Nanofluid Types, Their Synthesis, Properties and Incorporation in Direct Solar Thermal Collectors: A Review
Abstract
:1. Introduction
2. Solar Thermal Collector Types
2.1. Solar Collectors
2.2. Non-Concentrating Solar Collectors
2.3. Concentrating Solar Collectors
3. Direct Solar Absorption Collectors and the Use of Nanofluids to Improve Efficiency
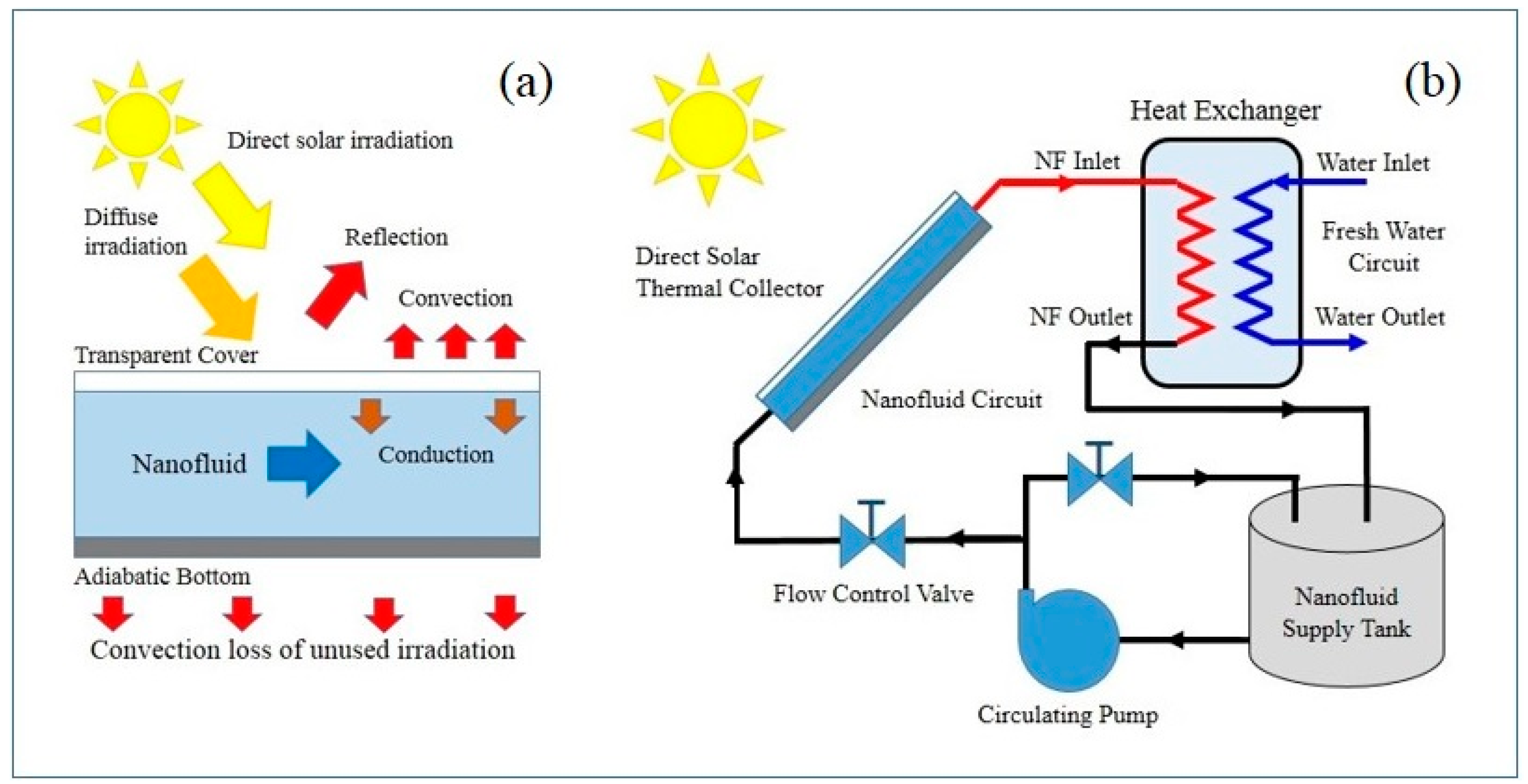
3.1. Direct Solar Absorption Collectors
3.2. Improving the Thermal Properties of the Working Fluid
4. Nanofluids for Use in Direct Solar Absorption Collectors
4.1. Synthesis and Stabilization of Nanofluids
4.2. Synthesis Techniques
4.3. Nanofluid Stabilization Methods and Stability Evaluation Methods
4.3.1. Methods to Enhance Nanofluid Stability
Physical Methods
Effect of Surfactants
Surface Functionalization of Nanoparticles
pH Control of Nanofluid Stability
4.3.2. Evaluation Methods for Determining Nanofluid Stability
Zeta Potential Measurement
Sedimentation and Centrifugation Methods
UV–Visible Spectroscopy Analysis
Dynamic Light Scattering (DLS) Method
5. Types of Nanofluids
5.1. Pure Metals, Metal Oxides and Carbide Based Nanofluids
5.2. Carbon Nanomaterial Based Nanofluids
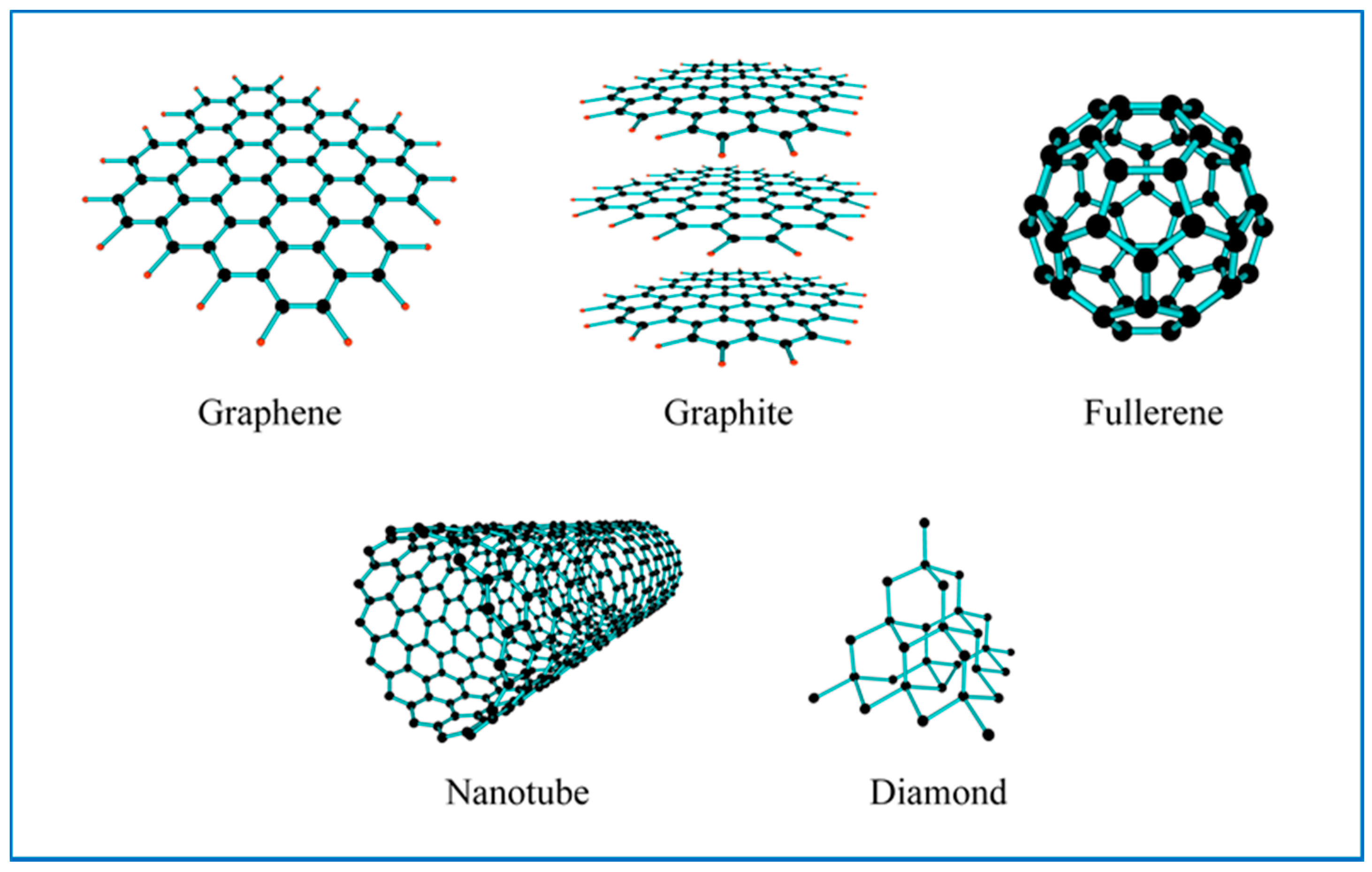
5.2.1. Carbon Nanomaterials
5.2.2. Carbon-Based Nanofluids for Direct Solar Absorption Collectors
Carbon Nanotube-Based Nanofluids
Nano-Diamond Nanofluids
Graphite and Graphene Nanofluids
Carbon Black and Other Carbon-Based Nanofluids
6. Modelling Nanofluid Thermal Conductivity
7. Challenges Facing the Use of Nanofluids in Direct Solar Thermal Absorption Collectors
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- BP Statistical Review of World Energy June 2016. Available online: https://www.bp.com/content/dam/bp/pdf/energy-economics/statistical-review-2016/bp-statistical-review-of-world-energy-2016-full-report.pdf (accessed on 19 August 2016).
- Banos, R.; Manzano-Agugliaro, F.; Montoya, F.G.; Gil, C.; Alcayde, A.; Gomez, J. Optimization methods applied to renewable and sustainable energy: A review. Renew. Sustain. Energy Rev. 2011, 15, 1753–1766. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable energy resources: Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Karcher, H.J.; Baars, J.W.M. Design of the large millimeter telescope/gran telescopio millimetrico (LMT/GTM). In Proceedings of the SPIE 4015, Radio Telescopes, Munich, Germany, 3 July 2000; pp. 155–168. [Google Scholar]
- Duffie, J.A.; Beckman, W.A. Solar Engineering of Thermal Processes; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Lewis, N.S. Toward cost-effective solar energy use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Smil, V. General Energetics: Energy in the Biosphere and Civilization; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Johansson, T.B.; Kelly, H.; Reddy, A.K.N.; Williams, R.H. Renewable Energy—Sources for Fuels and Electricity; Island Press: Washington, DC, USA, 1992. [Google Scholar]
- Ibrahim, A.; Othman, M.Y.; Ruslan, M.H.; Mat, S.; Sopian, K. Recent advances in flat plate photovoltaic/thermal solar collectors. Renew. Sustain. Energy Rev. 2011, 15, 352–365. [Google Scholar] [CrossRef]
- Serrano, E.; Guillermo, R.; García-Martínez, J. Nanotechnology for sustainable energy. Renew. Sustain. Energy Rev. 2009, 13, 2373–2384. [Google Scholar] [CrossRef]
- Green, M. Thin-film solar cells: Review of materials, technologies and commercial statue. J. Mater. Sci. Mater. Electron. 2007, 18, S15–S19. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, C.Y. A review of solar collectors and thermal energy storage in solar thermal applications. Appl. Energy. 2013, 104, 538–553. [Google Scholar] [CrossRef]
- Kalogirou, S.A. Solar thermal collectors and applications. Prog. Energy Combust. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Zalba, B.; Marin, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Tyagi, H.; Phelan, P.; Prasher, R. Predicted efficiency of a low-temperature Nanofluid-based direct absorption solar collector. J. Sol. Energy Eng. 2009, 131, 041004. [Google Scholar] [CrossRef]
- Otanicar, T.P.; Phelan, P.E.; Golden, J.S. Optical properties of liquids for direct absorption solar thermal energy systems. J. Sol. Energy 2009, 83, 969–977. [Google Scholar] [CrossRef]
- Taylor, R.A.; Phelan, P.E.; Otanicar, T.P.; Adrian, R.; Parsher, R. Nanofluid optical property characterisation: Towards efficient direct absorption solar collectors. Nanoscale Res. Lett. 2011, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandam, M.; Iniyan, S.; Goic, R. A review of solar thermal technologies. Renew. Sustain. Energy Rev. 2010, 14, 312–322. [Google Scholar] [CrossRef]
- Sukhatme, K.; Sukhatme, S.P. Solar Energy—Principles of Thermal Collection and Storage; Tata McGraw-Hill Education: New Delhi, India, 1996. [Google Scholar]
- Alghoul, M.A.; Sulaiman, M.Y.; Azmi, B.Z.; Wahab, M.A. Review of materials for solar thermal collectors. Anti Corros. Methods Mater. 2005, 52, 199–206. [Google Scholar] [CrossRef]
- Rabl, A. Optical and thermal properties of compound parabolic collectors. Sol. Energy 1976, 18, 497–511. [Google Scholar] [CrossRef]
- Gouthamraj, K.; Jamuna Rani, K.; Satyanarayana, G. Design and Analysis of Rooftop Linear Fresnel Reflector Solar Concentrator. Int. J. Eng. Innov. Technol. 2013, 2, 66–69. [Google Scholar]
- Kalogirou, S.A. A detailed thermal model of a parabolic trough collector receiver. Energy 2012, 48, 298–306. [Google Scholar] [CrossRef]
- Bakos, G.C. Design and construction of a two-axis Sun tracking system for parabolic trough collector (PTC) efficiency improvement. Renew. Energy 2006, 31, 2411–2421. [Google Scholar] [CrossRef]
- Zhang, H.L.; Baeyens, J.; Degreve, J.; Caceres, G. Concentrated solar power plants: A review and design methodology. Renew. Sustain. Energy Rev. 2013, 22, 466–481. [Google Scholar] [CrossRef]
- Wei, X.D.; Lu, Z.W.; Wang, Z.F.; Yu, W.X.; Zhang, H.X.; Yao, Z.H. A new method for the design of the heliostat field layout for solar tower power plant. Renew. Energy 2010, 35, 1970–1975. [Google Scholar] [CrossRef]
- Kainth, M.; Sharma, V.K. Latest Evolutions in Flat Plate Solar Collectors Technology. Int. J. Mech. Eng. 2014, 1, 7–11. [Google Scholar]
- Hellstrom, B.; Adsten, M.; Nostell, P.; Karlsson, B.; Wackelgard, E. The impact of optical and thermal properties on the performance of flat plate solar collectors. Renew. Energy 2003, 28, 331–344. [Google Scholar] [CrossRef]
- Konttinen, P.; Lund, P.D.; Kilpi, R.J. Mechanically manufactured selective solar absorber surfaces. Sol. Energy Mater. Sol. Cells 2003, 79, 273–283. [Google Scholar] [CrossRef]
- Vasiliev, L.L. Heat pipes in modern heat exchangers. Appl. Therm. Eng. 2005, 25, 1–19. [Google Scholar] [CrossRef]
- Al-Madani, H. The performance of a cylindrical solar water heater. Renew. Energy 2006, 31, 1751–1763. [Google Scholar] [CrossRef]
- Nixon, J.D.; Dey, P.K.; Davies, P.A. Which is the best solar thermal collection technology for electricity generation in north-west India? Evaluation of options using the analytical hierarchy process. Energy 2010, 35, 5230–5240. [Google Scholar] [CrossRef]
- Behar, O.; Khellaf, A.; Mohammedi, K. A review on central receiver solar thermal power plants. Renew. Sustain. Energy Rev. 2013, 23, 12–39. [Google Scholar] [CrossRef]
- Minardi, J.E.; Chuang, H.N. Performance of a “black” liquid flat-plate solar collector. Sol. Energy 1975, 17, 179–183. [Google Scholar] [CrossRef]
- Grasse, W.; Hertlein, H.; Winter, C.J.; Braun, G. Thermal solar power plants experience. In Solar Power Plants; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Lee, D.W.; Sharma, A. Thermal performances of the active and passive water heating systems based on annual operation. Sol. Energy 2007, 81, 207–215. [Google Scholar] [CrossRef]
- Eastop, T.D.; McConkey, A. Applied Thermodynamics for Engineering Technologists; Longman: London, UK, 1977. [Google Scholar]
- Sureshkumar, R.; Mohideen, S.T.; Nethaji, N. Heat transfer characteristics of nanofluids in heat pipes: A review. Renew. Sustain. Energy Rev. 2013, 20, 397–410. [Google Scholar] [CrossRef]
- Ram, M.K.; Myers, P.D.; Jotshi, C.; Goswami, D.Y.; Stefanakos, E.K.; Arvanitis, K.D.; Papanicolaou, E.; Belessiotis, V. Microencapsulated dimethyl terephthalate phase change material for heat transfer fluid performance enhancement. Int. J. Energy Res. 2017, 41, 252–262. [Google Scholar] [CrossRef]
- Kasaeian, A.; Eshghi, A.T.; Sameti, M. A review on the applications of nanofluids in solar energy systems. Renew. Sustain. Energy Rev. 2015, 43, 584–598. [Google Scholar] [CrossRef]
- Zhu, Q.Z.; Cui, Y.; Mu, L.J.; Tang, L.Q. Characterization of thermal radiative properties of nanofluids for selective absorption of solar radiation. Int. J. Thermophys. 2013, 34, 2307–2321. [Google Scholar] [CrossRef]
- Verma, S.K.; Tiwari, A.K. Progress of nanofluid application in solar collectors: A review. Energy Convers. Manag. 2015, 100, 324–346. [Google Scholar] [CrossRef]
- Lee, B.J.; Park, K.; Walsh, T.; Xu, L. Radiative heat transfer analysis in plasmonic nanofluid for direct solar thermal absorption. J. Sol. Energy Eng. 2012, 134, 021009. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Wang, C.; Wei, W.; Xiao, G.; Ni, M.J. Performance improvement of a nanofluid solar collector based on direct absorption collection (DAC) concepts. Int. J. Heat Mass Transf. 2014, 75, 262–271. [Google Scholar] [CrossRef]
- Duncan, A.B.; Peterson, G.P. Review of micro-scale heat transfer. Appl. Mech. Rev. 1994, 47, 397–428. [Google Scholar] [CrossRef]
- Cheng, X.M.; Zhu, C.; Zhang, H.; Yang, X.J. Research and application of heat transfer fluids in solar thermal power. Adv. Mater. Res. 2013, 815, 415–422. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.S.; Patel, H.P. Heat transfer in nanofluids: A review. Heat Transf. Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Wang, X.Q.; Majumdar, A.S. Heat transfer characteristics of nanofluids: A review. Int. J. Therm. Sci. 2007, 46, 1–19. [Google Scholar] [CrossRef]
- Godson, L.; Raja, B.; Mohan Lal, D.; Wongwises, S. Experimental investigation on the thermal conductivity and viscosity of silver-deionized water nanofluid. Exp. Heat Transf. 2010, 23, 317–332. [Google Scholar] [CrossRef]
- Sani, E.; Mercatelli, L.; Barison, S.; Pagura, C.; Agresti, F.; Colla, L.; Sansoni, P. Potential of carbon nanohorn-based suspensions for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2011, 95, 2994–3000. [Google Scholar] [CrossRef]
- Wen, D.; Ding, Y. Effective thermal conductivity of aqueous suspensions of carbon nanotubes (carbon nanotube nanofluids). J. Thermophys. Heat Transf. 2004, 18, 481–485. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.; Mohammad, H. A review on applications and challenges of nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Mu, L.J.; Zhu, Q.Z.; Si, L.L. Radiative properties of nanofluids and performance of a direct solar absorber using nanofluids. In Proceedings of the 2nd International Conference on ASME Micro/Nanoscale Heat & Mass Transfer, Shanghai, China, 18–21 December 2010; Volume 1, pp. 549–553. [Google Scholar]
- Han, D.; Meng, Z.; Wu, D.; Zhang, C.; Zhu, H. Thermal properties of carbon black aqueous nanofluids for solar absorption. Nanoscale Res. Lett. 2011, 6, 457. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.B.; Zhang, R.Y.; Ke, X.F. The photo-thermal properties of copper nanofluids. J. Guangdong Univ. Technol. 2008, 25, 13–17. [Google Scholar]
- Hwang, Y.; Lee, J.K.; Lee, C.H.; Jung, Y.M.; Cheong, S.I.; Lee, C.G.; Ku, B.C.; Jang, S.P. Stability and thermal conductivity characteristics of nanofluids. Thermochim. Acta 2007, 455, 70–74. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Z.H. A kind of nanofluid consisting of surface-functionalized nanoparticles. Nanoscale Res. Lett. 2010, 5, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Mujumdar, A.S. A review on nanofluids—Part II: Experiments and applications. Braz. J. Chem. Eng. 2008, 25, 631–648. [Google Scholar] [CrossRef]
- Wang, L.Q.; Fan, J. Nanofluids research: Key issues. Nanoscale Res. Lett. 2010, 5, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Choi, S.U.S.; Yu, W.; Pradeep, T. Nanofluids: Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Shou, C.H.; Luo, Z.Y.; Wang, T.; Cai, J.C.; Zhao, J.F.; Ni, M.J.; Cen, K.F. Research on the application of nano-fluids into the solar photoelectric utilization. Shanghai Electr. Power 2009, 16, 8–12. [Google Scholar]
- Singh, A. Thermal conductivity of nanofluids. Def. Sci. J. 2008, 58, 600–607. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.U.S.; Li, S.; Eastman, J.A. Measuring thermal conductivity of fluids containing oxide nanoparticles. ASME J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thomson, L.J. Anomalously increased effective thermal conductivities of ethylene glycol based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Yoo, D.; Hong, K.; Yang, H. Study of thermal conductivity of nanofluids for the application of heat transfer fluids. Thermochim. Acta 2007, 455, 66–69. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Chen, L.; Li, Y. Investigation of thermal conductivity and viscosity of ethylene glycol based ZnO nanofluid. Thermochim. Acta 2009, 491, 92–96. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Thiesen, P.; Roetzel, W. Temperature dependence of thermal conductivity enhancement for nanofluids. J. Heat Transf. 2003, 125, 567–574. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Routbort, J.L.; Choi, S.U.S. Review and comparison of nanofluid thermal conductivity and heat transfer enhancements. Heat Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Drotning, W.D. Optical properties of solar-absorbing oxide particles suspended in a molten salt heat transfer fluid. Sol. Energy 1978, 20, 313–319. [Google Scholar] [CrossRef]
- Palombo, N.; Park, K. Investigation of dynamic near-field radiation between quantum dots and plasmonic nanoparticles for effective tailoring of the solar spectrum. In Proceedings of the ASME 2011 International Mechanical Engineering Congress and Exposition, Denver, CO, USA, 11–17 November 2011; pp. 1–5. [Google Scholar]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw-Hill, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Shackelford, J.F.; Alexander, W. CRC Materials Science and Engineering Handbook, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Slack, G.A. Thermal conductivity of MgO, Al2O3, MgAl2O4, and FE3O4 crystals from 3 degrees to 300 °K. Phys. Rev. 1962, 126, 427–441. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Zhang, X.; Fujii, M. Stochastic thermal transport of nanoparticle suspensions. J. Appl. Phys. 2006, 100, 043507. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, S.R.; Kim, D. Thermal conductivity of metal-oxide nanofluids: Particle size dependence and effect of laser irradiation. J. Heat Transf. Trans. ASME 2007, 129, 298–307. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 14th ed.; McGraw-Hill, Inc.: New York, NY, USA, 1992. [Google Scholar]
- Yu, C.H.; Saha, S.; Zhou, J.H.; Shi, L.; Cassell, A.M.; Cruden, B.A.; Ngo, Q.; Li, J. Thermal contact resistance and thermal conductivity of a carbon nanofiber. J. Heat Transf. Trans. ASME 2006, 128, 234–239. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Zhang, Z.G.; Yu, W.; Lockwood, F.E.; Grulke, E.A. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wessel, D. ASHRAE Fundamentals Handbook; American Society of Heating, Refrigerating, and Air-Conditioning Engineers: Atlanta, GA, USA, 2001. [Google Scholar]
- Derjaguin, B.V.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim. URSS 1942, 14, 633–662. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Overbeek, J.T.G.; Mineola, N.Y. Theory of the Stability of Lyophobic Colloids; Dover Publications: Mineola, NY, USA, 1999. [Google Scholar]
- Treguer, M.; Cointet, C.; Remita, H.; Khatouri, J.; Mostafavi, M.; Amblard, J.; Belloni, J.J. Dose rate effect on radiolytic synthesis of gold-silver bimetallic clusters in solution. J. Phys. Chem. 1998, 102, 4310–4321. [Google Scholar] [CrossRef]
- Mafune, F.; Kohno, J.; Takeda, Y.; Kondow, T.J. Dissociation and aggregation of gold nanoparticles under laser irradiation. J. Phys. Chem. 2001, 105, 9050–9056. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, D.J. Fabrication of heterogeneous binary arrays of nanoparticles via colloidal lithography. J. Am. Chem. Soc. 2008, 130, 5616–5617. [Google Scholar] [CrossRef] [PubMed]
- Hummelgard, M.; Zhang, R.; Nilsson, H.E.; Olin, H. Electrical sintering of silver nanoparticle ink studied by In-Situ TEM probing. PLoS ONE 2011, 6, e17209. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cai, W.; Zhang, L.; Wang, G.; Zhang, L. Sonochemical processes and formation of gold nanoparticles within pores of mesoporous silica. J. Colloid Interface Sci. 2001, 238, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; Hsu, H.Y.; El-Sayed, M.A. Gold nanoparticle formation from photochemical reduction of Au3+ by continuous excitation in colloidal Solutions: A proposed molecular mechanism. J. Phys. Chem. 2005, 109, 4811–4815. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, L.; Blanco, M.C.; Lopez-Quintela, M.A. Electrochemical synthesis of silver nanoparticles. J. Phys. Chem. 2002, 104, 9683–9688. [Google Scholar] [CrossRef]
- Starowiicz, M.; Stypula, B.; Banas, J. Electrochemical synthesis of silver nanoparticles. Electrochem. Commun. 2006, 8, 227–230. [Google Scholar] [CrossRef]
- Alexandridis, P. Gold nanoparticle synthesis, morphology control, and stabilization by functional polymers. Chem. Eng. Technol. 2011, 14, 15–38. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhan, J.; Chai, Y.; Yang, C.; Xu, L.; Zhuang, W.; Jing, B. Synthesis of gold nano and microplates in hexagonal liquid crystals. J. Phys. Chem. 2005, 109, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Yang, S.; Ding, B.; Yang, H. Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem. Soc. Rev. 2013, 42, 2880–2904. [Google Scholar] [CrossRef] [PubMed]
- Otanicar, T.P.; Phelan, P.E.; Prasher, R.S.; Rosengarten, G.; Taylor, R.A. Nanofluid based direct absorption solar collector. J. Renew. Sustain. Energy 2010, 2, 033102. [Google Scholar] [CrossRef]
- Khullara, V.; Tyagia, H. A study on environmental impact of nanofluid-based concentrating solar water heating system. Int. J. Environ. Stud. 2012, 69, 220–232. [Google Scholar] [CrossRef]
- Saidur, R.; Meng, T.C.; Said, Z.; Hasanuzzaman, M.; Kamyar, A. Evaluation of the effect of nanofluid-based absorbers on direct solar collector. Int. J. Heat Mass Transf. 2012, 55, 5899–5907. [Google Scholar] [CrossRef]
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Masuda, H.; Ebata, A.; Teramae, K.; Hishinuma, N. Alteration of thermal conductivity and viscosity of liquid by dispersing ultra-fine particles. Netsu Bussei. 1993, 7, 227–233. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal conductivity of nanoparticle fluid mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Duan, F.; Dingtian, K.; Alexandru, C. Viscosity affected by nanoparticle aggregation in Al2O3-water nanofluids. Nanoscale Res. Lett. 2011, 6, 248. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.A.; Choi, U.S.; Li, S.; Thompson, L.J.; Lee, S. Enhanced thermal conductivity through the development of nanofluids. In Proceedings of the Materials Research Society Symposium; Materials Research Society: Pittsburgh, PA, USA, 1997; Volume 457, pp. 3–11. [Google Scholar]
- Hong, K.S.; Hong, T.K.; Yang, H.S. Thermal conductivity of Fe nanofluids depending on the cluster size of nanoparticles. Appl. Phys. Lett. 2006, 88, 031901. [Google Scholar] [CrossRef]
- Lotfizadeh Dehkordi, B.; Ghadimi, A.; Metselaar, H.S.C. Box-Behnken experimental design for investigation of stability and thermal conductivity of TiO2 nanofluids. J. Nanopart. Res. 2013, 15, 1369. [Google Scholar] [CrossRef]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. Surfactants and their applications. Annu. Rep. Prog. Chem. Sect. 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A Review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Li, Y.; Yu, W. Nanofluids containing carbon nanotubes treated by mechanochemical reaction. Thermochim. Acta 2008, 477, 21–24. [Google Scholar] [CrossRef]
- Li, Q.; Yimin, X.; Jian, W. Measurement of the viscosity of dilute magnetic fluids. Int. J. Thermophys. 2006, 27, 103–113. [Google Scholar] [CrossRef]
- Hung, Y.H.; Wen-Chieh, C. Chitosan for suspension performance and viscosity of MWCNTs. Int. J. Chem. Eng. 2012, 3, 347–353. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, H.; Wang, L.; Liu, L. Critical issues in nanofluids preparation, characterization and thermal conductivity. Curr. Nanosci. 2009, 5, 103–112. [Google Scholar] [CrossRef]
- He, Y.; Jin, Y.; Chen, H.; Ding, Y.; Cang, D.; Lu, H. Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int. J. Heat Mass Transf. 2007, 50, 2272–2281. [Google Scholar] [CrossRef]
- Ghadimi, A.; Metselaar, I.H. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 1–9. [Google Scholar] [CrossRef]
- Joni, I.M.; Ogi, T.; Iwaki, T.; Okuyama, K. Synthesis of a colourless suspension of TiO2 nanoparticles by nitrogen doping and the bead-mill dispersion process. Ind. Eng. Chem. Res. 2013, 52, 547–555. [Google Scholar] [CrossRef]
- Kulkarni, D.P.; Das, D.K.; Chukwu, G.A. Temperature dependent rheological property of copper oxide nanoparticles suspension (nanofluid). J. Nanosci. Nanotechnol. 2006, 6, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Baraton, M.I. Surface chemistry and functionalization of semiconducting nanosized particles. In Nanostructures: Synthesis, Functional Properties and Applications; Tsakalakos, T., Ovid‘ko, I., Vasudevan, A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 427–440. [Google Scholar]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites: A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Tang, E.; Cheng, G.; Ma, X.; Pang, X.; Zhao, Q. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system. Appl. Surf. Sci. 2006, 252, 5227–5232. [Google Scholar] [CrossRef]
- Choi, C.; Jung, M.; Choi, Y.; Lee, J.; Oh, J. Tribological properties of lubricating oil-based nanofluids with metal/carbon nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Vander Wal, R.L.; Mozes, S.D.; Pushkarev, V. Nanocarbon nanofluids: Morphology and nanostructure comparisons. Nanotechnology 2009, 20, 105702. [Google Scholar] [CrossRef] [PubMed]
- Halelfadl, S.; Mare, T.; Estelle, P. Efficiency of carbon nanotubes water based nanofluids as coolants. Exp. Therm. Fluid Sci. 2014, 53, 104–110. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H. Surfactant-free nanofluids containing double-and single-walled carbon nanotubes functionalized by a wet-mechanochemical reaction. Thermochim. Acta 2010, 497, 67–71. [Google Scholar] [CrossRef]
- Yu, Q.; Kim, Y.J.; Ma, H. Nanofluids with plasma treated diamond nanoparticles. Appl. Phys. Lett. 2008, 92, 103111. [Google Scholar] [CrossRef]
- Wang, X.-J.; Li, X.F. Influence of pH on nanofluids’ viscosity and thermal conductivity. Chin. Phys. Lett. 2009, 26, 056601. [Google Scholar] [CrossRef]
- Yang, H.G.; Li, C.Z.; Gu, H.C.; Fang, T.N. Rheological behaviour of titanium dioxide suspensions. J. Colloid Interface Sci. 2001, 236, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-F.; Luo, Z.-Y.; Ni, M.J.; Cen, K.F. Dependence of nanofluid viscosity on particle size and pH value. Chin. Phys. Lett. 2009, 26, 066202. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.W.; Kim, B.G. A new parameter to control heat transport in nanofluids: Surface charge state of the particle in suspension. J. Phys. Chem. 2006, 110, 4323–4328. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, J.; Xi, T.; Liu, Y.; Ai, F.; Wu, Q. Thermal conductivity enhancement of suspensions containing nanosized alumina particles. J. Appl. Phys. 2002, 91, 4568–4572. [Google Scholar] [CrossRef]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Fadhillahanafi, N.M.; Leong, K.Y.; Risby, M.S. Stability and thermal conductivity characteristics of carbon nanotube based nanofluids. Int. J. Automot. Mech. Eng. 2013, 8, 1376–1384. [Google Scholar] [CrossRef]
- Xie, H.; Lee, H.; Youn, W.; Choi, M. Nanofluids containing multi-walled carbon nanotubes and their enhanced thermal conductivities. J. Appl. Phys. 2003, 94, 4967–4971. [Google Scholar] [CrossRef]
- Amrollahia, A.; Rashidia, A.M.; Emami Meibodia, M.; Kashefia, K. Conduction heat transfer characteristics and dispersion behaviour of carbon nanofluids as a function of different parameters. J. Exp. Nanosci. 2009, 4, 347–363. [Google Scholar] [CrossRef]
- Yousefi, T.; Shojaeizadeh, E.; Veysi, F.; Zinadini, S. An experimental investigation on the effect of pH variation of MWCNT-H2O nanofluid on the efficiency of a flat-plate solar collector. Sol. Energy 2012, 86, 771–779. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.K.; Lee, J.K.; Jeong, Y.M.; Cheong, S.; Ahn, Y.C.; Kim, S.H. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Zhu, D.; Li, X.; Wang, N.; Wang, X.; Gao, J.; Li, H. Dispersion behaviour and thermal conductivity characteristics of Al2O3-H2O nanofluids. Curr. Appl. Phys. 2009, 9, 131–139. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, X.; Yang, S. Influence of pH and SDBS on the stability and thermal conductivity of nanofluids. Energy Fuels 2009, 23, 2684–2689. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Marneni, N. Preparation, sedimentation, and agglomeration of nanofluids. Chem. Eng. Technol. 2014, 37, 2011–2021. [Google Scholar] [CrossRef]
- Li, X.; Zhu, D.; Wang, X. Evaluation on dispersion behaviour of the aqueous copper nano-suspensions. J. Colloid Interface Sci. 2007, 310, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.S.; James, A.E. Settling and sediment bed behaviour of kaolinite in aqueous media. Sep. Purif. Technol. 2006, 51, 10–17. [Google Scholar] [CrossRef]
- Sen, S.; Govindarajan, V.; Pelliccione, C.J.; Wang, J.; Miller, D.J.; Timofeeva, E.V. Surface modification approach to TiO2 nanofluids with high particle concentration, low viscosity, and electrochemical activity. ACS Appl. Mater. Interfaces 2015, 7, 20538–20547. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, P.E.; Goodson, K.E. Aggregate fractal dimensions and thermal conduction in nanofluids. J. Appl. Phys. 2010, 108, 074309. [Google Scholar] [CrossRef]
- Hidehiro, K.; Motoyuki, I. Surface modification and characterization for dispersion stability of inorganic nanometre-scaled particles in liquid media. Sci. Technol. Adv. Mater. 2010, 11, 044304. [Google Scholar]
- Singh, A.K.; Raykar, V.S. Microwave synthesis of silver nanofluids with polyvinyl pyrrolidone (PVP) and their transport properties. Colloid Polym. Sci. 2008, 286, 1667–1673. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Casanova, C.; Paramo, R.; Barbes, B.; Legido, J.L.; Pineiro, M.M. A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J. Appl. Phys. 2009, 106, 064301-1–064301-8. [Google Scholar] [CrossRef]
- Kim, H.J.; Bang, I.C.; Onoe, J. Characteristic stability of bare Au-water nanofluids fabricated by pulsed laser ablation in liquids. Opt. Lasers Eng. 2009, 47, 532–538. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Sun, J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S.; Barison, S.; Agresti, F. Experimental stability analysis of different water-based nanofluids. Nanoscale Res. Lett. 2011, 6, 300. [Google Scholar] [CrossRef] [PubMed]
- Kole, M.; Dey, T.K. Effect of aggregation on the viscosity of copper oxide-gear oil nanofluids. Int. J. Therm. Sci. 2011, 50, 1741–1747. [Google Scholar] [CrossRef]
- Choi, S.U.S. Enhancing Thermal Conductivity of Fluids with Nanoparticles, Developments and Applications of Non-Newtonian Flows; Siginer, D.A., Wang, H.P., Eds.; ASME: New York, NY, USA, 1995. [Google Scholar]
- Prasher, R.; Song, D.; Wang, J.; Phelan, P. Measurements of nanofluid viscosity and its implications for thermal applications. Appl. Phys. Lett. 2006, 89, 133108–133110. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. J. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Murkherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1284–1306. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.W.; Holliday, R.J.; Thompson, D.T. Developing new industrial applications for gold: Gold nanotechnology. Gold Bull. 2002, 35, 111–117. [Google Scholar] [CrossRef]
- Filho, E.P.B.; Mendoza, O.S.H.; Beicker, C.L.L.; Menezes, A.; Wen, D. Experimental investigation of a silver nanoparticle-based direct absorption solar thermal system. Energy Convers. Manag. 2014, 84, 261–267. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Zhu, J.; Kim, D.R. Enhancement of photo-thermal conversion using gold nanofluids with different particle sizes. Energy Convers. Manag. 2016, 112, 21–30. [Google Scholar] [CrossRef]
- Hong, T.K.; Yang, H.S.; Choi, C.J. Study of the enhanced thermal conductivity of Fe nanofluids. J. Appl. Phys. 2005, 97, 064311. [Google Scholar] [CrossRef]
- Sokhansefat, T.; Kasaeian, A.B.; Kowsary, F. Heat transfer enhancement in parabolic trough collector tube using Al2O3/synthetic oil nanofluid. Renew. Sustain. Energy Rev. 2014, 33, 636–644. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, K.S.; Jang, S.P.; Lee, B.H.; Kim, J.H.; Choi, S.U.S.; Choi, C.J. Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int. J. Heat Mass Transf. 2008, 51, 2651–2656. [Google Scholar] [CrossRef]
- Murshed, S.; Leong, K.; Yang, C. Enhanced thermal conductivity of TiO2—Water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Bozorgan, N.; Shafahi, M. Performance evaluation of nanofluids in solar energy: A review of the recent literature. Micro Nano Syst. Lett. 2015, 3, 1–15. [Google Scholar] [CrossRef]
- Reddy, K.S.; Kamnapure, N.R.; Srivastava, S. Nanofluid and nanocomposite applications in solar energy conversion systems for performance enhancement: A review. Int. J. Low Carbon Technol. 2017, 12, 1–23. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminister fullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Yang, Z.P.; Ci, L.; Bur, J.A.; Lin, S.Y.; Ajayan, P.M. Experimental observation of an extremely dark material made by a low density nano-tube array. Nano Lett. 2008, 8, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Singer, P. CNTs an attractive alternative for interconnects (carbon nano-tubes). Semicond. Int. 2007, 30, 26–33. [Google Scholar]
- Poncharal, P.; Berger, C.; Yi, Y.; Wang, Z.L.; de Heer Walt, A. Room temperature ballistic conduction in carbon nanotubes. J. Phys. Chem. 2002, 106, 12104–12118. [Google Scholar] [CrossRef]
- Iijima, S. Helical micro-tubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Hordy, N.; Rabilloud, D.; Meunier, J.L.; Coulombe, S. High temperature and long-term stability of carbon nanotube nanofluids for direct absorption solar thermal collectors. Sol. Energy 2014, 105, 82–90. [Google Scholar] [CrossRef]
- Karami, M.; Raisee, M.; Delfani, S.; Akhavan Bahabadi, M.A.; Rashidi, A.M. Sunlight absorbing potential of carbon nanoball water and ethylene glycol-based nanofluids. Opt. Spectrosc. 2013, 115, 400–405. [Google Scholar] [CrossRef]
- Dorset, D.L.; Fryer, J.R. Quantitative electron crystallographic determinations of higher fullerenes in the hexagonal close packed polymorph. J. Phys. Chem. 2001, 105, 2356–2359. [Google Scholar] [CrossRef]
- Kuzuo, R.; Terauchi, M.; Tanaka, M.; Saito, Y.; Shinohara, H. Electron-energy-loss spectra of crystalline C 84. Phys. Rev. 1994, 49, 5054–5057. [Google Scholar] [CrossRef]
- Ge, M.; Sattler, K. Observation of fullerene cones. Chem. Phys. Lett. 1994, 220, 192–196. [Google Scholar] [CrossRef]
- Yoshitake, T.; Shimakawa, Y.; Kuroshima, S.; Kimura, H.; Ichihashi, T.; Kubo, Y.; Kasuya, D.; Takahashi, K.; Kokai, F.; Yudasaka, M.; et al. Preparation of fine platinum catalyst supported on single-wall carbon nanohorns for fuel cell application. Phys. Condens. Matter 2002, 323, 124–126. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yamada, M.; Miyake, M. Synthesis of carbon nano-tubes and carbon nanofilaments over palladium supported catalysts. Sci. Technol. Adv. Mater. 2005, 6, 420–426. [Google Scholar] [CrossRef]
- Sano, N.; Akazawa, H.; Kikuchi, T.; Kanki, T. Separated synthesis of iron-included carbon nanocapsules and nanotubes by pyrolysis of ferrocene in pure hydrogen. Carbon 2003, 41, 2159–2162. [Google Scholar] [CrossRef]
- Ladjevardi, S.M.; Asnaghi, A.; Izadkhast, P.S.; Kashani, A.H. Applicability of graphite nanofluids in direct solar energy absorption. Sol. Energy 2013, 94, 327–334. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Chen, J.; Zeng, J.; Tan, S. Preparation of monodisperse carbon nanospheres for electrochemical capacitors. Electrochem. Commun. 2008, 10, 1067–1070. [Google Scholar] [CrossRef]
- Yang, R.; Qiu, X.; Zhang, H.; Li, J.; Zhu, W.; Wang, Z.; Huang, X.; Chen, L. Mono-dispersed hard carbon spherules as a catalyst support for the electro-oxidation of methanol. Carbon 2005, 43, 11–16. [Google Scholar] [CrossRef]
- Wang, Y.; Su, F.; Wood, C.D.; Lee, J.Y.; Zhao, X.S. Preparation and characterization of carbon nanospheres as anode materials in lithium-ion secondary batteries. Ind. Eng. Chem. Res. 2008, 47, 2294–2300. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Brundavanam, S.; Shah, M.; Laava, I.; Fawcett, D. Photothermal response of CVD synthesised carbon (nano) spheres/aqueous nanofluids for potential application in direct solar absorption collectors: A preliminary investigation. Nanotechnol. Sci. Appl. 2012, 5, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Harish, S.; Ishikawa, K.; Einarsson, E.; Aikawa, S.; Chiashi, S.; Shiomi, J.; Maruyama, S. Enhanced thermal conductivity of ethylene glycol with single-walled carbon nanotube inclusions. Int. J. Heat Mass Transf. 2012, 55, 3885–3890. [Google Scholar] [CrossRef]
- Harish, S.; Ishikawa, K.; Einarsson, E.; Aikawa, S.; Inoue, T.; Zhao, P.; Watanabe, M.; Chiashi, S.; Shiomi, J.; Maruyama, S. Temperature dependent thermal conductivity increase of aqueous nanofluid with single walled carbon nanotube inclusion. Mater. Express 2012, 2, 213–223. [Google Scholar] [CrossRef]
- Glory, J.; Bonetti, M.; Helezen, M.; Mayne-L’Hermite, M.; Reynaud, C. Thermal and electrical conductivities of water-based nanofluids prepared with long multi-walled carbon nanotubes. J. Appl. Phys. 2008, 103, 094309. [Google Scholar] [CrossRef]
- Wusiman, K.; Jeong, H.; Tulugan, K.; Afrianto, H.; Chung, H. Thermal performance of multi-walled carbon nanotubes (MWCNTs) in aqueous suspensions with surfactants SDBS and SDS. Int. Commun. Heat Mass Transf. 2013, 41, 28–33. [Google Scholar] [CrossRef]
- Lotfi, R.; Rashidi, A.M.; Amrollahi, A. Experimental study on the heat transfer enhancement of MWNT-water nanofluid in a shell and tube heat exchanger. Int. Commun. Heat Mass Transf. 2012, 39, 108–111. [Google Scholar] [CrossRef]
- Natarajan, E.; Sathish, R. Role of nanofluids in solar water heater. Int. J. Adv. Manuf. Technol. 2009, 1, 3–7. [Google Scholar] [CrossRef]
- Karami, M.; Bahabadi, M.A.A.; Delfani, S.; Ghozatloo, A. A new application of carbon nanotubes nanofluid as working fluid of low-temperature direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2014, 121, 114–118. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, S.P. Extinction coefficient of aqueous nanofluids containing multi-walled carbon nanotubes. Int. J. Heat Mass Transf. 2013, 67, 930–935. [Google Scholar] [CrossRef]
- Mare, T.; Halelfadl, S.; Van Vaerenbergh, S.; Estelle, P. Unexpected sharp peak in thermal conductivity of carbon nanotubes water-based nanofluids. Int. Commun. Heat Mass Transf. 2015, 66, 80–83. [Google Scholar] [CrossRef]
- Branson, B.T.; Beauchamp, P.S.; Beam, J.C.; Lukehart, C.M.; Davidson, J.L. Nanodiamond nanofluids for enhanced thermal conductivity. ACS Nano 2013, 7, 3183–3189. [Google Scholar] [CrossRef] [PubMed]
- Taha-Tijerina, J.J.; Narayanan, T.N.; Tiwary, C.S.; Lozano, K.; Chipara, M.; Ajayan, P.M. Nanodiamond-based thermal fluids. ACS Appl. Mater. Interfaces 2014, 6, 4778–4785. [Google Scholar] [CrossRef] [PubMed]
- Ghazvini, M.; Akhavan-Behabadi, M.A.; Rasouli, E.; Raisee, M. Heat transfer properties of nanodiamond–engine oil nanofluid in laminar flow. Heat Transf. Eng. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Ma, H.B.; Wilson, C.; Borgmeyer, B.; Park, K.; Yu, Q.; Choi, S.U.S.; Tirumala, M. Effect of nanofluid on the heat transport capability in an oscillating heat pipe. Appl. Phys. Lett. 2006, 88, 143116–143119. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Li, Y.; Chen, L.; Wang, Q. Experimental investigation on the thermal transport properties of ethylene glycol based nanofluids containing low volume concentration diamond nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2011, 380, 1–5. [Google Scholar] [CrossRef]
- Assael, M.J.; Metaxa, I.N.; Kakosimos, K.; Constantinou, D. Thermal conductivity of nanofluids—Experimental and theoretical. Int. J. Thermophys. 2006, 27, 999–1017. [Google Scholar] [CrossRef]
- Choi, M.Y.; Kim, D.S.; Hong, D.S.; Kim, J.H.; Kim, Y.T. Ultrastable aqueous graphite nanofluids prepared by single-step liquid-phase pulsed laser ablation (LP-PLA). Chem. Lett. 2011, 40, 768–769. [Google Scholar] [CrossRef]
- Gupta, S.S.; Siva, V.M.; Krishnan, S.; Sreeprasad, T.S.; Singh, P.K.; Pradeep, T.; Das, S.K. Thermal conductivity enhancement of nanofluids containing graphene nanosheets. J. Appl. Phys. 2011, 110, 084302. [Google Scholar] [CrossRef]
- Dhar, P.; Gupta, S.S.; Chakraborty, S.; Pattamatta, A.; Das, S.K. The role of percolation and sheet dynamics during heat conduction in poly-dispersed graphene nanofluids. Appl. Phys. Lett. 2013, 102, 163114. [Google Scholar]
- Taylor, R.A.; Phelan, P.E.; Otanicar, T.P.; Walker, C.A.; Nguyen, M.; Trimble, S.; Prasher, R. Applicability of nanofluids in high flux solar collectors. J. Renew. Sustain. Energy 2011, 3, 023104-1–023104-15. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Sankaranarayanan, V.; Ramaprabhu, S. Graphene-based engine oil nanofluids for tribological applications. ACS Appl. Mater. Interfaces 2011, 3, 4221–4227. [Google Scholar] [CrossRef] [PubMed]
- Ghozatloo, A.; Rashidi, A.; Shariaty-Niassar, M. Convective heat transfer enhancement of graphene nanofluids in shell and tube heat exchanger. Exp. Therm. Fluid Sci. 2014, 53, 136–141. [Google Scholar] [CrossRef]
- Kirilov, R.; Girginov, C.; Stefchev, P. Black nanofluids for solar absorption on the basis of hydrogen peroxide treated carbon particles. Adv. Nat. Sci. Theory Appl. 2013, 2, 31–40. [Google Scholar]
- Meng, Z.; Han, D.; Wu, D.; Zhu, H.; Li, Q. Thermal Conductivities, Rheological Behaviours and Photothermal Properties of Ethylene Glycol-based Nanofluids Containing Carbon Black Nanoparticles. Proc. Eng. 2012, 36, 521–527. [Google Scholar] [CrossRef]
- Sharma, P.; Baek, I.H.; Cho, T.; Park, S.; Lee, K.B. Enhancement of thermal conductivity of ethylene glycol based silver nanofluids. Powder Technol. 2011, 208, 7–19. [Google Scholar] [CrossRef]
- Liu, M.S.; Lin, M.C.C.; Huang, I.T.; Wang, C.C. Enhancement of thermal conductivity with CuO for nanofluids. Chem. Eng. Technol. 2006, 29, 72–77. [Google Scholar] [CrossRef]
- Mintsa, H.A.; Roy, G.; Nguyen, C.T.; Doucet, D. New temperature dependent thermal conductivity data for water-based nanofluids. Int. J. Therm. Sci. 2009, 48, 363–371. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, H.S.; Oh, J.M. Preparation and heat transfer properties of nanoparticle in transformer oil dispersions as advanced energy-efficient coolants. Curr. Appl. Phys. 2008, 8, 710–712. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Fujii, M. Experimental study on the effective thermal conductivity and thermal diffusivity of nanofluid. Int. J. Thermophys. 2006, 27, 569–580. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Hosseinizadeh, S.F.; Alipanah, M.; Dehghani, A.; Vakilinejad, G.R. Numerical simulation of free convection based on experimental measured conductivity in a square cavity using water/SiO2 nanofluid. Int. Commun. Heat Mass Transf. 2010, 37, 687–694. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Amiri, A.; Montazer, E.; Hamed Khajeh Arzani, H.K.; Sadri, R.; Mahidzal Dahari, M.; Kazi, S.N. Nanofluid based on activated hybrid of biomass carbon/graphene oxide: Synthesis, thermo-physical and electrical properties. Int. Commun. Heat Mass Transf. 2016, 72, 10–15. [Google Scholar] [CrossRef]
- Marquis, F.; Chibante, L. Improving the heat transfer of nanofluids and nanolubricants with carbon nanotubes. JOM 2005, 57, 32–43. [Google Scholar] [CrossRef]
- Indhuja, A.; Suganthi, K.S.; Manikandan, S.; Rajan, K.S. Viscosity and thermal conductivity of dispersions of gum arabic capped MWCNT in water: Influence of MWCNT concentration and temperature. J. Taiwan Inst. Chem. Eng. 2013, 44, 474–479. [Google Scholar]
- Maxwell, J.C. A Treatise on Electricity and Magnetism, 3rd ed.; Oxford University Press: London, UK, 1892; Volume 2. [Google Scholar]
- Azari, A.; Kalbasi, M.; Moazzeni, A.; Rahman, A. A Thermal conductivity model for nanofluids heat transfer enhancement. Pet. Sci. Technol. 2014, 32, 91–99. [Google Scholar] [CrossRef]
- Koo, J.; Kleinstreuer, C. A new thermal conductivity model for nanofluids. J. Nanopart. Res. 2005, 6, 577–588. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U.S. Effects of various parameters on nanofluid thermal conductivity. J. Heat Transf. 2007, 129, 617–623. [Google Scholar] [CrossRef]
- Evans, W.; Fish, J.; Keblinski, P. Role of Brownian motion hydrodynamics on nanofluid thermal conductivity. Appl. Phys. Lett. 2006, 88, 093116. [Google Scholar] [CrossRef]
- Kumar, D.H.; Patel, H.E.; Kumar, R.V.R.; Sundararajan, T.; Pradeep, T.; Das, S.K. Model for heat conduction in nanofluids. Phys. Rev. Lett. 2004, 93, 144301. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.; Crosser, O.K. Thermal conductivity of heterogeneous two-component systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Evans, W.; Prasher, R.; Fish, J.; Meakin, P.; Phelan, P.; Keblinski, P. Effect of Aggregation and Interfacial Thermal Resistance on Thermal Conductivity of Nanocomposites and Colloidal Nanofluids. Int. J. Heat Mass Transf. 2008, 51, 1431–1438. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U.S. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Li, C.H.; Williams, W.; Buongiorno, J.; Hu, L.; Peterson, G.P. Transient and steady-state experimental comparison study of effective thermal conductivity of Al2O3/Water Nanofluids. J. Heat Transf. 2008, 130, 42407-1–42407-7. [Google Scholar] [CrossRef]
- Wojnar, R. The Brownian motion in a Thermal Field. Acta Phys. Pol. 2001, 32, 333–349. [Google Scholar]
- Patel, H.E.; Sundararajan, T.; Pradeep, T.; Dasgupta, A.; Dasgupta, N.; Das, S.K. A micro-convection model for thermal conductivity of nanofluids. Pramana J. Phys. 2005, 65, 863–869. [Google Scholar] [CrossRef]
- Prasher, R.; Bhattachara, P.; Phelan, P.E. Thermal conductivity of nanoscale colloidal solutions (Nanofluids). Am. Phys. Soc. 2005, 94, 025901. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Saha, S.K.; Yadav, A.; Phelan, P.E.; Prasher, R.S. Brownian dynamics simulation to determine the effective thermal conductivity of nanofluids. J. Appl. Phys. 2004, 95, 6492–6494. [Google Scholar] [CrossRef]
- Ren, Y.; Xie, H.; Cai, A. Effective thermal conductivity of nanofluids containing spherical nanoparticles. J. Phys. Appl. Phys. 2005, 38, 3958–3961. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.R.; Choi, U.S.U.; Eastman, J.A. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass Transf. 2002, 45, 855–863. [Google Scholar] [CrossRef]
- Xie, H.Q.; Fujii, M.; Zhang, X. Effect of interfacial layer on the effective thermal conductivity of nanoparticle-fluid mixture. Int. J. Heat Mass Transf. 2005, 48, 2926–2932. [Google Scholar] [CrossRef]
- Yu, C.J.; Richter, A.G.; Datta, A.; Durbin, M.K.; Dutta, P. Molecular layering in a liquid on a solid substrate: an X-ray reflectivity study. Physica 2000, 283, 27–31. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Hamilton-Crosser model. J. Nanopart. Res. 2004, 6, 355–361. [Google Scholar] [CrossRef]
- Leong, K.C.; Yang, C.; Murshed, S.M.S. A model for the thermal conductivity of nanofluids—The effect of interfacial layer. J. Nanopart. Res. 2006, 8, 245–254. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Hu, W. Aggregation structure and thermal conductivity of nanofluids. AIChE J. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Wang, B.X.; Zhou, L.P.; Peng, X.F. A fractal model for predicting the effective thermal conductivity of liquid with suspension of nanoparticles. Int. J. Heat Mass Transf. 2003, 46, 2665–2672. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, B.; Xu, P.; Zou, M. The effective thermal conductivity of nanofluids based on the nanolayer and the aggregation of nanoparticles. J. Phys. Appl. Phys. 2007, 40, 3164–3171. [Google Scholar] [CrossRef]
- Xu, J.; Yu, B.-M.; Yun, M.-J. Effect of clusters on thermal conductivity in nanofluids. Chin. Phys. Lett. 2006, 23, 2819–2822. [Google Scholar]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Thermophysical and electrokinetic properties of nanofluids: A critical review. Appl. Therm. Eng. 2008, 28, 2109–2125. [Google Scholar] [CrossRef]
- Mishra, P.C.; Mukherjee, S.; Nayak, S.K.; Panda, A. A brief review on viscosity of nanofluids. Int. Nano Lett. 2014, 4, 109–120. [Google Scholar] [CrossRef]
- Yousefi, T.; Veysi, F.; Shojaeizadeh, E.; Zinadini, S. An experimental investigation on the effect of Al2O3-H2O nanofluid on the efficiency of flat plate solar collectors. Renew. Energy 2012, 39, 293–298. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Zeng, S.; Zheng, Z. Experimental investigation on photothermal properties of nanofluids for direct absorption solar thermal energy systems. Energy Convers. Manag. 2013, 73, 150–157. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Effect of morphology of carbon nanomaterials on thermo-physical characteristics, optical properties and photo-thermal conversion performance of nanofluids. Renew. Energy 2016, 99, 888–897. [Google Scholar] [CrossRef]
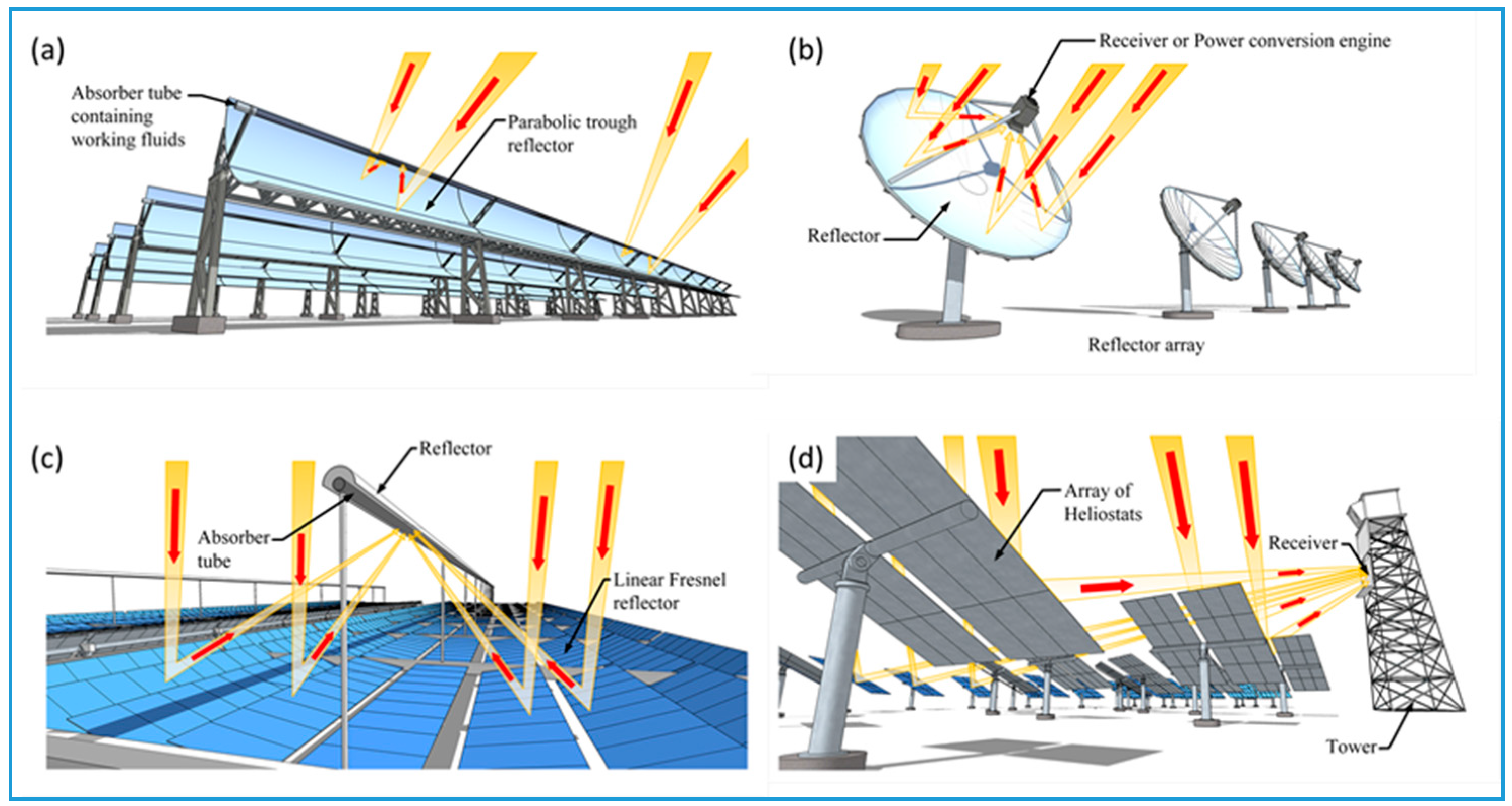
| Motion | Collector Configuration | Concentration Ratio | Temperature Range (°C) | Reference | |||
|---|---|---|---|---|---|---|---|
| Name/Absorber | |||||||
| Stationary | Flat plate collector | FPC | Flat | C ≤ 1 | 30 ≤ T ≤ 80 | [20] | |
| Evacuated tube collector | ETC | C ≤ 1 | 50 ≤ T ≤ 230 | [21] | |||
| Compound parabolic | CPC | Tubular | 1 ≤ C ≤ 5 | 60 ≤ T ≤ 240 | [22] | ||
| Sun Tracking | Single axis | Collector | 5 ≤ C ≤ 15 | 60 ≤ T ≤ 290 | |||
| Fresnel lens collector | FLC | 10 ≤ C ≤ 40 | 60 ≤ T ≤ 270 | [23] | |||
| Parabolic trough collector | PTC | 15 ≤ C ≤ 45 | 60 ≤ T ≤ 400 | [24] | |||
| Cylindrical trough collector | CTC | 10 ≤ C ≤ 50 | 60 ≤ T ≤ 400 | [25] | |||
| Two axis | Spherical Bowl Reflector | SBR | Point | 100 ≤ C ≤ 300 | 70 ≤ T ≤ 700 | [26] | |
| Parabolic dish reflector | PDR | 100 ≤ C ≤ 1000 | 100 ≤ T ≤ 900 | [26] | |||
| Heliostat field collector | HFC | 100 ≤ C ≤ 1500 | 150 ≤ T ≤ 2000 | [27] | |||
| Material | Thermal Conductivity (Wm−1K−1) | Reference |
|---|---|---|
| Metals | ||
| Gold | 315 | [72] |
| Silver | 424 | [72] |
| Copper | 398 | [72] |
| Aluminum | 273 | [72] |
| Iron | 80 | [72] |
| Steel | 46 | [21] |
| Stainless Steel | 16 | [21] |
| Metal Oxides | ||
| Alumina (Al2O3) | 40 | [73] |
| Cupric Oxide | 77 | [57] |
| Iron (II, III) Oxide | 7 | [74] |
| Titanium dioxide | 8.37 | [75] |
| Zinc Oxide | 29 | [76] |
| Carbons | ||
| Amorphous Carbon | 1.59 | [77] |
| Diamond | 900–2320 | [77] |
| Carbon Nano-fibers | 13 | [78] |
| Carbon Nanotubes | 2000 | [79] |
| C60–C70 (Fullerenes) | 0.4 | [57] |
| Graphite | 2000 | [80] |
| Working Fluids | ||
| Water | 0.608 | [81] |
| Ethylene Glycol | 0.257 | [72] |
| Nanoparticle | Particle Size (nm) | Working Fluid | Fraction | Thermal Enhancement (%) | Reference |
|---|---|---|---|---|---|
| Metals | |||||
| Ag | <100 | Water | 0.3–0.9 vol % | 30 at 50 °C | [50] |
| Ag | 100–500 | Ethylene Glycol | 0.1–1.0 vol % | 18 | [205] |
| Cu | 50–100 | Water | 0.1 vol % | 24 | [206] |
| Cu | <10 | Ethylene Glycol | 0.01–0.05 vol % | 41 | [67] |
| Fe | 10 | Ethylene Glycol | 0.1–0.55 vol % | 18 | [103] |
| Metal Oxides | |||||
| Al2O3 | 9 | Water | 2–10 vol % | 29 | [207] |
| Al2O3 | 28 | Water/Ethylene Glycol | 3–8 vol % | 41 | [100] |
| Al2O3 | 650–1000 | Transformer oil | 0.5–4 vol % | 20 | [208] |
| CuO | 100 | Water | 7.5 vol % | 52 | [209] |
| TiO2 | 15 | Water | 0.5–5 vol % | 30 | [159] |
| SiO2 | 12 | Ethylene Glycol | 1–4 vol % | 23 | [210] |
| Carbons | |||||
| Carbon Black | 190 | Water | 4.4–7.7 vol % | 10 at 35 °C | [55] |
| carbon/graphene oxide | Not specified | Ethylene Glycol | 0–0.06 wt % | 6.47 at 40 °C | [211] |
| SWCNT | Dia. 10–50 Len. 0.3–10 µm | Diesel Oil | 0.25–1 vol % | 10–46 | [212] |
| MWCNT | 25 nm × 50 µm | Oil | 1 vol % | 150 | [79] |
| MWCNT | Dia. 10 Len. 5–15 µm | Gum Arabic & Water | 0.14-0.24 vol % | 10 | [213] |
| Nanoparticle Size (nm) | Working Fluid | Fraction | Major Result of Study | Reference |
|---|---|---|---|---|
| Al2O3 Below 20 | Pure water | 1.0 vol % | Collector efficiency increases partially with increasing in particle size | [97] |
| Al2O3 15 | Distilled Water + Triton X-100 | 0.2 wt % 0.4 wt % | Collector efficiency at 0.2 wt % is 28.3% above working fluid of water. With surfactant efficiency was 15.63% | [241] |
| CuO 25 & 50 | Deionized water (surfactant) | 0.01, 0.02, 0.04, 0.1, 0.2 wt % | Good absorption while transmittance decreases with increasing nanoparticle size and mass fraction. | [242] |
| TiO2 ,10; Al2O3, 20 Ag, 50; Cu, 50, SiO2, 50 | Texatherm Oil | 0.1, 1.0, 2.0, 3.0 vol % | Collector outlet temperature and efficiency were improved using Ag, Cu, SiO2 and Al2O3, but not for TiO2. | [45] |
| Graphite, 35 | Texatherm Oil | 0.05, 3.0, 5.0 vol % | 5.0 vol % significantly improved thermal conductivity compare to Texatherm oil | [45] |
| CNT Dia. 10–20 nm Length 10–30 µm | Texatherm Oil | 1.0 vol % | Minor improvement in thermal conductivity of base working fluid | [45] |
| CNT Dia. 10–20 nm Length 0.5–2 µm | Texatherm Oil | 1.0 vol % | Minor improvement in thermal conductivity of base working fluid | [45] |
| Carbon nanomaterials | * Ionic Liquid (BMIM)BF4 | 0.005 wt % 0.01 wt % | Temperature range 20 to 145 °C Thermal conductivity performance | [243] |
| Graphite 30 | (BMIM)BF4 | 0.005 wt % 0.01 wt % | 1.0 to 3.4% 9.4 to 10.7% | [243] |
| SWCNT, Dia. 2 nm Length 5 to 30 µm | (BMIM)BF4 | 0.005 wt % 0.01 wt % | 6.2 to 5.8% 14.7 to 14.7% | [243] |
| Graphene 0.8 nm single layer Range: 0.5 to 2 µm | (BMIM)BF4 | 0.005 wt % 0.01 wt % | 13.9 to 14.5% 16.3 to 15.4% Carbons also displayed significant temperature enhancement compared to base working fluid | [243] |
| Functionalized CNT | Deionized water | 150 ppm | Thermal conductivity enhancement of 32.2% Also displayed good optical properties | [188] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamsa-ard, W.; Brundavanam, S.; Fung, C.C.; Fawcett, D.; Poinern, G. Nanofluid Types, Their Synthesis, Properties and Incorporation in Direct Solar Thermal Collectors: A Review. Nanomaterials 2017, 7, 131. https://doi.org/10.3390/nano7060131
Chamsa-ard W, Brundavanam S, Fung CC, Fawcett D, Poinern G. Nanofluid Types, Their Synthesis, Properties and Incorporation in Direct Solar Thermal Collectors: A Review. Nanomaterials. 2017; 7(6):131. https://doi.org/10.3390/nano7060131
Chicago/Turabian StyleChamsa-ard, Wisut, Sridevi Brundavanam, Chun Che Fung, Derek Fawcett, and Gerrard Poinern. 2017. "Nanofluid Types, Their Synthesis, Properties and Incorporation in Direct Solar Thermal Collectors: A Review" Nanomaterials 7, no. 6: 131. https://doi.org/10.3390/nano7060131
APA StyleChamsa-ard, W., Brundavanam, S., Fung, C. C., Fawcett, D., & Poinern, G. (2017). Nanofluid Types, Their Synthesis, Properties and Incorporation in Direct Solar Thermal Collectors: A Review. Nanomaterials, 7(6), 131. https://doi.org/10.3390/nano7060131





