The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation
Abstract
1. Introduction
2. Biomedical Application of Metallic NPs
2.1. Diagnostics
2.2. Drug Delivery
2.3. Additional Therapeutic Activities
2.3.1. Anti-Cancer Activity
2.3.2. Anti-Microbial Activity
2.3.3. Anti-Inflammatory Activity
2.3.4. Disease Therapy
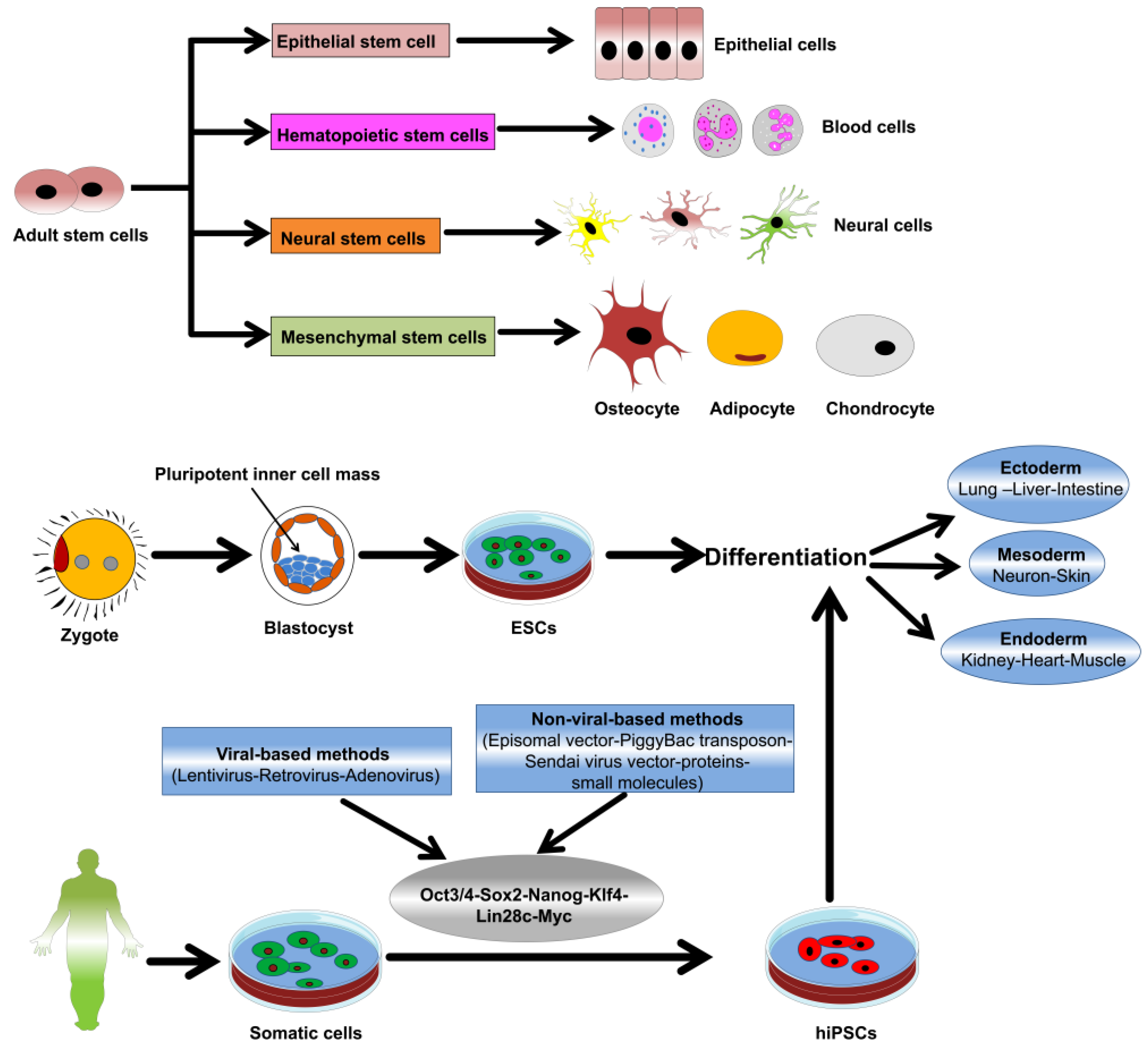
3. Nanomaterial-Stem Cell Communication
4. Metallic NPs and Stem Cell Differentiation and Proliferation
4.1. AuNP
4.2. AgNPs
4.3. TiO2
4.4. IONPs
4.5. Other Metallic NPs
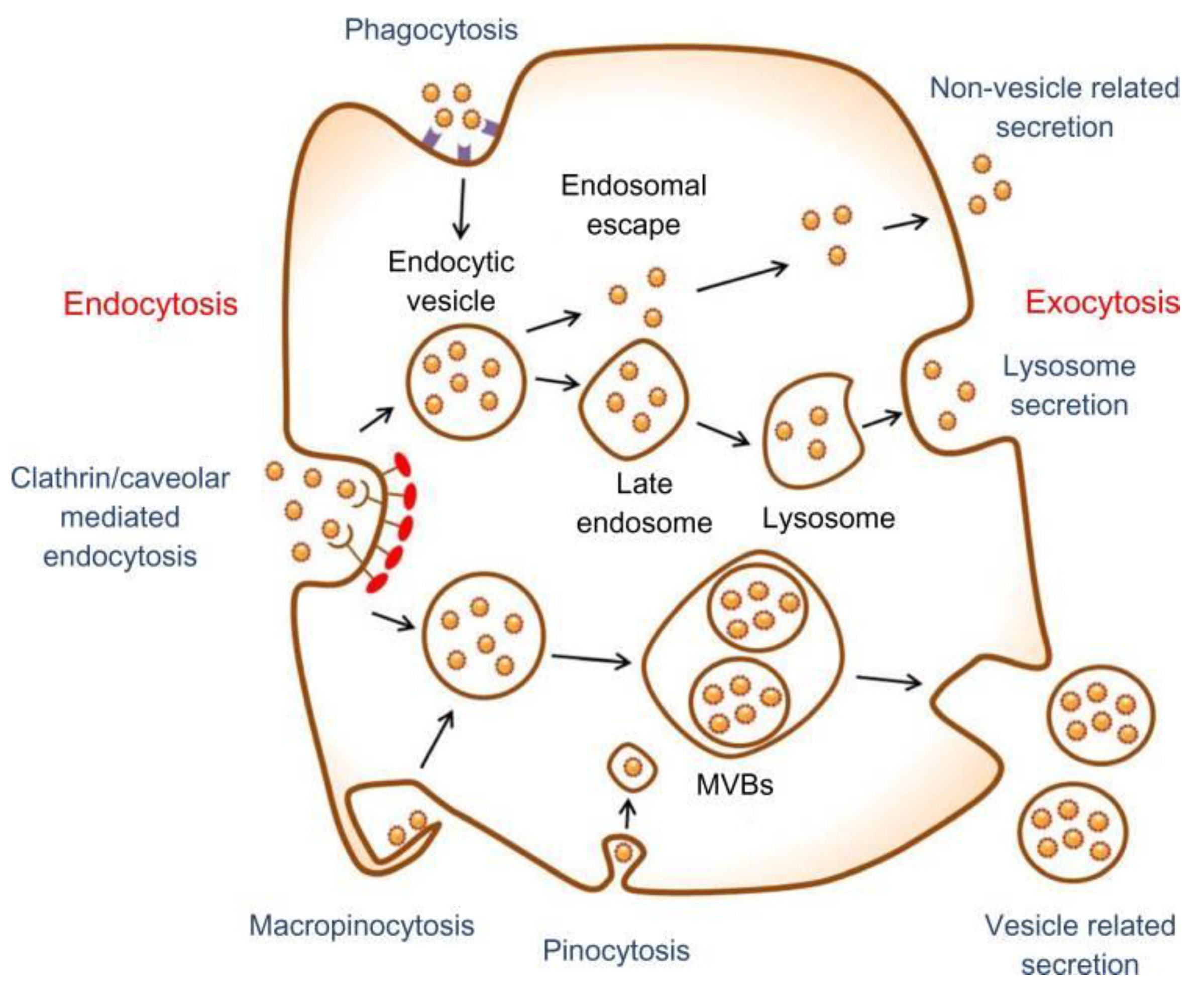
5. Metallic NPs and Stem Cell Toxicity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ESCs | Embryonic stem cells |
| ASCs | Adult stem cells |
| iPSCs | Induced pluripotent stem cells |
| MSC | Mesenchymal stem cells |
| SPR | Surface plasmon resonances |
| NP | Nanoparticle |
| TiO2 | Titanium oxide |
| IONPs | Iron oxide NPs |
| AgNP | Silver NP |
| AuNP | Gold NP |
| NIR | Near infrared |
| SPIO | Superparamagnetic iron oxide |
| Nanoceria | Cerium oxide NPs |
| CuO NPs | Copper oxide NPs |
| DOX | Doxorubicin |
| ROS | Reactive oxygen species |
| ZnO | Zinc oxide |
| CuS | Copper sulfide |
| E. coli | Escherichia coli |
| SiNPs | Silicate NPs |
| FACS | Fluorescence-activated cell sorting |
| VEGF | Vascular endothelial growth factor |
| TEM | Transmission electron microscopy |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| DA | Dopaminergic |
| RA | Retinoic acid |
| ALP | Alkaline phosphatase |
| OPN | Osteopontin |
| OCN | Osteocalcin |
| RUNX2 | Runt-related transcription factor 2 |
| COL I | Collagen type I |
| BSA | Bovine serum albumin |
| PVA | Polyvinyl alcohol |
| AgNO3 | Silver nitrate |
| TGF-β | Transforming growth factor-beta |
| BMP | Bone morphogenic protein |
| hASCs | Human autologous adipose derived mesenchymal stromal/stem cells |
| CSC | Cancer stem cell |
| FGF-2 | Fibroblast growth factor |
| FAK | Focal adhesion kinase |
| Rb | Retinoblastoma |
| RBP2 | Retinoblastoma binding protein 2 |
| TNTs | Titania nanotubes |
| MAP3K11 | Mitogen-activated protein kinase kinase kinase 11 |
| ATP1A2 | Alpha 2 polypeptide |
| ATP1A3 | Alpha 3 polypeptide |
| siRNA | Small interference RNA |
| DEX | Dextran |
| HSA | Human serum albumin |
| PES-PEG | Polyethersulfone-polyethylene glycol |
| PEI | Polyethyleneimine |
| GFAP | Glial fibrillary acidic protein |
| ERK | Extracellular signal–regulated kinase |
| MAPK | Mitogen-activated protein kinase |
| YAP | Yes-associated protein |
| 6-OHDA | 6-hydroxydopamine |
| SNpc | Substantia nigra pars compacta |
| TH | Tyrosine hydroxylase |
| BMP2 | Bone morphogenetic protein 2 |
| OMD | Osteomodulin |
| FOXO1 | Forkhead box protein O1 |
| ATF4 | Activating transcription factor 4 |
| MCAM | Melanoma cell adhesion molecule |
| CDK4 | Cyclin-dependent kinase 4 |
| ENG | Endoglin |
| CLCF | Cardiotrophin-like cytokine factor 1 |
| TPM1 | Tropomyosin 1 |
| FGFR1 | Fibroblast growth factor receptor 1 |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| RPS6KA1 | Ribosomal protein S6 kinase, 90 kDa, polypeptide 1 |
| CXCR4 | Chemokine receptor type 4 |
| EGFR | Epidermal growth factor receptor |
| GAP43 | growth associated protein 43 |
| MMP2 | Matrix metalloproteinase 2 |
| PCR | polymerase chain reaction |
| GO | Graphene oxide |
References
- Watt, F.M.; Driskell, R.R. The therapeutic potential of stem cells. Philos. Trans. R. Soc. Lond. Ser. B 2010, 365, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. A fresh look at ips cells. Cell 2009, 137, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Malgieri, A.; Kantzari, E.; Patrizi, M.P.; Gambardella, S. Bone marrow and umbilical cord blood human mesenchymal stem cells: State of the art. Int. J. Clin. Exp. Med. 2010, 3, 248. [Google Scholar] [PubMed]
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228. [Google Scholar] [PubMed]
- Kerativitayanan, P.; Carrow, J.K.; Gaharwar, A.K. Nanomaterials for engineering stem cell responses. Adv. Healthc. Mater. 2015, 4, 1600–1627. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Liechty, W.B.; Khademhosseini, A.; Peppas, N.A. Designing biomaterials to direct stem cell fate. ACS Nano 2012, 6, 9353–9358. [Google Scholar] [PubMed]
- Wei, M.; Li, S.; Le, W. Nanomaterials modulate stem cell differentiation: Biological interaction and underlying mechanisms. J. Nanobiotechnol. 2017, 15, 75. [Google Scholar]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for in vivo imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yuan, L.; Wu, C.; Luo, G.; Deng, J.; Mao, Z. Recent review of the effect of nanomaterials on stem cells. RSC Adv. 2018, 8, 17656–17676. [Google Scholar] [CrossRef]
- Faraday, M.X. The bakerian lecture.—experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.A.D.S.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A., Jr.; Santos, L.S.S.; Gonçalves, M.D.C.; Nogueira, A.F. Preparation of silver and gold nanoparticles: A simple method to introduce nanotechnology into teaching laboratories. Quim. Nova 2012, 35, 1872–1878. [Google Scholar]
- Iravani, S. Metal Nanoparticles: Synthesis and Applications in Pharmaceutical Sciences, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; Chapter 2; pp. 15–31. [Google Scholar]
- Klabunde, K.J. Nanoscale Materials in Chemistry; John Wiley & Sons, Inc.: New York, NY, USA, 2001; Chapter 1; pp. 1–13. [Google Scholar]
- Kumar, M.; Varshney, L.; Francis, S. Radiolytic formation of ag clusters in aqueous polyvinyl alcohol solution and hydrogel matrix. Radiat. Phys. Chem. 2005, 73, 21–27. [Google Scholar] [CrossRef]
- Mody, V.V.; Nounou, M.I.; Bikram, M. Novel nanomedicine-based mri contrast agents for gynecological malignancies. Adv. Drug Deliv. Rev. 2009, 61, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Xing, Y.; Kim, G.J.; Simons, J.W. Nanotechnology applications in cancer. Annu. Rev. Biomed. Eng. 2007, 9, 257–288. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.L.; Gutierres, M.G.; Thesing, A.; Lattuada, R.M.; Ferreira, J. Spr biosensors based on gold and silver nanoparticle multilayer films. J. Braz. Chem. Soc. 2014, 25, 928–934. [Google Scholar] [CrossRef]
- Nietzold, C.; Lisdat, F. Fast protein detection using absorption properties of gold nanoparticles. Analyst 2012, 137, 2821–2826. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Gurung, A.; Xu, H.; Baloda, M.; He, Y.; Liu, G. Simultaneous detection of nucleic acid and protein using gold nanoparticles and lateral flow device. Anal. Sci. 2014, 30, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Sagadevan, S.; Periasamy, M. Recent trends in nanobiosensors and their applications-a review. Rev. Adv. Mater. Sci. 2014, 36, 62–69. [Google Scholar]
- Ensafi, A.A.; Taei, M.; Rahmani, H.; Khayamian, T. Sensitive DNA impedance biosensor for detection of cancer, chronic lymphocytic leukemia, based on gold nanoparticles/gold modified electrode. Electrochim. Acta 2011, 56, 8176–8183. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, Z.-K.; Tang, H.; Tang, L.-J.; Jiang, J.-H. Activity-based DNA-gold nanoparticle probe as colorimetric biosensor for DNA methyltransferase/glycosylase assay. Anal. Chem. 2013, 85, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, J.; Nie, M.; Lu, S.; Wu, X. Diabetes insipidus as the first symptom caused by lung cancer metastasis to the pituitary glands: Clinical presentations, diagnosis, and management. J. Postgrad. Med. 2011, 57, 302. [Google Scholar] [PubMed]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.; Seo, J.; Jang, J.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gao, J.; Gambhir, S.S.; Cheng, Z. Near-infrared fluorescent nanoprobes for cancer molecular imaging: Status and challenges. Trends Mol. Med. 2010, 16, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Altınoğlu, E.İ.; Adair, J.H. Near infrared imaging with nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, S.; Lee, J.H.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo x-ray computed tomography imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Kneipp, H.; Wittig, B.; Kneipp, K. Novel optical nanosensors for probing and imaging live cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Stein, E.W.; Ashkenazi, S.; Wang, L.V. Nanoparticles for photoacoustic imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Vartholomeos, P.; Fruchard, M.; Ferreira, A.; Mavroidis, C. Mri-guided nanorobotic systems for therapeutic and diagnostic applications. Annu. Rev. Biomed. Eng. 2011, 13, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.D.; Yao, W.W.; Chen, T.W.; Zhu, J.; Zhang, J.J.; Pu, Y.; Liu, G.; Zhang, X.M. The effect of superparamagnetic iron oxide with irgd peptide on the labeling of pancreatic cancer cells in vitro: A preliminary study. Biomed. Res. Int. 2014, 2014, 852352. [Google Scholar] [CrossRef] [PubMed]
- Irure, A.; Marradi, M.; Arnáiz, B.; Genicio, N.; Padro, D.; Penadés, S. Sugar/gadolinium-loaded gold nanoparticles for labelling and imaging cells by magnetic resonance imaging. Biomater. Sci. 2013, 1, 658–668. [Google Scholar] [CrossRef]
- Chien, C.-C.; Chen, H.-H.; Lai, S.-F.; Wu, K.-C.; Cai, X.; Hwu, Y.; Petibois, C.; Chu, Y.; Margaritondo, G. Gold nanoparticles as high-resolution x-ray imaging contrast agents for the analysis of tumor-related micro-vasculature. J. Nanobiotechnol. 2012, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Glaus, C.; Chen, J.; Welch, M.J.; Xia, Y. Inorganic nanoparticle-based contrast agents for molecular imaging. Trends Mol. Med. 2010, 16, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwar, Y. Metal Nanoparticles: Synthesis and Applications in Pharmaceutical Sciences, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; Chapter 4; pp. 49–81. [Google Scholar]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Jang, E.; Lee, K.; Haam, S.; Huh, Y.-M. Delivery of cancer therapeutics using nanotechnology. Pharmaceutics 2013, 5, 294–317. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Tsai, C.-Y.; Huang, P.-Y.; Chang, M.-Y.; Cheng, P.-C.; Chou, C.-H.; Chen, D.-H.; Wang, C.-R.; Shiau, A.-L.; Wu, C.-L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharmaceutics 2007, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic nanoparticles: Technology overview & drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942. [Google Scholar] [PubMed]
- Satapathy, S.R.; Mohapatra, P.; Preet, R.; Das, D.; Sarkar, B.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Silver-based nanoparticles induce apoptosis in human colon cancer cells mediated through p53. Nanomedicine 2013, 8, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Nallathamby, P.D.; Xu, X.-H.N. Study of cytotoxic and therapeutic effects of stable and purified silver nanoparticles on tumor cells. Nanoscale 2010, 2, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y. Anti-leukemia activity of pvp-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- Fageria, L.; Pareek, V.; Dilip, R.V.; Bhargava, A.; Pasha, S.S.; Laskar, I.R.; Saini, H.; Dash, S.; Chowdhury, R.; Panwar, J. Biosynthesized protein-capped silver nanoparticles induce ros-dependent proapoptotic signals and prosurvival autophagy in cancer cells. ACS Omega 2017, 2, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ros) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Razmjou, A. Gold nanoparticles as potent anticancer agent: Green synthesis, characterization, and in vitro study. RSC Adv. 2016, 6, 63973–63983. [Google Scholar] [CrossRef]
- Geetha, R.; Ashokkumar, T.; Tamilselvan, S.; Govindaraju, K.; Sadiq, M.; Singaravelu, G. Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nanotechnol. 2013, 4, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold nanoparticles-enabled efficient dual delivery of anticancer therapeutics to hela cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Raja, M.; Ahmad, I.; Siddiqui, M.K.J.; AlSalhi, M.S.; Alrokayan, S.A. Zno nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin and bax/bcl-2 pathways: Role of oxidative stress. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 904–913. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, B.; Civitelli, G.; Condello, M.; Lista, P.; Pozzi, R.; Arancia, G.; Meschini, S. Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol. Appl. Pharmacol. 2010, 246, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Huang, Q.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-Y.; Lee, W.-C. Killing of cancer cell line by photoexcitation of folic acid-modified titanium dioxide nanoparticles. J. Photochem. Photobiol. A 2009, 204, 148–153. [Google Scholar] [CrossRef]
- Yacoby, I.; Benhar, I. Antibacterial nanomedicine. Nanomedicine, 2008; 3, 329–341. [Google Scholar]
- Klasen, H. A historical review of the use of silver in the treatment of burns. Ii. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; You-Sheng, O.-Y.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Wagh, P.; Wadhwani, S.; Gaidhani, S.; Kumbhar, A.; Bellare, J.; Chopade, B.A. Synthesis, optimization, and characterization of silver nanoparticles from acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013, 8, 4277. [Google Scholar]
- Dutta, R.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A. Studies on antibacterial activity of ZnO nanoparticles by ros induced lipid peroxidation. Colloids Surf. B 2012, 94, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of escherichia coli. Free Radical Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Skorb, E.; Antonouskaya, L.; Belyasova, N.; Shchukin, D.; Möhwald, H.; Sviridov, D. Antibacterial activity of thin-film photocatalysts based on metal-modified TiO2 and TiO2: In2O3 nanocomposite. Appl. Catal. B 2008, 84, 94–99. [Google Scholar] [CrossRef]
- Surassmo, S.; Lauruengtana, V.; Kangwansupamongkol, W.; Ruktanonchai, U. In Proceedings of the Antibacterial Effect of Apatite Coated Titanium Dioxide for Textiles and Coating Applications, 2nd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Bangkok, Thailand, 16–19 January 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 1012–1015. [Google Scholar]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Innovative TiO2/Cu nanosurfaces inactivating bacteria in the minute range under low-intensity actinic light. ACS Appl. Mater. Interfaces 2012, 4, 5234–5240. [Google Scholar] [CrossRef] [PubMed]
- Armelao, L.; Barreca, D.; Bottaro, G.; Gasparotto, A.; Maccato, C.; Maragno, C.; Tondello, E.; Štangar, U.L.; Bergant, M.; Mahne, D. Photocatalytic and antibacterial activity of TiO2 and au/TiO2 nanosystems. Nanotechnology 2007, 18, 375709. [Google Scholar] [CrossRef]
- Uchiyama, M.K.; Deda, D.K.; Rodrigues, S.F.D.P.; Drewes, C.C.; Bolonheis, S.M.; Kiyohara, P.K.; Toledo, S.P.d.; Colli, W.; Araki, K.; Farsky, S.H.P. In vivo and in vitro toxicity and anti-inflammatory properties of gold nanoparticle bioconjugates to the vascular system. Toxicol. Sci. 2014, 142, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Yoshihisa, Y.; Miyamoto, Y.; Shimizu, T. The anti-inflammatory effects of platinum nanoparticles on the lipopolysaccharide-induced inflammatory response in raw 264.7 macrophages. Inflamm. Res. 2012, 61, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Cheung, S.O.; Huang, L.; Niu, J.; Tao, C.; Ho, C.M.; Che, C.M.; Tam, P.K. Further evidence of the anti-inflammatory effects of silver nanoparticles. ChemMedChem 2009, 4, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.M.; Foster, K.N.; Blome-Eberwein, S.A.; Twomey, J.A.; Herndon, D.N.; Luterman, A.; Silverstein, P.; Antimarino, J.R.; Bauer, G.J. Randomized clinical study of hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns. J. Burn Care Res. 2006, 27, 298–309. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using european black elderberry fruits extract. Colloids Surf. B 2014, 122, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, E.R. Nanoparticles as anti-inflammatory and pro-regenerative therapeutic molecules. In Perspectives in Translational Research in Life Sciences and Biomedicine; Springer: Berlin, Germany, 2017; pp. 57–88. [Google Scholar]
- Karthick, V.; Kumar, V.G.; Dhas, T.S.; Singaravelu, G.; Sadiq, A.M.; Govindaraju, K. Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats—An in vivo approach. Colloids Surf. B 2014, 122, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Umrani, R.D.; Paknikar, K.M. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced type 1 and 2 diabetic rats. Nanomedicine 2014, 9, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Alkaladi, A.; Abdelazim, A.M.; Afifi, M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2014, 15, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, M.; Hashemi, H.; Jabbarvand, M.; Delrish, E. Penetration of silicate nanoparticles into the corneal stroma and intraocular fluids. Cornea 2014, 33, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.H.; Jo, D.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. The inhibition of retinal neovascularization by gold nanoparticles via suppression of vegfr-2 activation. Biomaterials 2011, 32, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Son, J.G.; Song, N.W.; Kim, Y.-I.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Anti-angiogenic effect of bare titanium dioxide nanoparticles on pathologic neovascularization without unbearable toxicity. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Soo Lee, S.; Savini, M.; Popp, L.; Colvin, V.L.; Segatori, L. Ceria nanoparticles stabilized by organic surface coatings activate the lysosome-autophagy system and enhance autophagic clearance. ACS Nano 2014, 8, 10328–10342. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.Y.; Erlichman, J.S. The potential of cerium oxide nanoparticles (nanoceria) for neurodegenerative disease therapy. Nanomedicine 2014, 9, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. 2012, 124, 11201–11205. [Google Scholar] [CrossRef]
- Ilie, I.; Ilie, R.; Mocan, T.; Bartos, D.; Mocan, L. Influence of nanomaterials on stem cell differentiation: Designing an appropriate nanobiointerface. Int. J. Nanomed. 2012, 7, 2211. [Google Scholar]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: Effect of size and surface charge. Mol. Pharmaceutics 2014, 11, 4363–4373. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51. [Google Scholar]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tiruppathi, C.; Minshall, R.D.; Malik, A.B. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano 2009, 3, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the er. Nat. Cell Biol. 2001, 3, 473. [Google Scholar] [CrossRef] [PubMed]
- Adjei, I.M.; Sharma, B.; Labhasetwar, V. Nanoparticles: Cellular uptake and cytotoxicity. In Nanomaterial; Springer: Berlin, Germany, 2014; pp. 73–91. [Google Scholar]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-K.; Heo, D.N.; Moon, H.-J.; Lee, S.J.; Bae, M.S.; Lee, J.B.; Sun, I.-C.; Jeon, H.B.; Park, H.K.; Kwon, I.K. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J. Colloid Interface Sci. 2015, 438, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Florez, L.; Herrmann, C.; Cramer, J.M.; Hauser, C.P.; Koynov, K.; Landfester, K.; Crespy, D.; Mailänder, V. How shape influences uptake: Interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small 2012, 8, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, S.; Hauser, C.P.; Autenrieth, B.; Weiss, C.K.; Landfester, K.; Mailänder, V. The softer and more hydrophobic the better: Influence of the side chain of polymethacrylate nanoparticles for cellular uptake. Macromol. Biosci. 2010, 10, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
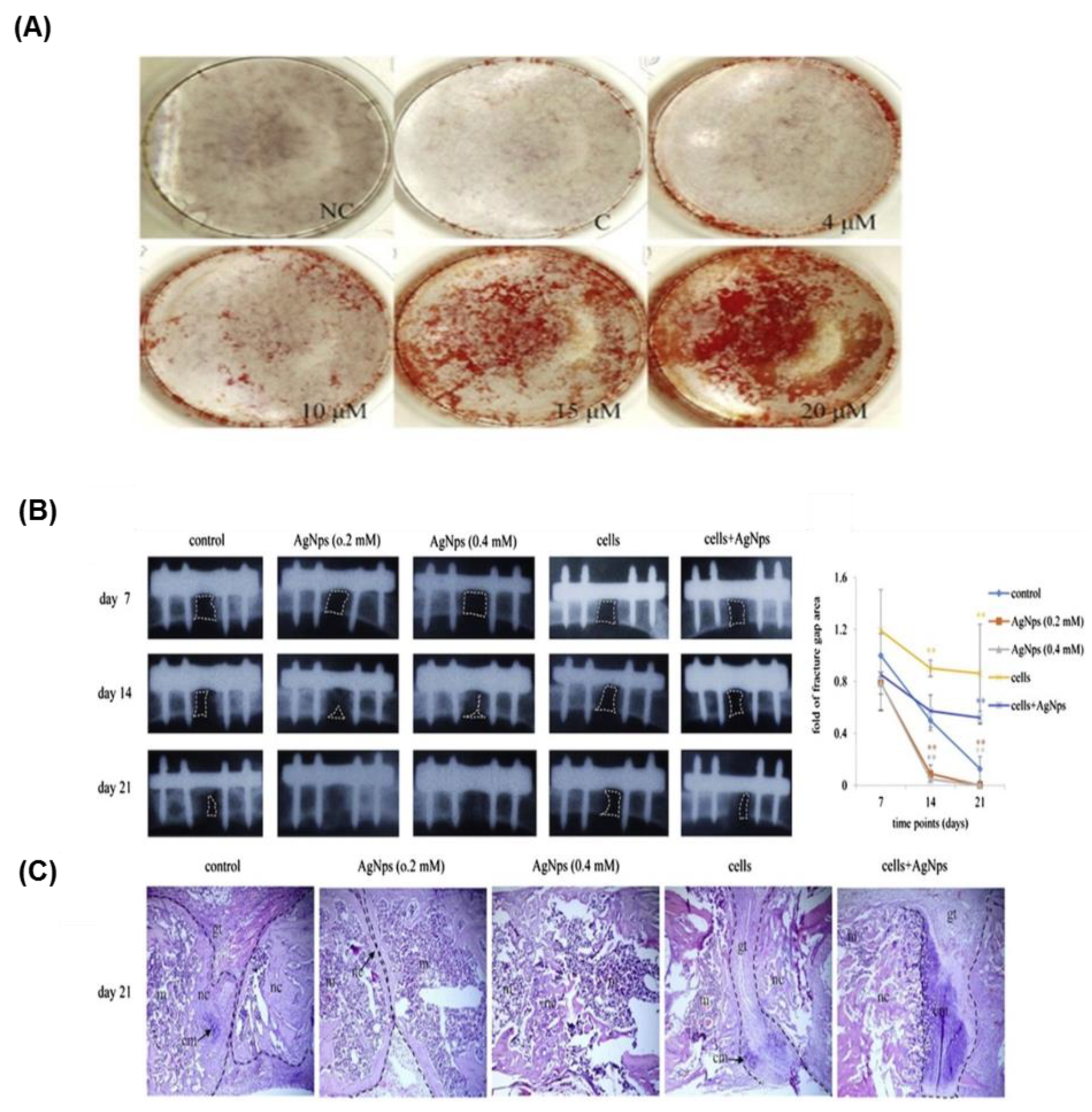
- Zhang, R.; Lee, P.; Lui, V.C.; Chen, Y.; Liu, X.; Lok, C.N.; To, M.; Yeung, K.W.; Wong, K.K. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Tekin, H.; Sanchez, J.G.; Landeros, C.; Dubbin, K.; Langer, R.; Khademhosseini, A. Controlling spatial organization of multiple cell types in defined 3d geometries. Adv. Mater. 2012, 24, 5543–5547. [Google Scholar] [CrossRef] [PubMed]
- Zorlutuna, P.; Annabi, N.; Camci-Unal, G.; Nikkhah, M.; Cha, J.M.; Nichol, J.W.; Manbachi, A.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabricated biomaterials for engineering 3d tissues. Adv. Mater. 2012, 24, 1782–1804. [Google Scholar] [CrossRef] [PubMed]
- Van, V.T.; Ponsaerts, P.; Berneman, Z.N. Mrna-based gene transfer as a tool for gene and cell therapy. Curr. Opin. Mol. Ther. 2007, 9, 423–431. [Google Scholar]
- Shrestha, S.; Mao, Z.; Fedutik, Y.; Gao, C. Influence of titanium dioxide nanorods with different surface chemistry on the differentiation of rat bone marrow mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 6955–6966. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Zhu, C.; Zhang, W.; Mao, Z.; Gao, C. Fe3O4/bsa particles induce osteogenic differentiation of mesenchymal stem cells under static magnetic field. Acta Biomater. 2016, 46, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radical Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Wei, M.; Li, S.; Yang, Z.; Zheng, W.; Le, W. Gold nanoparticles enhance the differentiation of embryonic stem cells into dopaminergic neurons via mtor/p70s6k pathway. Nanomedicine 2017, 12, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold nanoparticle-decorated scaffolds promote neuronal differentiation and maturation. Nano Lett. 2015, 16, 2916–2920. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, J. Biocompatible gold nanoparticles ameliorate retinoic acid-induced cell death and induce differentiation in f9 teratocarcinoma stem cells. Nanomaterials 2018, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Wang, K.; Zhang, W.; Teh, S.W.; Peli, A.; Mok, P.L.; Higuchi, A.; Kumar, S.S. Gold nanoparticles inducing osteogenic differentiation of stem cells: A review. J. Cluster Sci. 2018, 29, 1–7. [Google Scholar] [CrossRef]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.-C.; Jung, B.-Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.N.; Joo, S.-W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the wnt/β-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383. [Google Scholar]
- Zhang, D.; Liu, D.; Zhang, J.; Fong, C.; Yang, M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the erk/mapk signaling pathway. Mater. Sci. Eng. C 2014, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Song, W.; Gao, H.; Li, T.; Cao, X.; Zhong, S.; Wang, Y. Mir-29b-loaded gold nanoparticles targeting to the endoplasmic reticulum for synergistic promotion of osteogenic differentiation. ACS Appl. Mater. Interfaces 2016, 8, 19217–19227. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zheng, H.; Zheng, X.; Yao, M.; Li, Z.; Gao, C. Gold nanoparticles with surface-anchored chiral poly (acryloyl-L(D)-valine) induce differential response on mesenchymal stem cell osteogenesis. Nano Res. 2016, 9, 3683–3694. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Yang, G.M.; Kim, K.; Saha, S.K.; Kim, J.H.; Cho, S.G. The potential of nanoparticles in stem cell differentiation and further therapeutic applications. Biotechnol. J. 2016, 11, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Venugopal, J.R.; Sridhar, R.; Ramakrishna, S. Cardiogenic differentiation of mesenchymal stem cells with gold nanoparticle loaded functionalized nanofibers. Colloids Surf. B 2015, 134, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Sridhar, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Gold nanoparticle loaded hybrid nanofibers for cardiogenic differentiation of stem cells for infarcted myocardium regeneration. Macromol. Biosci. 2014, 14, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Lee, K.-B.; Choi, J.-W. 3D graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials 2013, 34, 8660–8670. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.N.; Chang, L.; San Thian, E. Development of nanosized silver-substituted apatite for biomedical applications: A review. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Samberg, M.E.; Loboa, E.G.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Silver nanoparticles do not influence stem cell differentiation but cause minimal toxicity. Nanomedicine 2012, 7, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Kienzle, A.; Liu, X.; Müller, W.E.; Elkhooly, T.A.; Feng, Q. In vitro effect of 30 nm silver nanoparticles on adipogenic differentiation of human mesenchymal stem cells. J. Biomed. Nanotechnol. 2016, 12, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, W.; Fang, Z.; Kienzle, A.; Feng, Q. Influence of silver nanoparticles on osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Nanotechnol. 2014, 10, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomed. 2014, 9, 2469. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.T.; Monroe, W.T.; Dasa, V.; Gimble, J.M.; Hayes, D.J. Mir-148b–nanoparticle conjugates for light mediated osteogenesis of human adipose stromal/stem cells. Biomaterials 2013, 34, 7799–7810. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Elkhooly, T.A.; Liu, X.; Cavallaro, A.; Taheri, S.; Vasilev, K.; Feng, Q. Silver nanoparticle based coatings enhance adipogenesis compared to osteogenesis in human mesenchymal stem cells through oxidative stress. J. Mater. Chem. B 2016, 4, 1466–1479. [Google Scholar] [CrossRef]
- Han, J.W.; Gurunathan, S.; Choi, Y.-J.; Kim, J.-H. Dual functions of silver nanoparticles in f9 teratocarcinoma stem cells, a suitable model for evaluating cytotoxicity-and differentiation-mediated cancer therapy. Int. J. Nanomed. 2017, 12, 7529. [Google Scholar] [CrossRef] [PubMed]
- Carinci, F.; Guidi, R.; Franco, M.; Viscioni, A.; Rigo, L.; De Santis, B.; Tropina, E. Implants inserted in fresh-frozen bone: A retrospective analysis of 88 implants loaded 4 months after insertion. Quintessence Int. 2009, 40, 413–419. [Google Scholar] [PubMed]
- Gapski, R.; Wang, H.L.; Mascarenhas, P.; Lang, N.P. Critical review of immediate implant loading. Clin. Oral Implants Res. 2003, 14, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, X.; Deng, X.; Huo, Y.; Xie, J.; Huang, H.; Jiao, Z.; Wu, M.; Liu, Y.; Wen, T. A protein interaction network for the analysis of the neuronal differentiation of neural stem cells in response to titanium dioxide nanoparticles. Biomaterials 2010, 31, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; von der Mark, K.; Schmuki, P. Size selective behavior of mesenchymal stem cells on ZrO2 and TiO2 nanotube arrays. Integr. Biol. 2009, 1, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Li, Y.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Pozio, A.; Palmieri, A.; Girardi, A.; Cura, F.; Carinci, F. Titanium nanotubes stimulate osteoblast differentiation of stem cells from pulp and adipose tissue. Dent. Res. J. 2012, 9, S169–S174. [Google Scholar]
- Vercellino, M.; Ceccarelli, G.; Cristofaro, F.; Balli, M.; Bertoglio, F.; Bruni, G.; Benedetti, L.; Avanzini, M.A.; Imbriani, M.; Visai, L. Nanostructured TiO2 surfaces promote human bone marrow mesenchymal stem cells differentiation to osteoblasts. Nanomaterials 2016, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, Y.; Zhang, P.; Zhang, X.; Liu, J.; Chen, T.; Su, P.; Li, H.; Zhou, Y. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating h3k4 trimethylation. Biomaterials 2015, 39, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Su, P.; Chen, S.; Wang, N.; Wang, J.; Liu, Y.; Ma, Y.; Li, H.; Zhang, Z.; Webster, T.J. Antibacterial and osteogenic stem cell differentiation properties of photoinduced TiO2 nanoparticle-decorated TiO2 nanotubes. Nanomedicine 2015, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Qian, C.; Jiang, X.; Zhang, F.; Weng, W. Mechanisms of stem cell osteogenic differentiation on TiO2 nanotubes. Colloids Surf. B 2015, 136, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cai, K.; Luo, Z.; Xu, D.; Xie, D.; Huang, Y.; Yang, W.; Liu, P. TiO2 nanotubes as drug nanoreservoirs for the regulation of mobility and differentiation of mesenchymal stem cells. Acta Biomater. 2012, 8, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.-Y.; Hsiao, J.-K.; Hsu, S.-C.; Yao, M.; Lu, C.-W.; Liu, H.-M.; Chen, Y.-C.; Yang, C.-S.; Huang, D.-M. In vivo magnetic resonance imaging of cell tropsim, trafficking mechanism, and therapeutic impact of human mesenchymal stem cells in a murine glioma model. Biomaterials 2011, 32, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Dong, J.; Zhang, T.; Su, Z.; Ding, J.; Zhang, Y.; Mao, X. Polyethyleneimine-functionalized iron oxide nanoparticles for systemic sirna delivery in experimental arthritis. Nanomedicine 2014, 9, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Miranda-Nieves, D.; Ankrum, J.A.; Matthiesen, M.E.; Phillips, J.A.; Roes, I.; Wojtkiewicz, G.R.; Juneja, V.; Kultima, J.R.; Zhao, W. Tracking mesenchymal stem cells with iron oxide nanoparticle loaded poly (lactide-co-glycolide) microparticles. Nano Lett. 2012, 12, 4131–4139. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Chen, G.; Zhang, H.; Tian, F.; Tan, B.; Dai, J.; Wang, Q.; Deng, Z. Magnetic resonance imaging of Fe3O4@ SiO2-labeled human mesenchymal stem cells in mice at 11.7 t. Biomaterials 2013, 34, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- LaConte, L.; Nitin, N.; Bao, G. Magnetic nanoparticle probes. Mater. Today 2005, 8, 32–38. [Google Scholar] [CrossRef]
- Bull, E.; Madani, S.Y.; Sheth, R.; Seifalian, A.; Green, M.; Seifalian, A.M. Stem cell tracking using iron oxide nanoparticles. Int. J. Nanomed. 2014, 9, 1641. [Google Scholar]
- Norizadeh-Abbariki, T.; Mashinchian, O.; Shokrgozar, M.A.; Haghighipour, N.; Sen, T.; Mahmoudi, M. Superparamagnetic nanoparticles direct differentiation of embryonic stem cells into skeletal muscle cells. J. Biomater. Tissue Eng. 2014, 4, 579–585. [Google Scholar] [CrossRef]
- Chung, T.-H.; Hsu, S.-C.; Wu, S.-H.; Hsiao, J.-K.; Lin, C.-P.; Yao, M.; Huang, D.-M. Dextran-coated iron oxide nanoparticle-improved therapeutic effects of human mesenchymal stem cells in a mouse model of parkinson’s disease. Nanoscale 2018, 10, 2998–3007. [Google Scholar] [CrossRef] [PubMed]
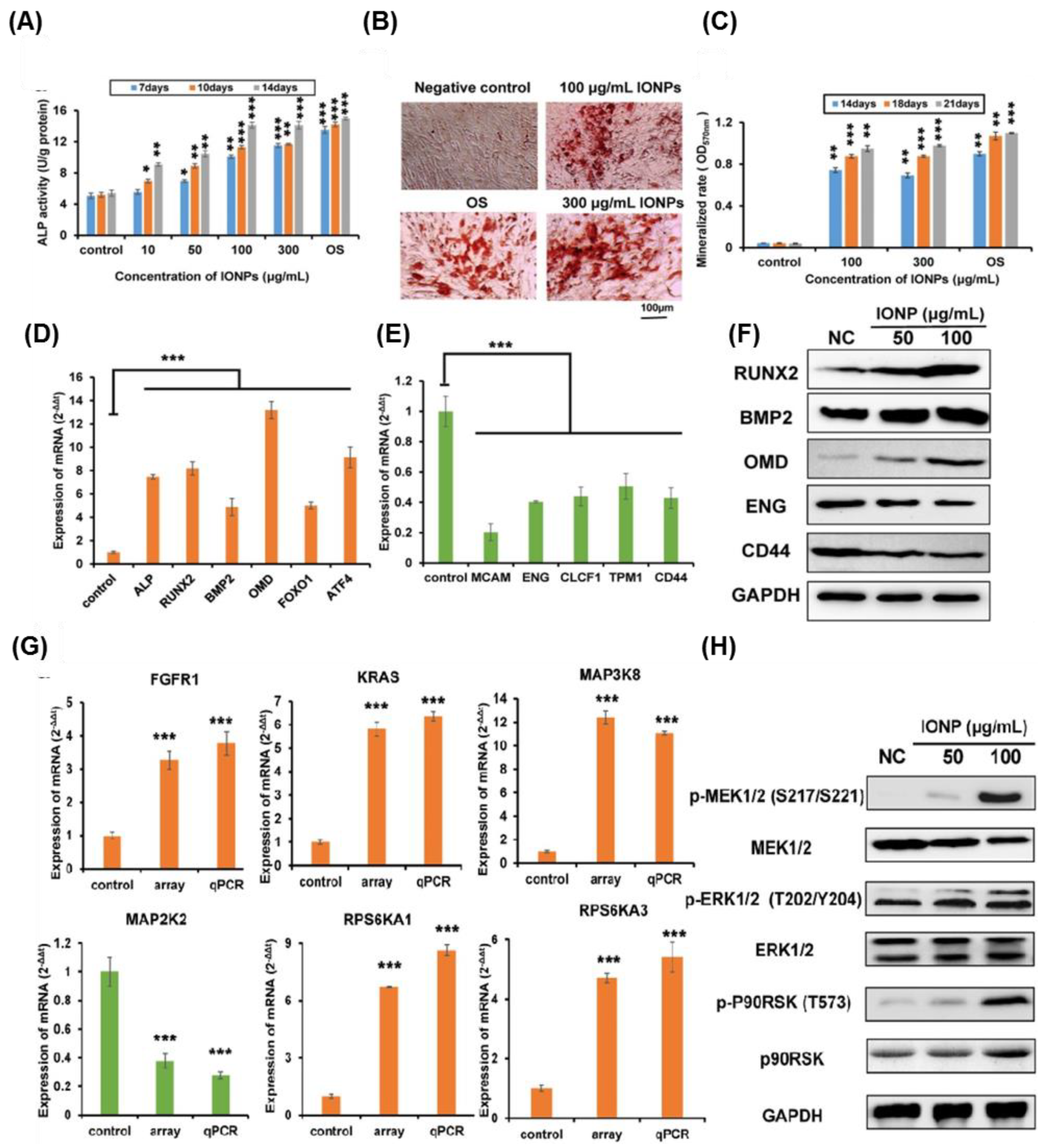
- Wang, Q.; Chen, B.; Cao, M.; Sun, J.; Wu, H.; Zhao, P.; Xing, J.; Yang, Y.; Zhang, X.; Ji, M. Response of mapk pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hbmscs. Biomaterials 2016, 86, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, X.; Huang, J.; Song, L.; Chen, Z.; Liu, H.; Li, Y.; Zhang, Y.; Gu, N. Magnetic assembly-mediated enhancement of differentiation of mouse bone marrow cells cultured on magnetic colloidal assemblies. Sci. Rep. 2014, 4, 5125. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Ma, F.; Lin, S.; Cao, M.; Li, Y.; Gu, N. Magnetic iron oxide nanoparticles accelerate osteogenic differentiation of mesenchymal stem cells via modulation of long noncoding rna inzeb2. Nano Res. 2017, 10, 626–642. [Google Scholar] [CrossRef]
- Levy, I.; Sher, I.; Corem-Salkmon, E.; Ziv-Polat, O.; Meir, A.; Treves, A.J.; Nagler, A.; Kalter-Leibovici, O.; Margel, S.; Rotenstreich, Y. Bioactive magnetic near infra-red fluorescent core-shell iron oxide/human serum albumin nanoparticles for controlled release of growth factors for augmentation of human mesenchymal stem cell growth and differentiation. J. Nanobiotechnol. 2015, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, B.; Shin, J.-Y.; Ryu, S.; Noh, M.; Woo, J.; Park, J.-S.; Lee, Y.; Lee, N.; Hyeon, T. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano 2015, 9, 2805–2819. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-M.; Hsiao, J.-K.; Chen, Y.-C.; Chien, L.-Y.; Yao, M.; Chen, Y.-K.; Ko, B.-S.; Hsu, S.-C.; Tai, L.-A.; Cheng, H.-Y. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Amiri, B.; Ghollasi, M.; Shahrousvand, M.; Kamali, M.; Salimi, A. Osteoblast differentiation of mesenchymal stem cells on modified pes-peg electrospun fibrous composites loaded with Zn2SiO4 bioceramic nanoparticles. Differentiation 2016, 92, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Njuguna, J.; Kavosh, N.; Briscoe, J. Correlation between stem cell differentiation and the topography of zinc oxide nanorods. J. Bionanosci. 2015, 9, 73–76. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Huang, Q.; He, W.; Zhang, R.; Feng, Q.; Benayahu, D. The stimulatory effect of silica nanoparticles on osteogenic differentiation of human mesenchymal stem cells. Biomed. Mater. 2016, 12, 015001. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Joe, Y.A.; Kim, M.K.; Lee, S.J.; Ryu, Y.H.; Cho, D.-W.; Rhie, J.W. Silica nanoparticles increase human adipose tissue-derived stem cell proliferation through erk1/2 activation. Int. J. Nanomed. 2015, 10, 2261. [Google Scholar] [CrossRef] [PubMed]
- Popara, J.; Accomasso, L.; Vitale, E.; Gallina, C.; Roggio, D.; Iannuzzi, A.; Raimondo, S.; Rastaldo, R.; Alberto, G.; Catalano, F. Silica nanoparticles actively engage with mesenchymal stem cells in improving acute functional cardiac integration. Nanomedicine 2018, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, X.; Wang, K.; He, C.; Shi, H.; Jian, L. Biocompatible silica nanoparticles− insulin conjugates for mesenchymal stem cell adipogenic differentiation. Bioconjugate Chem. 2010, 21, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-H.; Tsai, P.-H.; Chen, W.; Chiou, S.-H.; Mou, C.-Y. Dual delivery of sirna and plasmid DNA using mesoporous silica nanoparticles to differentiate induced pluripotent stem cells into dopaminergic neurons. J. Mater. Chem. B 2017, 5, 3012–3023. [Google Scholar] [CrossRef]
- Solanki, A.; Chueng, S.T.D.; Yin, P.T.; Kappera, R.; Chhowalla, M.; Lee, K.B. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv. Mater. 2013, 25, 5477–5482. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shen, Q.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Incorporation of cerium oxide into hydroxyapatite coating regulates osteogenic activity of mesenchymal stem cell and macrophage polarization. J. Biomater. Appl. 2017, 31, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Mattoli, V.; Mazzolai, B.; Ciofani, G. Cerium oxide nanoparticles inhibit adipogenesis in rat mesenchymal stem cells: Potential therapeutic implications. Pharm. Res. 2014, 31, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Ermakov, A.; Savintseva, I.; Selezneva, I.; Poltavtseva, R.; Zaraisky, E.; Poltavtsev, A.; Stepanov, A.; Ivanov, V.; Sukhikh, G. Citrate-stabilized nanoparticles of ceo 2 stimulate proliferation of human mesenchymal stem cells in vitro. Nanosci. Technol. Int. J. 2016, 7, 235–246. [Google Scholar]
- Xiang, J.; Li, J.; He, J.; Tang, X.; Dou, C.; Cao, Z.; Yu, B.; Zhao, C.; Kang, F.; Yang, L. Cerium oxide nanoparticle modified scaffold interface enhances vascularization of bone grafts by activating calcium channel of mesenchymal stem cells. ACS Appl. Mater. Interfaces 2016, 8, 4489–4499. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Ermakov, A.; Savintseva, I.; Selezneva, I.; Poltavtseva, R.; Zaraisky, E.; Poltavtsev, A.; Stepanova, I.; Ivanov, V.; Sukhikh, G. Biosafety and effect of nanoparticles of ceo 2 on metabolic and proliferative activity of human mesenchymal stem cells in vitro. Nanosci. Technol. Int. J. 2016, 7, 165–175. [Google Scholar]
- Gliga, A.R.; Edoff, K.; Caputo, F.; Källman, T.; Blom, H.; Karlsson, H.L.; Ghibelli, L.; Traversa, E.; Ceccatelli, S.; Fadeel, B. Cerium oxide nanoparticles inhibit differentiation of neural stem cells. Sci. Rep. 2017, 7, 9284. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Ricotti, L.; Canale, C.; D’Alessandro, D.; Berrettini, S.; Mazzolai, B.; Mattoli, V. Effects of barium titanate nanoparticles on proliferation and differentiation of rat mesenchymal stem cells. Colloids Surf. B 2013, 102, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mahmood, M.; Xu, Y.; Watanabe, F.; Biris, A.S.; Hansen, D.K.; Inselman, A.; Casciano, D.; Patterson, T.A.; Paule, M.G. Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front. Neurosci. 2015, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Rajanahalli, P.; Stucke, C.J.; Hong, Y. The effects of silver nanoparticles on mouse embryonic stem cell self-renewal and proliferation. Toxicol. Rep. 2015, 2, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Repar, N.; Li, H.; Aguilar, J.S.; Li, Q.Q.; Drobne, D.; Hong, Y. Silver nanoparticles induce neurotoxicity in a human embryonic stem cell-derived neuron and astrocyte network. Nanotoxicology 2018, 12, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Sengstock, C.; Diendorf, J.; Epple, M.; Schildhauer, T.A.; Köller, M. Effect of silver nanoparticles on human mesenchymal stem cell differentiation. Beilstein J. Nanotechnol. 2014, 5, 2058. [Google Scholar] [CrossRef] [PubMed]
- Söderstjerna, E.; Johansson, F.; Klefbohm, B.; Johansson, U.E. Gold-and silver nanoparticles affect the growth characteristics of human embryonic neural precursor cells. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Cai, K.; Li, J.; Chen, X.; Lai, M.; Hu, Y.; Luo, Z.; Ding, X.; Xu, D. Effects of titanium nanoparticles on adhesion, migration, proliferation, and differentiation of mesenchymal stem cells. Int. J. Nanomed. 2013, 8, 3619. [Google Scholar]
- Zhang, W.; Jiang, P.; Chen, W.; Zheng, B.; Mao, Z.; Antipov, A.; Correia, M.; Larsen, E.H.; Gao, C. Genotoxicity of copper oxide nanoparticles with different surface chemistry on rat bone marrow mesenchymal stem cells. J. Nanosci. Nanotechnol. 2016, 16, 5489–5497. [Google Scholar] [CrossRef] [PubMed]
- Murgia, A.; Mancuso, L.; Manis, C.; Caboni, P.; Cao, G. Gc-ms metabolomics analysis of mesenchymal stem cells treated with copper oxide nanoparticles. Toxicol. Mech. Methods 2016, 26, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Luan, Q.; Chen, W.; Wang, Y.; Wu, M.; Zhang, H.; Jiao, Z. Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 2009, 20, 115101. [Google Scholar] [CrossRef] [PubMed]
- Ickrath, P.; Wagner, M.; Scherzad, A.; Gehrke, T.; Burghartz, M.; Hagen, R.; Radeloff, K.; Kleinsasser, N.; Hackenberg, S. Time-dependent toxic and genotoxic effects of zinc oxide nanoparticles after long-term and repetitive exposure to human mesenchymal stem cells. Int. J. Environ. Res. Public Health 2017, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Syama, S.; Sreekanth, P.; Varma, H.; Mohanan, P. Zinc oxide nanoparticles induced oxidative stress in mouse bone marrow mesenchymal stem cells. Toxicol. Mech. Methods 2014, 24, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (spion). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Jiang, P.; Sousa, M.H.; Morais, P.C.; Mao, Z.; Gao, C. Citrate-capped iron oxide nanoparticles impair the osteogenic differentiation potential of rat mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 245–256. [Google Scholar] [CrossRef]
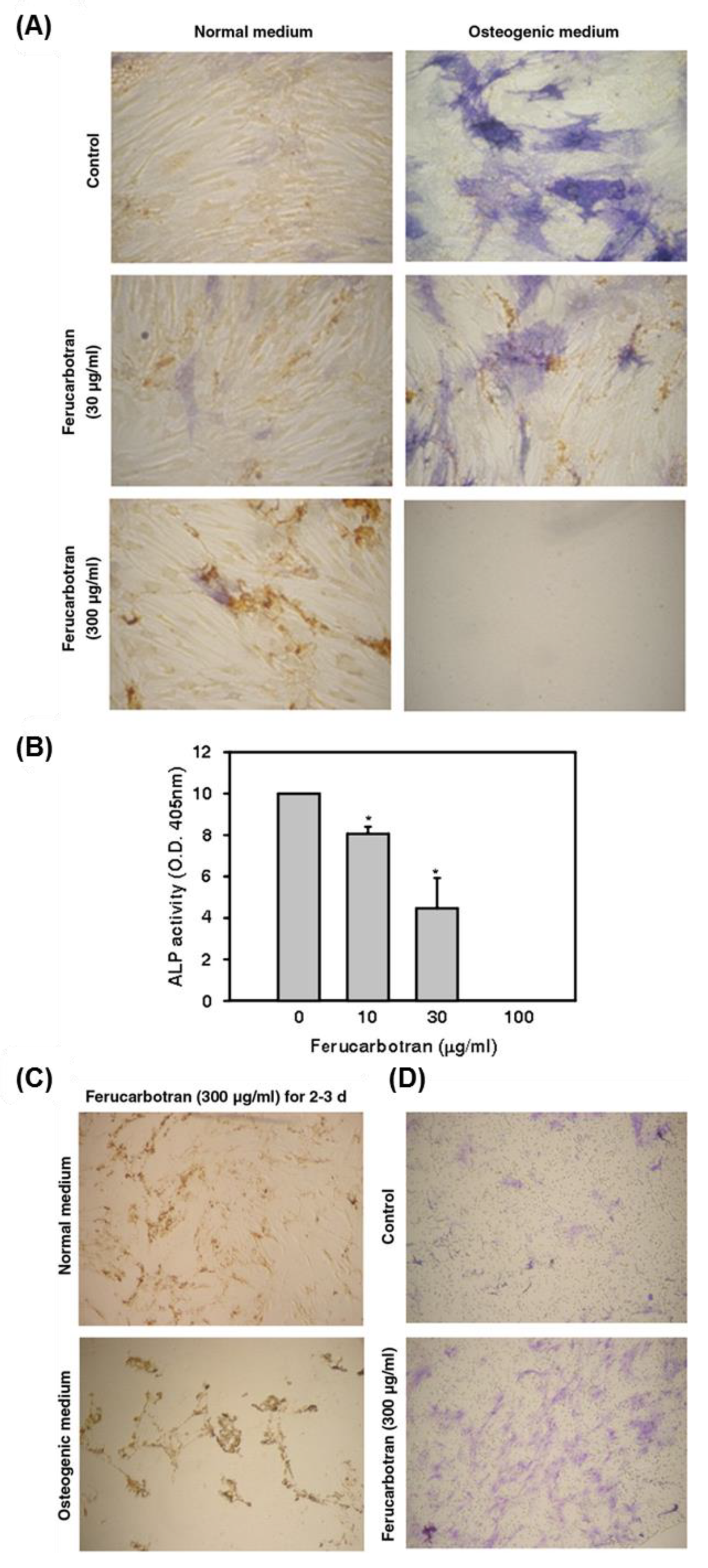
- Chen, Y.-C.; Hsiao, J.-K.; Liu, H.-M.; Lai, I.-Y.; Yao, M.; Hsu, S.-C.; Ko, B.-S.; Chen, Y.-C.; Yang, C.-S.; Huang, D.-M. The inhibitory effect of superparamagnetic iron oxide nanoparticle (ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2010, 245, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kostura, L.; Kraitchman, D.L.; Mackay, A.M.; Pittenger, M.F.; Bulte, J.W. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004, 17, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Yang, Y.; Kawazoe, N.; Chen, G. Sub-10 nm gold nanoparticles promote adipogenesis and inhibit osteogenesis of mesenchymal stem cells. J. Mater. Chem. B 2017, 5, 1353–1362. [Google Scholar] [CrossRef]
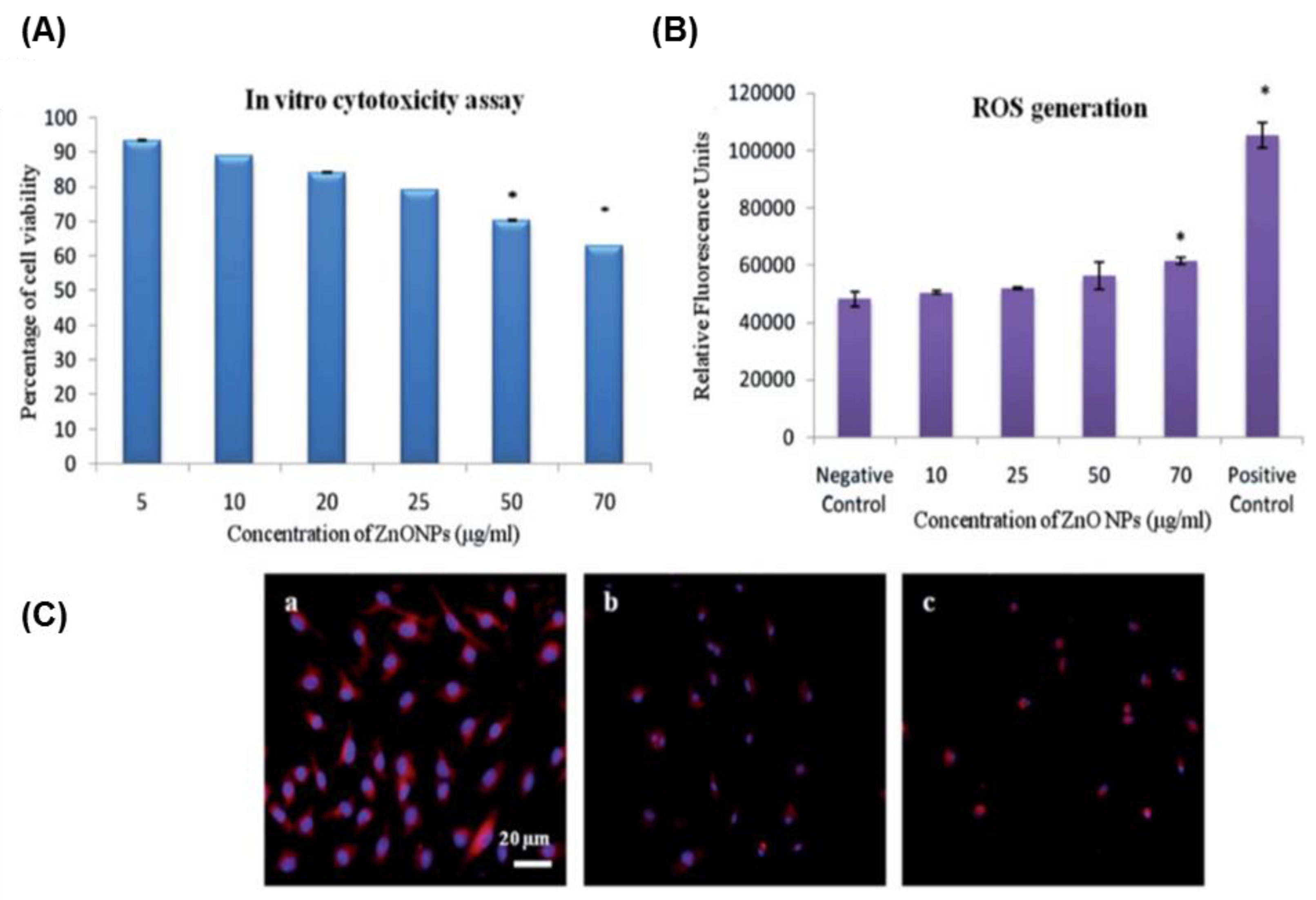
| Cellular Effect | Nanomaterial (Name/Size) | Effect | Cell Type | Mechanism | References | |
|---|---|---|---|---|---|---|
| Differentiation | Neural | AuNP | Enhance | mESC | Targeting mTOR/p70S6K signaling pathway | [112] |
| AuNP | Enhance | F9 teratocarcinoma stem cells | -Upregulation of, RA binding protein, collagen type IV, Gata 6 & Laminin 1 | [114] | ||
| AgNP | Enhance | F9 teratocarcinoma stem cells | Upregulation of the expression levels of neural-specific markers | [133] | ||
| DEX-IONP | Enhance | hMSCs | -DA-like neurons differentiation -Enhance the paracrine action | [153] | ||
| IONP/HSA | Enhance | hBM-MSCs | -Covalent conjugation to FGF2 -Upregulation of MAP2 and GFAP expressions | [157] | ||
| SiNP | Enhance | miPSC | Co-delivery of pNurr1 and siRex1 | [166] | ||
| Osteogenic | Nanoceria | Suppress | Neural stem cells | Suppression the expression levels of βIII-tubulin and GFAP genes | [173] | |
| AuNP (70 nm) | Enhance | hMSC | YAP activity regulation | [101] | ||
| AuNP (30 & 50 nm) | Enhance | hADSC | Increase ALP activity | [102] | ||
| AuNP | Enhance | hADSC | Wnt/β-catenin signaling pathway | [117] | ||
| AuNP | Enhance | hADSC | ERK/MAPK signaling pathway | [118] | ||
| AuNP | Enhance | hBM-MSC | Delivery of miR-29b | [120] | ||
| AuNP | Enhance | rBM-MSCs | MAPK/p38 pathway activation | [121] | ||
| AuNP (4 nm) | Suppress | hBM-MSC | Increase ROS generation | [190] | ||
| Differentiation | Osteogenic | AgNP | Enhance | Human Urine-derived stem cells | -RhoA activation -Cytoskeleton tension -Actin polymerization | [130] |
| AgNP | Enhance | mMSC | TGF-β/BMP signaling activation | [105] | ||
| AgNP (80 nm) | Suppress | hMSC | Agglomeration in endo-lysosomal cell compartment | [178] | ||
| AgNP | Enhance | hADSC | Delivery of photo-activated miR-148b mimic | [131] | ||
| AgNP | Suppress | hBM-MSCs | Enhance ROS generation | [132] | ||
| TiO2 | Enhance | rBM-MSC | Promote cell adhesion and spreading | [139] | ||
| TiO2 | Enhance | Human pulp- and adipose tissue-derived stem cells | Enhance the expression levels of bone-related genes RUNX2, FOSL1, and SPP1 | [140] | ||
| TiO2–COOH NRs | Suppress | rBM-MSCs | Upregulation of the expression level of FGF-2 and TGF-β1 | [109] | ||
| TiO2 | Enhance | hBM-MSCs | High phosphorylation of FAK-mediated cell adhesion | [141] | ||
| TiO2 nanotube (70 nm) | Enhance | hASCs | Promote the methylation of the histone H3 at lysine 4 in the promoter regions of the osteogenic differentiation markers | [142] | ||
| TNT-TiO2 | Enhance | hBM-MSCs | High surface area and the photocatalysis | [143] | ||
| TiO2 nanotube (100 nm) | Enhance | rBM-MSC | Activation of MAP3K11, Na+/K+ transporting ATPases ATP1A2, and ATP1A3 | [144] | ||
| TiO2 | Enhance | rBM-MSC | Activation of the motogenic response of MSC and release of BMP2 | [145] | ||
| Differentiation | Osteogenic | IONPs | Enhance | hBM-MSCs | Activation of MAPK signaling | [154] |
| IONPs | Enhance | Primary mouse bone marrow cells | Magentic field-mediated osteogenic induction | [155] | ||
| IONPs | Enhance | hBM-MSCs | Upregulation of long noncoding RNA INZEB2 | [156] | ||
| Citrate-capped IONPs | Suppress | rBM-MSC | -Suppression of calcium deposition -Downregulation the expression levels of collagen type I and osteocalcin | [187] | ||
| Fe3O4/BSA-loaded IONP | Enhance | rBM-MSC | Static magnetic field-mediated particle uptake and activation of osteogenic differentiation | [110] | ||
| IONP/HSA | Enhance | hBM-MSCs | Covalent conjugation to FGF2 | [157] | ||
| PES-PEG electrospun composites coated with Zn2SiO4 bioceramic NPs | Enhance | hMSC | -Promotion of cell proliferation -Upregulation of ALP and the osteogenesis marker | [160] | ||
| SiNPs | Enhance | hMSC | Activation of ALP | [162] | ||
| SiNPs | Enhance | hADSCs | Phosphorylation of ERK1/2 | [163] | ||
| Nanoceria | Enhance | rBM-MSC | Activation of BMP signaling | [164] | ||
| Glycol–chitosan-coated barium titanate NPs | Enhance | rMSC | -Significant increase of hydroxyapatite deposit formation -Rearrangement of f-actin based structure | [174] | ||
| SPIO NPs | Suppress | hMSCs | High release of the free iron | [188] | ||
| Differentiation | Adipogenic | AuNP (4 nm) | Enhance | hBM-MSCs | Increase ROS generation | [190] |
| AgNP (80 nm) | Supress | hMSC | Agglomeration in endo-lysosomal cell compartment | [178] | ||
| AgNP | Enhance | hBM-MSCs | Increase of intracellular ROS | [132] | ||
| IONP/HSA | Enhance | hBM-MSCs | Covalent conjugation to FGF2 | [157] | ||
| SiNP-conjugated insulin | Enhance | rBM-MSC | Insulin delivery | [165] | ||
| Nanoceria | Suppress | rBM-MSC | Suppression of ROS generation | [169] | ||
| Glycol–chitosan-coated barium titanate NPs | Enhance | rMSC | Cytoskeleton organization | [174] | ||
| Cardiogenic | AuNP (16 nm) | Enhance | hBM-MSCs | -Formation of contractile proteins -upregulation of the cardiogenic differentiation markers | [123] | |
| IONPs | Enhance | hBM-MSCs | Enhance the link between MSC and the cardiomyblast via activation of connexin-43 | [158] | ||
| SiNP (50–120 nm) | Enhance | hMSC | -High focal adhesion and upregulation of connexin-43 -Promote the interaction of hMSC with cardiac myoblasts in ischemic condition | [164] | ||
| AuNP-loaded BSA/PVA scaffolds | Enhance | hBM-MSCs | -Increase cell proliferation -Upregulation of cardiomyocyte-related protein markers | [124] | ||
| Differentiation | Myogenic | IONPs | Enhance | ESCs | Upregulation of MyoG and Myh2 | [152] |
| Angiogenic | Nanoceria | Enhance | Murine MSC | Upregulation of the expression of angiogenic factor VEGF | [171] | |
| Proliferation | TiO2 (> 50 nm) | Suppress | rBM-MSC | Activation of the programmed cell death | [139] | |
| SiNPs (50–120 nm) | Enhance | hADSCs | Increase the phosphorylation of ERK1/2 signaling | [163] | ||
| Nanoceria | Enhance | rBM-MSC | Activation of BMP signaling | [168] | ||
| Citrate-stabilized nanoceria | Enhance | hMSC | Enhance the transcription level for mRNA of proliferation- and cell cycle-associated genes | [170] | ||
| Nanoceria | Enhance | Human dental pulp-derived MSCs | Modulation of proliferation- and cell cycle- related gene expression | [172] | ||
| AgNP | Suppress | Human- and rat-derived embryonic NSCs | -High ROS generation -Mitochondrial dysfunction -Activation of BAX protein -Release of the lactate dehydrogenase | [175] | ||
| AgNP | Suppress | mESC | Cell cycle arrest via inhibition of the phosphorylation of the retinoblastoma protein | [176] | ||
| SPIO NPs | Enhance | hMSCs | Upregulation of the cell cycle related proteins including cyclin B, cyclin D1, and CDK4 | [159] | ||
| Proliferation | AgNP | Suppress | hESC-derived neuron and astrocyte | -Activation of Akt/glycogen synthase kinase-3/caspase-3 signaling -High ROS generation | [177] | |
| AgNP | Suppress | human embryonic neural precursor cells | Activation of apoptosis | [179] | ||
| TiO2 | Suppress | rBM-MSC | Negative impacts of cell membrane integrity and cytoskeleton | [180] | ||
| CuO NPs | Suppress | rBM-MSC | High ROS generation | [181] | ||
| CuO NPs | Suppress | hBM-MSCs | Upregulation of Serine, glyceric acid, and succinic | [182] | ||
| ZnO NP | Suppress | mNSC | Inhibit mitochondrial respiration | [183] | ||
| ZnO NP | Suppress | mBM-MSCs | -High ROS production -Activation of the apoptotic factors, | [185] | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdal Dayem, A.; Lee, S.B.; Cho, S.-G. The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials 2018, 8, 761. https://doi.org/10.3390/nano8100761
Abdal Dayem A, Lee SB, Cho S-G. The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials. 2018; 8(10):761. https://doi.org/10.3390/nano8100761
Chicago/Turabian StyleAbdal Dayem, Ahmed, Soo Bin Lee, and Ssang-Goo Cho. 2018. "The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation" Nanomaterials 8, no. 10: 761. https://doi.org/10.3390/nano8100761
APA StyleAbdal Dayem, A., Lee, S. B., & Cho, S.-G. (2018). The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials, 8(10), 761. https://doi.org/10.3390/nano8100761







