Research Progress of NiMn Layered Double Hydroxides for Supercapacitors: A Review
Abstract
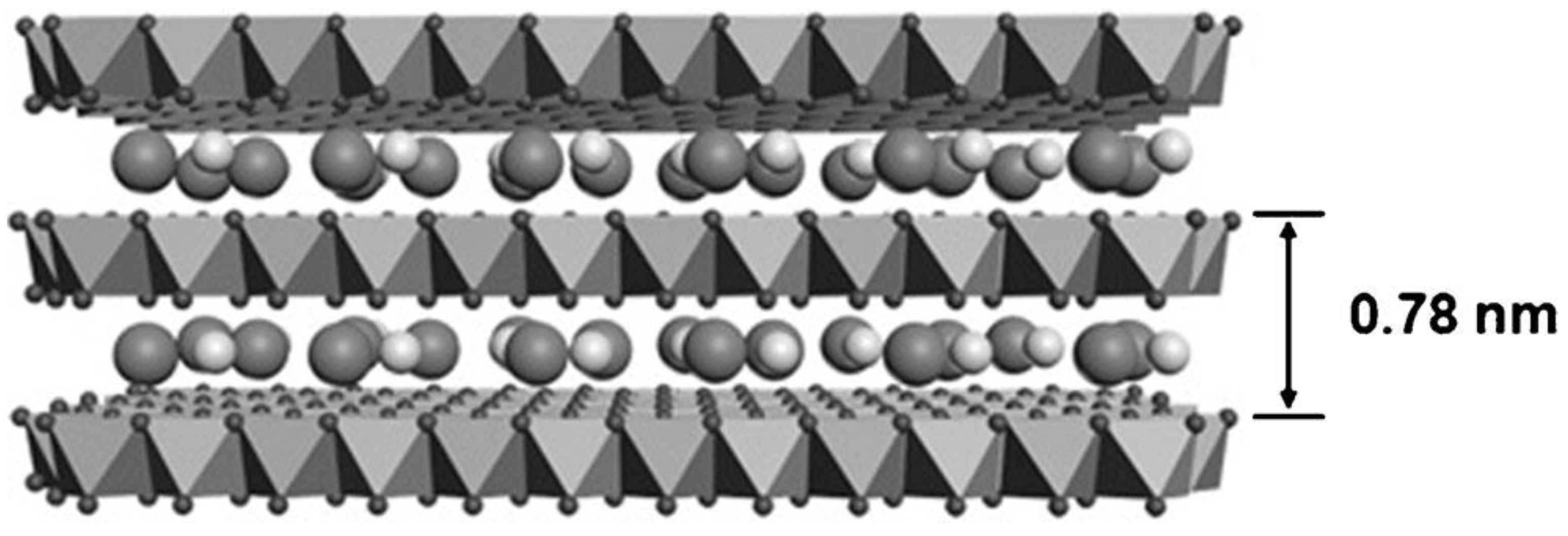
1. Introduction
2. The Synthesis Method of NiMn LDHs
3. Electrochemical Performances of NiMn LDH-Based Electrode Materials
3.1. Energy Storage Mechanism for SCs
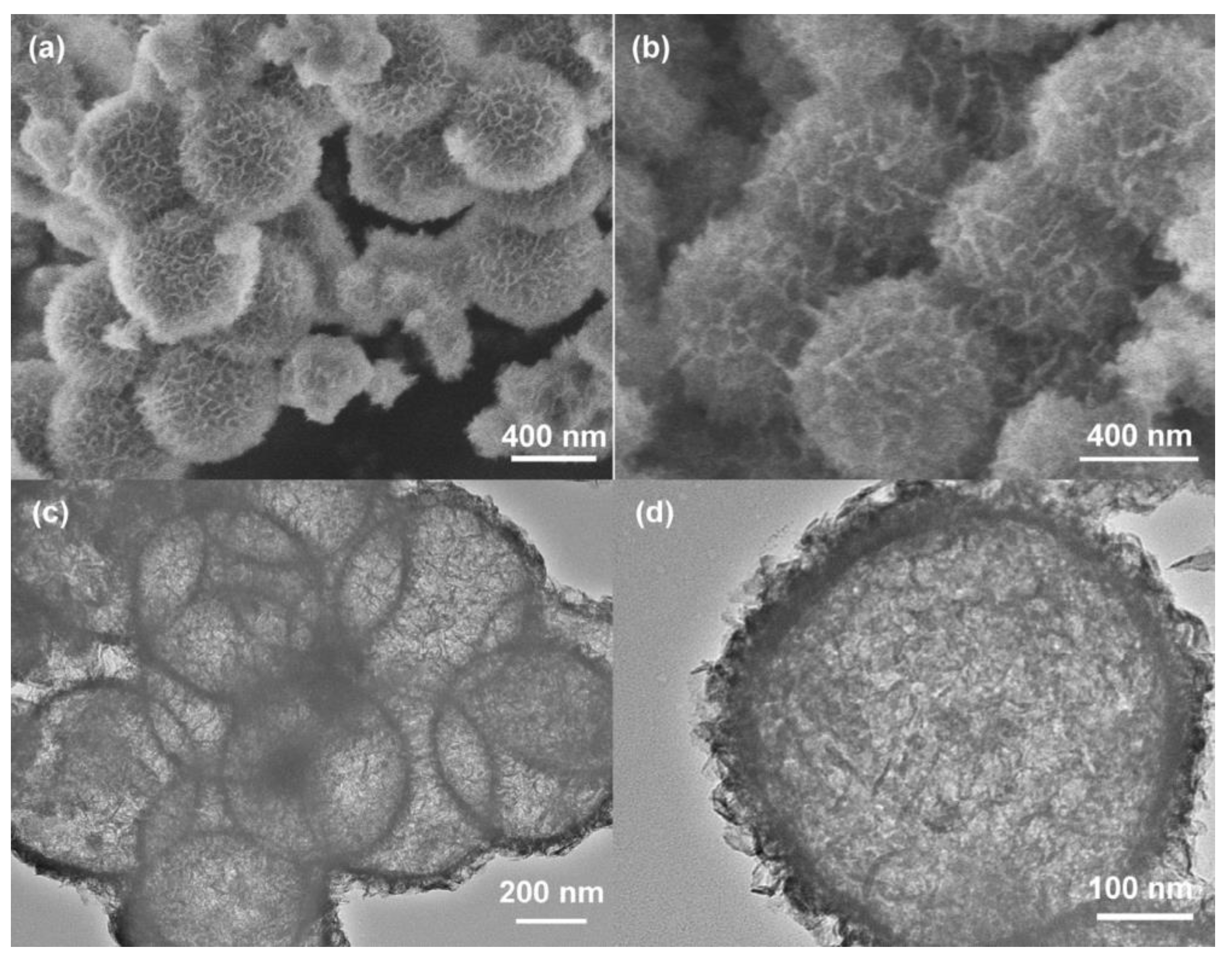
3.2. Designing and Chemical Modification of NiMn LDHs
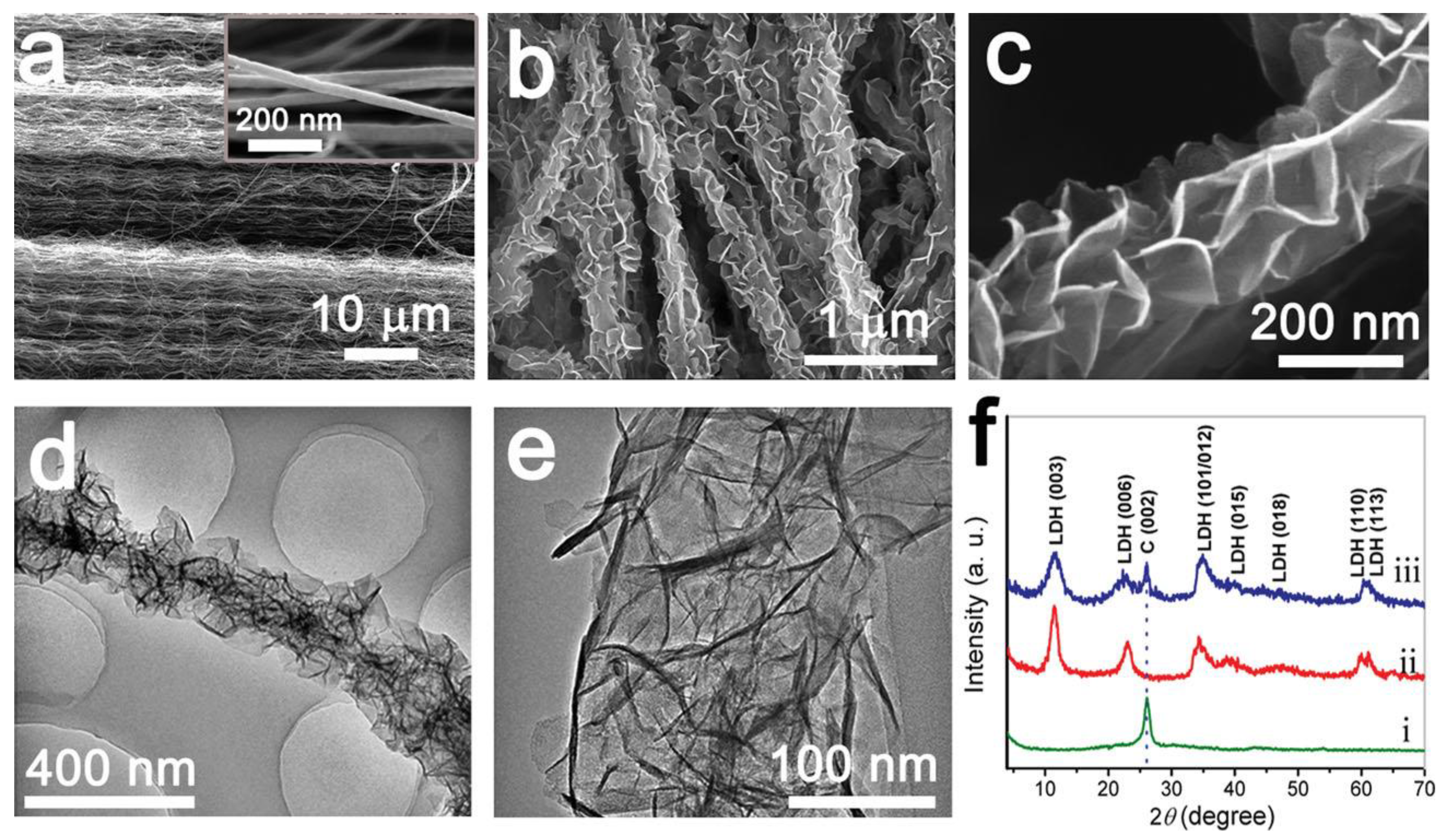
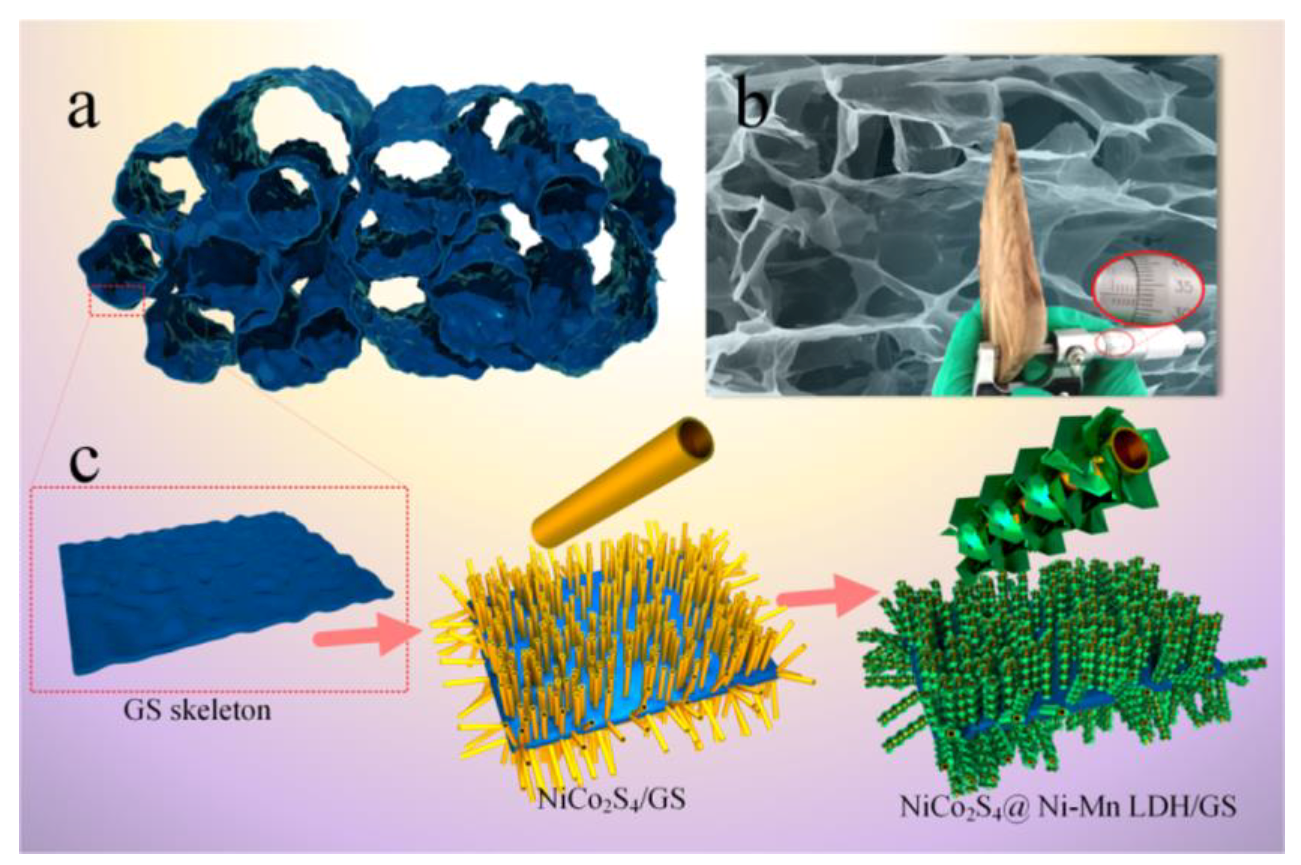
3.3. NiMn LDHs Composites
3.4. NiMn LDHs Film Electrodes
3.5. Chemical Modification of NiMn LDHs Composites
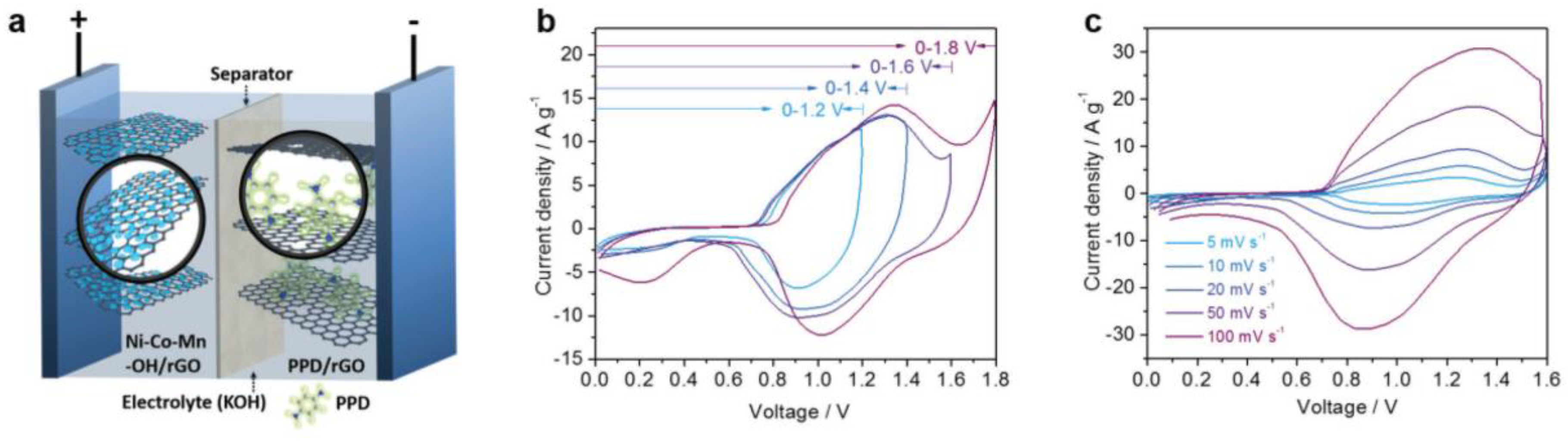
3.6. NiMn LDH Materials for Asymmetric Capacitor
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Piao, H.Y.; Kim, M.H.; Choy, J.H. Enabling nanohybrid drug discovery through the soft chemistry telescope. Ind. Eng. Chem. Res. 2016, 55, 11211–11224. [Google Scholar] [CrossRef]
- Gu, Z.; Atherton, J.J.; Xu, Z.P. Hierarchical layered double hydroxide nanocomposites: Structure, synthesis and applications. Chem. Commun. 2015, 51, 3024–3036. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.F.; Zeng, H.Y.; Huang, Q.J.; Zhang, W.; Du, J.Z.; Duan, H.Z.; Chen, C.R. Facile preparation of modifying layered double hydroxide nanoparticles for drug delivery. J. Nanosci. Nanotechnol. 2018, 18, 5256–5265. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, M.; Rouster, D.P.; Oncsik, T.; Szilagyi, D.I. Tuning colloidal stability of layered double hydroxides: From monovalent ions to polyelectrolytes. Chem. Plus Chem. 2017, 82, 121–131. [Google Scholar] [CrossRef]
- Conterosito, E.; Gianotti, V.; Palin, L.; Boccaleri, E.; Viterbo, D.; Milanesio, M. Facile preparation methods of hydrotalcite layered materials and their structural characterization by combined techniques. Inog. Chim. Acta 2018, 470, 36–50. [Google Scholar] [CrossRef]
- Ay, A.N.; Zumroglu-Karan, B.; Mafra, L. A simple mechanochemical route to layered double hydroxides: Synthesis of hydrotalcite-like Mg-Al-NO3-LDH by manual grinding in a mortar. Zeitschrift für Anorganische und Allgemeine Chemie ZAAC 2009, 635, 1470–1475. [Google Scholar] [CrossRef]
- Shou, Q.; Cheng, J.; Zhang, L.; Nelson, B.J.; Zhang, X. Synthesis and characterization of a nanocomposite of goethite nanorods and reduced graphene oxide for electrochemical capacitors. J. Solid State Chem. 2012, 185, 191–197. [Google Scholar] [CrossRef]
- Li, X.; Du, D.; Zhang, Y.; Xing, W.; Xue, Q.; Yan, Z. Layered double hydroxides toward high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 15460–15485. [Google Scholar] [CrossRef]
- Fang, J.; Li, M.; Li, Q.; Zhang, W.; Shou, Q.; Liu, F.; Zhang, X.B.; Cheng, J.P. Microwave-assisted synthesis of CoAl-layered double hydroxide/graphene oxide composite and its application in supercapacitors. Electrochim. Acta 2012, 85, 248–255. [Google Scholar] [CrossRef]
- Ma, K.Y.; Zhao, W.J.; Cheng, J.P.; Liu, F.; Zhang, X.B. Free-standing α-Co(OH)2/graphene oxide thin films fabricated through delamination and reassembling of acetate anions intercalated α-Co(OH)2 and graphene oxide in water. J. Colloid Interface Sci. 2016, 468, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, G.; Li, X. Graphene/layered double hydroxide nanocomposite: Properties, synthesis, and applications. Chem. Eng. J. 2016, 292, 207–223. [Google Scholar] [CrossRef]
- Wang, H.Y.; Shi, G.Q. Layered double hydroxide/graphene composites and their applications for energy storage and conversion. Acta Phys. Chim. Sin. 2018, 34, 22–35. [Google Scholar]
- Patel, R.; Park, J.T.; Patel, M.; Dash, J.K.; Gowd, E.B.; Karpoormath, R.; Mishra, A.; Kwak, J.; Kim, J.H. Transition-metal-based layered double hydroxides tailored for energy conversion and storage. J. Mater. Chem. A 2018, 6, 12–29. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Q.; Li, B.; Xue, H.; Pang, H.; Chen, C. Recent progress in layered double hydroxide based materials for electrochemical capacitors: Design, synthesis and performance. Nanoscale 2017, 9, 15206–15225. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.P.; Zhang, J.; Liu, F. Recent development of metal hydroxides as electrode material of electrochemical capacitors. RSC Adv. 2014, 4, 38893–38917. [Google Scholar] [CrossRef]
- Kovanda, F.; Grygar, T.; Dorničák, V. Thermal behaviour of Ni–Mn layered double hydroxide and characterization of formed oxides. Solid State Sci. 2003, 5, 1019–1026. [Google Scholar] [CrossRef]
- Jayashree, R.S.; Kamath, P.V. Layered double hydroxides of Ni with Cr and Mn as candidate electrode materials for alkaline secondary cells. J. Power Sources 2002, 107, 120–124. [Google Scholar] [CrossRef]
- Guerlou-Demourgues, L.; Denage, C.; Delmas, C. New manganese-substituted nickel hydroxides: Part 1. Crystal chemistry and physical characterization. J. Power Sources 1994, 52, 269–274. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Zhang, X.B.; Cheng, J.P. A comparative study of Ni-Mn layered double hydroxide/carbon composites with different morphologies for supercapacitors. Phys. Chem. Chem. Phys. 2016, 18, 30068–30078. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, J.P.; Wang, J.; Liu, F.; Zhang, X.B. The growth of nickel-manganese and cobalt-manganese layered double hydroxides on reduced graphene oxide for supercapacitor. Electrochim. Acta 2016, 206, 108–115. [Google Scholar] [CrossRef]
- Zhou, J.; Dai, S.; Li, Y.; Han, F.; Yuan, Y.; Tang, J.; Tang, W. Earth-abundant nanotubes with layered assembly for battery-type supercapacitors. Chem. Eng. J. 2018, 350, 835–843. [Google Scholar] [CrossRef]
- Jia, G.; Hu, Y.; Qian, Q.; Yao, Y.; Zhang, S.; Li, Z.; Zou, Z. Formation of hierarchical structure composed of (Co/Ni)Mn-LDH nanosheets on MWCNT backbones for efficient electrocatalytic water oxidation. ACS Appl. Mater. Interfaces 2016, 8, 14527–14534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, F.; Dahn, J.R. Phases formed in Al-doped Ni1/3Mn1/3Co1/3(OH)2 prepared by coprecipitation: Formation of layered double hydroxide. J. Electrochem. Soc. 2008, 155, A642–A647. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, L.; Zhang, Q.; Chen, D.; Cheng, Y.; Deng, X.; Chen, Y.; Murphy, R.; Xiong, X.; Song, B.; et al. Rational design of nickel hydroxide-based nanocrystals on graphene for ultrafast energy storage. Adv. Energy Mater. 2018, 8, 1702247. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, T.; Ji, G.; Hu, N.; Xu, C.; Zhang, Y. Core/shell design of efficient electrocatalysts based on NiCo2O4 nanowires and NiMn LDH nanosheets for rechargeable zinc–air batteries. J. Mater. Chem. A 2018, 6, 10243–10252. [Google Scholar] [CrossRef]
- Guo, X.L.; Zhang, J.M.; Xu, W.N.; Hu, C.G.; Sun, L.; Zhang, Y.X. Growth of NiMn LDH nanosheet arrays on KCu7S4 microwires for hybrid supercapacitors with enhanced electrochemical performance. J. Mater. Chem. A 2017, 5, 20579–20587. [Google Scholar] [CrossRef]
- Barriga, C.; Fernández, J.M.; Ulibarri, M.A.; Labajos, F.M.; Rives, V. Synthesis and characterization of new hydrotalcite-like compounds containing Ni(II) and Mn(III) in the hydroxide layers and of their calcination products. J. Solid State Chem. 1996, 124, 205–213. [Google Scholar] [CrossRef]
- Latorre-Sanchez, M.; Atienzar, P.; Abellán, G.; Puche, M.; Fornés, V.; Ribera, A.; García, H. The synthesis of a hybrid graphene–nickel/manganese mixed oxide and its performance in lithium-ion batteries. Carbon 2012, 50, 518–525. [Google Scholar] [CrossRef]
- Li, M.; Yuan, P.; Guo, S.; Liu, F.; Cheng, J.P. Design and synthesis of Ni-Co and Ni-Mn layered double hydroxides hollow microspheres for supercapacitor. Int. J. Hydrogen Energy 2017, 42, 28797–28806. [Google Scholar] [CrossRef]
- Li, X.; Xin, M.; Guo, S.; Cai, T.; Du, D.; Xing, W.; Zhao, L.; Guo, W.; Xue, Q.; Yan, Z. Insight of synergistic effect of different active metal ions in layered double hydroxides on their electrochemical behaviors. Electrochim. Acta 2017, 253, 302–310. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Li, X.; Xin, M.; Cai, T.; Xing, W.; Chai, Y.; Xue, Q.; Yan, Z. Bifuntional petaloid nickel manganese layered double hydroxides decorated on a freestanding carbon foam for flexible asymmetric supercapacitor and oxygen evolution. Electrochim. Acta 2017, 252, 275–285. [Google Scholar] [CrossRef]
- Chen, H.; Ai, Y.; Liu, F.; Chang, X.; Xue, Y.; Huang, Q.; Wang, C.; Lin, H.; Han, S. Carbon-coated hierarchical Ni–Mn layered double hydroxide nanoarrays on Ni foam for flexible high-capacitance supercapacitors. Electrochim. Acta 2016, 213, 55–65. [Google Scholar] [CrossRef]
- Huang, L.; Liu, B.; Hou, H.; Wu, L.; Zhu, X.; Hu, J.; Yang, J. Facile preparation of flower-like NiMn layered double hydroxide/reduced graphene oxide microsphere composite for high-performance asymmetric supercapacitors. J. Alloys Compd. 2018, 730, 71–80. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, X.; Chen, N.; Wang, K.; Kang, L.; Liu, Z.H. Oxidizing synthesis of Ni2+–Mn3+ layered double hydroxide with good crystallinity. Mater. Res. Bull. 2011, 46, 1843–1847. [Google Scholar] [CrossRef]
- Singh, S.; Shinde, N.M.; Xia, Q.X.; Gopi, C.; Yun, J.M.; Mane, R.S.; Kim, K.H. Tailoring the morphology followed by the electrochemical performance of NiMn-LDH nanosheet arrays through controlled Co-doping for high-energy and power asymmetric supercapacitors. Dalton Trans. 2017, 46, 12876–12883. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.; Jo, C.; Yu, T.; Lim, E.; Yoon, S.; Lee, J.H.; Yoo, J.; Lee, J.; Lim, B. Reverse micelle synthesis of colloidal nickel-manganese layered double hydroxide nanosheets and their pseudocapacitive properties. Chem. Eur. J. 2014, 20, 14880–14884. [Google Scholar] [CrossRef] [PubMed]
- Vialat, P.; Leroux, F.; Mousty, C. Electrochemical properties of layered double hydroxides containing 3d metal cations. J. Solid State Electrochem. 2015, 19, 1975–1983. [Google Scholar] [CrossRef]
- Cheng, J.P.; Liu, L.; Zhang, J.; Liu, F.; Zhang, X.B. Influences of anion exchange and phase transformation on the supercapacitive properties of α-Co(OH)2. J. Electroanal. Chem. 2014, 722–723, 23–31. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, J.P.; Zhang, J.; Liu, F.; Zhang, X.B. Effects of dodecyl sulfate and nitrate anions on the supercapacitive properties of α-Co(OH)2. J. Alloys Compd. 2014, 615, 868–874. [Google Scholar] [CrossRef]
- Lv, L.; Xu, K.; Wang, C.; Wan, H.; Ruan, Y.; Liu, J.; Zou, R.; Miao, L.; Ostrikov, K.; Lan, Y.; et al. Intercalation of glucose in NiMn-layered double hydroxide nanosheets: An effective path way towards battery-type electrodes with enhanced performance. Electrochim. Acta 2016, 216, 35–43. [Google Scholar] [CrossRef]
- Cheng, J.P.; Liu, L.; Ma, K.Y.; Wang, X.; Li, Q.Q.; Wu, J.S.; Liu, F. Hybrid nanomaterial of α-Co(OH)2 nanosheets and few-layer graphene as an enhanced electrode material for supercapacitors. J. Colloid Interface Sci. 2017, 486, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ma, R.; Wu, J.; Sun, P.; Liu, X.; Zhou, K.; Sasaki, T. Development of efficient electrocatalysts via molecular hybridization of NiMn layered double hydroxide nanosheets and graphene. Nanoscale 2016, 8, 10425–10432. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Jiang, C.; Wang, S.; Li, Y.; Zhang, Z.; Tang, Z.; Favier, F. New nanocomposite material as supercapacitor electrode prepared via restacking of Ni-Mn LDH and MnO2 nanosheets. Electrochim. Acta 2017, 247, 1072–1079. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Ma, J.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical NiMn layered double hydroxide/carbon nanotubes architecture with superb energy density for flexible supercapacitors. Adv. Funct. Mater. 2014, 24, 2938–2946. [Google Scholar] [CrossRef]
- Padmini, M.; Kiran, S.K.; Lakshminarasimhan, N.; Sathish, M.; Elumalai, P. High-performance solid-state hybrid energy-storage device consisting of reduced graphene-oxide anchored with NiMn-layered double hydroxide. Electrochim. Acta 2017, 236, 359–370. [Google Scholar] [CrossRef]
- Lee, I.; Jeong, G.H.; An, S.; Kim, S.W.; Yoon, S. Facile synthesis of 3D MnNi-layered double hydroxides (LDH)/graphene composites from directly graphites for pseudocapacitor and their electrochemical analysis. Appl. Surf. Sci. 2018, 429, 196–202. [Google Scholar] [CrossRef]
- Yu, M.; Liu, R.; Liu, J.; Li, S.; Ma, Y. Polyhedral-like NiMn-layered double hydroxide/porous carbon as electrode for enhanced electrochemical performance supercapacitors. Small 2017, 13, 1702616. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Liu, X.Y.; Hao, X.D.; Zhu, S.J.; Dong, F.; Wen, Z.Q.; Zhang, Y.X. Nickel-manganese layered double hydroxide nanosheets supported on nickel foam for high-performance supercapacitor electrode materials. Electrochim. Acta 2016, 194, 179–186. [Google Scholar] [CrossRef]
- Liu, P.F.; Zhou, J.J.; Li, G.C.; Wu, M.K.; Tao, K.; Yi, F.Y.; Zhao, W.N.; Han, L. A hierarchical NiO/NiMn-layered double hydroxide nanosheet array on Ni foam for high performance supercapacitors. Dalton Trans. 2017, 46, 7388–7391. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Liu, F.; Zhang, M.; Zhang, X.; Cheng, J.P. Core/shell microrod arrays of NiO/Co-Fe layered double hydroxides deposited on nickel foam for energy storage and conversion. Electrochim. Acta 2017, 225, 425–434. [Google Scholar] [CrossRef]
- Guo, W.; Yu, C.; Li, S.; Yang, J.; Liu, Z.; Zhao, C.; Huang, H.; Zhang, M.; Han, X.; Niu, Y.; et al. High-stacking-density, superior-roughness LDH bridged with vertically aligned graphene for high-performance asymmetric supercapacitors. Small 2017, 13, 1701288. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, Y.; Lou, G.; Wu, Y.; Zhu, X.; Chen, H.; Shen, Z.; Fu, S.; Bao, B.; Wu, L. Synthesis of NiMn-LDH nanosheet@Ni3S2 nanorod hybrid structures for supercapacitor electrode materials with ultrahigh specific capacitance. Sci. Rep. 2018, 8, 5246. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Jia, H.; Liang, H.; Chen, S.; Cai, Y.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. Hierarchical CuCo2S4@NiMn-layered double hydroxide core-shell hybrid arrays as electrodes for supercapacitors. Chem. Eng. J. 2018, 336, 562–569. [Google Scholar] [CrossRef]
- Shi, L.; Sun, P.; Du, L.; Xu, R.; He, H.; Tan, S.; Zhao, C.; Huang, L.; Mai, W. Flexible honeycomb-like NiMn layered double hydroxide/carbon cloth architecture for electrochemical energy storage. Mater. Lett. 2016, 175, 275–278. [Google Scholar] [CrossRef]
- Wan, H.; Liu, J.; Ruan, Y.; Lv, L.; Peng, L.; Ji, X.; Miao, L.; Jiang, J. Hierarchical configuration of NiCo2S4 nanotube@Ni-Mn layered double hydroxide arrays/three-dimensional graphene sponge as electrode materials for high-capacitance supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 15840–15847. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Tang, Z.L.; Wang, S.T.; Hong, Y.; Zhang, Z.T. Facile preparation of free-standing rGO paper-based Ni-Mn LDH/graphene superlattice composites as a pseudocapacitive electrode. Chem. Commun. 2016, 52, 3694–3696. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, J.P.; Liu, F.; Zhang, X.B. 3D-architectured nickel–cobalt–manganese layered double hydroxide/reduced graphene oxide composite for high-performance supercapacitor. Chem. Phys. Lett. 2015, 640, 5–10. [Google Scholar] [CrossRef]
- Xiong, G.; He, P.; Liu, L.; Chen, T.; Fisher, T.S. Plasma-grown graphene petals templating Ni–Co–Mn hydroxide nanoneedles for high-rate and long-cycle-life pseudocapacitive electrodes. J. Mater. Chem. A 2015, 3, 22940–22948. [Google Scholar] [CrossRef]
- Oliver-Tolentino, M.A.; Ramos-Sánchez, G.; Manzo-Robledo, A.; Ramírez-Rosales, D.; Flores-Moreno, J.L.; Lima, E.; Guzmán-Vargas, A. Some attributes of Mn3+ sites in nickel-based layered double hydroxides during methanol electro-oxidation in alkaline media. Chem. Electron. Chem. 2018, 5, 708–716. [Google Scholar] [CrossRef]
- Zheng, X.; Han, X.; Zhao, X.; Qi, J.; Ma, Q.; Tao, K.; Han, L. Construction of Ni-Co-Mn layered double hydroxide nanoflakes assembled hollow nanocages from bimetallic imidazolate frameworks for supercapacitors. Mater. Res. Bull. 2018, 106, 243–249. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Wang, J.; Lee, P.S. Sulfidation of NiMn-layered double hydroxides/graphene oxide composites toward supercapacitor electrodes with enhanced performance. Adv. Energy Mater. 2016, 6, 1501745. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, C.; Fang, J.; Cheng, J.; Zhang, X.; Dong, S.; Zhang, L. Asymmetric electrochemical capacitors with high energy and power density based on graphene/CoAl-LDH and activated carbon electrodes. RSC Adv. 2013, 3, 2483–2490. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, H. Nanostructured electrode materials for electrochemical capacitor applications. Nanomaterials 2015, 5, 906–936. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, Y.; Yu, M.; Liu, P.; Tong, Y.; Cheng, F.; Lu, X. Recent smart methods for achieving high-energy asymmetric supercapacitors. Small Methods 2018, 2, 1700230. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.; Chen, L.; Dai, B.; Yu, F. Three-dimensional honeycomb-like porous carbon with both interconnected hierarchical porosity and nitrogen self-doping from cotton seed husk for supercapacitor electrode. Nanomaterials 2018, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, F.; Cheng, J.P.; Zhang, X.B. Binary nickel-cobalt oxides electrode materials for high-performance supercapacitors: Influence of its composition and porous nature. ACS Appl. Mater. Interfaces 2015, 7, 17630–17640. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Lu, S.; Peng, L.; Zhou, J.; Kong, J.; Jia, D.; Li, F. Hierarchical Mn2O3 microspheres in-situ coated with carbon for supercapacitors with highly enhanced performances. Nanomaterials 2017, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, K.Y.; Zhang, J.; Liu, F.; Cheng, J.P. Template-free synthesis of hierarchical hollow NiSx microspheres for supercapacitor. J. Colloid Interface Sci. 2017, 507, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Samal, R.; Dash, B.; Sarangi, C.K.; Sanjay, K.; Subbaiah, T.; Senanayake, G.; Minakshi, M. Influence of synthesis temperature on the growth and surface morphology of Co3O4 nanocubes for supercapacitor applications. Nanomaterials 2017, 7, 356. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Adelowo, E.; Baboukani, A.R.; Villegas, M.F.; Henriques, A.; Wang, C. Electrostatic spray deposition-based manganese oxide films-from pseudocapacitive charge storage materials to three-dimensional microelectrode integrands. Nanomaterials 2017, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, X.; Chen, D.; Liu, J.; Liu, P.; Xue, Y.; Lin, H.; Han, S. Graphene-Karst cave flower-like Ni–Mn layered double oxides nanoarrays with energy storage electrode. Electrochim. Acta 2016, 220, 36–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Yang, J.; Bai, Y.; Qiu, H.; Wang, Y. Unique sandwiched carbon sheets@Ni-Mn nanoparticles for enhanced oxygen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 11396–11402. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, W.; Li, M.; Wang, J.; Liu, F.; Cheng, J.P. Effect of reaction temperature on the amorphous-crystalline transition of copper cobalt sulfide for supercapacitors. Electrochim. Acta 2018, 271, 498–506. [Google Scholar] [CrossRef]

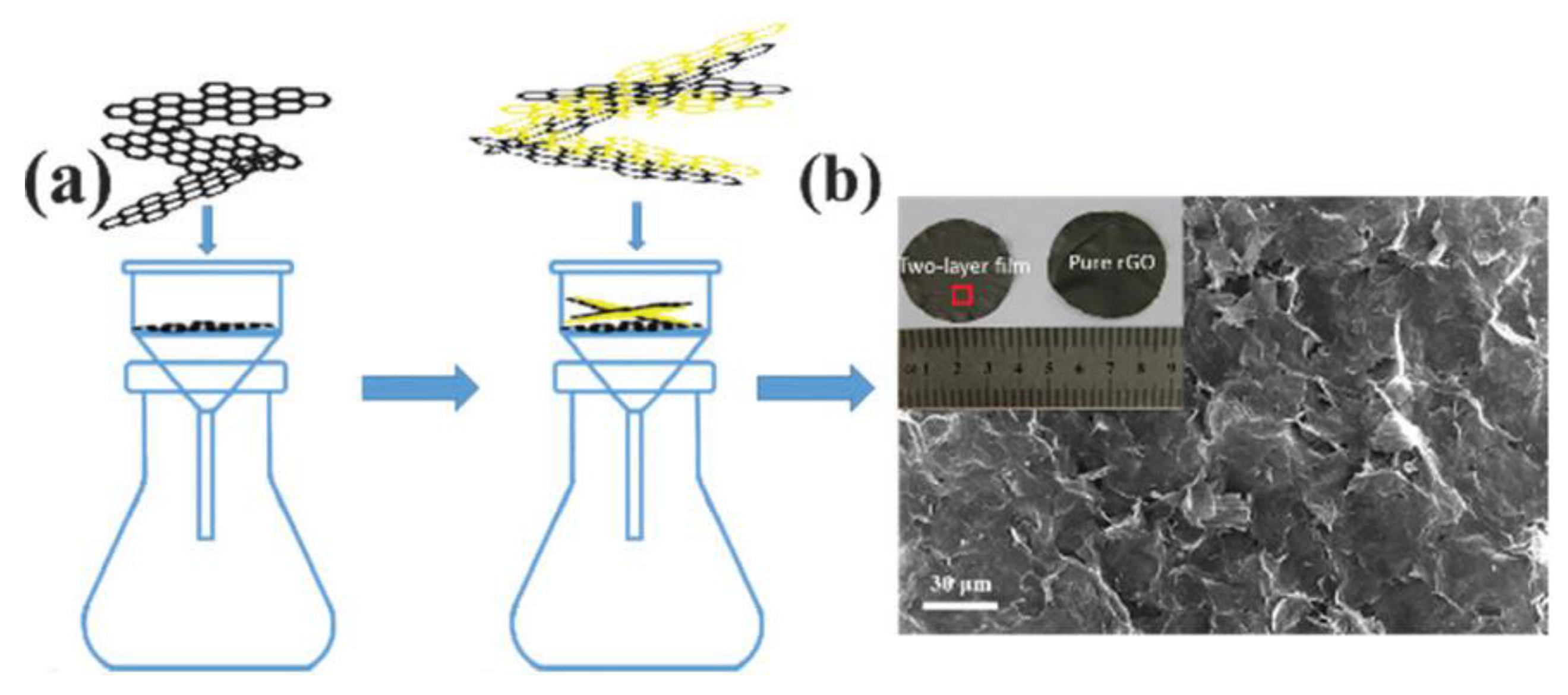
| Method | Materials | Electrolyte | Specific Capacitance (F g−1) | Current Density (A g−1) | References |
|---|---|---|---|---|---|
| Precipitation | Ni Mn LDH/CNTs/rGO | 2 M KOH | 1268 | 1 | [23] |
| Precipitation | Ni-Mn LDHs hollow spheres | 2 M KOH | 595.6 | 1 | [33] |
| Reverse micelle | NiMn LDH nanosheets | 1 M KOH | 881 | 1 | [40] |
| Precipitation | NiMn LDHs/halloysite | 2 M KOH | 1665 | 1 | [25] |
| Hydrothermal | Flower-like NiMn LDH/rGO | 6 M KOH | 1500 | 1 | [37] |
| Precipitation | NiMn-LDH/rGO | 3 M KOH | 1250 | 1 | [49] |
| Precipitation | NiMn-LDH/rGO | 2 M KOH | 1635 | 1 | [24] |
| Hydrothermal | NiMn LDH/graphene | 2 M KOH | 2219 | 0.73 | [50] |
| Precipitation | NiMn LDH/CNTs | 1M KOH | 2960 | 1 | [48] |
| Hydrothermal | Glucose intercalated NiMn LDH | 6 M KOH | 1464 | 0.5 | [44] |
| Precipitation | NiMn LDH/MnO2 nanosheets | 1 M KOH | 380 | 10 m v−1 | [47] |
| Hydrothermal | Polyhedral NiMn LDH@carbon | 6 M KOH | 1634 | 1 | [51] |
| Precipitation | NiMn LDH/rGO | 2 M KOH | 1209 | 2 | [28] |
| Hydrothermal | NiMn LDH/GO | 2 M KOH | 1188 | 1 | [65] |
| Precipitation | NiMn LDH@Ni foam | 1 M KOH | 1511 | 2.5 | [52] |
| Hydrothermal | NiO/NiMn LDH@Ni foam | 3 M KOH | 937 | 0.5 | [53] |
| Hydrothermal | Carbon coated NiMn LDH @Ni foam | 1 M KOH | 1863 | 1 | [36] |
| Hydrothermal | Free-standing NiMn LDH/rGO | 1 M KOH | 421 | 36 | [60] |
| Precipitation | KCu7S4/NiMn LDHs@Ni foam | 1 M LiOH | 773.8 | 1 | [30] |
| Hydrothermal | NiCo2S4/NiMn LDH@graphene sponge | 6 M KOH | 1740 mF cm−2 | 1 mA cm−2 | [59] |
| Hydrothermal | CuCo2S4/NiMn LDH@Ni foam | 6 M KOH | 2250 | 2 | [57] |
| Hydrothermal | Ni3S2/NiMn LDH@Ni foam | 1 M KOH | 2703 | 3 | [56] |
| Hydrothermal | LDH/vertical graphene@Ni foam | 6 M KOH | 2920 | 2 | [55] |
| Precipitation | NiCoMn LDH/rGO | 2 M KOH | 912 | 1 | [61] |
| Hydrothermal | Carbon/NiCoMn LDH@carbon cloth | 2 M KOH | 1400 | 0.5 mA cm−2 | [62] |
| Hydrothermal | NiCoMn LDH (10% Co) | 3 M KOH | 2420 | 1 | [39] |
| Precipitation | NiCoMn LDH nanocages | 1 M KOH. | 2012.5 | 1 | [64] |
| Negative Electrode | Positive Electrode | Power Density (kW kg−1) | Energy Density (Wh kg−1) | Potential (V) | References |
|---|---|---|---|---|---|
| rGO | Flower-like NiMn LDH/rGO | 0.4 | 29.3 | 1.6 | [37] |
| rGO | NiMn-LDH/rGO | 1 | 22.5 | 1.8 | [49] |
| AC | NiMn-LDH/rGO | 0.85 | 33.8 | 1.7 | [24] |
| rGO/CNT | NiMn LDH/CNTs | 0.85 | 88.3 | 1.7 | [48] |
| AC | Polyhedral NiMn LDH@carbon | 0.225 | 18.6 | 1.5 | [51] |
| rGO | NiMn-LDH/rGO | 1.68 | 74.7 | 1.6 | [28] |
| AC | NiO/NiMn LDH@Ni foam | 0.41 | 27.8 | 1.6 | [53] |
| AC | Carbon coated NiMn LDH @Ni foam | 0.378 | 37.7 | 1.5 | [36] |
| Graphene | KCu7S4/NiMn LDHs@Ni foam | 0.65 | 15.9 | 1.5 | [30] |
| AC | CuCo2S4/NiMn LDH@Ni foam | 1.499 | 45.8 | 1.5 | [57] |
| AC | Ni3S2/NiMn LDH@Ni foam | 0.6 | 68 | 1.6 | [56] |
| AC | LDH/vertical graphene@Ni foam | 0.26 | 56.8 | 1.6 | [55] |
| AC | NiCoMn LDH/rGO | 4.24 | 42.4 | 1.8 | [61] |
| rGO | NiCoMn-(LDH) (10% Co) | 0.75 | 57.4 | 1.5 | [39] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, A.-L.; Wang, X.-C.; Cheng, J.-P. Research Progress of NiMn Layered Double Hydroxides for Supercapacitors: A Review. Nanomaterials 2018, 8, 747. https://doi.org/10.3390/nano8100747
Yan A-L, Wang X-C, Cheng J-P. Research Progress of NiMn Layered Double Hydroxides for Supercapacitors: A Review. Nanomaterials. 2018; 8(10):747. https://doi.org/10.3390/nano8100747
Chicago/Turabian StyleYan, Ai-Lan, Xin-Chang Wang, and Ji-Peng Cheng. 2018. "Research Progress of NiMn Layered Double Hydroxides for Supercapacitors: A Review" Nanomaterials 8, no. 10: 747. https://doi.org/10.3390/nano8100747
APA StyleYan, A.-L., Wang, X.-C., & Cheng, J.-P. (2018). Research Progress of NiMn Layered Double Hydroxides for Supercapacitors: A Review. Nanomaterials, 8(10), 747. https://doi.org/10.3390/nano8100747






