A Novel Tb@Sr-MOF as Self-Calibrating Luminescent Sensor for Nutritional Antioxidant
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of [Sr(BDC)·DMAC·H2O]n (Sr-MOF)
2.2. Synthesis of Tb(3+)@Sr-MOF
2.3. Detection of Sesamol
2.4. Materials and Characterization
2.5. X-ray Crystallography
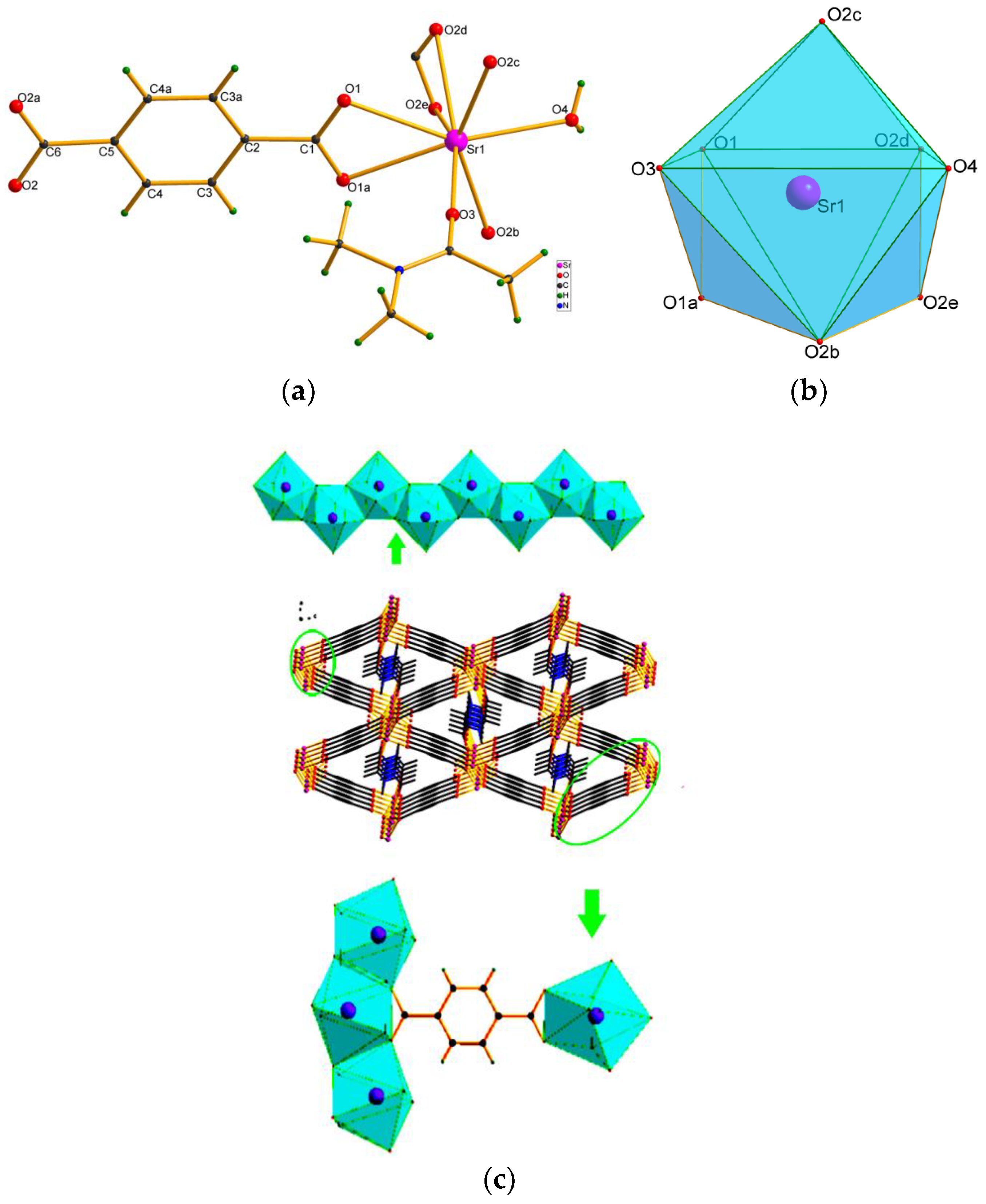
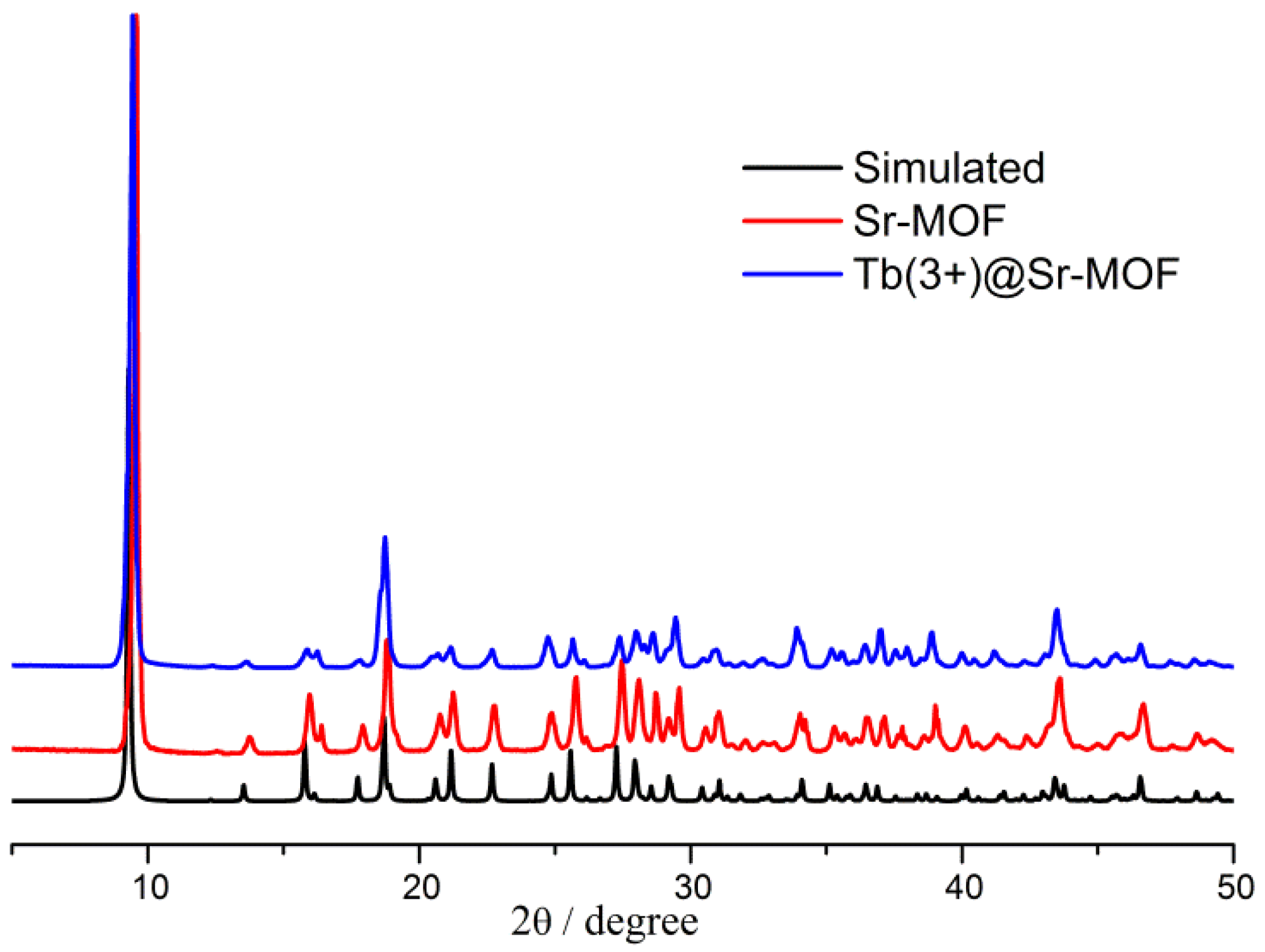
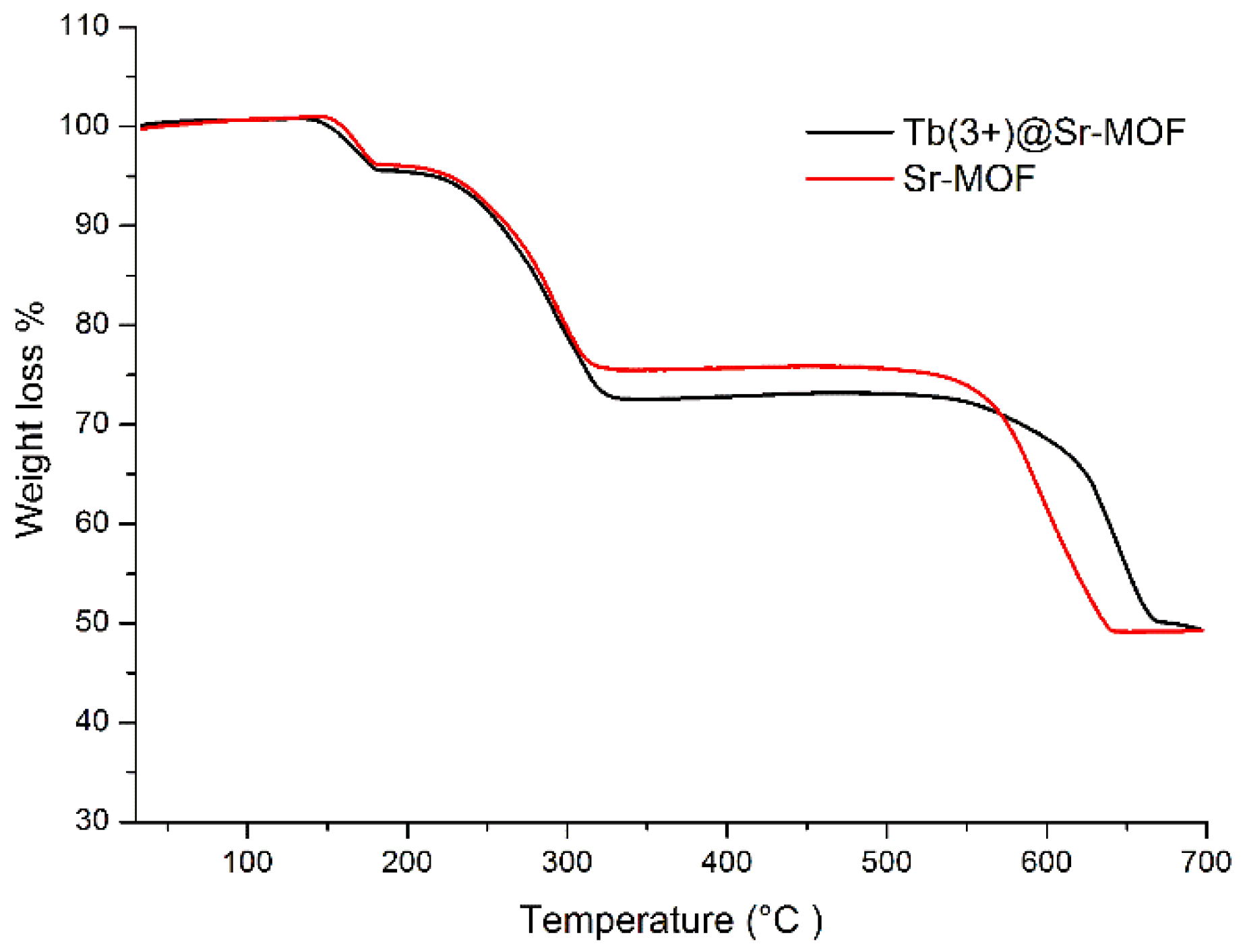
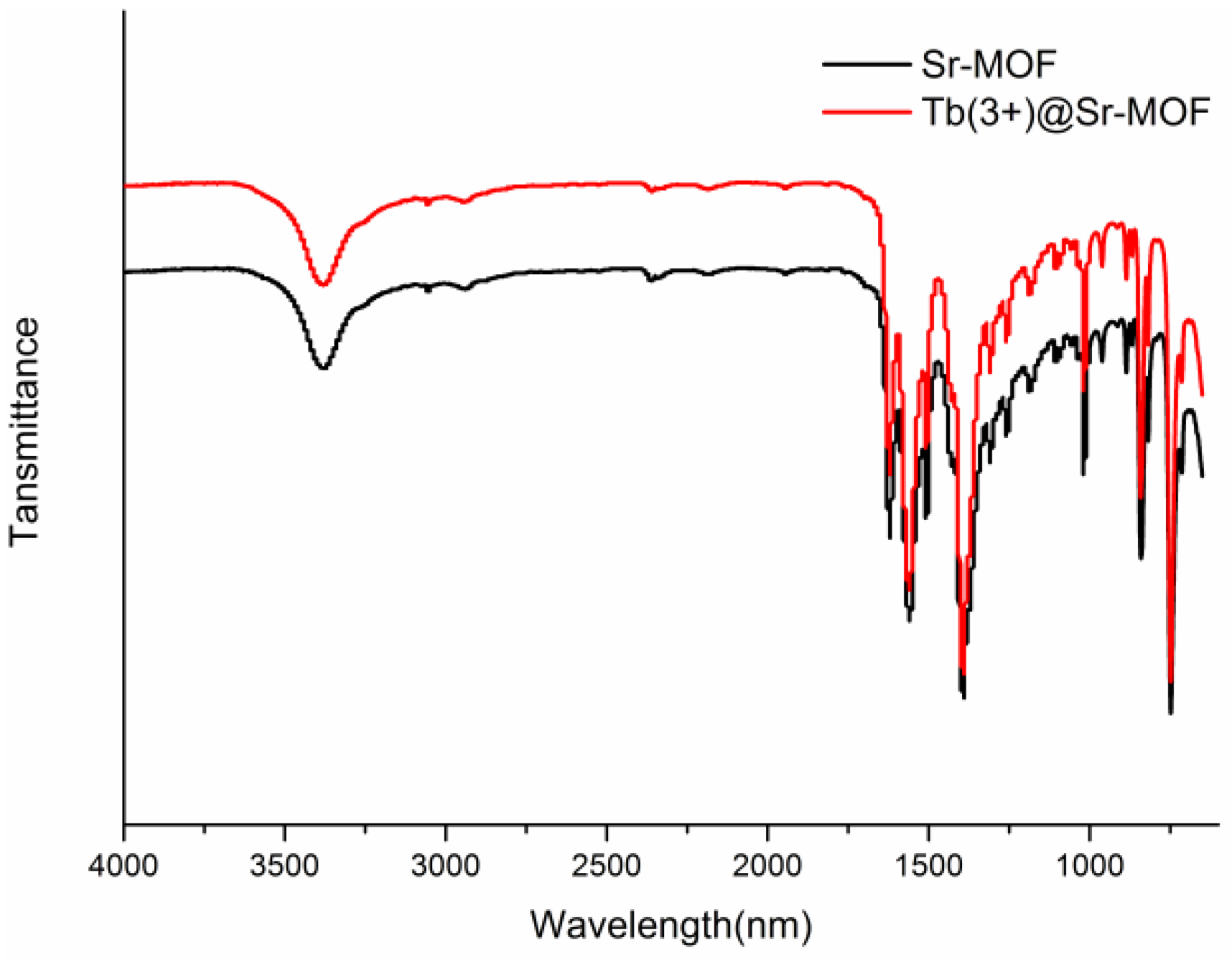
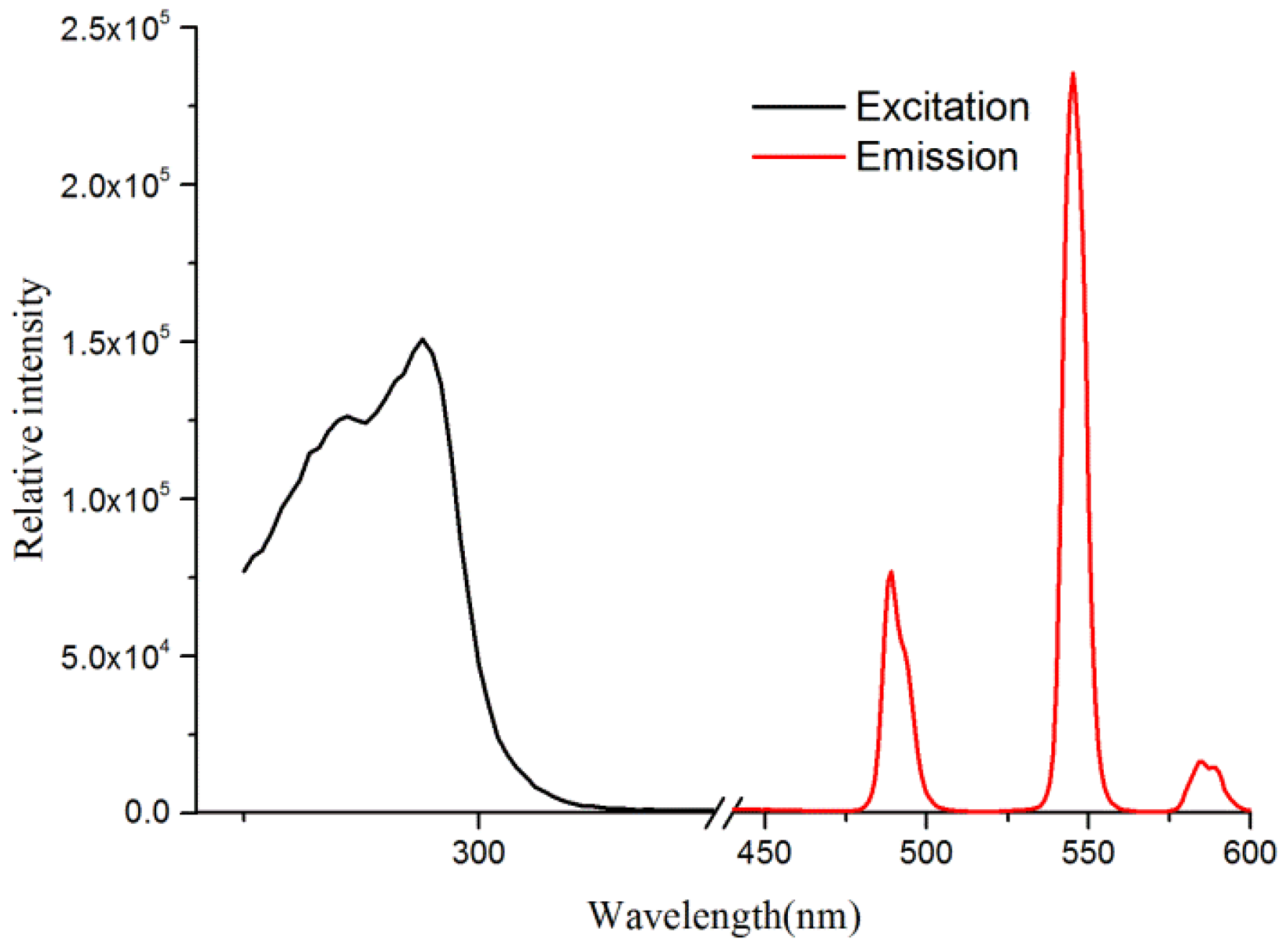
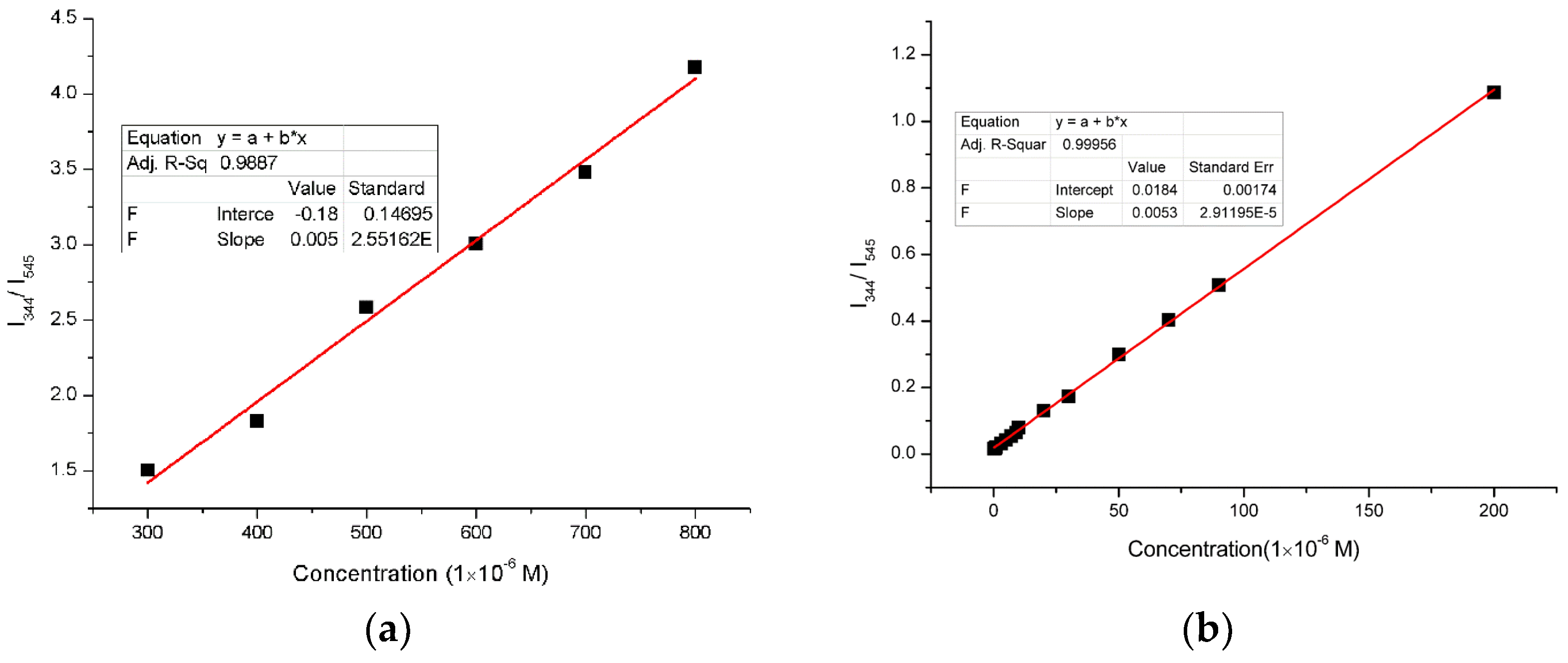
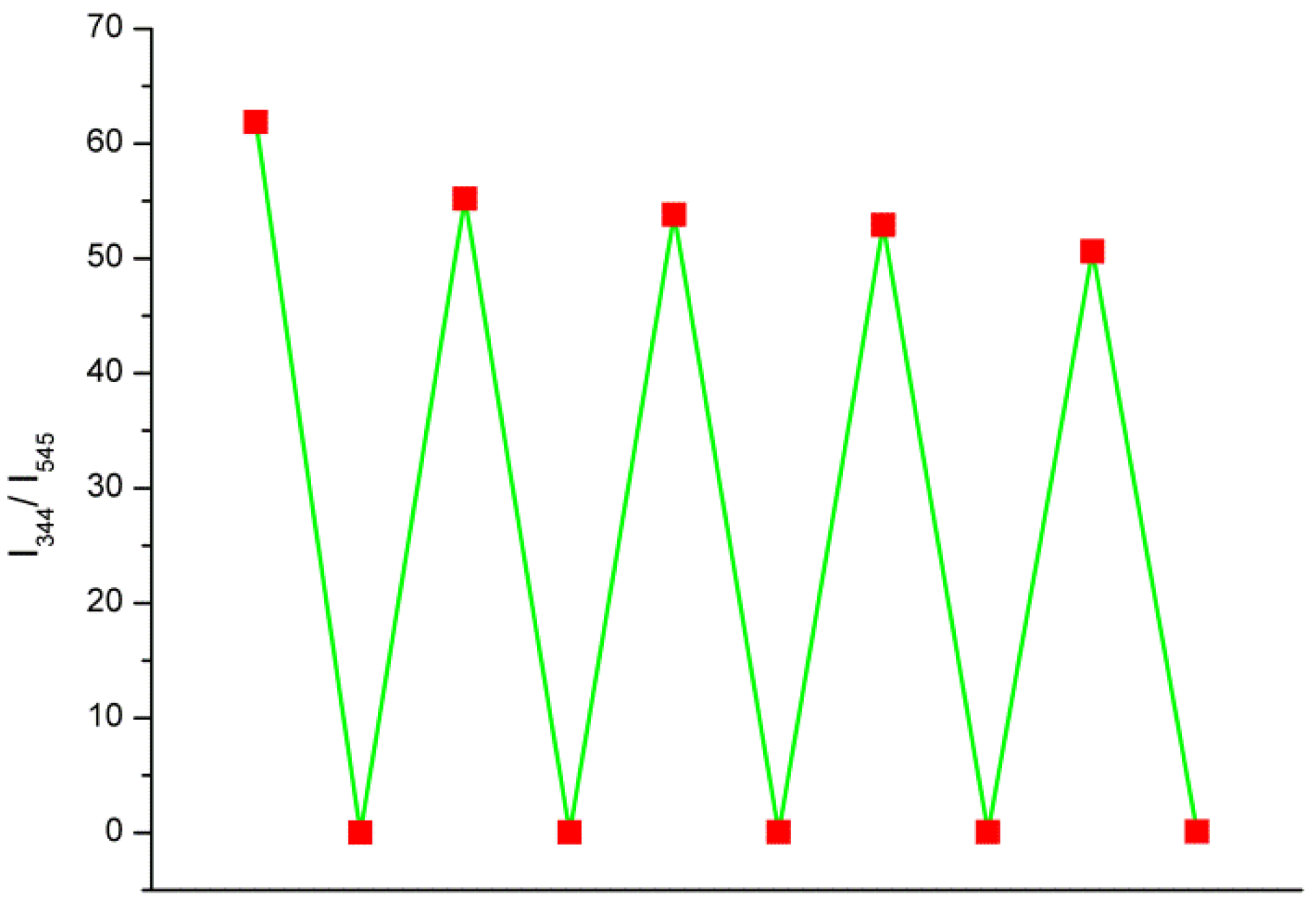
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yashaswini, P.S.; Kurrey, N.K.; Singh, S.A. Encapsulation of sesamol in phosphatidyl choline micelles: Enhanced bioavailability and anti-inflammatory activity. Food Chem. 2017, 228, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Sun, Y.L.; Qiao, Q.L.; Zhao, T.; Zhang, W.T.; Ren, B.; Liu, X.B. Sesamol ameliorates high-fat and high-fructose induced cognitive defects via improving insulin signaling disruption in the central nervous system. Food Funct. 2017, 8, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Ren, B.; Wang, Y.H.; Zou, C.; Qiao, Q.L.; Diao, Z.J.; Mi, Y.S.; Zhu, D.; Liu, X.B. Sesamol induces human hepatocellular carcinoma cells apoptosis by impairing mitochondrial function and suppressing autophagy. Sci. Rep. 2017, 7, 47528. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Sun, P.P.; Ma, X.N.; Jiang, Y.; Chen, F. Sesamol enhances cell growth and the biosynthesis and accumulation of docosahexaenoic acid in the microalga crypthecodinium cohnii. J. Agric. Food Chem. 2015, 63, 5640–5645. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, D.; Liu, Y.; Zhang, H.; Ma, T.; Aidaerhan, A.; Wang, J.; Sun, B. Application of an optosensing chip based on molecularly imprinted polymer coated quantum dots for the highly selective and sensitive determination of sesamol in sesame oils. J. Agric. Food Chem. 2015, 11, 2545–2549. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.L.; Wang, Y.; Bai, D.J.; He, M.H.; Gao, X.X.; He, Y.B. Selective adsorption of C2H2 and CO2 from CH4 in an isoreticular series of MOFs constructed from unsymmetrical diisophthalate linkers and the effect of alkoxy group functionalization on gas adsorption. J. Mater. Chem. A 2018, 6, 3471–3478. [Google Scholar] [CrossRef]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional metal–organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Z.B.; Chang, G.G.; Xing, H.B.; Krishna, R.; Ren, Q.L.; Chen, B.L. Potential of microporous metal–organic frameworks for separation of hydrocarbon mixtures. Energy Environ. Sci. 2016, 9, 3612–3641. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Cui, Y.J.; Zhou, W.; Qian, G.D.; Chen, B.L. Emerging multifunctional metal–organic framework materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Voorde, B.V.; Bueken, B.; Denayer, J.; Vos, D.D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed]
- Modena, M.M.; Hirschle, P.; Wuttke, S.; Burg, T.P. Mass measurements reveal preferential sorption of mixed solvent components in porous nanoparticles. Small 2018, 14, 1800826. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Chen, L.F.; Cui, H.; Zhang, J.Y.; Su, C.Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepúlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin., P.; Kapteijn, F.; et al. Metal–organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Jin, R.M.; Wu, Y.; Zheng, J.; Li, X.G. Chemical induced fragmentation of MOFs for highly efficient Ni-based hydrogen evolution catalysts. Nanoscale Horiz. 2018, 3, 218–225. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Wöll, C. Surface-supported metal–organic framework thin films: Fabrication methods, applications, and challenges. Chem. Soc. Rev. 2017, 46, 5730–5770. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.; Lächelt, U.; Gruber, T.; Rühle, B.; Wuttke, S. Multifunctional efficiency: Extending the concept of atom economy to functional nanomaterials. ACS Nano 2018, 12, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, C.S. Synthesis, functionalization, and applications of metal–organic frameworks in biomedicine. Dalton Trans. 2018, 47, 2114–2133. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, S.; Lismont, M.; Escudero, A.; Rungtaweevoranit, B.; Parak, W.J. Positioning metal-organic framework nanoparticles within the context of drug delivery—A comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials 2017, 123, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of metal-organic frameworks: On the road to in vivo efficacy in biomedicine. Adv. Mater. 2018, 111, 1707365. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Z.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–organic framework nanoparticles. Adv. Mater. 2018, 423, 1800202. [Google Scholar] [CrossRef] [PubMed]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal–Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Meyer, L.V.; Schönfeld, F.; Müller-Buschbaum, K. Lanthanide based tuning of luminescence in MOFs and dense frameworks–from mono- and multimetal systems to sensors and films. Chem. Commun. 2014, 50, 8093–8108. [Google Scholar] [CrossRef] [PubMed]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide probes for bioresponsive imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Liu, X.W.; Huang, W.; Bettinelli, M.; Liu, X.G. Lanthanide-activated phosphors based on 4f-5d optical transitions: Theoretical and experimental aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.H.; Shi, F.; Zhang, W.M.; Zang, S.Q.; Mak, T.C.W. Selective sensing of Fe3+ and Al3+ ions and detection of 2,4,6-trinitrophenol by a water-stable terbium-based metal–organic framework. Chem. A Eur. J. 2015, 21, 15705–15712. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, C.L.; Chen, S.G.; Han, L.J.; Zheng, H.G. Two lanthanide metal–organic frameworks as remarkably selective and sensitive bifunctional luminescence sensor for metal ions and small organic molecules. ACS Appl. Mater. Interfaces 2017, 9, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Hinterholzinger, F.M.; Rühle, B.; Wuttke, S.; Karaghiosoff, K.; Bein, T. Highly sensitive and selective fluoride detection in water through fluorophore release from a metal-organic framework. Sci. Rep. 2013, 3, 2562. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Liu, S.X.; Cheng, Y.; Guo, C.; Wu, X.X.; Guo, J.H.; Liu, Y.Y.; Li, Y. Heterometallic alkaline earth–lanthanide BaII–LaIII microporous metal–organic framework as bifunctional luminescent probes of Al3+ and MnO4–. Inorg. Chem. 2016, 55, 4391–4402. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Wang, L.B.; Zapata, F.; Qian, G.D.; Lobkovsky, E.B. A luminescent microporous metal−organic framework for the recognition and sensing of anions. J. Am. Chem. Soc. 2008, 130, 6718–6719. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.S.; Zhang, F.; Wang, Y.; Yang, Y.Y.; Ng, S.W. A novel electrophoretic deposited coordination supramolecular network film for detecting phosphate and biophosphate. Chem. A Eur. J. 2017, 32, 7748–7754. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.S.; Dong, W.W.; Zhao, J.; Wu, Y.P.; Zhang, Q.C.; Li, D.S. A water-stable Tb(III)-based metal-organic gel(MOG) for detection of antibiotics and explosives. Inorg. Chem. Front. 2017, 5, 120–126. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Shi, W.; Cheng, P.; Zaworotko, M.J. A mixed-crystal lanthanide Zeolite-like metal–organic framework as a fluorescent indicator for Lysophosphatidic Acid, a cancer biomarker. J. Am. Chem. Soc. 2015, 137, 12203–12206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yao, H.; Chu, T.S.; Zhang, G.W.; Wang, Y.; Yang, Y.Y. The Co3O4 nano-anchors fixed Ln-MOF thin film as a highly efficient luminescent sensor for nitrofuran antibiotics. Chem. Eur. J. 2017, 23, 10293–10300. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, S.; Dietl, C.; Hinterholzinger, F.M.; Hintz, H.; Langhalsa, H.; Bein, T. Turn-on fluorescence triggered by selective internal dye replacement in MOFs. Chem. Commun. 2014, 50, 3599–3601. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.F.; Gao, S.Y.; Liu, T.F.; Shi, J.L.; Cao, R. Preparation of dual-emitting Ln@UiO-66-Hybrid films via electrophoretic deposition for ratiometric temperature sensing. ACS Appl. Mater. Interfaces 2018, 10, 6014–6023. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.T.; Song, T.; Gao, J.K.; Cui, Y.J.; Yang, Y.; Wu, C.D.; Chen, B.L.; Qian, G.D. A highly sensitive mixed lanthanide metal organic framework self-calibrated luminescent thermometer. J. Am. Chem. Soc. 2013, 135, 15559–15564. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Yan, B. Intelligent molecular searcher from logic computing network based on Eu(III) functionalized UMOFs for environmental monitoring. Adv. Funct. Mater. 2017, 27, 1700247. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-functionalized metal–organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.S.; Yu, J.C.; Cui, Y.J.; Yang, Y.; Wang, Z.Y.; Yang, D.R.; Qian, G.D. Luminescent metal–organic framework films as highly sensitive and fast-response oxygen sensors. J. Am. Chem. Soc. 2014, 136, 5527–5530. [Google Scholar] [CrossRef] [PubMed]
- Douvali, A.; Tsipis, A.C.; Eliseeva, S.V.; Petoud, S.; Papaefstathiou, G.S.; Malliakas, C.D.; Papadas, I.; Armatas, G.S.; Margiolaki, I.; Kanatzidis, M.G.; et al. Turn-on luminescence sensing and real-time detection of traces of water in organic solvents by a flexible metal-organic framework. Angew. Chem. Int. Ed. Engl. 2015, 54, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.; Hidalgo, T.; Gorman, M.; Lozano-Fernández, T.; Simón-Vázquez, R.; Olivier, C.; Guillou, N.; Serre, C.; Martineau, C.; Taulelle, F.; et al. A biocompatible porous Mg-gallate metal–organic framework as an antioxidant carrier. Chem. Commun. 2015, 51, 5848–5851. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Li, H.H.; Zhang, H.; Li, H.M.; Shi, W.; Cheng, P.A. Bimetallic lanthanide metal-organic material as a self-calibrating color-gradient luminescent sensor. Adv. Mater. 2015, 27, 7072–7077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ni, J.; Zhang, J.J.; Liu, S.Q.; Sun, Y.J.; Zhou, H.J.; Li, Y.Q.; Duan, C.Y. A trichromatic MOF composite for multidimensional ratiometric luminescent sensing. Chem. Sci. 2018, 9, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, C.H.; Tan, H.L. Dual-emissive polystyrene@zeolitic imidazolate framework-8 composite for ratiometric detection of singlet oxygen. J. Mater. Chem. B 2017, 5, 9175–9182. [Google Scholar] [CrossRef]
- Schrimpf, W.; Jiang, J.; Ji, Z.; Hirschle, P.; Lamb, D.C.; Yaghi, O.M.; Wuttke, S. Chemical diversity in a metal–organic framework revealed by fluorescence lifetime imaging. Nat. Commun. 2018, 9, 1647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, R.; Zhang, J.J.; Cheng, G.; Yang, H. Lanthanide(Tb 3+, Eu3+)-functionalized a new one dimensional Zn-MOF composite as luminescent probe for highly selectively sensing Fe3+. Polyhedron 2018, 148, 178–183. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Cheng, G.; Wu, Y.Y.; Lin, S.M. A new Tb(III )-functionalized layer-like Cd MOF as luminescent probe for high-selectively sensing of Cr3+. CrystEngComm 2017, 19, 7270–7276. [Google Scholar] [CrossRef]
- Yue, D.; Huang, Y.K.; Zhang, L.; Jiang, K.; Zhang, X.; Cui, Y.J.; Yu, Y.; Qian, G.D. Ratiometric luminescence sensing based on a mixed Ce/Eu metal–organic framework. J. Mater. Chem. C 2018, 6, 2054–2059. [Google Scholar] [CrossRef]






| Compound | Sr-MOF |
|---|---|
| Chemical Formula | C12H15NO6Sr |
| Formula weight | 356.87 |
| Crystal system | Orthorhombic |
| Space group | Pnma |
| a (Å) | 10.9304 (3) |
| b (Å) | 6.96110 (10) |
| c (Å) | 19.0421 (4) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| V (Å3) | 1488.87 |
| Z | 4 |
| Dc (g/cm3) | 1.636 |
| μ (mm−1) | 1.347 |
| T (K) | 293 (2) |
| Mo Kalpha | 1.54178 |
| F (000) | 720 |
| Crystal size (mm) | 0.23 × 0.19 × 0.15 |
| θ range (°) | 4.64 to 67.08 |
| Index ranges | −11 ≤ h ≤ 12 |
| −6 ≤ k ≤ 8 | |
| −22 ≤ l ≤ 22 | |
| Reflections collected | 6263 |
| Independent reflections | 1398[Rint = 0.0326] |
| Parameters | 109 |
| Goodness-of-fit on F2 | 1.112 |
| R1 indices [I > 2σ(I)] | 0.0340 |
| wR2 indices [I > 2σ(I)] | 0.0878 |
| R1 indices [all data] | 0.0369 |
| wR2 indices [all data] | 0.0902 |
| Main Coordination Modes Bond Length |
|---|
| Sr(1)-O(2)#1 2.490(2) |
| Sr(1)-O(2)#2 2.490(2) |
| Sr(1)-O(3) 2.496(4) |
| Sr(1)-O(4) 2.570(3) |
| Sr(1)-O(1) 2.642(2) |
| Sr(1)-O(1)#3 2.642(2) |
| Sr(1)-O(2)#4 2.689(2) |
| Sr(1)-O(2)#5 2.689(2) |
| Sr(1)-C(6)#4 2.971(5) |
| O(2)-Sr(1)#8 2.490(2) |
| O(2)-Sr(1)#9 2.689(2) |
| Symmetry transformations used to generate equivalent atoms: |
| #1 −x + 1/2, −y + 1, z − 1/2 #2 −x + 1/2, y + 1/2, z − 1/2 |
| #3 x, −y + 3/2, z #4 x − 1/2, y, −z + 1/2 #5 x − 1/2, −y + 3/2, −z + 1/2 |
| #6 −x, −y + 1, −z #7 −x, −y + 2, −z #8 −x + 1/2, −y + 1, z + 1/2 |
| #9 x + 1/2, y, −z + 1/2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lin, S.; Luo, J.; Huang, R.; Cai, H.; Yan, W.; Yang, H. A Novel Tb@Sr-MOF as Self-Calibrating Luminescent Sensor for Nutritional Antioxidant. Nanomaterials 2018, 8, 796. https://doi.org/10.3390/nano8100796
Wang Y, Lin S, Luo J, Huang R, Cai H, Yan W, Yang H. A Novel Tb@Sr-MOF as Self-Calibrating Luminescent Sensor for Nutritional Antioxidant. Nanomaterials. 2018; 8(10):796. https://doi.org/10.3390/nano8100796
Chicago/Turabian StyleWang, Yi, Shaomin Lin, Jun Luo, Rui Huang, Hong Cai, Wei Yan, and Huan Yang. 2018. "A Novel Tb@Sr-MOF as Self-Calibrating Luminescent Sensor for Nutritional Antioxidant" Nanomaterials 8, no. 10: 796. https://doi.org/10.3390/nano8100796
APA StyleWang, Y., Lin, S., Luo, J., Huang, R., Cai, H., Yan, W., & Yang, H. (2018). A Novel Tb@Sr-MOF as Self-Calibrating Luminescent Sensor for Nutritional Antioxidant. Nanomaterials, 8(10), 796. https://doi.org/10.3390/nano8100796