Applications of SERS in the Detection of Stress-Related Substances
Abstract
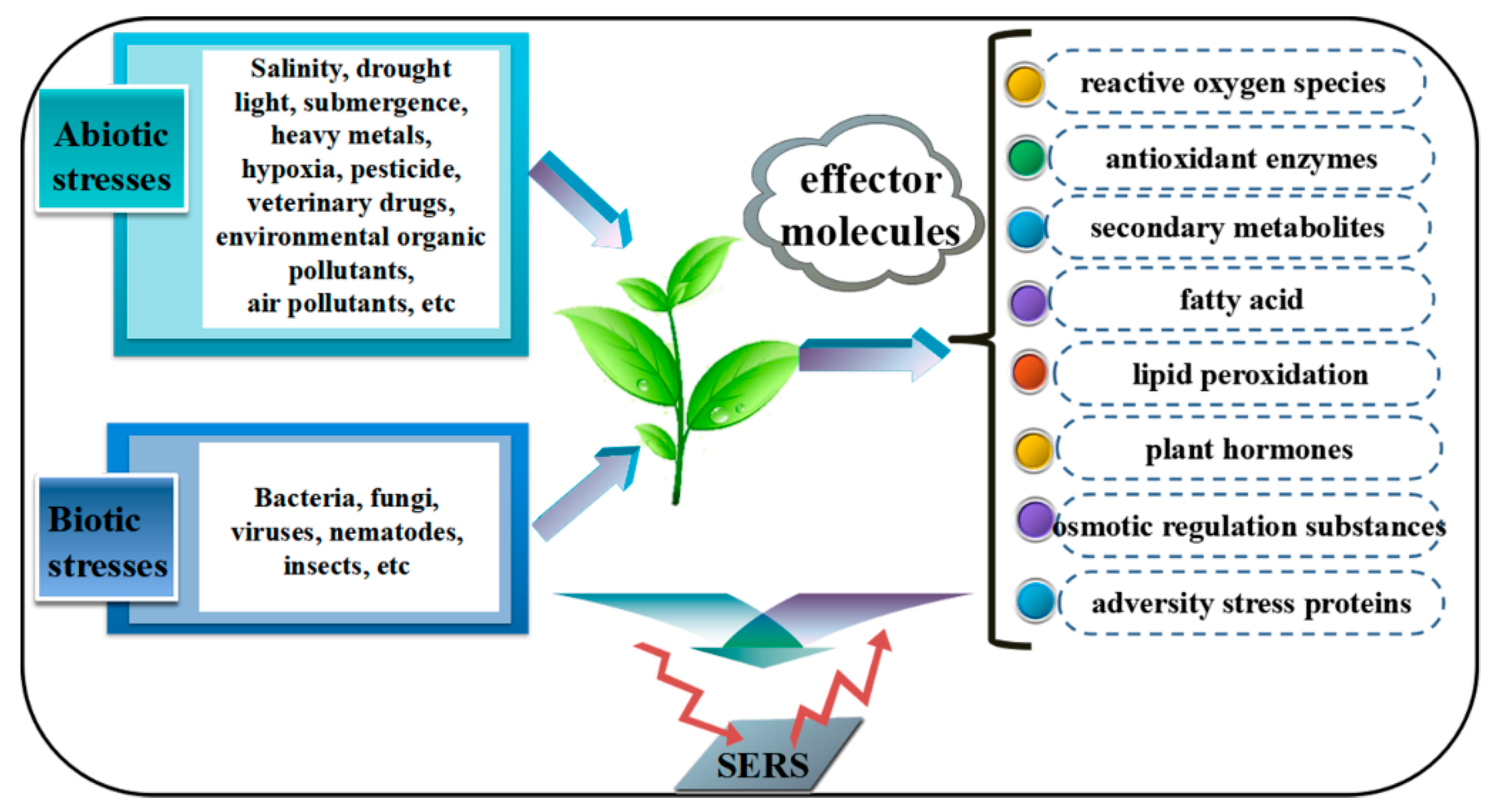
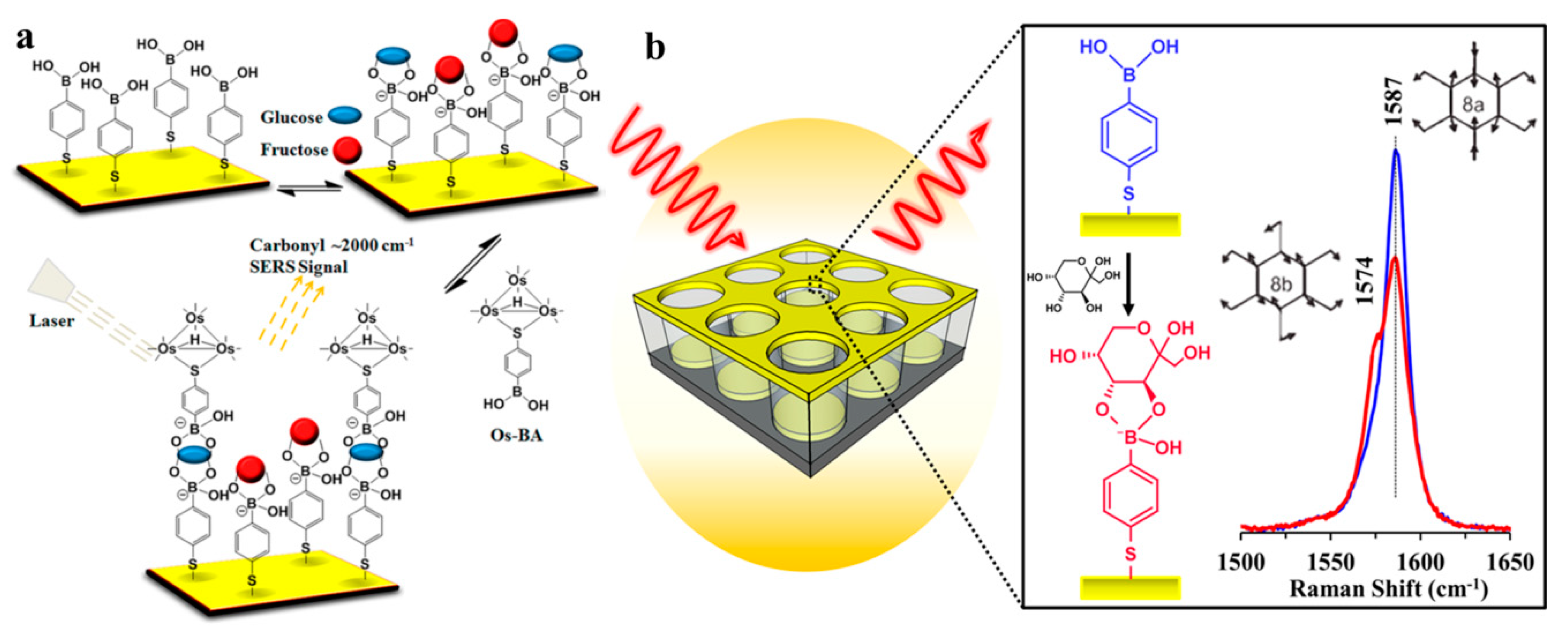
1. Introduction
2. Principle of Surface-enhanced Raman Spectroscopy (SERS)
3. SERS Applications in the Detection of Stress Factors
3.1. Inorganic Ions
3.1.1. Heavy Metal Ions
3.1.2. Anions
3.2. Organic Pollutants
3.2.1. Pesticides and Veterinary Drug Residues
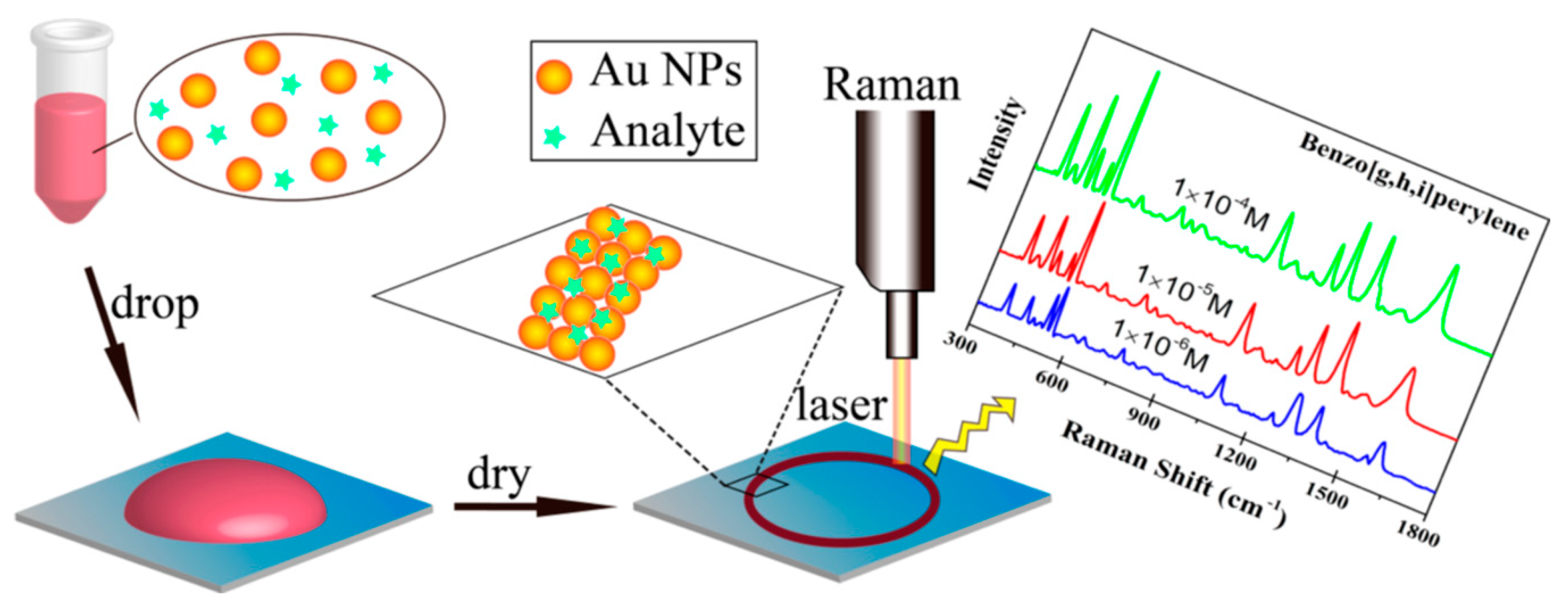
3.2.2. Polycyclic Aromatic Hydrocarbons
3.2.3. Formaldehyde
3.3. Microorganism
3.3.1. Bacteria
3.3.2. Virus
3.4. Air Pollutants
4. SERS Applications in the Detection of Effector Molecules
4.1. Reactive Oxygen Species (ROS)
4.2. Enzymatic Antioxidants
4.3. Nonenzymatic Antioxidants
4.4. Plant Hormones
4.5. Production of Lipid Peroxidation
4.6. Osmotic Regulation Substances
4.7. Others
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Li, L.; Yang, H.J.; Liu, D.C.; He, H.B.; Wang, C.F.; Zhong, J.F.; Gao, T.D.; Zeng, Y. Analysis of biofilms formation and associated genes detection in staphylococcus isolates from bovine mastitis. Intern. J. Appl. Res. Vet. Med. 2012, 10, 62–68. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wang, X.J.; Zhang, F.; Huo, X.B.; Fu, R.S.; Liu, J.J.; Sun, W.B.; Kang, D.M.; Jing, X. The identification of a bacterial strain BGI-1 isolated from the intestinal flora of Blattella germanica, and its anti-entomopathogenic fungi activity. J. Econ. Entomol. 2013, 106, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shi, W.; Liu, R.; Xu, Y.; Sui, N.; Zhou, J.; Feng, G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Spec. Biol. 2017, 32, 107–114. [Google Scholar] [CrossRef]
- He, C.Q.; Liu, Y.X.; Wang, H.M.; Hou, P.L.; He, H.B.; Ding, N.Z. New genetic mechanism, origin and population dynamic of bovine ephemeral fever virus. Vet. Microbiol. 2016, 182, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi-Dehkordi, E.; Alemzadeh, A.; Tanaka, N.; Razi, H. Meta-analysis of transcriptomic responses to biotic and abiotic stress in tomato. PeerJ 2018, 6, 4631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Song, J.; Fan, J.; Feng, G. Effects of saline-waterlogging and dryness/moist alternations on seed germination of halophyte and xerophyte. Plant Spec. Biol. 2015, 30, 231–236. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.Y.; Zhang, J.; Zhao, L.; Miao, M.S.; Huang, H. Responses of zooplankton community to environmental factors and phytoplankton biomass in Lake Nansihu, China. Pak. J. Zool. 2017, 49, 493–504. [Google Scholar] [CrossRef]
- Song, J.; Shi, G.; Gao, B.; Fan, H.; Wang, B. Waterlogging and salinity effects on two Suaeda salsa populations. Physiol. Plant. 2011, 141, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Lou, M.F.; Shen, W.; Fu, R.S.; Wang, D.H. A maternal low-fiber diet predisposes offspring to improved metabolic phenotypes in adulthood in an herbivorous rodent. Physiol. Biochem. Zool. 2017, 90, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Chen, M.; Yang, J.; Song, J.; Wang, B. The optimal dosage of 60co gamma irradiation for obtaining salt gland mutants of exo-recretohalophyte Limonium bicolor (Bunge) O. Kuntze. Pak. J. Bot. 2015, 47, 71–76. [Google Scholar]
- Cheng, S.; Yang, Z.; Wang, M.; Song, J.; Sui, N.; Fan, H. Salinity improves chilling resistance in Suaeda salsa. Acta Physiol. Plant. 2014, 36, 1823–1830. [Google Scholar] [CrossRef]
- Qin, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 2011, 52, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Perez-Clemente, R.M.; Vives, V.; Zandalinas, S.I.; Lopez-Climent, M.F.; Munoz, V.; Gomez-Cadenas, A. Biotechnological approaches to study plant responses to stress. Biomed. Res. Int. 2013, 2013, 654120. [Google Scholar] [CrossRef] [PubMed]
- Sui, N. Photoinhibition of Suaeda salsa to chilling stress is related to energy dissipation and water-water cycle. Photosynthetica 2015, 53, 207–212. [Google Scholar] [CrossRef]
- Zhao, S.Z.; Sun, H.Z.; Chen, M.; Wang, B.S. Light-regulated betacyanin accumulation in euhalophyte Suaeda salsa calli. Plant Cell Tissue Org. Cult. 2010, 102, 99–107. [Google Scholar] [CrossRef]
- Zhao, S.Z.; Sun, H.Z.; Gao, Y.; Sui, N.; Wang, B.S. Growth regulator-induced betacyanin accumulation and dopa-4,5-dioxygenase (DODA) gene expression in euhalophyte Suaeda salsa calli. In Vitro Cell. Dev. Biol. Plant 2011, 47, 391–398. [Google Scholar] [CrossRef]
- Sui, N.; Tian, S.; Wang, W.; Wang, M.; Fan, H. Overexpression of glycerol-3-phosphate acyltransferase from Suaeda salsa improves salt tolerance in arabidopsis. Front. Plant Sci. 2017, 8, 1337. [Google Scholar] [CrossRef] [PubMed]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Li, M.; Wan, S.; Sui, N. Antioxidants and unsaturated fatty acids are involved in salt tolerance in peanut. Acta Physiol. Plant. 2017, 39, 207. [Google Scholar] [CrossRef]
- Sui, N.; Wang, Y.; Liu, S.; Yang, Z.; Wang, F.; Wan, S. Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in peanut. Front. Plant Sci. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Chen, M.; Yang, J.; Leng, B.; Wang, B. A system for the transformation and regeneration of the recretohalophyte Limonium bicolor. In Vitro Cell. Dev. Biol. Plant 2014, 50, 610–617. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, C.; Yang, C.Y.; Wang, J.Y.; Chen, L.; Bao, X.M.; Zhao, Y.X.; Zhang, H.; Liu, J. The role of arabidopsis AtFes1A in cytosolic Hsp70 stability and abiotic stress tolerance. Plant J. 2010, 62, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Leng, B.; Wang, B. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Leterrier, M.; Valderrama, R.; Airaki, M.; Chaki, M.; Palma, J.M.; Barroso, J.B. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011, 181, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Perumal, J.; Balasundaram, G.; Mahyuddin, A.P.; Choolani, M.; Olivo, M. SERS-based quantitative detection of ovarian cancer prognostic factor haptoglobin. Int. J. Nanomed. 2015, 10, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hao, R.; Wang, Z.; Zhang, H.; Hao, Y.; Zhang, C.; Liu, Y. Green synthesis of multi-dimensional plasmonic coupling structures: Graphene oxide gapped gold nanostars for highly intensified surface enhanced raman scattering. Chem. Eng. J. 2018, 349, 581–587. [Google Scholar] [CrossRef]
- Breitman, M.; Ruiz-Moreno, S.; Gil, A.L. Experimental problems in Raman spectroscopy applied to pigment identification in mixtures. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 68, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Azbej, T.; Severs, M.J.; Rusk, B.G.; Bodnar, R.J. In situ quantitative analysis of individual H2O–CO2 fluid inclusions by laser Raman spectroscopy. Chem. Geol. 2007, 237, 255–263. [Google Scholar] [CrossRef]
- Mitchell, B.L.; Patwardhan, A.J.; Ngola, S.M.; Chan, S.; Sundararajan, N. Experimental and statistical analysis methods for peptide detection using surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2010, 39, 380–388. [Google Scholar] [CrossRef]
- And, M.G.; Feng, M.L. SERS substrates fabricated by island lithography: The silver/pyridine system. J. Phys. Chem. B 2003, 107, 13015–13021. [Google Scholar]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with SERS hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.S.; Itoh, T. Why and how do the shapes of surface-enhanced raman scattering spectra change? Recent progress from mechanistic studies. J. Raman Spectrosc. 2016, 47, 78–88. [Google Scholar] [CrossRef]
- Schlucker, S. Surface-enhanced raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. Engl. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, R.; Liu, G.K.; Wang, Y.H.; Liu, J.Y.; Ding, S.Y.; Li, J.F.; Wu, D.Y.; Tian, Z.Q. Surface-enhanced raman spectroscopy: Bottlenecks and future directions. Chem. Commun. 2017, 54, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Yamamoto, Y.S.; Ozaki, Y. Plasmon-enhanced spectroscopy of absorption and spontaneous emissions explained using cavity quantum optics. Chem. Soc. Rev. 2017, 46, 3904–3921. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Yang, T.; He, L. Review of surface enhanced raman spectroscopic (sers) detection of synthetic chemical pesticides. TrAC Trends Anal. Chem. 2016, 85, 73–82. [Google Scholar] [CrossRef]
- Cheng, J.M.; Liu, Y.Z.; Wang, H.W. Effects of surface-modified nano-scale carbon black on Cu and Zn fractionations in contaminated soil. Int. J. Phytoremediat. 2014, 16, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Gasic, K.; Korban, S.S. Heavy metal stress. In Physiology & Molecular Biology of Stress Tolerance in Plants; Madhava Rao, K.V., Raghavendra, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 8, pp. 219–254. [Google Scholar]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, X.; Wang, H.; Song, B.; Zhu, Y.; Wang, H.; Su, Y.; He, Y. Surface-enhancement Raman scattering sensing strategy for discriminating trace mercuric ion (II) from real water samples in sensitive, specific, recyclable, and reproducible manners. Anal. Chem. 2015, 87, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.L.; Zhong, J.; Yao, G.H.; Huang, Q. A label-free sers approach to quantitative and selective detection of mercury (II) based on DNA aptamer-modified SiO2@Au core/shell nanoparticles. Sens. Actuator B-Chem. 2018, 258, 365–372. [Google Scholar] [CrossRef]
- Makam, P.; Shilpa, R.; Kandjani, A.E.; Periasamy, S.R.; Sabri, Y.M.; Madhu, C.; Bhargava, S.K.; Govindaraju, T. SERS and fluorescence-based ultrasensitive detection of mercury in water. Biosens. Bioelectron. 2018, 100, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, L.H.; Chen, Y.H.; Bi, N.; Zheng, X.; Qi, H.B.; Qin, M.H.; Liao, X.; Zhang, H.Q.; Tian, Y. Determination of mercury(II) by surface-enhanced Raman scattering spectroscopy based on thiol-functionalized silver nanoparticles. Microchim. Acta 2012, 177, 341–348. [Google Scholar] [CrossRef]
- Ma, P.Y.; Liang, F.H.; Yang, Q.Q.; Wang, D.; Sun, Y.; Wang, X.H.; Gao, D.J.; Song, D.Q. Highly sensitive SERS probe for mercury(II) using cyclodextrin-protected silver nanoparticles functionalized with methimazole. Microchim. Acta 2014, 181, 975–981. [Google Scholar] [CrossRef]
- Xing, N.; Ji, L.Z.; Song, J.; Ma, J.C.; Li, S.G.; Ren, Z.M.; Xu, F.; Zhu, J.P. Cadmium stress assessment based on the electrocardiogram characteristics of zebra fish (Danio rerio): QRS complex could play an important role. Aquat. Toxicol. 2017, 191, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, T.; Song, J.B.; Zhang, Q.; Liu, S.Y.; Xu, R.; Duan, H.W. SERS-active nanoparticles for sensitive and selective detection of cadmium ion (Cd2+). Chem. Mater. 2011, 23, 4756–4764. [Google Scholar] [CrossRef]
- Thatai, S.; Khurana, P.; Prasad, S.; Kumar, D. Plasmonic detection of Cd2+ ions using surface-enhanced Raman scattering active core-shell nanocomposite. Talanta 2015, 134, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Irudayaraj, J. A SERS dnazyme biosensor for lead ion detection. Chem. Commun. 2011, 47, 4394–4396. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Fan, P.D.; Liang, A.H.; Liu, Q.Y.; Jiang, Z.L. Aptamer based determination of pb(II) by SERS and by exploiting the reduction of HAuCl4 by H2O2 as catalyzed by graphene oxide nanoribbons. Microchim. Acta 2018, 185, 177. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.S.; Dempsey, M.J.; Whitehead, D.E. Highly sensitive SERS detection of pb2+ ions in aqueous media using citrate functionalised gold nanoparticles. Sens. Actuator B-Chem. 2015, 221, 1003–1008. [Google Scholar] [CrossRef]
- Sarfo, D.K.; Izake, E.L.; O’Mullane, A.P.; Ayoko, G.A. Molecular recognition and detection of pb(II) ions in water by aminobenzo-18-crown-6 immobilised onto a nanostructured SERS substrate. Sens. Actuator B-Chem. 2018, 255, 1945–1952. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Gu, W.; Zhang, C.L.; Shi, X.H.; Xian, Y.Z. In situ regulation nanoarchitecture of Au nanoparticles/reduced graphene oxide colloid for sensitive and selective SERS detection of lead ions. J. Colloid Interface Sci. 2016, 465, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, N.; Su, Y.Y.; Wang, H.Y.; He, Y. Silicon nanohybrid-based SERS chips armed with an internal standard for broad-range, sensitive and reproducible simultaneous quantification of lead(II) and mercury(II) in real systems. Nanoscale 2018, 10, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Chen, L.X.; Lou, T.T.; Wang, Y.Q. Highly sensitiveSERS detection of As3+ ions in aqueous media using glutathione functionalized silver nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 3936–3941. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sun, J.F.; Cao, D.; Zhang, L.Q.; Liu, J.F.; Jiang, G.B. Fabrication of highly-specific SERS substrates by co-precipitation of functional nanomaterials during the self-sedimentation of silver nanowires into a nanoporous film. Chem. Commun. 2015, 51, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Song, L.L.; Mao, K.; Zhou, X.D.; Hu, J.M. A novel biosensor based on Au@Ag core-shell nanoparticles for SERS detection of arsenic (III). Talanta 2016, 146, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Hao, J.M.; Li, F.S.; Meng, X.G. Surface-enhanced Raman spectroscopy of arsenate and arsenite using ag nanofilm prepared by modified mirror reaction. J. Colloid Interface Sci. 2010, 347, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.Q.; Wang, J. Gold nanorod@nanoparticle seed-SERS nanotags/graphene oxide plasmonic superstructured nanocomposities as an “on-off” SERS aptasensor. Carbon 2018, 133, 209–217. [Google Scholar] [CrossRef]
- Ndokoye, P.; Ke, J.; Liu, J.; Zhao, Q.D.; Li, X.Y. l-cysteine-modified gold nanostars for SERS-based copper ions detection in aqueous media. Langmuir 2014, 30, 13491–13497. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.L.; She, G.W.; Mu, L.X.; Shi, W.S. Highly uniform indicator-mediated SERS sensor platform for the detection of Zn2+. RSC Adv. 2016, 6, 16555–16560. [Google Scholar] [CrossRef]
- Zhao, K.F.; Song, J.; Fan, H.; Zhou, S.; Zhao, M. Growth response to ionic and osmotic stress of NaCl in salt-tolerant and salt-sensitive maize. J. Integr. Plant Biol. 2010, 52, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.U.; Shu, S.; Zhu, W.; Guo, S. Effects of exogenous spermidine on the growth and quality of pakchoi seedlings under calcium nitrate stress. Acta Bot. Boreali-Occident. Sin. 2015, 49, 696–699. [Google Scholar]
- Han, F.U.; Shi, Q.Q.; Ming, Z.; Yu, L.; Biochemistry, D.O.; University, J.; Company, D.B. Effect of copper sulphate stress during germination on the growth of mung bean and the intracellular density change response. J. Plant Physiol. 2016, 52, 1891–1900. [Google Scholar]
- Tsoutsi, D.; Montenegro, J.M.; Dommershausen, F.; Koert, U.; Liz-Marzán, L.M.; Parak, W.J.; Alvarez-Puebla, R.A. Quantitative surface-enhanced Raman scattering ultradetection of atomic inorganic ions: The case of chloride. ACS Nano 2011, 5, 7539–7546. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.K.T.; Tran, H.T.T.; Bui, H.T.T.; Dang, T.Q.; Nguyen, L.Q. Determination of low level nitrate/nitrite contamination using SERS-active Ag/ITO substrates coupled to a self-designed Raman spectroscopy system. J. Sci. Adv. Mater. 2017, 2, 172–177. [Google Scholar]
- Ravindranath, S.P.; Kadam, U.S.; Thompson, D.K.; Irudayaraj, J. Intracellularly grown gold nanoislands as SERS substrates for monitoring chromate, sulfate and nitrate localization sites in remediating bacteria biofilms by Raman chemical imaging. Anal. Chim. Acta 2012, 745, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shakir, S.K.; Irfan, S.; Akhtar, B.; Rehman, S.U.; Daud, M.K.; Taimur, N.; Azizullah, A. Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, D.-W.; Pu, H.; Wei, Q. Surface enhanced Raman spectroscopy (SERS): A novel reliable technique for rapid detection of common harmful chemical residues. Trends Food Sci. Technol. 2018, 75, 10–22. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and adhesive surface enhance Raman scattering active tape for rapid detection of pesticide residues in fruits and vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor materials in analytical applications of surface-enhanced Raman scattering. J. Raman Spectrosc. 2016, 47, 51–58. [Google Scholar] [CrossRef]
- Xu, M.L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of pesticide residues in food using surface-enhanced Raman spectroscopy: A review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Wang, M.; Zhu, Y.; Wang, Y.; Ma, W. Synthesis of flexible and stable SERS substrate based on Au nanofilms/cicada wing array for rapid detection of pesticide residues. Opt. Commun. 2018, 425, 49–57. [Google Scholar] [CrossRef]
- Wijaya, W.; Pang, S.; Labuza, T.P.; He, L. Rapid detection of acetamiprid in foods using surface-enhanced Raman spectroscopy (SERS). J. Sci. Food Agric. 2014, 79, T743–T747. [Google Scholar]
- Weisman, D.; Alkio, M.; Colon-Carmona, A. Transcriptional responses to polycyclic aromatic hydrocarbon-induced stress in Arabidopsis thaliana reveal the involvement of hormone and defense signaling pathways. BMC Plant Biol. 2010, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Weisman, D.; Tang, L.; Tan, L.; Zhang, W.K.; Wang, Z.H.; Huang, Y.H.; Lin, W.X.; Liu, X.M.; Colon-Carmona, A. Stress signaling in response to polycyclic aromatic hydrocarbon exposure in Arabidopsis thaliana involves a nucleoside diphosphate kinase, NDPK-3. Planta 2015, 241, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Gen. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Barreira, L.A.; Mudge, S.M.; Bebianno, M.J. Oxidative stress in the Clam Ruditapes decussatus (linnaeus, 1758) in relation to polycyclic aromatic hydrocarbon body burden. Environ. Toxicol. 2007, 22, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, X.; Han, X.; Song, W.; Ruan, W.; Liu, J.; Zhao, B.; Ozaki, Y. Selective sers detection of each polycyclic aromatic hydrocarbon (PAH) in a mixture of five kinds of PAHs. J. Raman Spectrosc. 2011, 42, 945–950. [Google Scholar] [CrossRef]
- Du, J.; Xu, J.; Sun, Z.; Jing, C. Au nanoparticles grafted on Fe3O4 as effective sers substrates for label-free detection of the 16 EPA priority polycyclic aromatic hydrocarbons. Anal. Chim. Acta 2016, 915, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Tian, S.; Zhou, Q.; Adkins, J.; Gu, Z.; Li, X.; Zheng, J. SERS detection of polycyclic aromatic hydrocarbons on a bowl-shaped silver cavity substrate. RSC Adv. 2013, 3, 25989–25996. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Han, X.; Xue, X.; Ji, W.; Qi, Z.; Liu, J.; Zhao, B.; Ozaki, Y. Sensing of polycyclic aromatic hydrocarbons with cyclodextrin inclusion complexes on silver nanoparticles by surface-enhanced Raman scattering. Analyst 2010, 135, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-H.; Sowoidnich, K.; Schmidt, H.; Kronfeldt, H.-D. Application of calixarene to high active surface-enhanced Raman scattering (SERS) substrates suitable for in situ detection of polycyclic aromatic hydrocarbons (PAHs) in seawater. J. Raman Spectrosc. 2012, 43, 1003–1009. [Google Scholar] [CrossRef]
- Sheng, P.; Wu, S.; Bao, L.; Wang, X.; Chen, Z.; Cai, Q. Surface enhanced Raman scattering detecting polycyclic aromatic hydrocarbons with gold nanoparticle-modified TiO2 nanotube arrays. New J. Chem. 2012, 36, 2501–2505. [Google Scholar] [CrossRef]
- Xu, J.; Du, J.; Jing, C.; Zhang, Y.; Cui, J. Facile detection of polycyclic aromatic hydrocarbons by a surface-enhanced Raman scattering sensor based on the Au coffee ring effect. ACS Appl. Mater. Interfaces 2014, 6, 6891–6897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jin, J.; Tian, W.; Li, R.; Yu, Z.; Song, W.; Cong, Q.; Zhao, B.; Ozaki, Y. Three-dimensional superhydrophobic surface-enhanced Raman spectroscopy substrate for sensitive detection of pollutants in real environments. J. Mater. Chem. A 2015, 3, 4330–4337. [Google Scholar] [CrossRef]
- Wang, X.; Hao, W.; Zhang, H.; Pan, Y.; Kang, Y.; Zhang, X.; Zou, M.; Tong, P.; Du, Y. Analysis of polycyclic aromatic hydrocarbons in water with gold nanoparticles decorated hydrophobic porous polymer as surface-enhanced Raman spectroscopy substrate. Spectrochim. Acta A 2015, 139, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Erofeeva, E.A. Hormesis and paradoxical effects of pea (Pisum sativum L.) parameters upon exposure to formaldehyde in a wide range of doses. Ecotoxicology 2018, 27, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ji, Z.; Wei, C.; McHale, C.M.; Ding, S.; Thomas, R.; Yang, X.; Zhang, L. Inhaled formaldehyde induces DNA-protein crosslinks and oxidative stress in bone marrow and other distant organs of exposed mice. Environ. Mol. Mutagen. 2013, 54, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Musto, C.J.; Suslick, K.S. A simple and highly sensitive colorimetric detection method for gaseous formaldehyde. J. Am. Chem. Soc. 2010, 132, 4046–4047. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.S.; Sousa, E.T.; de Andrade, J.B. A sensitive flow analysis system for the fluorimetric determination of low levels of formaldehyde in alcoholic beverages. Talanta 2007, 73, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Peng, J.F.; Chi, Y.G.; Jiang, G.B. Determination of formaldehyde in shiitake mushroom by ionic liquid-based liquid-phase microextraction coupled with liquid chromatography. Talanta 2005, 65, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.G.; Lu, L.Q.; Lin, L.; Xu, A.W. A silver nanoparticle based surface enhanced resonance Raman scattering (SERS) probe for the ultrasensitive and selective detection of formaldehyde. Nanoscale 2012, 4, 7358–7361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, C.; Ma, Y.; Li, G. Rapid analysis of trace volatile formaldehyde in aquatic products by derivatization reaction-based surface enhanced Raman spectroscopy. Analyst 2014, 139, 3614–3621. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Liang, F.; Wang, D.; Yang, Q.; Ding, Y.; Yu, Y.; Gao, D.; Song, D.; Wang, X. Ultrasensitive determination of formaldehyde in environmental waters and food samples after derivatization and using silver nanoparticle assisted SERS. Microchim. Acta 2014, 182, 863–869. [Google Scholar] [CrossRef]
- Lv, Z.Y.; Mei, L.P.; Chen, W.Y.; Feng, J.J.; Chen, J.Y.; Wang, A.J. Shaped-controlled electrosynthesis of gold nanodendrites for highly selective and sensitive SERS detection of formaldehyde. Sens. Actuator B-Chem. 2014, 201, 92–99. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Sun, L.; Lian, J.J.; Gao, X.L.; Zhao, L.; Ding, M.Y.; Li, J.; Liang, Y.C. The phospholipase c (FgPLC1) is involved in regulation of development, pathogenicity, and stress responses in Fusarium graminearum. Fungal Genet. Biol. 2016, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, F.; Guo, H.; Zhu, Y.; Yuan, J.; Yang, G.; An, L. Molecular characterization of hepcidin gene in common carp (Cyprinus carpio L.) and its expression pattern responding to bacterial challenge. Fish Shellfish Immunol. 2013, 35, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Guo, H.; Li, H.; Shan, S.; Zhang, X.; Rombout, J.H.; An, L. Molecular characterization of LEAP-2 cDNA in common carp (Cyprinus carpio L.) and the differential expression upon a Vibrio anguillarum stimulus; indications for a significant immune role in skin. Fish Shellfish Immunol. 2014, 37, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.J.; Liu, D.Z.; Wang, L.; Zhu, Y.Y.; Zhang, F.M.; Li, T.; An, L.G.; Yang, G.W. Identification and expression analysis of irak1 gene in common carp Cyprinus carpio L.: Indications for a role of antibacterial and antiviral immunity. J. Fish Biol. 2015, 87, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, Y.H.; Liu, S.Z.; Zhang, L.; Li, B.T.; Zhao, X.X.; Fu, Y.; Liu, J.J.; Zhang, X.X. Pseudomonas reactans, a Bacterial strain isolated from the intestinal flora of Blattella germanica with anti-Beauveria bassiana activity. Environ. Entomol. 2013, 42, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, H.J.; He, H.B.; Wang, C.F.; Gao, Y.D.; Zhong, Q.F.; Wang, X.H.; Zeng, Y.J. Study on the hemolysin phenotype and the genetype distribution of staphyloccocus aureus caused bovine mastitis in shandong dairy farms. Int. J. Appl. Res. Vet. Med. 2011, 9, 416–421. [Google Scholar]
- Wang, J.; Wu, X.; Wang, C.; Shao, N.; Dong, P.; Xiao, R.; Wang, S. Magnetically assisted surface-enhanced Raman spectroscopy for the detection of Staphylococcus aureus based on aptamer recognition. ACS Appl. Mater. Interfaces 2015, 7, 20919–20929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, X.; Liu, Y.; Duan, N.; Wu, S.; Wang, Z.; Xu, B. Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens. Bioelectron. 2015, 74, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.W.; Tao, G.Q.; Wang, J. Assembly of bioconjugated rod-nanotags and multilayer plasmonic nanorod-array for ultrasensitive SERS detection of S. aureus bacteria. J. Nanopart. Res. 2018, 20, 1–10. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, Y.M.; Liao, P.H.; Chen, D.W.; Lin, H.P.; Chang, H.C. A filter-like AuNPs@MS SERS substrate for Staphylococcus aureus detection. Biosens. Bioelectron. 2014, 53, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.A.; Kaminska, A.; Adamkiewicz, W.; Witkowska, E.; Tkacz, M. Novel highly sensitive Cu-based SERS platforms for biosensing applications. J. Raman Spectrosc. 2015, 46, 428–433. [Google Scholar] [CrossRef]
- Duan, N.; Shen, M.F.; Wu, S.J.; Zhao, C.X.; Ma, X.Y.; Wang, Z.P. Graphene oxide wrapped Fe3O4@Au nanostructures as substrates for aptamer-based detection of Vibrio parahaemolyticus by surface-enhanced Raman spectroscopy. Microchim. Acta 2017, 184, 2653–2660. [Google Scholar] [CrossRef]
- Xu, J.J.; Turner, J.W.; Idso, M.; Biryukov, S.V.; Rognstad, L.; Gong, H.; Trainer, V.L.; Wells, M.L.; Strom, M.S.; Yu, Q.M. In situ strain-level detection and identification of Vibrio parahaemolyticus using surface-enhanced Raman spectroscopy. Anal. Chem. 2013, 85, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- He, L.L.; Deen, B.D.; Pagel, A.H.; Diez-Gonzalez, F.; Labuza, T.P. Concentration, detection and discrimination of Bacillus anthracis spores in orange juice using aptamer based surface enhanced Raman spectroscopy. Analyst 2013, 138, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Wang, H.S.; Lu, L.H.; Ai, K.L.; Zhang, G.; Cheng, X.L. Large-area silver-coated silicon nanowire arrays for molecular sensing using surface-enhanced Raman spectroscopy. Adv. Funct. Mater. 2008, 18, 2348–2355. [Google Scholar] [CrossRef]
- Farquharson, S.; Shende, C.; Smith, W.; Huang, H.; Inscore, F.; Sengupta, A.; Sperry, J.; Sickler, T.; Prugh, A.; Guicheteau, J. Selective detection of 1000 B. anthracis spores within 15 minutes using a peptide functionalized SERS assay. Analyst 2014, 139, 6366–6370. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.; Lee, W.W.Y.; Cowcher, D.P.; Goodacre, R.; Bell, S.E.J. SERS of meso-droplets supported on superhydrophobic wires allows exquisitely sensitive detection of dipicolinic acid, an anthrax biomarker, considerably below the infective close. Chem. Commun. 2016, 52, 9925–9928. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.R.; Zeng, Y.; Zhou, X.D.; Wang, X.H.; Shen, A.G.; Hu, J.M. Environmentally safe mercury(II) ions aided zero-background and ultrasensitive SERS detection of dipicolinic acid. Anal. Chem. 2017, 89, 10335–10342. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhao, G.; He, C.; Wang, H.; He, H. Biopanning of polypeptides binding to bovine ephemeral fever virus G1 protein from phage display peptide library. BMC Vet. Res. 2018, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Wang, H.; Zhao, G.; He, C.; He, H. Rapid detection of infectious bovine Rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet. Res. 2017, 13, 386. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.W.; Tian, F.L.; Lan, Z.R.; Huang, B.; Zhuang, W.Z. Selection characterization on overlapping reading frame of multiple-protein-encoding P gene in Newcastle disease virus. Vet. Microbiol. 2010, 144, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, J.Q.; Zhang, Y.Y.; Guo, L.H.; Cong, X.Y.; Du, Y.J.; Li, J.; Sun, W.B.; Shi, J.L.; Peng, J.; et al. Concurrent highly pathogenic porcine reproductive and respiratory syndrome virus infection accelerates Haemophilus parasuis infection in conventional pigs. Vet. Microbiol. 2012, 158, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Qi, C.; Zhu, Y.; Li, H.; An, L.; Yang, G. Expression profile of carp IFN correlate with the up-regulation of interferon regulatory factor-1 (IRF-1) in vivo and in vitro: The pivotal molecules in antiviral defense. Fish Shellfish Immunol. 2016, 52, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qi, C.; Shan, S.; Zhang, F.; Li, H.; An, L.; Yang, G. Characterization of common carp (Cyprinus carpio L.) interferon regulatory factor 5 (IRF5) and its expression in response to viral and bacterial challenges. BMC Vet. Res. 2016, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, H.; Peng, S.; Zhang, F.; An, L.; Yang, G. Molecular characterization and expression pattern of X box-binding protein-1 (XBP1) in common carp (Cyprinus carpio L.): Indications for a role of XBP1 in antibacterial and antiviral immunity. Fish Shellfish Immunol. 2017, 67, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wu, X.; Zhang, L.; Xin, C.; Liu, Y.; Shi, J.; Peng, Z.; Xu, S.; Fu, F.; Yu, J.; et al. The occurrence of porcine circovirus 3 without clinical infection signs in shandong province. Transbound. Emerg. Dis. 2017, 64, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Liao, J.D.; You, J.W.; Wu, C.L. Focused-ion-beam-fabricated Au/Ag multilayered nanorod array as SERS-active substrate for virus strain detection. Sens. Actuator B-Chem. 2013, 181, 361–367. [Google Scholar] [CrossRef]
- Lim, J.Y.; Nam, J.S.; Yang, S.E.; Shin, H.; Jang, Y.H.; Bae, G.U.; Kang, T.; Lim, K.I.; Choi, Y. Identification of newly emerging influenza viruses by surface-enhanced Raman spectroscopy. Anal. Chem. 2015, 87, 11652–11659. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, L.; Zhang, F.D.; Song, Z.G.; Hu, Y.W.; Ji, Y.J.; Shen, J.Y.; Li, B.; Lu, H.Z.; Yang, H.F. A promising magnetic SERS immunosensor for sensitive detection of avian influenza virus. Biosens. Bioelectron. 2017, 89, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.H.; Li, W.T.; Lu, D.L.; Chen, K.; He, Q.G.; Han, H.Y.; Zou, M.Q. A SERS-based immunoassay for porcine circovirus type 2 using multi-branched gold nanoparticles. Microchim. Acta 2013, 180, 1501–1507. [Google Scholar] [CrossRef]
- Serra, A.; Manno, D.; Filippo, E.; Buccolieri, A.; Urso, E.; Rizzello, A.; Maffia, M. SERS based optical sensor to detect prion protein in neurodegenerate living cells. Sens. Actuator B-Chem. 2011, 156, 479–485. [Google Scholar] [CrossRef]
- Ashmore, M.; Toet, S.; Emberson, L. Ozone-a significant threat to future world food production? New Phytol. 2010, 170, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Pina, J.M.; Moraes, R.M. Gas exchange, antioxidants and foliar injuries in saplings of a tropical woody species exposed to ozone. Ecotoxicol. Environ. Saf. 2010, 73, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Tausz, M.; Grulke, N.E.; Wieser, G. Defense and avoidance of ozone under global change. Environ. Pollut. 2007, 147, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, C.; Liu, Q.; Liang, A. An ultrasensitive SERS method for the determination of ozone using a nanogold sol as substrate and rhodamines as probe. RSC Adv. 2014, 4, 959–962. [Google Scholar] [CrossRef]
- Wu, B.; Shao, H.; Wang, Z.; Hu, Y.; Tang, Y.J.; Jun, Y.S. Viability and metal reduction of shewanella oneidensis MR-1 under CO2 stress: Implications for ecological effects of CO2 leakage from geologic CO2 sequestration. Environ. Sci. Technol. 2010, 44, 9213–9218. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, I.; Kurano, N.; Miyachi, S. Effects of high-CO2 stress on photosystem II in a green alga, chlorococcum littorale, which has a tolerance to high CO2. J. Photochem. Photobiol. B 1996, 36, 327–332. [Google Scholar] [CrossRef]
- Lust, M.; Pucci, A.; Akemann, W.; Otto, A. SERS of CO2 on cold-deposited Cu: An electronic effect at a minority of surface sites. J. Phys. Chem. C 2008, 112, 11075–11077. [Google Scholar] [CrossRef]
- Bontempi, N.; Carletti, L.; De Angelis, C.; Alessandri, I. Plasmon-free SERS detection of environmental CO2 on TiO2 surfaces. Nanoscale 2016, 8, 3226–3231. [Google Scholar] [CrossRef] [PubMed]
- Jun-Cheol, M.; Woncheol, Y.; Sungdon, L.; Song, K.T.; Byung-Moo, L. Differentially expressed genes and in silico analysis in response to ozone (O3) stress of soybean leaves. Aust. J. Sci. 2014, 8, 276–283. [Google Scholar]
- Li, K.; Pang, C.H.; Ding, F.; Sui, N.; Feng, Z.T.; Wang, B.S. Overexpression of Suaeda salsa stroma ascorbate peroxidase in Arabidopsis chloroplasts enhances salt tolerance of plants. S. Afr. J. Bot. 2012, 78, 235–245. [Google Scholar] [CrossRef]
- Chen, Q.F.; Rao, Y.Y.; Ma, X.Y.; Dong, J.A.; Qian, W.P. Raman spectroscopy for hydrogen peroxide scavenging activity assay using gold nanoshell precursor nanocomposites as SERS probes. Anal. Methods 2011, 3, 274–279. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, J.H.; Lin, Y.G.; Hsu, Y.K. Silver nanowires on coffee filter as dual-sensing functionality for efficient and low-cost SERS substrate and electrochemical detection. Sens. Actuator B-Chem. 2017, 245, 189–195. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, J.H.; Hsu, Y.K. Branched silver nanowires on fluorine-doped tin oxide glass for simultaneous amperometric detection of H2O2 and of 4-aminothiophenol by SERS. Microchim. Acta 2018, 185, 106. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Li, X.; Kang, S.-Z.; Mu, J. Gold nanoparticles conjugated dopamine as sensing platform for SERS detection. Colloids Surf. B. Biointerfaces 2015, 126, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Guo, G.M.; Xie, W.; Li, Y.; Zhang, M.Y.; Qian, W.P. Free radical-quenched SERS probes for detecting H2O2 and glucose. Analyst 2015, 140, 2741–2746. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.H.; Li, K.; Wang, B. Overexpression of SsCHLAPXs confers protection against oxidative stress induced by high light in transgenic Arabidopsis thaliana. Physiol. Plant. 2011, 143, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.T.; Deng, Y.Q.; Fan, H.; Sun, Q.J.; Sui, N.; Wang, B.S. Effects of NaCl stress on the growth and photosynthetic characteristics of Ulmus pumila L. seedlings in sand culture. Photosynthetica 2014, 52, 313–320. [Google Scholar] [CrossRef]
- Han, N.; Lan, W.; He, X.; Shao, Q.; Wang, B.; Zhao, X. Expression of a Suaeda salsa vacuolar H+/Ca2+ transporter gene in Arabidopsis contributes to physiological changes in salinity. Plant Mol. Biol. Rep. 2011, 30, 470–477. [Google Scholar] [CrossRef]
- Sun, W.; Li, Y.; Zhao, Y.; Zhang, H. The TsnsLTP4, a nonspecific lipid transfer protein involved in wax deposition and stress tolerance. Plant Mol. Biol. Rep. 2014, 33, 962–974. [Google Scholar] [CrossRef]
- Sun, G.J.; Pan, J.; Liu, K.C.; Wang, S.F.; Wang, X.; Wang, X.M. Molecular cloning and expression analysis of P-selectin glycoprotein ligand-1 from zebrafish (Danio rerio). Fish Physiol. Biochem. 2012, 38, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Li, M.; Li, K.; Song, J.; Wang, B.S. Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. Enhances protection of photosystem ii under high salinity. Photosynthetica 2010, 48, 623–629. [Google Scholar] [CrossRef]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. Int. Rev. Hydrobiol. 2016, 101, 3–19. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, W.H.; Lv, Z.W.; Zhang, S.L.; Hidema, J.; Shi, F.M.; Liu, L.L. Abscisic acid is involved in the response of Arabidopsis mutant sad2-1 to ultraviolet-B radiation by enhancing antioxidant enzymes. S. Afr. J. Bot. 2013, 85, 79–86. [Google Scholar] [CrossRef]
- Cao, S.; Du, X.H.; Li, L.H.; Liu, Y.D.; Zhang, L.; Pan, X.; Li, Y.; Li, H.; Lu, H. Overexpression of populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Russ. J. Plant Physiol. 2017, 64, 224–234. [Google Scholar] [CrossRef]
- Qi, Y.-C.; Wang, F.-F.; Zhang, H.; Liu, W.-Q. Overexpression of Suadea salsa S-adenosylmethionine synthetase gene promotes salt tolerance in transgenic tobacco. Acta Physiol. Plant 2009, 32, 263–269. [Google Scholar] [CrossRef]
- Qi, Y.C.; Liu, W.Q.; Qiu, L.Y.; Zhang, S.M.; Ma, L.; Zhang, H. Overexpression of glutathione S-transferase gene increases salt tolerance of arabidopsis. Russ. J. Plant Physiol. 2010, 57, 233–240. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Wang, H.; Feng, G. Effect of salinity on seed germination, ion content and photosynthesis of cotyledons in halophytes or xerophyte growing in central Asia. J. Plant Ecol. 2010, 3, 259–267. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Liu, C.; Qiu, L.; Li, Z. A general and versatile fluorescence turn-on assay for detecting the activity of protein tyrosine kinases based on phosphorylation-inhibited tyrosyl oxidation. Chem. Commun. 2016, 52, 12570–12573. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Huang, B.; Lin, R.; Xu, P.; Liu, Y.; Zhao, Y. A “turn-off” SERS assay for kinase detection based on arginine N-phosphorylation process. Talanta 2018, 189, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Bjerneld, E.J.; Földes-Papp, Z.; Käll, M.A.; Rigler, R. Single-molecule surface-enhanced Raman and fluorescence correlation spectroscopy of horseradish peroxidase. J. Phys. Chem. B 2010, 106, 1213–1218. [Google Scholar] [CrossRef]
- Kahraman, M.; Sur, I.; Culha, M. Label-free detection of proteins from self-assembled protein-silver nanoparticle structures using surface-enhanced Raman scattering. Anal. Chem. 2010, 82, 7596–7602. [Google Scholar] [CrossRef] [PubMed]
- Giorgis, F.; Descrovi, E.; Chiodoni, A.; Froner, E.; Scarpa, M.; Venturello, A.; Geobaldo, F. Porous silicon as efficient surface enhanced Raman scattering (SERS) substrate. Appl. Surf. Sci. 2008, 254, 7494–7497. [Google Scholar] [CrossRef]
- Cottat, M.; D’Andrea, C.; Yasukuni, R.; Malashikhina, N.; Grinyte, R.; Lidgi-Guigui, N.; Fazio, B.; Sutton, A.; Oudar, O.; Charnaux, N.; et al. High sensitivity, high selectivity SERS detection of MnSOD using optical nanoantennas functionalized with aptamers. J. Phys. Chem. C 2015, 119, 15532–15540. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Chen, M.; Song, Y.P.; Song, J.; Wang, B.S.; Feng, G. Relationships between ion and chlorophyll accumulation in seeds and adaptation to saline environments in Suaeda salsa populations. Plant Biosyst. 2012, 146, 142–149. [Google Scholar] [CrossRef]
- Lian, W.N.; Shiue, J.; Wang, H.H.; Hong, W.C.; Shih, P.H.; Hsu, C.K.; Huang, C.Y.; Hsing, C.R.; Wei, C.M.; Wang, J.K.; et al. Rapid detection of copper chlorophyll in vegetable oils based on surface-enhanced Raman spectroscopy. Food Addit. Contam. A 2015, 32, 627–634. [Google Scholar]
- Aguilar-Hernández, I.; Afseth, N.K.; López-Luke, T.; Contreras-Torres, F.F.; Wold, J.P.; Ornelas-Soto, N. Surface enhanced Raman spectroscopy of phenolic antioxidants: A systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vib. Spectrosc. 2017, 89, 113–122. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Domingo, C.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12802–12811. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chen, W. A SERS method with attomolar sensitivity: A case study with the flavonoid catechin. Microchim. Acta 2018, 185, 120. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, Y.; Gao, J.; Ma, C.; Bi, Y. A comparative transcriptome analysis of a wild purple potato and its red mutant provides insight into the mechanism of anthocyanin transformation. PLoS ONE 2018, 13, e0191406. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, T.; Li, W.; Tang, W.; Zhang, D.; Dong, H. Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 2016, 38, 61. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Wei, X.; Zhao, X.; Wang, B.; Sui, N. Transcription profiles of genes related to hormonal regulations under salt stress in sweet sorghum. Plant Mol. Biol. Rep. 2017, 35, 586–599. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Fan, S. Meta-analysis of salt-related gene expression profiles identifies common signatures of salt stress responses in arabidopsis. Plant Syst. Evol. 2017, 303, 757–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Dai, J.; Luo, Z.; Li, Z.; Lu, H.; Xu, S.; Tang, W.; Zhang, D.; Li, W.; et al. Global gene expression in cotton (Gossypium hirsutum L.) leaves to waterlogging stress. PLoS ONE 2017, 12, e0185075. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Cui, F.; Hou, L.; Zhao, S.; Xia, H.; Qiu, J.; Li, T.; Zhang, Y.; Wang, X.; et al. Genome-wide analysis of gene expression provides new insights into cold responses in Thellungiella salsuginea. Front. Plant Sci. 2017, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Lyu, M.J.; Leng, B.Y.; Zhu, X.G.; Wang, B.S. The transcriptome of NaCl-treated Limonium bicolor leaves reveals the genes controlling salt secretion of salt gland. Plant Mol. Biol. 2016, 91, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, J.; Zhao, W.; Xu, H.; Wang, F.; Xu, Y.; Wang, L.; Tian, C. Effects of salinity and nitrate on production and germination of dimorphic seeds applied both through the mother plant and exogenously during germination in Suaeda salsa. Plant Spec. Biol. 2016, 31, 19–28. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.G.; Wang, S.; Shi, W.; Liu, R.; Feng, G.; Song, J. Salinity affects production and salt tolerance of dimorphic seeds of Suaeda salsa. Plant Physiol. Biochem. 2015, 95, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Dai, A.; Wei, H.; Yang, S.; Wang, B.; Jiang, N.; Feng, X. Arabidopsis KLU homologue GmCYP78A72 regulates seed size in soybean. Plant Mol. Biol. 2016, 90, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ji, S.; Fang, X.; Wang, Q.; Li, Z.; Yao, F.; Hou, L.; Dai, S. Protein kinase LTRPK1 influences cold adaptation and microtubule stability in rice. J. Plant Growth Regul. 2013, 32, 483–490. [Google Scholar] [CrossRef]
- Novak, O.; Napier, R.; Ljung, K. Zooming in on plant hormone analysis: Tissue- and cell-specific approaches. Annu. Rev. Plant Biol. 2017, 68, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Q.; Zhang, F.; Wang, Y.; Zhang, S.; Cheng, H.; Yan, L.; Li, L.; Chen, F.; Xie, X. Overexpression of OsPIL15, a phytochrome-interacting factor-like protein gene, represses etiolated seedling growth in rice. J. Integr. Plant Biol. 2014, 56, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.G.; Liu, R.; Sui, N.; Shi, W.; Wang, L.; Tian, C.; Song, J. Changes in endogenous hormones and seed-coat phenolics during seed storage of two Suaeda salsa populations. Aust. J. Bot. 2016, 64, 325–332. [Google Scholar] [CrossRef]
- Meng, X.; Yang, D.; Li, X.; Zhao, S.; Sui, N.; Meng, Q. Physiological changes in fruit ripening caused by overexpression of tomato SLAN2, an R2R3-MYB factor. Plant Physiol. Biochem. 2015, 89, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.X.; He, J.; Chen, J.L.; Yang, S.J.; Zha, D.S. Alleviation of exogenous 6-benzyladenine on two genotypes of eggplant (Solanum melongena Mill.) growth under salt stress. Protoplasma 2014, 251, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, L.M.; Zheng, D.W.; Lin, T.F.; Wei, X.D.; Liu, X.Y.; Wang, H.Q. Surface-enhanced Raman spectroscopic analysis of N6-benzylaminopurine residue quantity in sprouts with gold nanoparticles. J. Environ. Sci. Health B 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
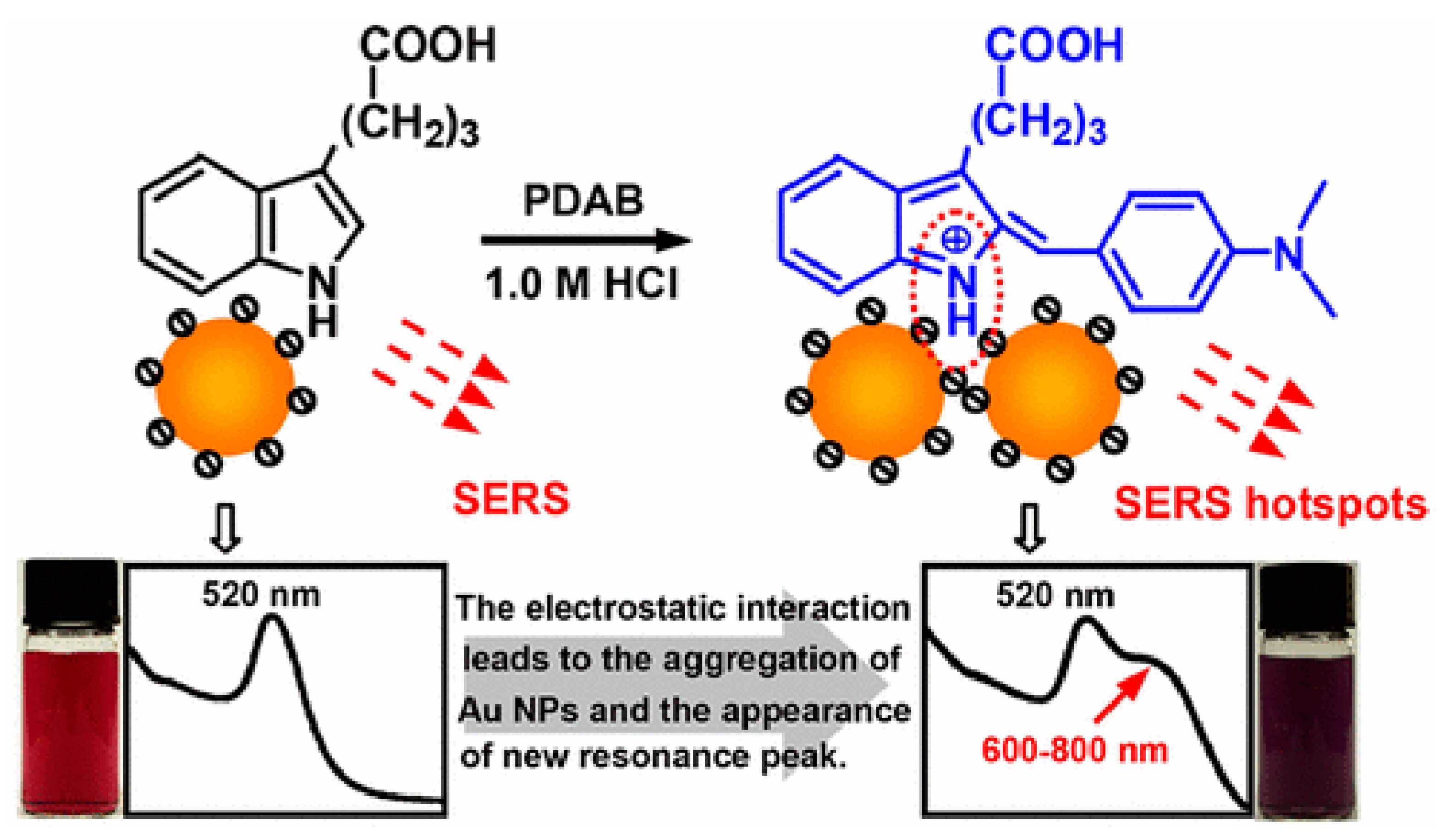
- Wang, F.; Gu, X.; Zheng, C.; Dong, F.; Zhang, L.; Cai, Y.; You, Z.; You, J.; Du, S.; Zhang, Z. Ehrlich reaction evoked multiple spectral resonances and gold nanoparticle hotspots for raman detection of plant hormone. Anal. Chem. 2017, 89, 8836–8843. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Zhang, Z.; Liu, M.; Chen, Q.; Hua, Y.; Chen, X. In situ fabrication of label-free optical sensing paper strips for the rapid surface-enhanced Raman scattering (SERS) detection of brassinosteroids in plant tissues. Talanta 2017, 165, 313–320. [Google Scholar]
- Chen, T.; Yuan, F.; Song, J.; Wang, B. Nitric oxide participates in waterlogging tolerance through enhanced adventitious root formation in the euhalophyte Suaeda salsa. Funct. Plant Biol. 2016, 43, 244. [Google Scholar] [CrossRef]
- Cui, J.; Hu, K.; Sun, J.J.; Qu, L.L.; Li, D.W. SERS nanoprobes for the monitoring of endogenous nitric oxide in living cells. Biosens. Bioelectron. 2016, 85, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, W.; Li, L.; Zhou, F.; Zhou, J.; Tian, Y. Ratiometric SERS imaging and selective biosensing of nitric oxide in live cells based on trisoctahedral gold nanostructures. Chem. Commun. 2017, 53, 1880–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Han, X.; Wang, Z.; Yang, Z.; Zhang, W.; Li, J.; Yang, H.; Ling, X.Y.; Xing, B. A live bacteria SERS platform for the in situ monitoring of nitric oxide release from a single mrsa. Chem. Commun. 2018, 54, 7022–7025. [Google Scholar] [CrossRef] [PubMed]
- Bobba, K.N.; Saranya, G.; Alex, S.M.; Velusamy, N.; Maiti, K.K.; Bhuniya, S. SERS-active multi-channel fluorescent probe for NO: Guide to discriminate intracellular biothiols. Sens. Actuator B-Chem. 2018, 260, 165–173. [Google Scholar] [CrossRef]
- Sui, N.; Han, G. Salt-induced photoinhibition of PSII is alleviated in halophyte Thellungiella halophila by increases of unsaturated fatty acids in membrane lipids. Acta Physiol. Plant. 2014, 36, 983–992. [Google Scholar] [CrossRef]
- Tang, G.Y.; Wei, L.Q.; Liu, Z.J.; Bi, Y.P.; Shan, L. Ectopic expression of peanut acyl carrier protein in tobacco alters fatty acid composition in the leaf and resistance to cold stress. Biol. Plant. 2012, 56, 493–501. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, W.; Song, J.; Wang, D.; Chen, M.; Wang, B. Thellungilla halophila is more adaptive to salinity than Arabidopsis thaliana at stages of seed germination and seedling establishment. Acta Physiol. Plant. 2012, 34, 1287–1294. [Google Scholar] [CrossRef]
- Ding, F.; Chen, M.; Sui, N.; Wang, B.S. Ca2+ significantly enhanced development and salt-secretion rate of salt glands of Limonium bicolor under NaCl treatment. S. Afr. J. Bot. 2010, 76, 95–101. [Google Scholar] [CrossRef]
- Sun, Y.L.; Li, F.; Su, N.; Sun, X.L.; Zhao, S.J.; Meng, Q.W. The increase in unsaturation of fatty acids of phosphatidylglycerol in thylakoid membrane enhanced salt tolerance in tomato. Photosynthetica 2010, 48, 400–408. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, Y.; Zhang, B.; Wang, X. Rapid prediction of fatty acid composition of vegetable oil by Raman spectroscopy coupled with least squares support vector machines. J. Raman Spectrosc. 2013, 44, 1739–1745. [Google Scholar] [CrossRef]
- He, X.N.; Gao, Y.; Mahjouri-Samani, M.; Black, P.N.; Allen, J.; Mitchell, M.; Xiong, W.; Zhou, Y.S.; Jiang, L.; Lu, Y.F. Surface-enhanced Raman spectroscopy using gold-coated horizontally aligned carbon nanotubes. Nanotechnology 2012, 23, 205702. [Google Scholar] [CrossRef] [PubMed]
- Šimáková, P.; Kočišová, E.; Procházka, M. Sensitive Raman spectroscopy of lipids based on drop deposition using DCDR and SERS. J. Raman Spectrosc. 2013, 44, 1479–1482. [Google Scholar] [CrossRef]
- Sweetenham, C.S.; Larraona-Puy, M.; Notingher, I. Simultaneous surface-enhanced Raman spectroscopy (SERS) and atomic force microscopy (AFM) for label-free physicochemical analysis of lipid bilayers. Appl. Spectrosc. 2011, 65, 1387–1392. [Google Scholar] [CrossRef]
- Kong, L.; Xie, Y.; Hu, L.; Si, J.; Wang, Z. Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Sci. Rep. 2017, 7, 43363. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Cui, L.; Xie, L.; Zheng, C.; Zhou, G.; Zhou, J.; Xie, X. Phytochrome B negatively affects cold tolerance by regulating OsDREB1 gene expression through phytochrome interacting factor-like protein OsPIL16 in rice. Front Plant Sci. 2016, 7, 1963. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.C.; Fu, T.T.; Sui, N.; Guo, J.R.; Feng, G.; Fan, J.L.; Song, J. The role of salinity in seed maturation of the euhalophyte Suaeda salsa. Plant Biosyst. 2014, 150, 83–90. [Google Scholar] [CrossRef]
- Zhang, D.; Haputhanthri, R.; Ansar, S.M.; Vangala, K.; De Silva, H.I.; Sygula, A.; Saebo, S.; Pittman, C.U., Jr. Ultrasensitive detection of malondialdehyde with surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2010, 398, 3193–3201. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, F.; Zhou, J.J.; Chen, F.; Wang, B.S.; Xie, X.Z. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol. Biol. 2012, 78, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Han, G.L.; Wang, M.J.; Yuan, F.; Sui, N.; Song, J.; Wang, B.S. The CCCH zinc finger protein gene ATZFP1 improves salt resistance in Arabidopsis thaliana. Plant Mol. Biol. 2014, 86, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.R.; Suo, S.S.; Wang, B.S. Sodium chloride improves seed vigour of the euhalophyte Suaeda salsa. Seed Sci. Res. 2015, 25, 335–344. [Google Scholar] [CrossRef]
- Sun, Z.B.; Qi, X.Y.; Wang, Z.L.; Li, P.H.; Wu, C.X.; Zhang, H.; Zhao, Y.X. Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiol. Biochem. 2013, 69, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Mary, Y.S.; Ushakumari, L.; Harikumar, B.; Varghese, H.T.; Panicker, C.Y. FT-IR, FT-raman and SERS spectra of L-proline. J. Iran. Chem. Soc. 2009, 6, 138–144. [Google Scholar] [CrossRef]
- Carcamo, J.J.; Aliaga, A.E.; Clavijo, E.; Garrido, C.; Gomez-Jeria, J.S.; Campos-Vallette, M.M. Proline and hydroxyproline deposited on silver nanoparticles. A Raman, SERS and theoretical study. J. Raman Spectrosc. 2012, 43, 750–755. [Google Scholar] [CrossRef]
- Kong, K.V.; Lam, Z.; Lau, W.K.; Leong, W.K.; Olivo, M. A transition metal carbonyl probe for use in a highly specific and sensitive SERS-based assay for glucose. J. Am. Chem. Soc. 2013, 135, 18028–18031. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Qi, G.H.; Xu, S.P.; Xu, W.Q. Construction of highly sensitive surface-enhanced raman scattering (SERS) nanosensor aimed for the testing of glucose in urine. RSC Adv. 2016, 6, 53800–53803. [Google Scholar] [CrossRef]
- Sun, F.; Bai, T.; Zhang, L.; Ellamenye, J.R.; Liu, S.; Nowinski, A.K.; Jiang, S.; Yu, Q. Sensitive and fast detection of fructose in complex media via symmetry breaking and signal amplification using surface-enhanced Raman spectroscopy. Anal. Chem. 2014, 86, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.H.; Jia, K.Q.; Fu, C.C.; Xu, S.P.; Xu, W.Q. A highly sensitive SERS sensor for quantitative analysis of glucose based on the chemical etching of silver nanoparticles. J. Opt. 2015, 17. [Google Scholar] [CrossRef]
- Gu, X.; Wang, H.; Schultz, Z.D.; Camden, J.P. Sensing glucose in urine and serum and hydrogen peroxide in living cells by use of a novel boronate nanoprobe based on surface-enhanced Raman spectroscopy. Anal. Chem. 2016, 88, 7191–7197. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jin, S.; Oh, J.; Xu, S.P.; Jung, Y.M. Facile detection of glucose in human serum employing silver-ion-guided surface-enhanced Raman spectroscopy signal amplification. Analyst 2017, 142, 2887–2891. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Huang, J.; Ge, M.; Iocozzia, J.; Lin, Z.; Zhang, K.Q.; Lai, Y. Immobilization of Pt nanoparticles via rapid and reusable electropolymerization of dopamine on TiO2 nanotube arrays for reversible SERS substrates and nonenzymatic glucose sensors. Small 2017, 13, 1604240. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Srinivasan, S.; J McGoron, A. AgNPs-based label-free colloidal SERS nanosensor for the rapid and sensitive detection of stress-proteins expressed in response to environmental-toxins. J. Biosens. Bioelectron. 2013, S12, 005. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Q. A SERS study of oxidation of glutathione under plasma irradiation. RSC Adv. 2015, 5, 57847–57852. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Yu, C.; Tang, L.; Lu, L. Applications of SERS in the Detection of Stress-Related Substances. Nanomaterials 2018, 8, 757. https://doi.org/10.3390/nano8100757
Du S, Yu C, Tang L, Lu L. Applications of SERS in the Detection of Stress-Related Substances. Nanomaterials. 2018; 8(10):757. https://doi.org/10.3390/nano8100757
Chicago/Turabian StyleDu, Shuyuan, Chundi Yu, Lin Tang, and Lixia Lu. 2018. "Applications of SERS in the Detection of Stress-Related Substances" Nanomaterials 8, no. 10: 757. https://doi.org/10.3390/nano8100757
APA StyleDu, S., Yu, C., Tang, L., & Lu, L. (2018). Applications of SERS in the Detection of Stress-Related Substances. Nanomaterials, 8(10), 757. https://doi.org/10.3390/nano8100757



