. Renew. Sustain. Energy Rev. 2016, 53, 779–791. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Gracia-Fernández, C.; Legido, J.L.; Lugo, L. Specific heat of metal oxide nanofluids at high concentrations for heat transfer. Int. J. Heat Mass Transf. 2015, 88, 872–879. [Google Scholar] [CrossRef]
- Susan Mousavi, N.S.; Kumar, S. Effective heat capacity of ferrofluids—Analytical approach. Int. J. Therm. Sci. 2014, 84, 267–274. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Suresh, S.; Senthilkumar, T. Mechanisms proposed through experimental investigations on thermophysical properties and forced convective heat transfer characteristics of various nanofluids—A review. Renew. Sustain. Energy Rev. 2012, 16, 3917–3938. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.R.; Choi, S.U.S.; Eastman, J.A. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass Transf. 2002, 45, 855–863. [Google Scholar] [CrossRef]
- Keblinski, P.; Eastman, J.A.; Cahill, D.G. Nanofluids for thermal transport. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Machrafi, H.; Lebon, G. The role of several heat transfer mechanisms on the enhancement of thermal conductivity in nanofluids. Contin. Mech. Thermodyn. 2016, 28, 1461–1475. [Google Scholar] [CrossRef]
- Milanese, M.; Iacobazzi, F.; Colangelo, G.; de Risi, A. An investigation of layering at the liquid-solid interface in Cu and CuO based nanofluids. Int. J. Heat Mass Transf. 2016, 103, 564–571. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Saha, S.K.; Yadav, A.; Phelan, P.E.; Prasher, R.S. Brownian dynamics simulation to determine the effective thermal conductivity of nanofluids. J. Appl. Phys. 2004, 95, 6492–6494. [Google Scholar] [CrossRef]
- Kapitza, P.L. The study of heat transfer in helium II. J. Phys. U.S.S.R. 1941, 4, 181. [Google Scholar]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; Lomascolo, M.; de Risi, A. An explanation of the Al2O3 nanofluid thermal conductivity based on the phonon theory of liquid. Energy 2016, 116, 786–794. [Google Scholar] [CrossRef]
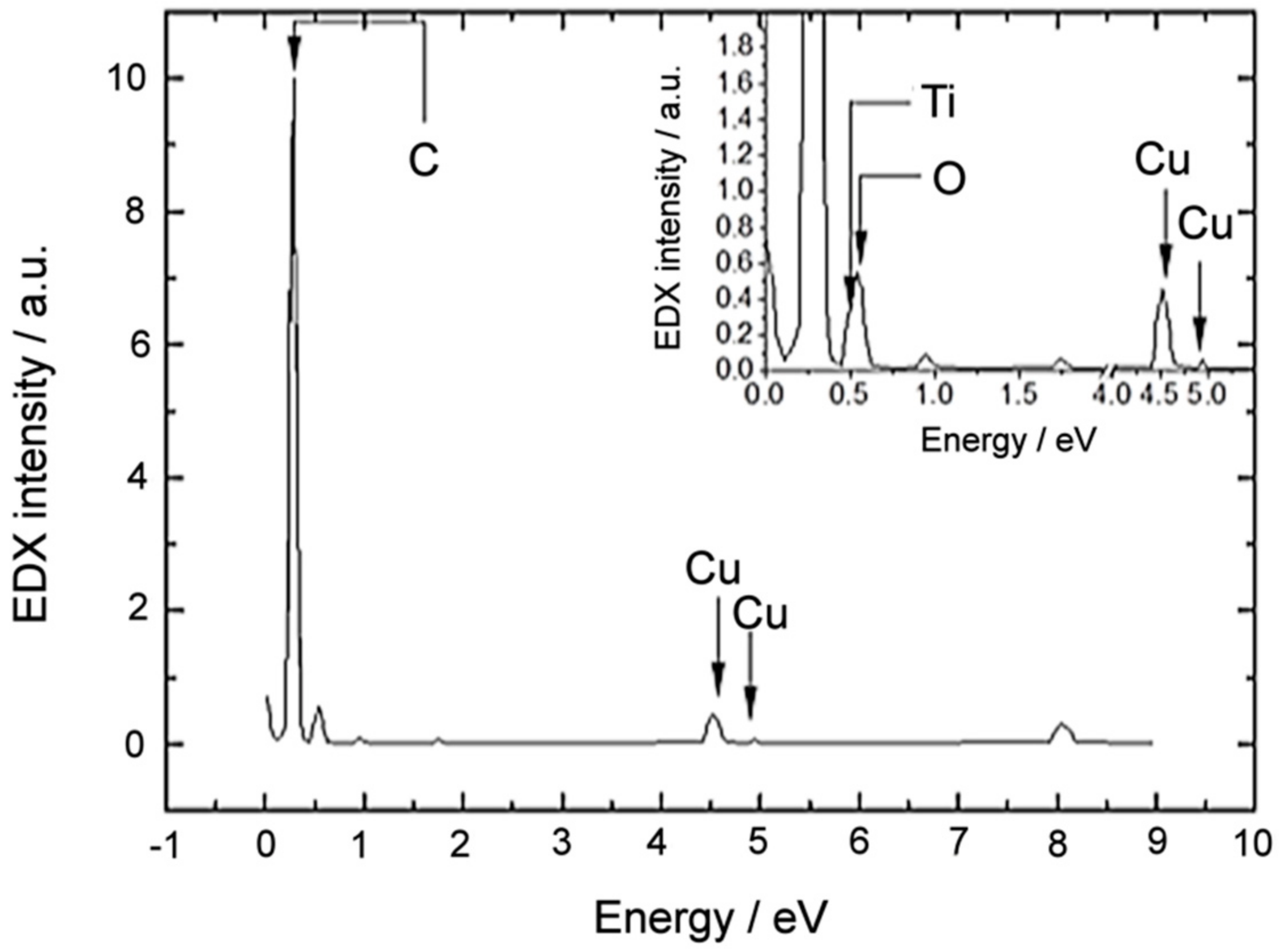
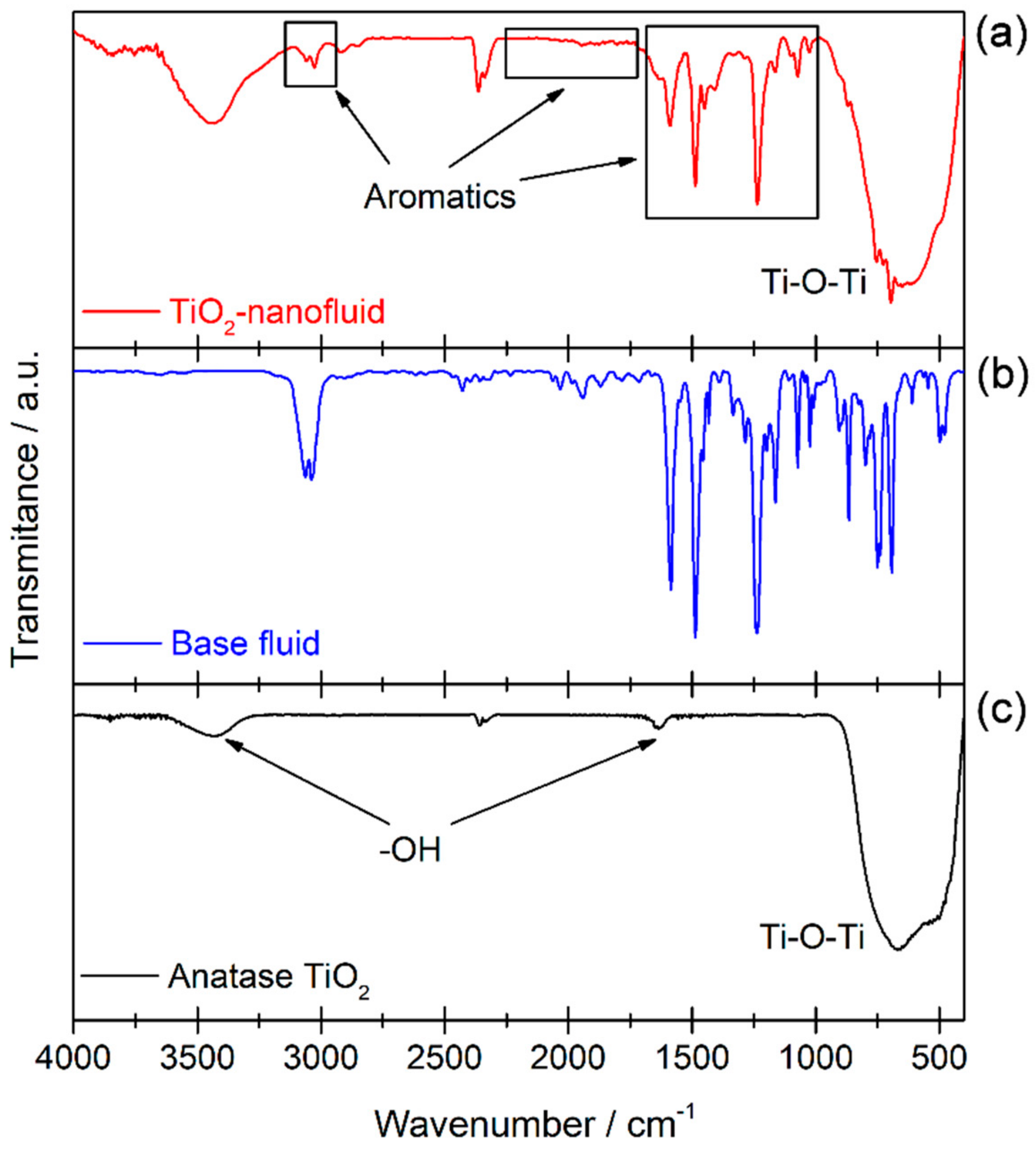
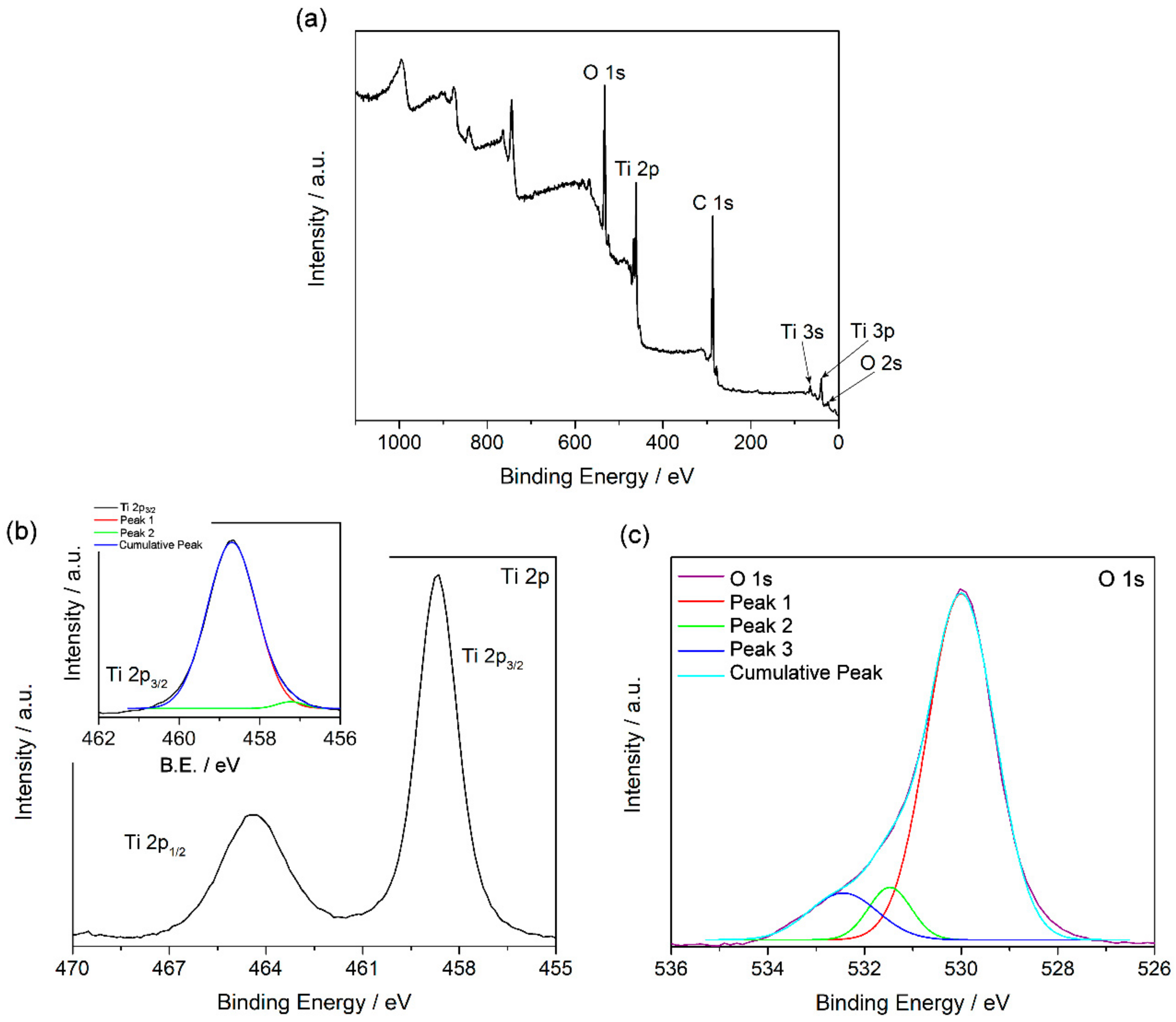

| Ti 2p3/2 | O 1s | ||||
|---|---|---|---|---|---|
| Peaks | B.E./eV | % | Peaks | B.E./eV | % |
| 1 | 458.7 | 97.5 | 1 | 529.9 | 71.8 |
| 2 | 457.2 | 2.5 | 2 | 531.5 | 12.7 |
| 3 | 532.5 | 15.5 | |||
| XPS Signal | C 1s | O 1s | Ti 2p |
|---|---|---|---|
| % | 62.7 | 26.6 | 10.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, T.; Carrillo-Berdugo, I.; Gómez-Villarejo, R.; Gallardo, J.J.; Martínez-Merino, P.; Piñero, J.C.; Alcántara, R.; Fernández-Lorenzo, C.; Navas, J. A Solvothermal Synthesis of TiO2 Nanoparticles in a Non-Polar Medium to Prepare Highly Stable Nanofluids with Improved Thermal Properties. Nanomaterials 2018, 8, 816. https://doi.org/10.3390/nano8100816
Aguilar T, Carrillo-Berdugo I, Gómez-Villarejo R, Gallardo JJ, Martínez-Merino P, Piñero JC, Alcántara R, Fernández-Lorenzo C, Navas J. A Solvothermal Synthesis of TiO2 Nanoparticles in a Non-Polar Medium to Prepare Highly Stable Nanofluids with Improved Thermal Properties. Nanomaterials. 2018; 8(10):816. https://doi.org/10.3390/nano8100816
Chicago/Turabian StyleAguilar, Teresa, Ivan Carrillo-Berdugo, Roberto Gómez-Villarejo, Juan Jesús Gallardo, Paloma Martínez-Merino, José Carlos Piñero, Rodrigo Alcántara, Concha Fernández-Lorenzo, and Javier Navas. 2018. "A Solvothermal Synthesis of TiO2 Nanoparticles in a Non-Polar Medium to Prepare Highly Stable Nanofluids with Improved Thermal Properties" Nanomaterials 8, no. 10: 816. https://doi.org/10.3390/nano8100816
APA StyleAguilar, T., Carrillo-Berdugo, I., Gómez-Villarejo, R., Gallardo, J. J., Martínez-Merino, P., Piñero, J. C., Alcántara, R., Fernández-Lorenzo, C., & Navas, J. (2018). A Solvothermal Synthesis of TiO2 Nanoparticles in a Non-Polar Medium to Prepare Highly Stable Nanofluids with Improved Thermal Properties. Nanomaterials, 8(10), 816. https://doi.org/10.3390/nano8100816