High Ultraviolet Absorption in Colloidal Gallium Nanoparticles Prepared from Thermal Evaporation
Abstract
:1. Introduction
2. Results and Discussion
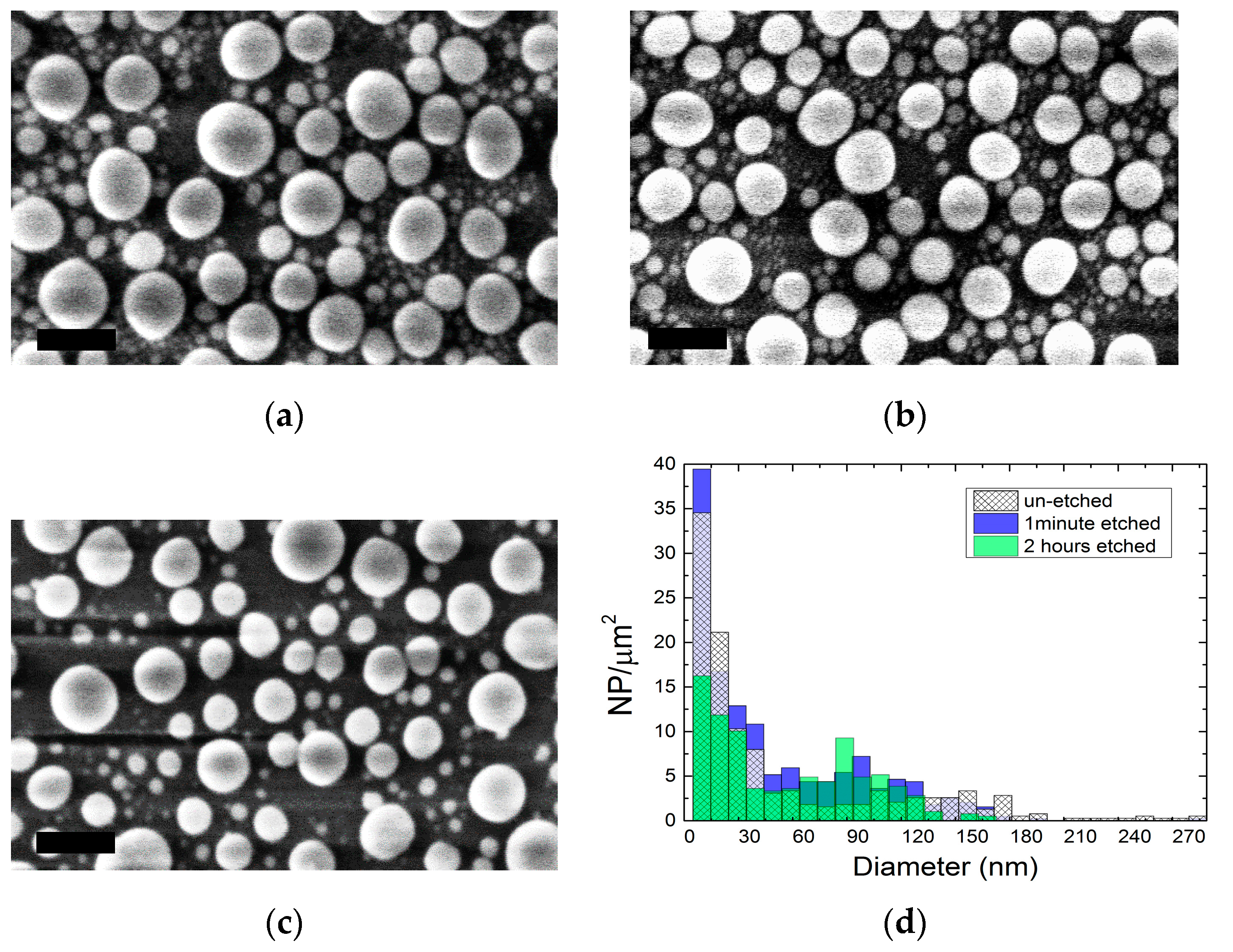
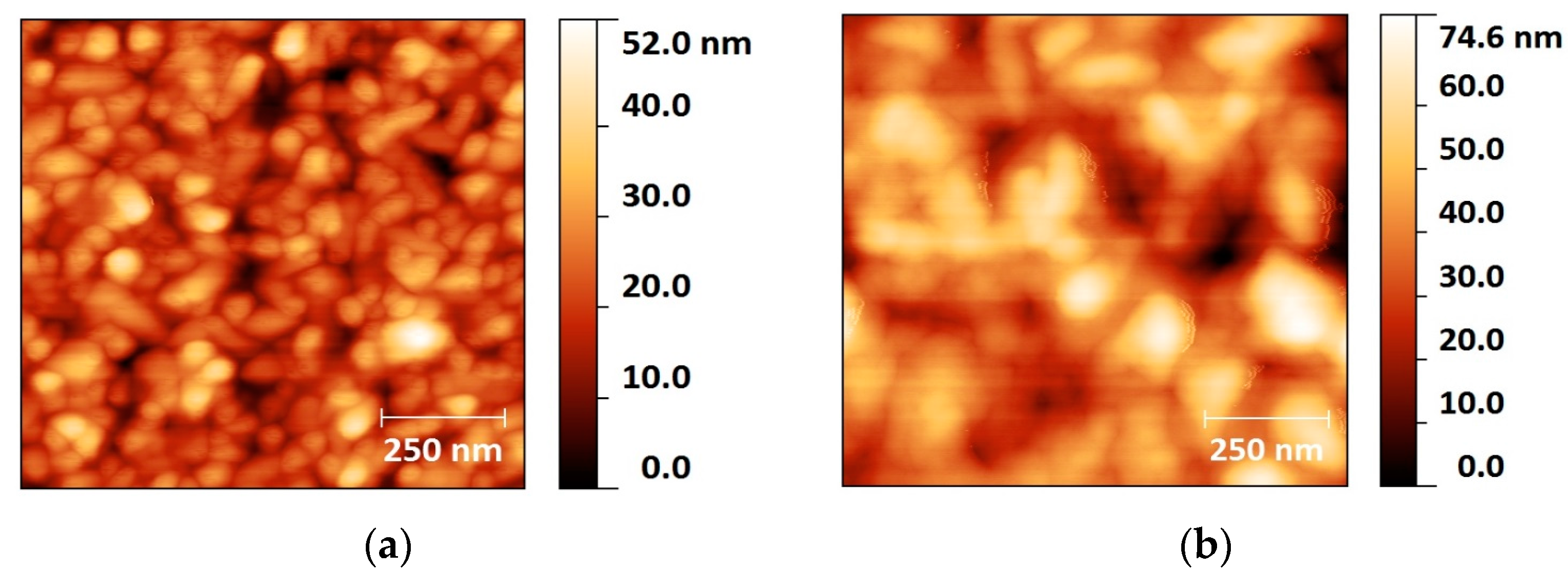
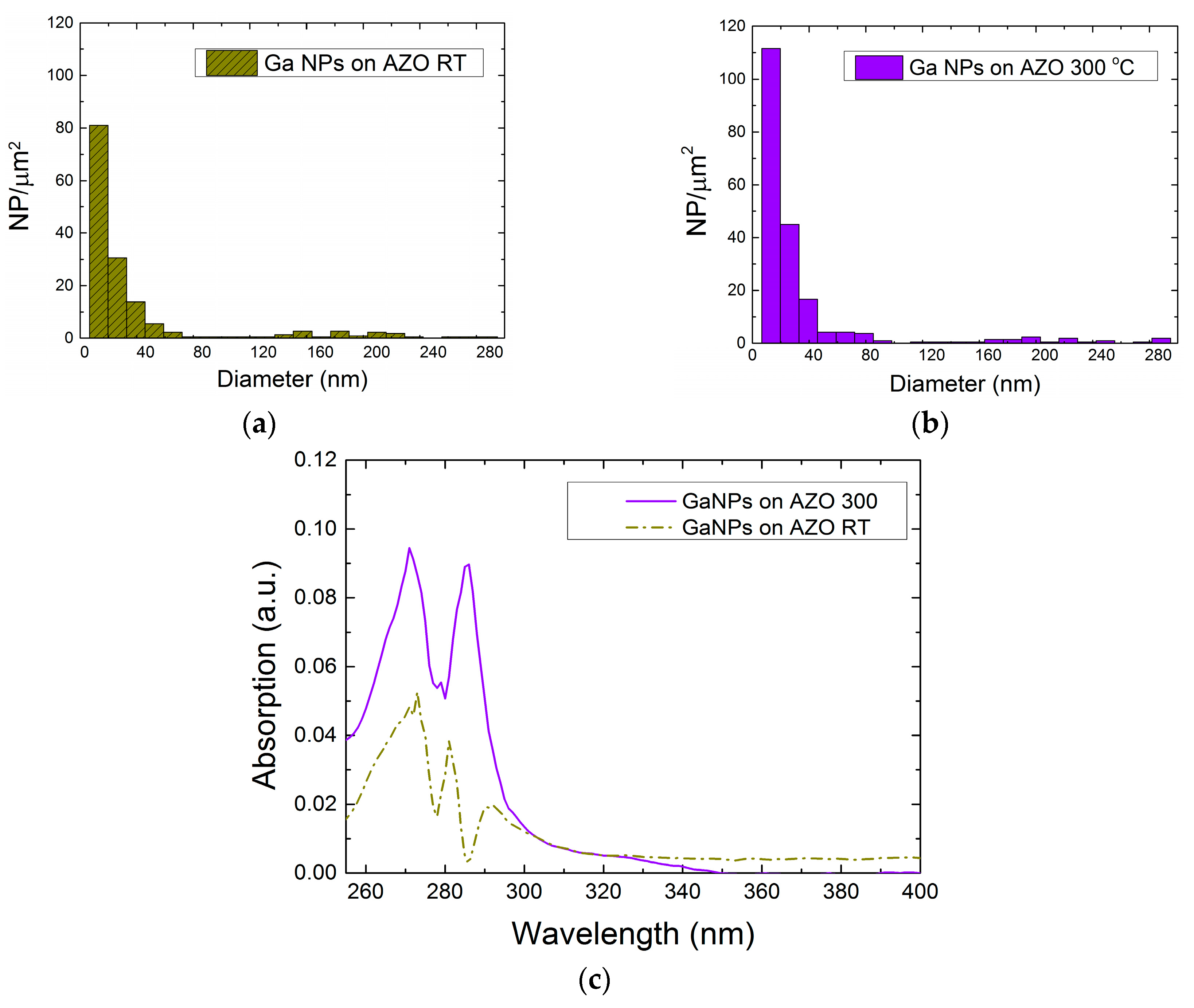
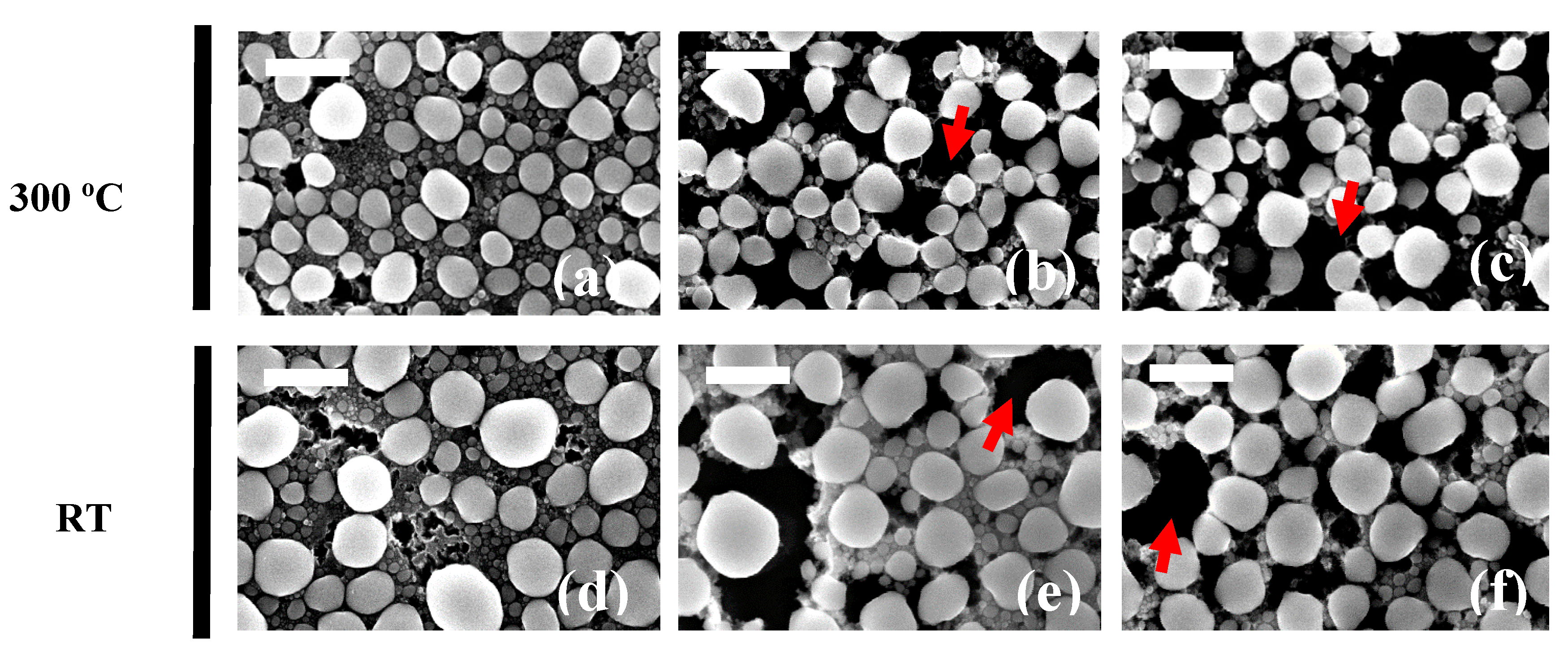
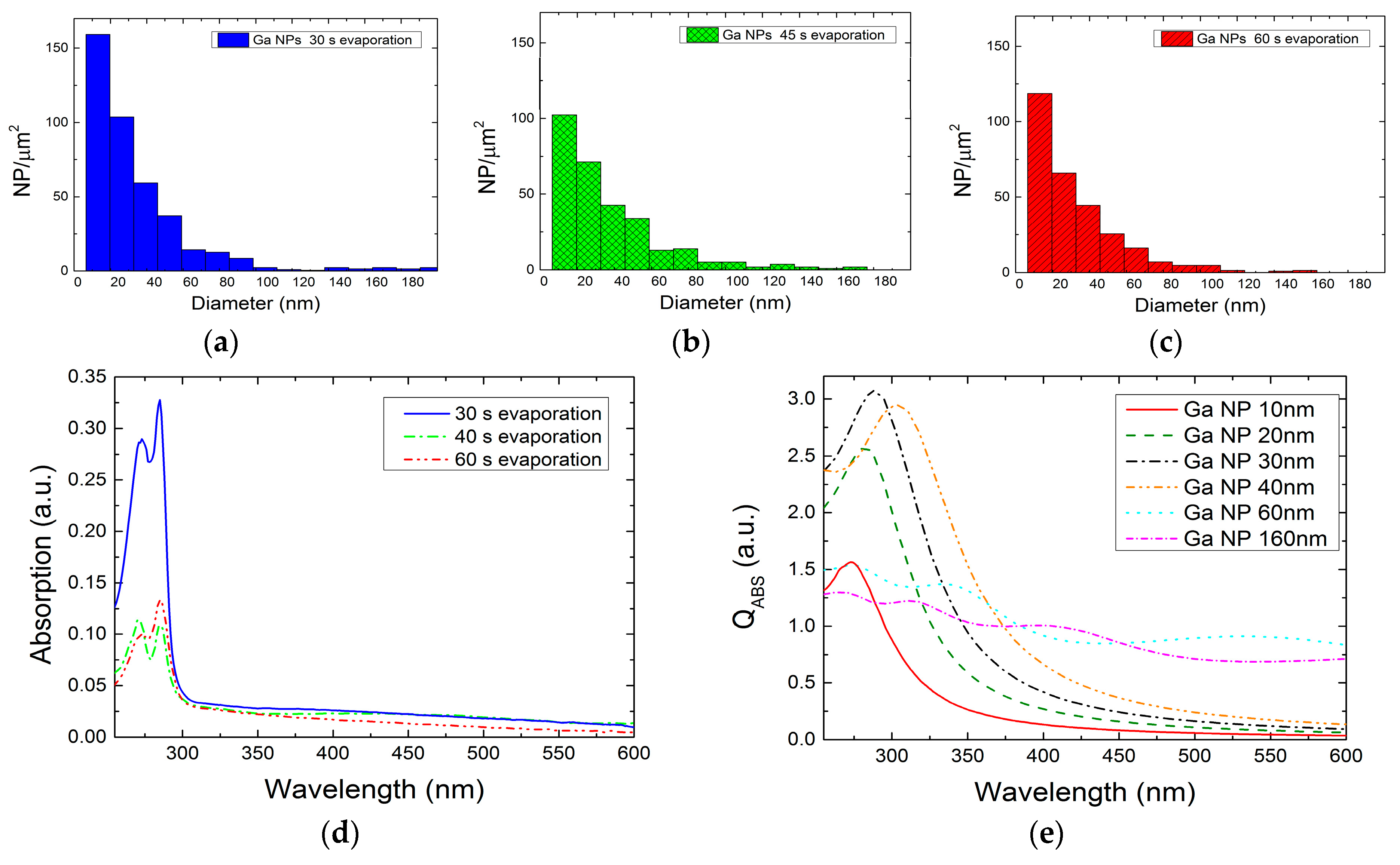
2.1. Colloidal Synthesis Optimization
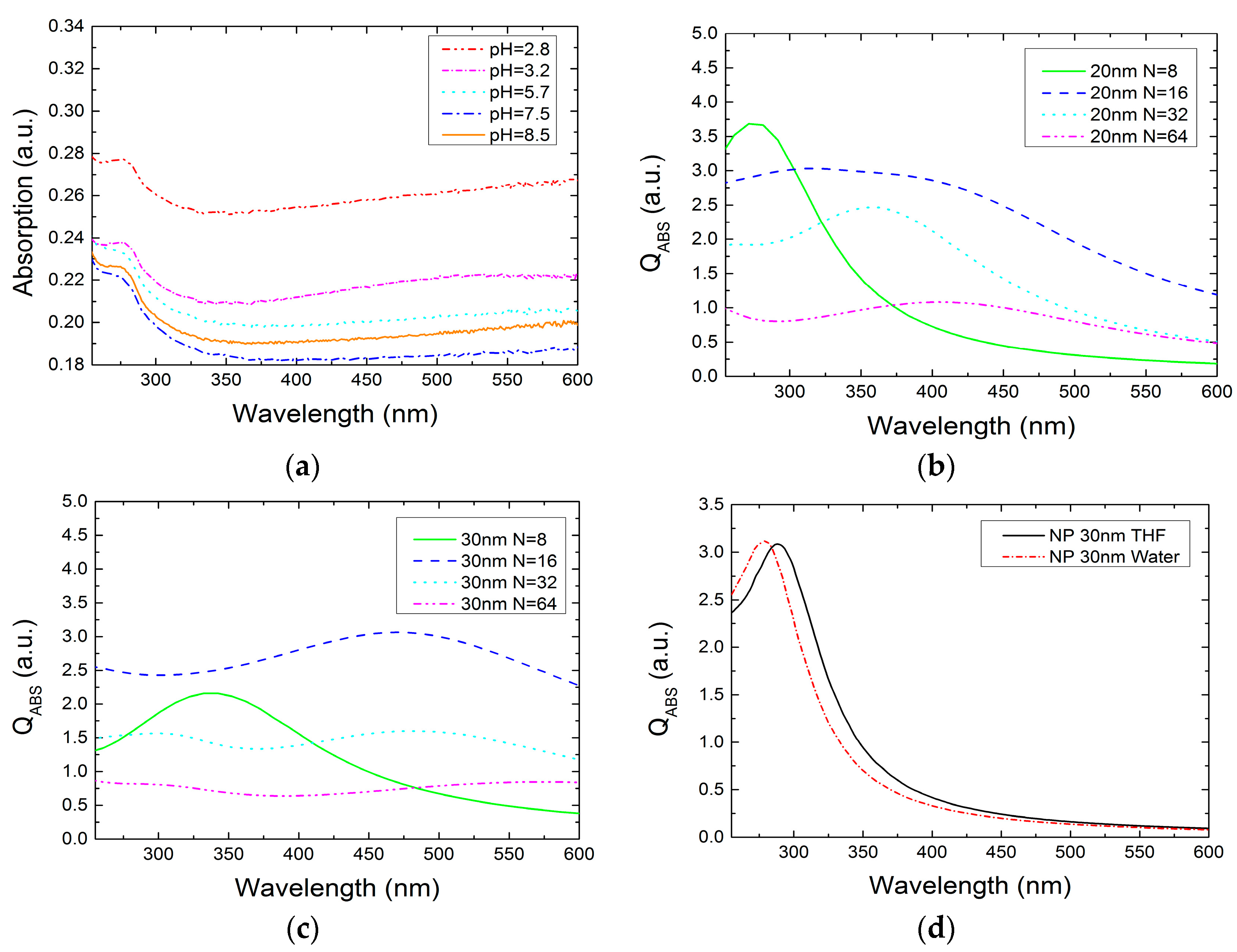
2.2. Optical Characteristics of Ga NPs
2.2.1. Ga NPs in THF Solvent
2.2.2. Ga NPs in Aqueous Solution
3. Materials and Methods
3.1. Ga NPs Synthesis
3.2. Scanning Electron Microscopy (SEM)
3.3. Atomic Force Microscopy (AFM)
3.4. pH Measurement
3.5. UV-Visible Spectrophotometry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold NPs in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, Y.; Li, T.; Yu, C.-P. Reaction of silver NPs in the disinfection process. Chemosphere 2013, 93, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Millard, M.; Brolo, A.G. Optimizing Plasmonic Silicon Photovoltaics with Ag and Au Nanoparticle Mixtures. J. Phys. Chem. 2014, 118, 5889–5895. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, M.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble Metal NPs for Biosensing Applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.W.; Holliday, R.J.; Thompson, D.T. Progress towards the commercial application of gold catalysts. Top. Catal. 2007, 44, 331–343. [Google Scholar] [CrossRef]
- Hudson, R.; Hamasaka, G.; Osako, T.; Yamada, Y.M.A.; Li, C.-J.; Uozumi, Y.; Moores, A. Highly efficient iron(0) nanoparticle-catalyzed hydrogenation in water in flow. Green Chem. 2013, 15, 2141–2148. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Chikan, V.; McLaurin, E.J. Rapid Nanoparticle Synthesis by Magnetic and Microwave Heating. Nanomaterials 2016, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, R.; Long, H.; Jin, Y.; Sanders, A.; Park, W.; Zhang, W. Template Synthesis of Gold NPs with an Organic Molecular Cage. J. Am. Chem. Soc. 2014, 136, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Hezard, T.; Fajerwerg, K.; Evrard, D.; Collière, V.; Behra, P.; Gros, P. Gold NPs electrodeposited on glassy carbon using cyclic voltammetry: Application to Hg(II) trace analysis. J. Electroanal. Chem. 2012, 664, 46–52. [Google Scholar] [CrossRef]
- Bhau, B.S.; Ghosh, S.; Puri, S.; Borah, B.; Sarmah, D.K.; Khan, R. Green synthesis of gold NPs from the leaf extract of Nepenthes khasiana and antimicrobial assay. Adv. Mater. Lett. 2015, 6, 55–58. [Google Scholar] [CrossRef]
- Kruis, F.E.; Fissan, H.; Peled, A. Synthesis of NPs in the gas phase for electronic, optical and magnetic applications—A review. J. Aerosol Sci. 1998, 29, 511–535. [Google Scholar] [CrossRef]
- Kowalczyk, B.; Lagzi, I.; Grzybowski, B.A. Nanoseparations: Strategies for size and/or shape-selective purification of NPs. Curr. Opin. Colloid Interface Sci. 2011, 16, 135–148. [Google Scholar] [CrossRef]
- Markovich, G.; Collier, C.P.; Henrichs, S.E.; Remacle, F.; Levine, R.D.; Heath, J.R. Architectonic Quantum Dot Solids. Acc. Chem. Res. 1999, 32, 415–423. [Google Scholar] [CrossRef]
- Collier, C.P.; Vossmeyer, T.; Heath, J.R. Nanocrystal superlattices. Annu. Rev. Phys. Chem. 1998, 49, 371–404. [Google Scholar] [CrossRef] [PubMed]
- Fendler, J.H. Colloid Chemical Approach to Nanotechnology. Korean J. Chem. Eng. 2001, 18, 1–13. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Khanna, S.N. A Systematic Framework and Nanoperiodic Concept for Unifying Nanoscience: Hard/Soft Nanoelements, Superatoms, Meta-Atoms, New Emerging Properties, Periodic Property Patterns, and Predictive Mendeleev-like Nanoperiodic Tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef] [PubMed]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Alexandridis, P. Single-Step Synthesis and Stabilization of Metal NPs in Aqueous Pluronic Block Copolymer Solutions at Ambient Temperature. Langmuir 2004, 20, 8426–8430. [Google Scholar] [CrossRef] [PubMed]
- Meléndrez, M.F.; Cárdenas, G.; Arbiol, J. Synthesis and characterization of gallium colloidal NPs. J. Colloid Interface Sci. 2010, 346, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Yarema, M.; Wörle, M.; Rossell, M.D.; Erni, R.; Caputo, R.; Protesescu, L.; Kravchyk, K.V.; Dirin, D.N.; Lienau, K.; von Rohr, F.; et al. Monodisperse Colloidal Gallium NPs: Synthesis, Low Temperature Crystallization, Surface Plasmon Resonance and Li-Ion Storage. J. Am. Chem. Soc. 2014, 136, 12422–12430. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2014, 136, 12422–12430. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Arai, N. Wet chemical etching behavior of β-Ga2O3 single crystal. Phys. Status Solidi C 2008, 5, 3116–3118. [Google Scholar] [CrossRef]
- Junga, Y.; Ahna, J.; Baikb, K.H.; Kimc, D.; Peartond, S.J.; Rene, F.; Kima, J. Chemical Etch Characteristics of N-Face and Ga-Face GaN by Phosphoric Acid and Potassium Hydroxide Solutions. J. Electrochem. Soc. 2012, 159, H117–H120. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Ring, T.A. Fundamentals of Ceramic Powder Processing and Synthesis; Academic Press Inc.: New York, NY, USA, 1996. [Google Scholar]
- Tsuda, A.; Henry, F.S.; Butler, J.P. Particle transport and deposition: Basic physics of particle kinetics. Compr. Physiol. 2013, 3, 1437–1471. [Google Scholar] [PubMed]
- Verwey, E.J.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids; Elsevier: Amsterdam, The Netherlands, 1948. [Google Scholar]
- Derjaguin, B.V.; Lanadau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solution of electrolytes. Acta Physicochim. USSR 1941, 14, 633–662. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, M.; Suvorova, A.; Rubanov, S.; Hingerl, K.; Brown, A.S. Thermally stable coexistence of liquid and solid phases in gallium nanoparticles. Nat. Mater. 2016, 15, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.W.; Coenen, T.; Yang, Y.; Brenny, B.J.M.; Losurdo, M.; Brown, A.S.; Everitt, H.O.; Polman, A. Gallium Plasmonics: Deep Subwavelength Spectroscopic Imaging of Single and Interacting Gallium Nanoparticles. ACS Nano 2015, 9, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Draine, B.T.; Flatau, P.J. Discrete dipole approximation for scattering calculations. J. Opt. Soc. Am. A 1994, 11, 1491–1499. [Google Scholar] [CrossRef]
- Rebiena, M.; Henrion, W.; Hong, M.; Mannaerts, J.P.; Fleischer, M. Optical properties of gallium oxide thin films. Appl. Phys. Lett. 2002, 81, 250. [Google Scholar] [CrossRef]
- Vasiliev, A.N.; Gulliver, E.A.; Khinast, J.G.; Riman, R.E. Highly dispersible polymer-coated silver Nanoparticle. Surf. Coat. Technol. 2009, 203, 2841–2844. [Google Scholar] [CrossRef]
- Kosmulski, M. Pristine Points of Zero Charge of Gallium and Indium Oxides. J. Colloid Interface Sci. 2001, 238, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Draine, B.T.; Johnson, E.T. Modeling Porous Dust Grains with Ballistic Aggregates. I. Geometry and Optical Properties. Astrophys. J. 2008, 689, 260–275. [Google Scholar] [CrossRef]
- Minami, T.; Nanot, H.; Takata, S. Highly Conductive and Transparent Aluminum Doped Zinc Oxide Thin Films Prepared by RF Magnetron Sputtering. Jpn. J. Appl. Phys. 1984, 23, 776–779. [Google Scholar] [CrossRef]
- Marín, A.G.; Hernández, M.J.; Ruiz, E.; Abad, J.M.; Lorenzo, E.; Piqueras, J.; Pau, J.L. Immunosensing platform based on gallium nanoparticle arrays on silicon substrates. Biosens. Bioelectron. 2015, 74, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]

| Solvent | Class | Refractive Index @ 270 nm | Solvent λ Cut-Off (nm) |
|---|---|---|---|
| Water | Polar protic | 1.33 | 190 |
| THF | Polar aprotic | 1.40 | 212 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nucciarelli, F.; Bravo, I.; Catalan-Gomez, S.; Vázquez, L.; Lorenzo, E.; Pau, J.L. High Ultraviolet Absorption in Colloidal Gallium Nanoparticles Prepared from Thermal Evaporation. Nanomaterials 2017, 7, 172. https://doi.org/10.3390/nano7070172
Nucciarelli F, Bravo I, Catalan-Gomez S, Vázquez L, Lorenzo E, Pau JL. High Ultraviolet Absorption in Colloidal Gallium Nanoparticles Prepared from Thermal Evaporation. Nanomaterials. 2017; 7(7):172. https://doi.org/10.3390/nano7070172
Chicago/Turabian StyleNucciarelli, Flavio, Iria Bravo, Sergio Catalan-Gomez, Luis Vázquez, Encarnación Lorenzo, and Jose Luis Pau. 2017. "High Ultraviolet Absorption in Colloidal Gallium Nanoparticles Prepared from Thermal Evaporation" Nanomaterials 7, no. 7: 172. https://doi.org/10.3390/nano7070172
APA StyleNucciarelli, F., Bravo, I., Catalan-Gomez, S., Vázquez, L., Lorenzo, E., & Pau, J. L. (2017). High Ultraviolet Absorption in Colloidal Gallium Nanoparticles Prepared from Thermal Evaporation. Nanomaterials, 7(7), 172. https://doi.org/10.3390/nano7070172






