Electrolytic Manganese Dioxide Coatings on High Aspect Ratio Micro-Pillar Arrays for 3D Thin Film Lithium Ion Batteries
Abstract
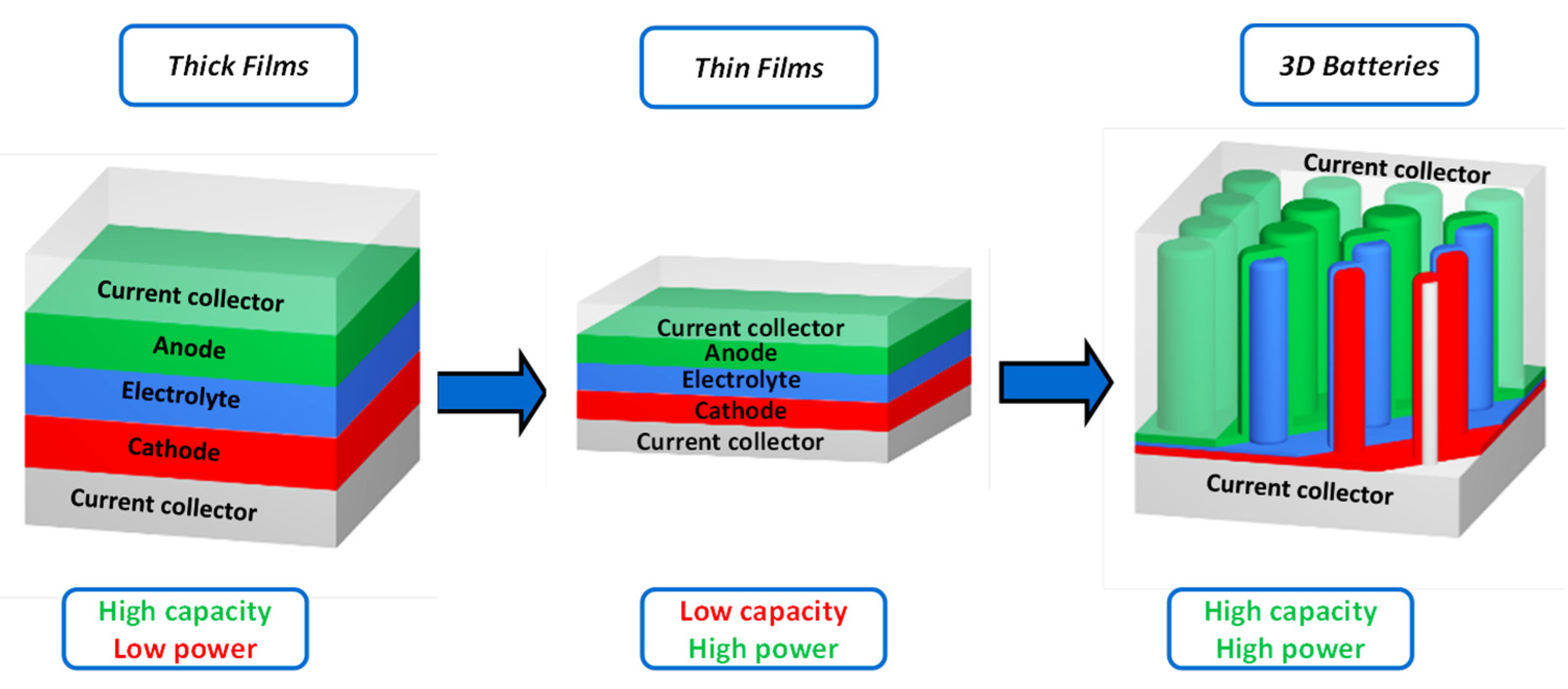
:1. Introduction
2. Experimental
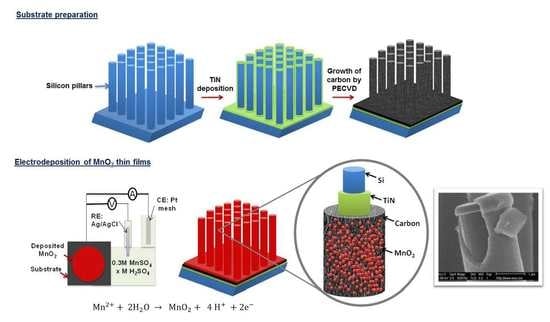
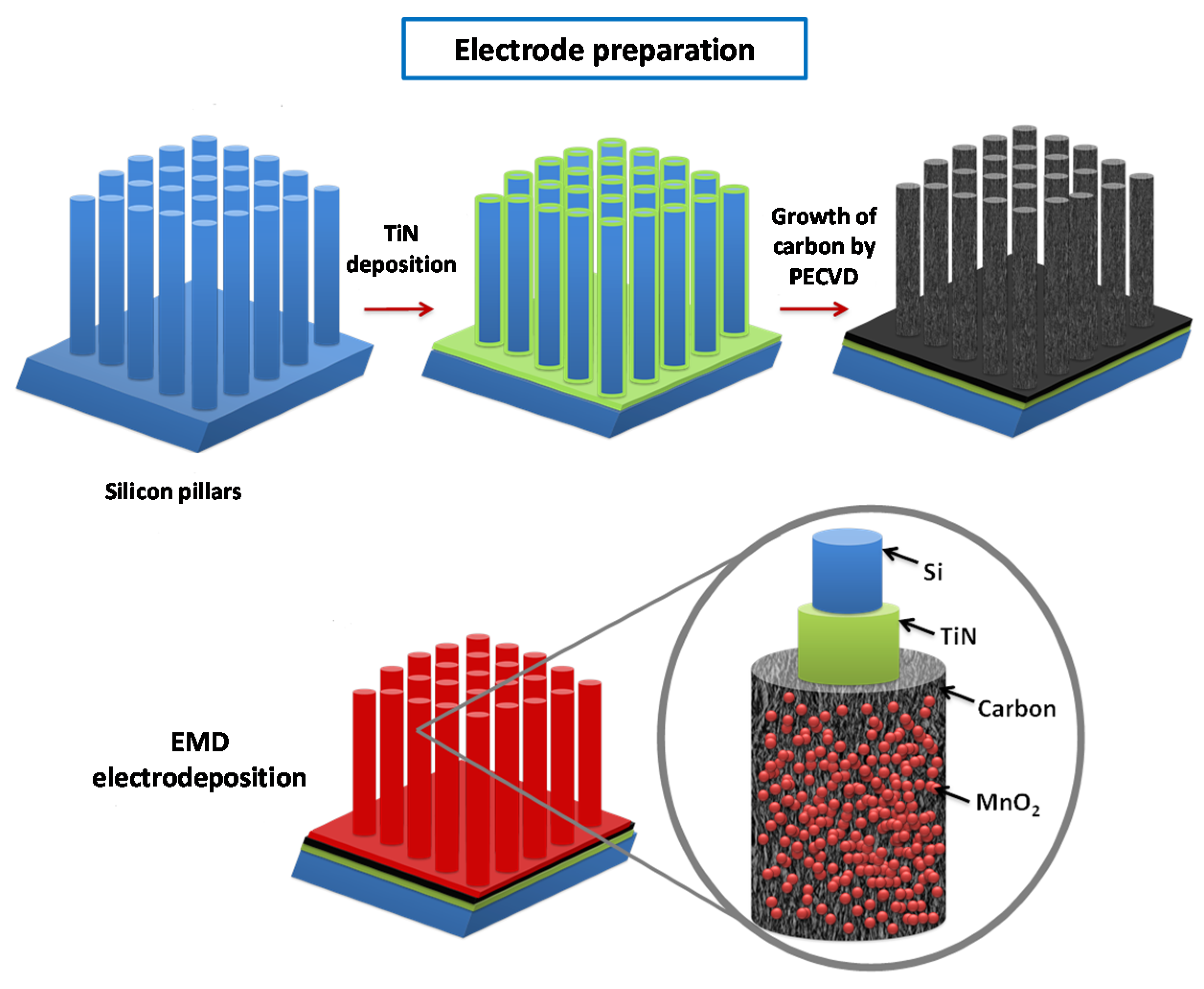
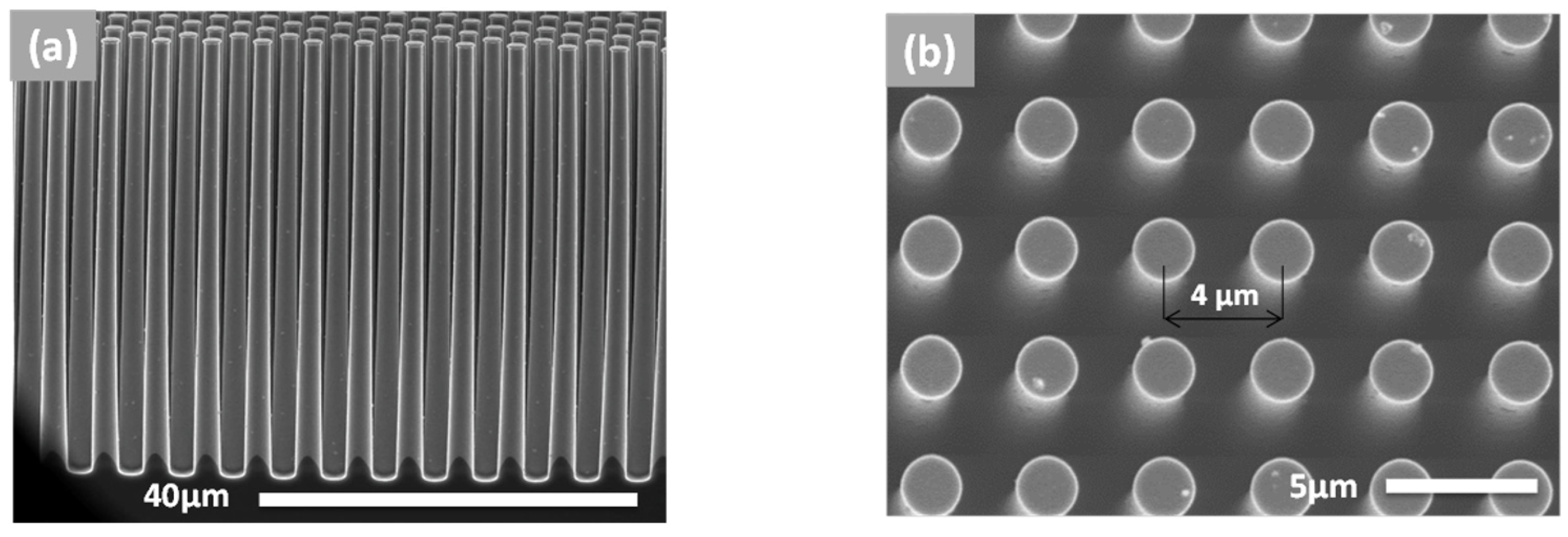
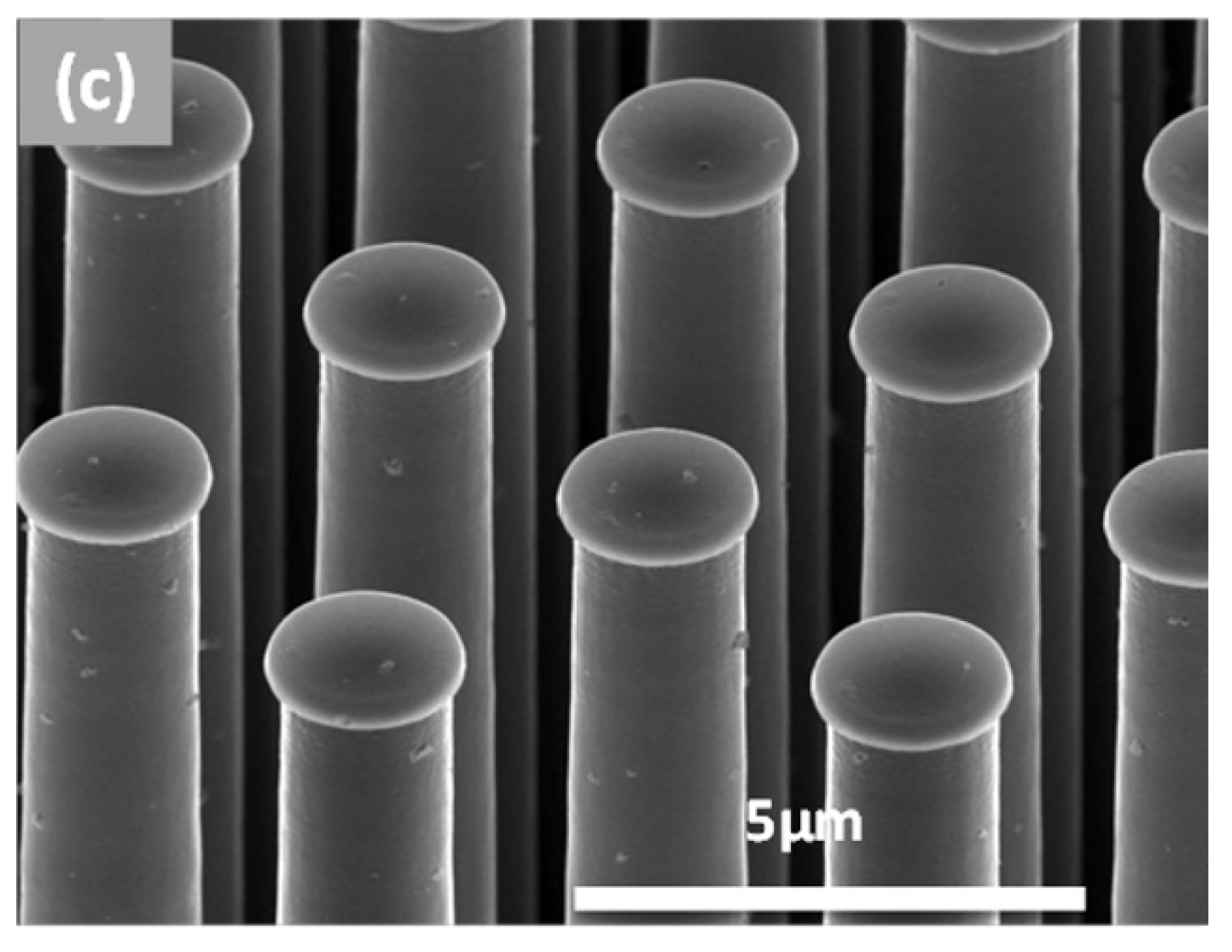
2.1. Fabrication of Silicon Pillar Arrays
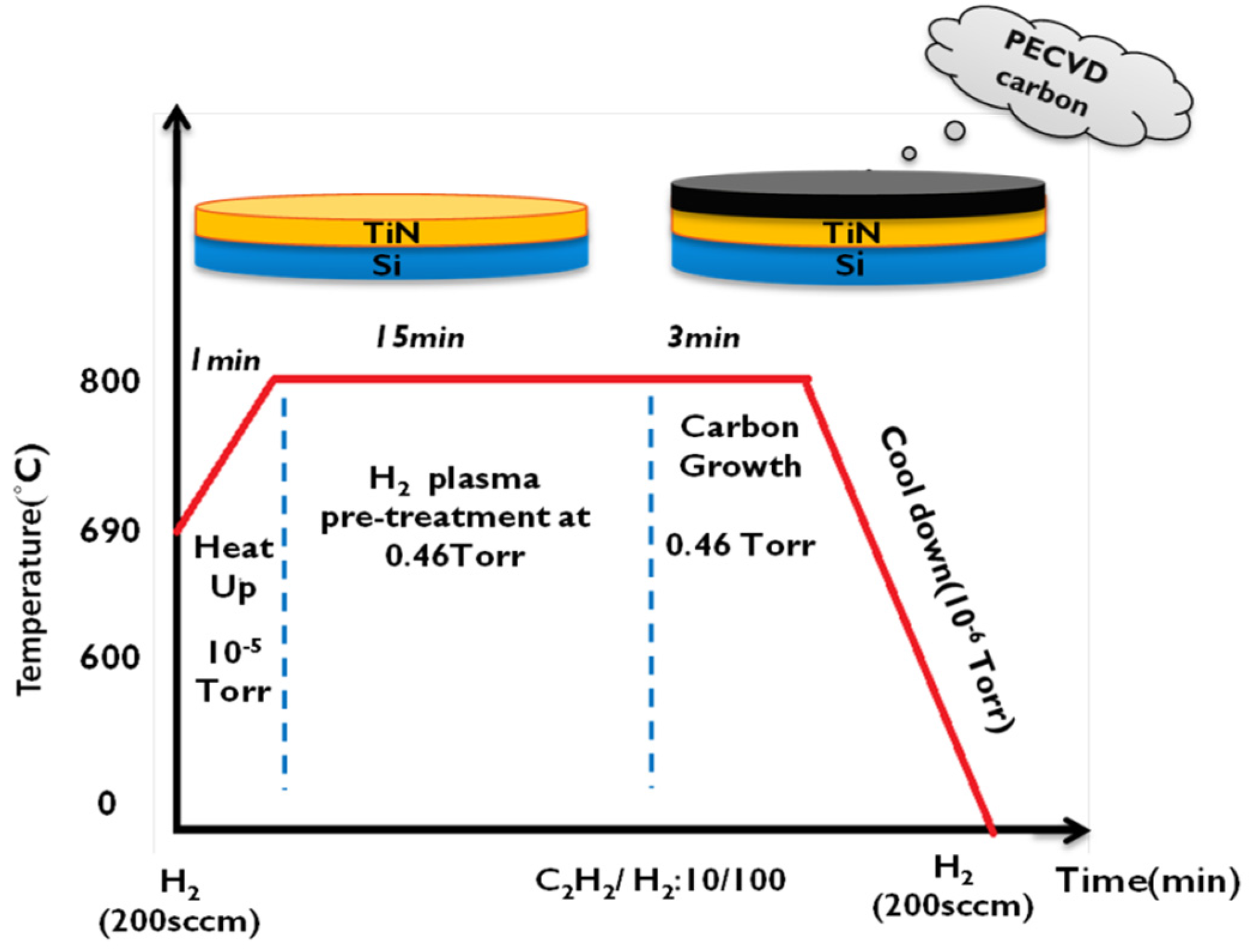
2.2. Carbon Coating on TiN/Si Pillars
2.3. Deposition of EMD Thin Films on Carbon Coated TiN/Si Pillars
2.4. Physical and Electrochemical Characterization of EMD Thin Films
3. Results and Discussion
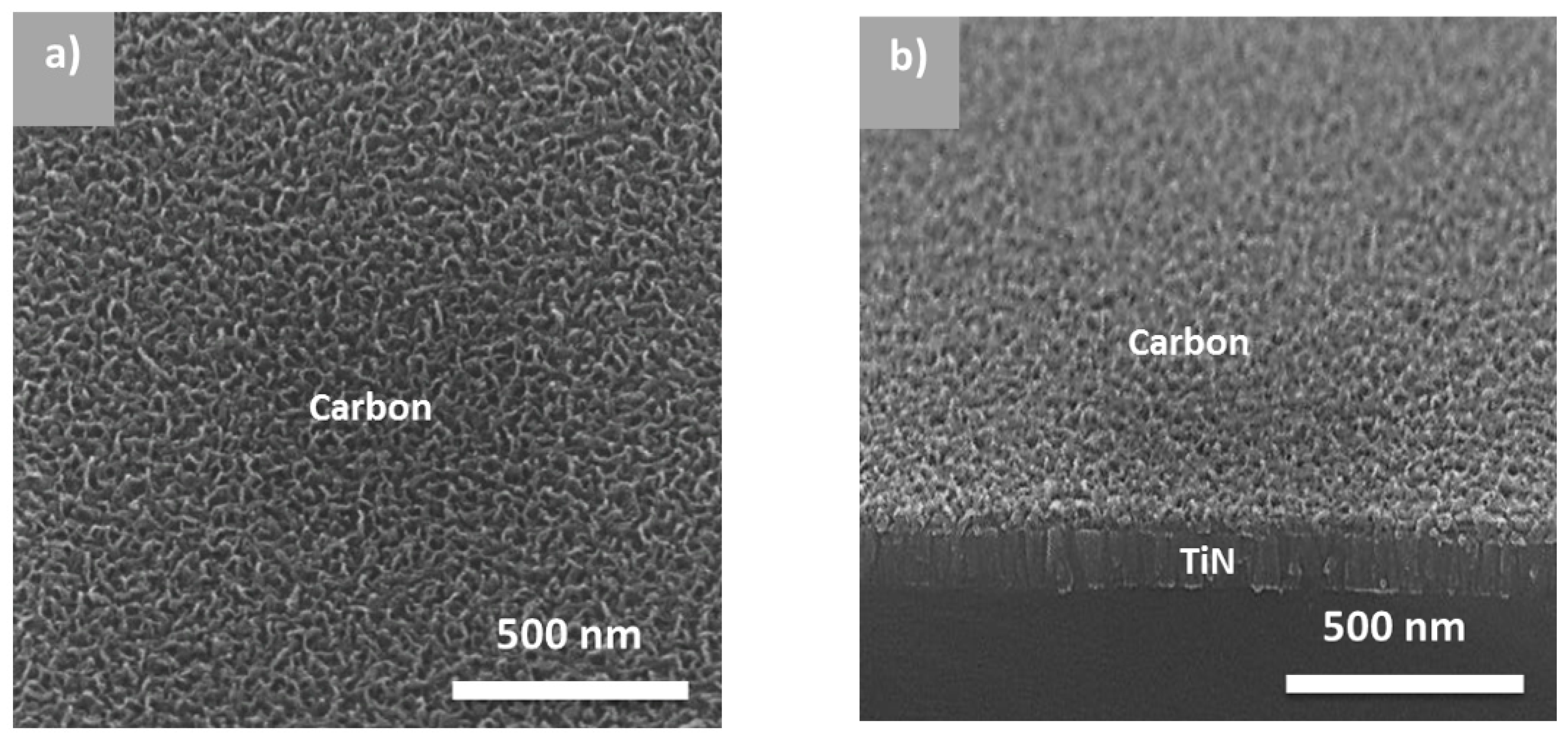
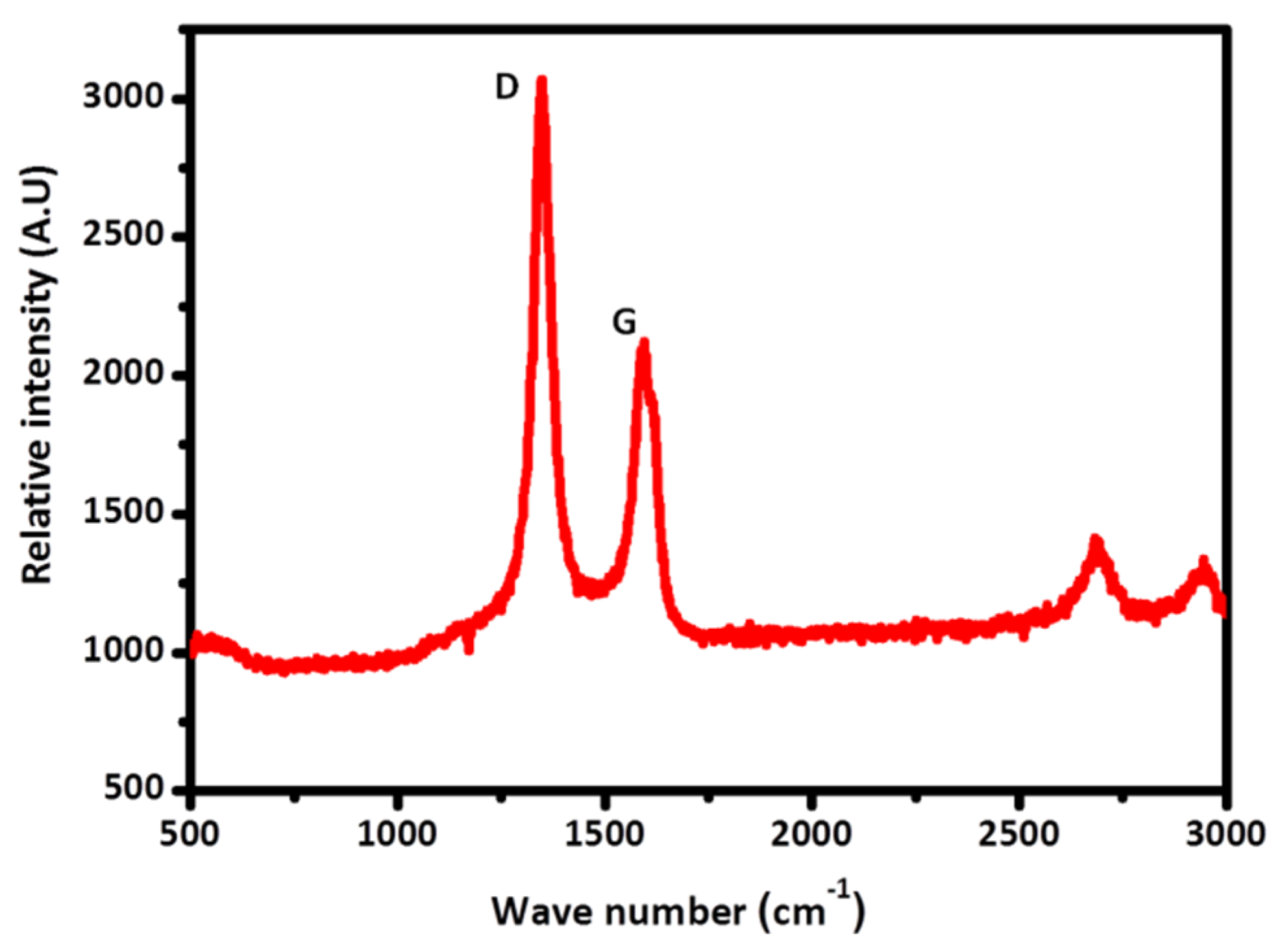
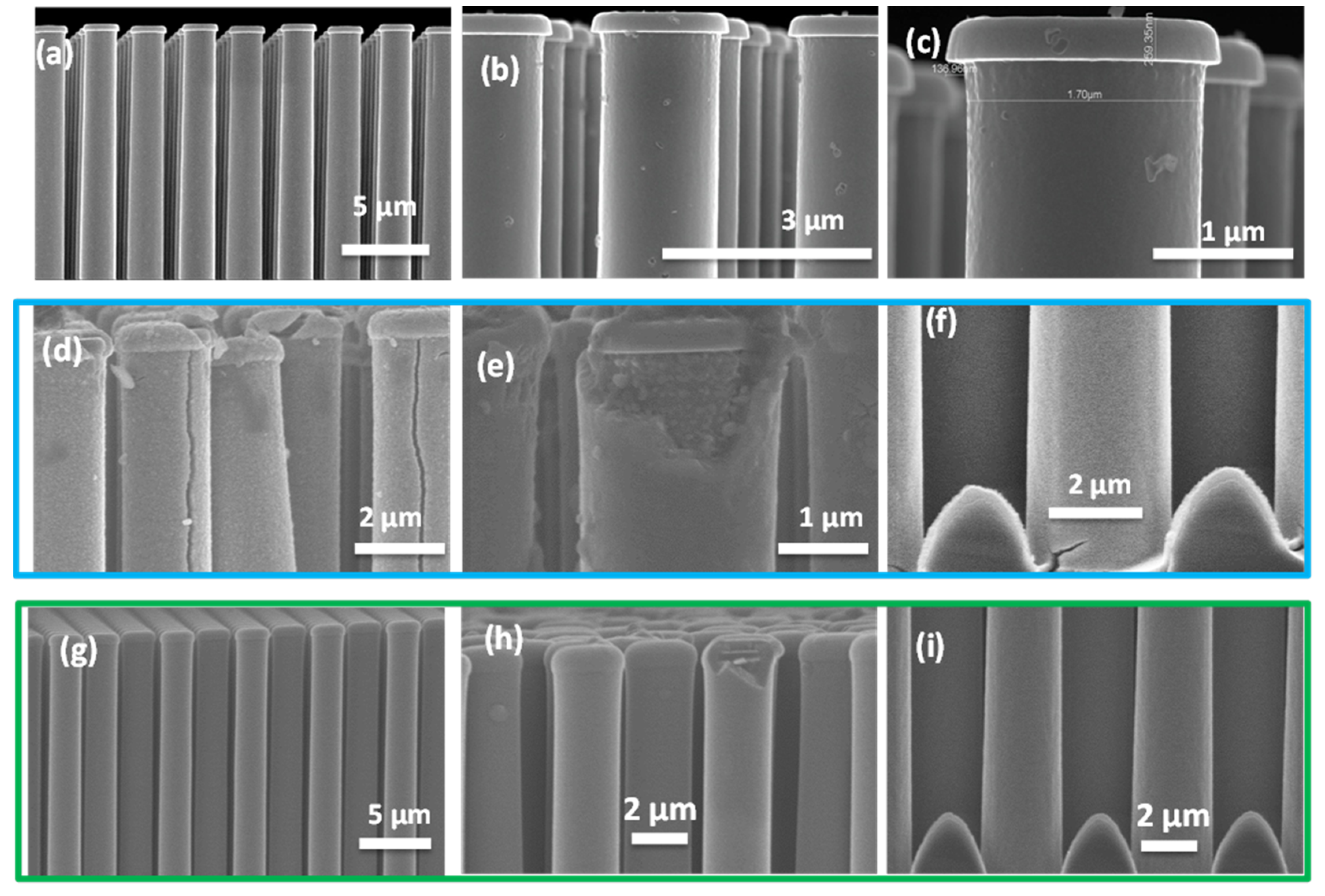
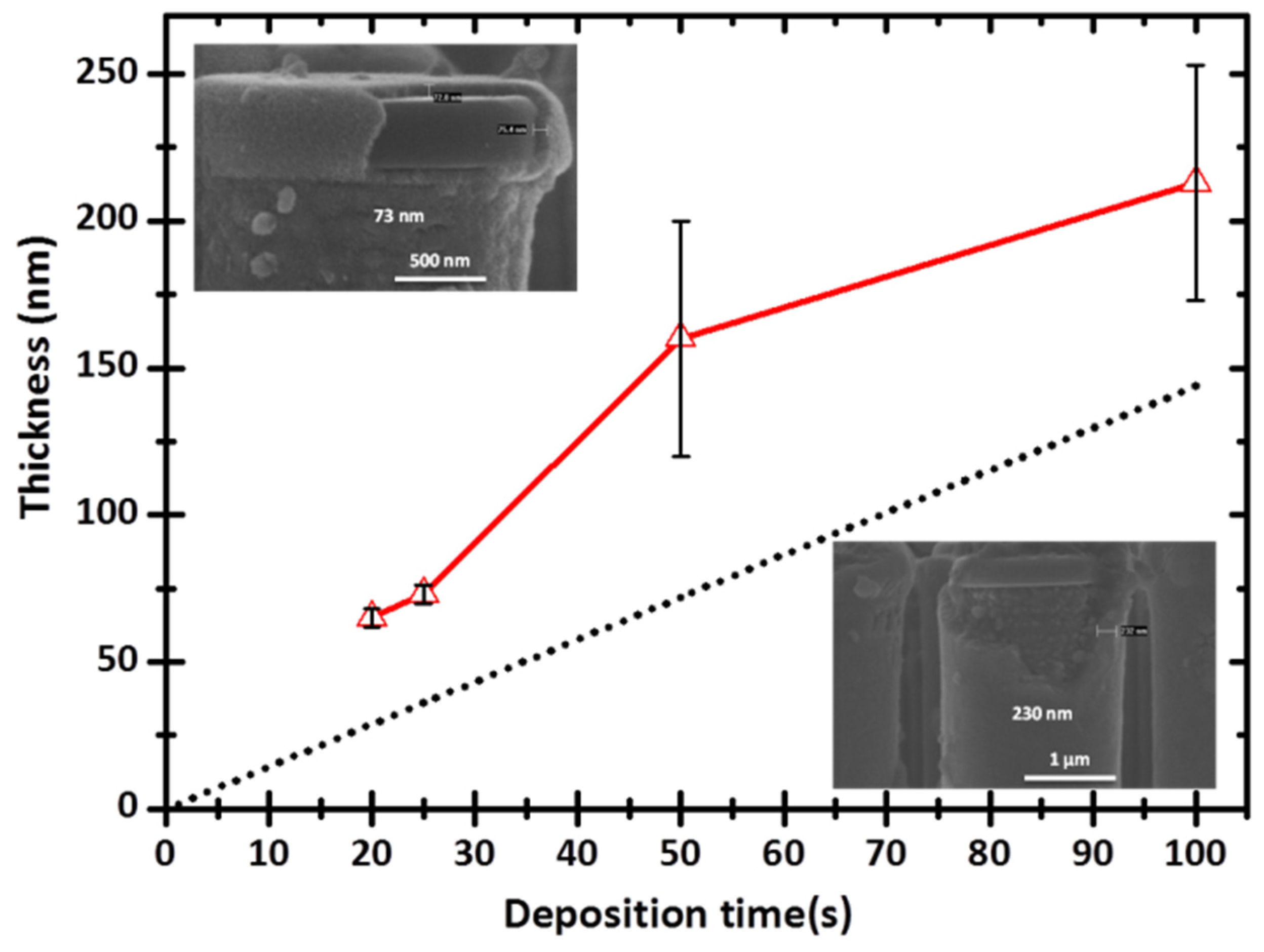
3.1. Conformal Electrodeposition of EMD Films on C-Coated TiN/Si Pillars
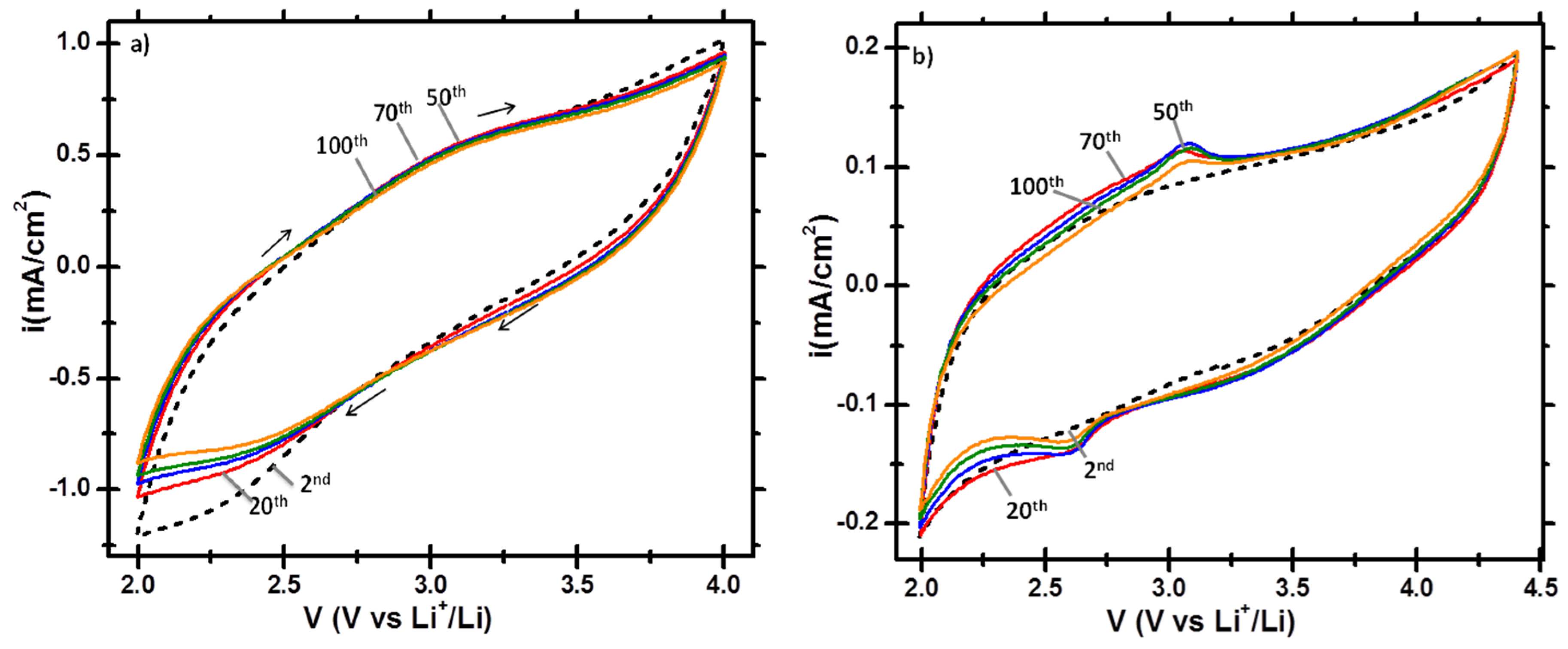
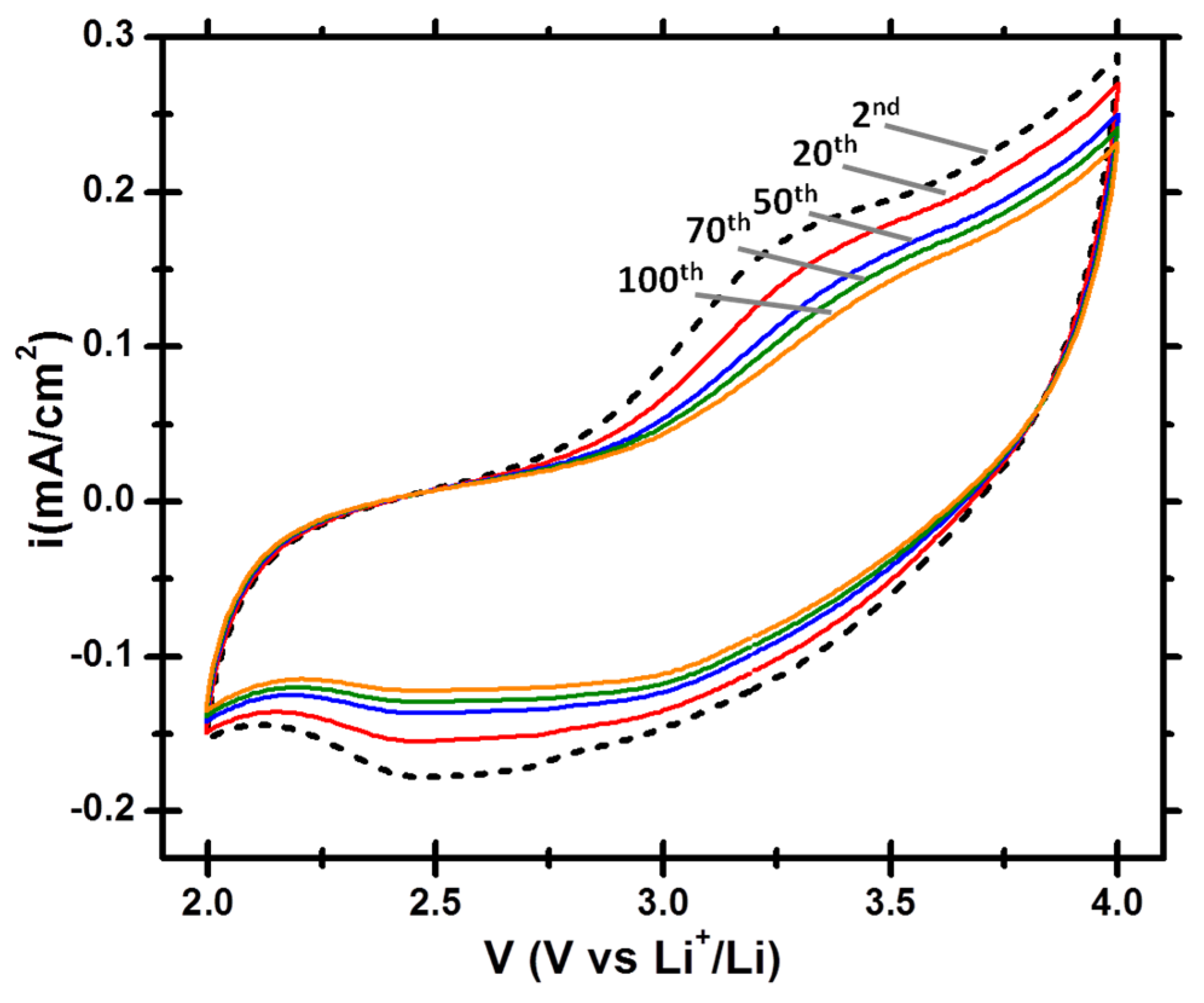
3.2. Electrochemical Lithiation/Delithiation Properties of EMD Deposited on C/TiN/Si Pillars
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roberts, M.; Johns, P.; Owen, J.; Brandell, D.; Edstrom, K.; El Enany, G.; Guery, C.; Golodnitsky, D.; Lacey, M.; Lecoeur, C.; et al. 3D Lithium Ion Batteries—From Fundamentals to Fabrication. J. Mater. Chem. 2011, 21, 9876–9890. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium Batteries: Status, Prospects and Future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; Schalkwijk, V.W. Nanostructured Materials for Advanced Energy Conversion and Storage Devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and Lithium Ion Batteries for Applications in Microelectronic Devices: A Review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Vereecken, P.M.; Huyghebaert, C. Conformal Deposition For 3D Thin-Film Batteries. ECS Trans. 2013, 58, 111–118. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Arthur, T.S.; Bates, D.J.; Cirigliano, N.; Johnson, D.C.; Malati, P.; Mosby, J.M.; Perre, E.; Rawls, M.T.; Prieto, A.L.; Dunn, B. Three-Dimensional Electrodes and Battery Architectures. MRS Bull. 2011, 36, 523–531. [Google Scholar] [CrossRef]
- Valvo, M.; Roberts, M.; Oltean, G.; Sun, B.; Rehnlund, D.; Brandell, D.; Nyholm, L.; Gustafsson, T.; Edström, K. Electrochemical Elaboration of Electrodes and Electrolytes for 3D Structured Batteries. J. Mater. Chem. A 2013, 1, 9281–9293. [Google Scholar] [CrossRef]
- Oltean, G.; Valvo, M.; Nyholm, L.; Edström, K. On the Electrophoretic and Sol-Gel Deposition of Active Materials on Aluminium Rod Current Collectors for Three-Dimensional Li-Ion Micro-Batteries. Thin Solid Films 2014, 562, 63–69. [Google Scholar] [CrossRef]
- Zargouni, Y.; Deheryan, S.; Radisic, A.; Cott, D.J.; Rochan, S.; Alouani, K.; Huyghebaert, C.; Vereecken, P.M. Electrodeposition and Characterization of Manganese Dioxide Thin Films on Silicon Pillar Arrays for 3D Thin-Film Lithium-Ion Batteries. ECS Trans. 2014, 61, 29–41. [Google Scholar] [CrossRef]
- Oltean, G.; Asfaw, H.D.; Nyholm, L.; Edstrom, K. A Li-Ion Microbattery with 3D Electrodes of Different Geometries. ECS Electrochem. Lett. 2014, 3, A54–A57. [Google Scholar] [CrossRef]
- Rehnlund, D.; Valvo, M.; Edstrom, K.; Nyholm, L. Electrodeposition of Vanadium Oxide/Manganese Oxide Hybrid Thin Films on Nanostructured Aluminum Substrates. J. Electrochem. Soc. 2014, 161, D515–D521. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1–23. [Google Scholar] [CrossRef]
- Gambino, J.P.; Adderly, S.A.; Knickerbocker, J.U. An Overview of Through-Silicon-via Technology and Manufacturing Challenges. Microelectron. Eng. 2015, 135, 73–106. [Google Scholar] [CrossRef]
- Chamran, F.; Yeh, Y.; Min, H.; Dunn, B.; Kim, C. Fabrication of High-Aspect-Ratio Electrode Arrays for Three-Dimensional Microbatteries. J. Microelectromechanical Syst. 2007, 16, 844–852. [Google Scholar] [CrossRef]
- Wang, C.; Taherabadi, L.; Jia, G.; Kassegne, S.; Zoval, J.V.; Madou, M.J. Carbon-MEMS Architectures for 3D Microbatteries. Proc. SPIE 2004, 5455, 295–302. [Google Scholar] [CrossRef]
- Notten, B.P.H.L.; Roozeboom, F.; Niessen, R.A.H.; Baggetto, L. 3-D Integrated All-Solid-State Rechargeable Batteries. Adv. Mater. 2007, 19, 4564–4567. [Google Scholar] [CrossRef]
- Whitehead, A.H.; Schreiber, M. Current Collectors for Positive Electrodes of Lithium-Based Batteries. J. Electrochem. Soc. 2005, 152, A2105. [Google Scholar] [CrossRef]
- Kim, S.W.; Cho, K.Y. Current Collectors for Flexible Lithium Ion Batteries: A Review of Materials. J. Electrochem. Sci. Technol. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Freixas, J.; Eustache, E.; Roussel, P.; Brillard, C.; Deresmes, D.; Nuns, N.; Rolland, N.; Brousse, T.; Lethien, C. Sputtered Titanium Nitride: A Bifunctional Material for Li-Ion Microbatteries. J. Electrochem. Soc. 2015, 162, 493–500. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.M.; Baggetto, L.; Notten, P.H.L. All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Architectures for Future Hybrid Energy Storage. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Golodnitsky, D.; Nathan, M.; Yufit, V.; Strauss, E.; Freedman, K.; Burstein, L.; Gladkich, A.; Peled, E. Progress in Three-Dimensional (3D) Li-Ion Microbatteries. Solid State Ionics 2006, 177, 2811–2819. [Google Scholar] [CrossRef]
- Taberna, P.L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.-M. High Rate Capabilities Fe3O4-Based Cu Nano-Architectured Electrodes for Lithium-Ion Battery Applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.R.; Leela Mohana Reddy, A.; Zhan, X.; Ajayan, P.M. Building Energy Storage Device on a Single Nanowire. Nano Lett. 2011, 11, 3329–3333. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Gnanaraj, J.; Liang, J. Synthesis and Rate Performance of Fe3O4-Based Cu Nanostructured Electrodes for Li-Ion Batteries. J. Power Sources 2011, 196, 4779–4784. [Google Scholar] [CrossRef]
- Moitzheim, S.; Nimisha, C.S.; Deng, S.; Cott, D.J.; Detavernier, C.; Vereecken, P.M. Nanostructured TiO2/carbon Nanosheet Hybrid Electrode for High-Rate Thin-Film Lithium-Ion Batteries. Nanotechnology 2014, 25, 504008. [Google Scholar] [CrossRef] [PubMed]
- Edström, K.; Brandell, D.; Gustafsson, T.; Nyholm, L. Electrodeposition as a Tool for 3D Microbattery Fabrication. Electrochem. Soc. Interface 2011, 20, 41–46. [Google Scholar] [CrossRef]
- Knoops, H.C.M.; Donders, M.E.; van de Sanden, M.C.M.; Notten, P.H.L.; Kessels, W.M.M. Atomic Layer Deposition for Nanostructured Li-Ion Batteries. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 30, 10801. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X. Elegant Design of Electrode and Electrode/electrolyte Interface in Lithium-Ion Batteries by Atomic Layer Deposition. Nanotechnology 2015, 26, 24001. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yushin, G. Chemical Vapor Deposition and Atomic Layer Deposition for Advanced Lithium Ion Batteries and Supercapacitors. Energy Environ. Sci. 2015, 8, 1889–1904. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Meng, X.; Tang, Y.; Banis, M.N.; Yang, J.; Hu, Y.; Li, R.; Cai, M.; Sun, X. Significant Impact on Cathode Performance of Lithium-Ion Batteries by Precisely Controlled Metal Oxide Nanocoatings via Atomic Layer Deposition. J. Power Sources 2014, 247, 57–69. [Google Scholar] [CrossRef]
- Aaltonen, T.; Nilsen, O.; Magras, A.; Fjellv, H. Atomic Layer Deposition of Li2O–Al2O3 Thin Films. Chem. Mater. 2011, 23, 4669–4675. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese Oxides for Lithium Batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Shaijumon, M.M.; Gowda, S.R.; Ajayan, P.M. Coaxial MnO2/carbon Nanotube Array Electrodes for High-Performance Lithium Batteries. Nano Lett. 2009, 9, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Lins, V.; Matencio, T. Metallic and Oxide Electrodeposition. Mod. Surf. Eng. Treat. 2013, 101–122. [Google Scholar] [CrossRef]
- He, X.; Wang, L.; Pu, W.; Zhang, G.; Jiang, C.; Wan, C. Synthesis of Spinel LiMn2O4 for Li-Ion Batteries via Sol–Gel Process. Int. J. Electrochem. Soc. 2006, 1, 12–16. [Google Scholar]
- Biswal, A.; Chandra Tripathy, B.; Sanjay, K.; Subbaiah, T.; Minakshi, M. Electrolytic Manganese Dioxide (EMD): A Perspective on Worldwide Production, Reserves and Its Role in Electrochemistry. RSC Adv. 2015, 5, 58255–58283. [Google Scholar] [CrossRef]
- Scrosati, B. Recent Advances in Lithium Solid State Batteries. J. Appl. Electrochem. 1972, 2, 231–238. [Google Scholar] [CrossRef]
- Chabre, Y.; Pannetier, J. Structural and Electrochemical Properties of the Proton/γ-MnO2 System. Prog. Solid State Chem. 1995, 23, 1–130. [Google Scholar] [CrossRef]
- Ammundsen, B.; Paulsen, J. Novel Lithium-Ion Cathode Materials Based on Layered Manganese Oxides. Adv. Mater. 2001, 13, 943–956. [Google Scholar] [CrossRef]
- Dupont, M.F.; Donne, S.W. Nucleation and Growth of Electrodeposited Manganese Dioxide for Electrochemical Capacitors. Electrochim. Acta 2014, 120, 219–225. [Google Scholar] [CrossRef]
- Minakshi Sundaram, M.; Biswal, A.; Mitchell, D.; Jones, R.; Fernandez, C. Correlation among Physical and Electrochemical Behaviour of Nanostructured Electrolytic Manganese Dioxide from Leach Liquor and Synthetic for Aqueous Asymmetric Capacitor. Phys. Chem. Chem. Phys. 2016, 18, 4711–4720. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Aradilla, D.; Bidan, G.; Gentile, P.; Schubert, T.J.S.; Wimberg, J.; Sadki, S.; Gomez-Romero, P. 3D Hierarchical Assembly of Ultrathin MnO2 Nanoflakes on Silicon Nanowires for High Performance Micro-Supercapacitors in Li-Doped Ionic Liquid. Sci. Rep. 2015, 5, 9771. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOX, Mn2O3, and Mn3O4 Electrodeposited Films for the Oxygen Evolution Reaction of Water. J. Phys. Chem. C 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Roche, I.; Chaînet, E.; Chatenet, M.; Vondrák, J. Durability of Carbon-Supported Manganese Oxide Nanoparticles for the Oxygen Reduction Reaction (ORR) in Alkaline Medium. J. Appl. Electrochem. 2008, 38, 1195–1201. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Rossouw, M.H.; de Kock, A.; de la Harpe, A.P.; Gummow, R.J.; Pearce, K.; Liles, D.C. The Versatility of MnO2 for Lithium Battery Applications. J. Power Sources 1993, 43, 289–300. [Google Scholar] [CrossRef]
- Dose, W.M.; Donne, S.W. Thermal Treatment Effects on Manganese Dioxide Structure, Morphology and Electrochemical Performance. J. Electrochem. Soc. 2011, 158, A905. [Google Scholar] [CrossRef]
- Dose, W.M.; Donne, S.W. Kinetic Analysis of γ-MnO2 Thermal Treatment. J. Therm. Anal. Calorim. 2011, 105, 113–122. [Google Scholar] [CrossRef]
- Paik, Y.; Bowden, W.; Richards, T.; Grey, C.P. The Effect of Heat-Treatment on Electrolytic Manganese Dioxide: A 2H and 6Li Magic Angle Spinning NMR Study. J. Electrochem. Soc. 2005, 152, A1539–A1547. [Google Scholar] [CrossRef]
- Etman, A.S.; Radisic, A.; Emara, M.M.; Huyghebaert, C.; Vereecken, P.M. Effect of Film Morphology on the Li-Ion Intercalation Kinetics in Anodic Porous Manganese Dioxide Thin Films. J. Phys. Chem. C 2014, 118, 9889–9898. [Google Scholar] [CrossRef]
- Deheryan, S.; Zargouni, Y.; Sinha, R.; Put, B.; Radisic, A.; Heyns, M.; Vereecken, P.M. Nanometer-Thin Graphitic Carbon Buffer Layers for Electrolytic MnO2 for Thin-Film Energy Storage Devices. J. Electrochem. Soc. 2017, 164, A538–A544. [Google Scholar] [CrossRef]
- Biswal, A.; Minakshi, M.; Tripathy, B.C. Electrodeposition of Sea Urchin and Cauliflower-like Nickel-/Cobalt-Doped Manganese Dioxide Hierarchical Nanostructures with Improved Energy-Storage Behavior. ChemElectroChem 2016, 3, 976–985. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Sun, P. Fabrication of Highly Ordered Metallic Arrays and Silicon Pillars with Controllable Size Using Nanosphere Lithography. Phys. E-Low-Dimensional Syst. Nanostruct. 2009, 41, 1600–1603. [Google Scholar] [CrossRef]
- Eustache, E.; Douard, C.; Retoux, R.; Lethien, C.; Brousse, T. MnO2 Thin Films on 3D Scaffold: Microsupercapacitor Electrodes Competing with “Bulk” Carbon Electrodes. Adv. Energy Mater. 2015, 5, 4564–4567. [Google Scholar] [CrossRef]
- Deheryan, S.; Cott, D.J.; Mertens, P.W.; Heyns, M.; Vereecken, P.M. Direct Correlation between the Measured Electrochemical Capacitance, Wettability and Surface Functional Groups of CarbonNanosheets. Electrochim. Acta 2014, 132, 574–582. [Google Scholar] [CrossRef]
- Cott, D.J.; Verheijen, M.; Richard, O.; Radu, I.; de Gendt, S.; van Elshocht, S.; Vereecken, P.M. Synthesis of Large Area Carbon Nanosheets for Energy Storage Applications. Carbon 2013, 58, 59–65. [Google Scholar] [CrossRef]
- Qaid, M.; Put, B.; Cott, D.J.; AlSalhi, M.S.; Stesmans, A.; Vereecken, P.M.; Radu, I.P. Large Area Carbon Nanosheet Capacitors. ECS Solid State Lett. 2014, 3, N8–N10. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Pereira-Ramos, J.-P. Raman Microspectrometry Applied to the Study of Electrode Materials for Lithium Batteries. Chem. Rev. 2010, 110, 1278–1319. [Google Scholar] [CrossRef] [PubMed]
- Perez, I.; Corso, B.L.; Khalap, V.R.; Collins, P.G. Conformal MnO2 Electrodeposition onto Defect-Free Graphitic Carbons. Electrochem. Commun. 2011, 13, 590–592. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A Review of Conduction Phenomena in Li-Ion Batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Yao, F.; Güneş, F.; Ta, H.Q.; Lee, S.M.; Chae, S.J.; Sheem, K.Y.; Cojocaru, C.S.; Xie, S.S.; Lee, Y.H. Diffusion Mechanism of Lithium Ion through Basal Plane of Layered Graphene. J. Am. Chem. Soc. 2012, 134, 8646–8654. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, M.; Biglari, Z.; Binder, L. Effect of Bath Temperature on Electrochemical Properties of the Anodically Deposited Manganese Dioxide. J. Power Sources 2001, 102, 29–34. [Google Scholar] [CrossRef]
- Jacob, G.M.; Zhitomirsky, I. Microstructure and Properties of Manganese Dioxide Films Prepared by Electrodeposition. Appl. Surf. Sci. 2008, 254, 6671–6676. [Google Scholar] [CrossRef]
- Johns, P.; Roberts, M.; Owen, J. Conformal Electrodeposition of Manganese Dioxide onto Reticulated Vitreous Carbon for 3D Microbattery Applications. J. Mater. Chem. 2011, 21, 10153–10159. [Google Scholar] [CrossRef]
- Gibson, A.J.; Burns, R.C.; Dupont, M.F.; Donne, S.W. Mesoscale Morphological Control of Electrodeposited Manganese Dioxide Films. Electrochim. Acta 2015, 170, 343–352. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Ghodbane, O.; Pascal, J.L.; Favier, F. Microstructural Effects on Charge-Storage Properties in MnO2-Based Electrochemical Supercapacitors. ACS Appl. Mater. Interfaces 2009, 1, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Sarciaux, S.; la Salle, A.L.G.; Verbaere, A.; Piffard, Y.; Guyomard, D. γ-MnO2 for Li Batteries: Part I. γ-MnO2: Relationships between Synthesis Conditions, Material Characteristics and Performances in Lithium Batteries. J. Power Sources 1999, 81–82, 656–660. [Google Scholar] [CrossRef]
- Sarciaux, S.; la Salle, A.L.G.; Verbaere, A.; Piffard, Y.; Guyomard, D. γ-MnO2 for Li Batteries Part II. Some Aspects of the Lithium Insertion Process into γ-MnO2 and Electrochemically Lithiated γ-LixMnO2 Compounds. J. Power Sources 1999, 81–82, 661–665. [Google Scholar] [CrossRef]
- Hausbrand, R.; Cherkashinin, G.; Ehrenberg, H.; Gröting, M.; Albe, K.; Hess, C.; Jaegermann, W. Fundamental Degradation Mechanisms of Layered Oxide Li-Ion Battery Cathode Materials: Methodology, Insights and Novel Approaches. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2015, 192, 3–25. [Google Scholar] [CrossRef]
- Jiang, F.; Peng, P. Elucidating the Performance Limitations of Lithium-Ion Batteries due to Species and Charge Transport through Five Characteristic Parameters. Sci. Rep. 2016, 6, 32639. [Google Scholar] [CrossRef] [PubMed]
- Dose, W.M.; Donne, S.W. Optimizing Li/MnO2 Batteries: Relating Manganese Dioxide Properties and Electrochemical Performance. J. Power Sources 2013, 221, 261–265. [Google Scholar] [CrossRef]
- Bélanger, D.; Brousse, T.; Long, J.W. Manganese Oxides: Battery Materials Make the Leap to Electrochemical Capacitors. Electrochem. Soc. Interface 2008, 17, 49–52. [Google Scholar]
- Minakshi, M.; Mitchell, D.R.G.; Prince, K. Incorporation of TiB2 Additive into MnO2 Cathode and Its Influence on Rechargeability in an Aqueous Battery System. Solid State Ionics 2008, 179, 355–361. [Google Scholar] [CrossRef]
- Dose, W.M.; Donne, S.W. Optimising Heat Treatment Environment and Atmosphere of Electrolytic Manganese Dioxide for Primary Li/MnO2 Batteries. J. Power Sources 2014, 247, 852–857. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zargouni, Y.; Deheryan, S.; Radisic, A.; Alouani, K.; Vereecken, P.M. Electrolytic Manganese Dioxide Coatings on High Aspect Ratio Micro-Pillar Arrays for 3D Thin Film Lithium Ion Batteries. Nanomaterials 2017, 7, 126. https://doi.org/10.3390/nano7060126
Zargouni Y, Deheryan S, Radisic A, Alouani K, Vereecken PM. Electrolytic Manganese Dioxide Coatings on High Aspect Ratio Micro-Pillar Arrays for 3D Thin Film Lithium Ion Batteries. Nanomaterials. 2017; 7(6):126. https://doi.org/10.3390/nano7060126
Chicago/Turabian StyleZargouni, Yafa, Stella Deheryan, Alex Radisic, Khaled Alouani, and Philippe M. Vereecken. 2017. "Electrolytic Manganese Dioxide Coatings on High Aspect Ratio Micro-Pillar Arrays for 3D Thin Film Lithium Ion Batteries" Nanomaterials 7, no. 6: 126. https://doi.org/10.3390/nano7060126
APA StyleZargouni, Y., Deheryan, S., Radisic, A., Alouani, K., & Vereecken, P. M. (2017). Electrolytic Manganese Dioxide Coatings on High Aspect Ratio Micro-Pillar Arrays for 3D Thin Film Lithium Ion Batteries. Nanomaterials, 7(6), 126. https://doi.org/10.3390/nano7060126