Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer
Abstract
:1. Introduction
2. Results and Discussion
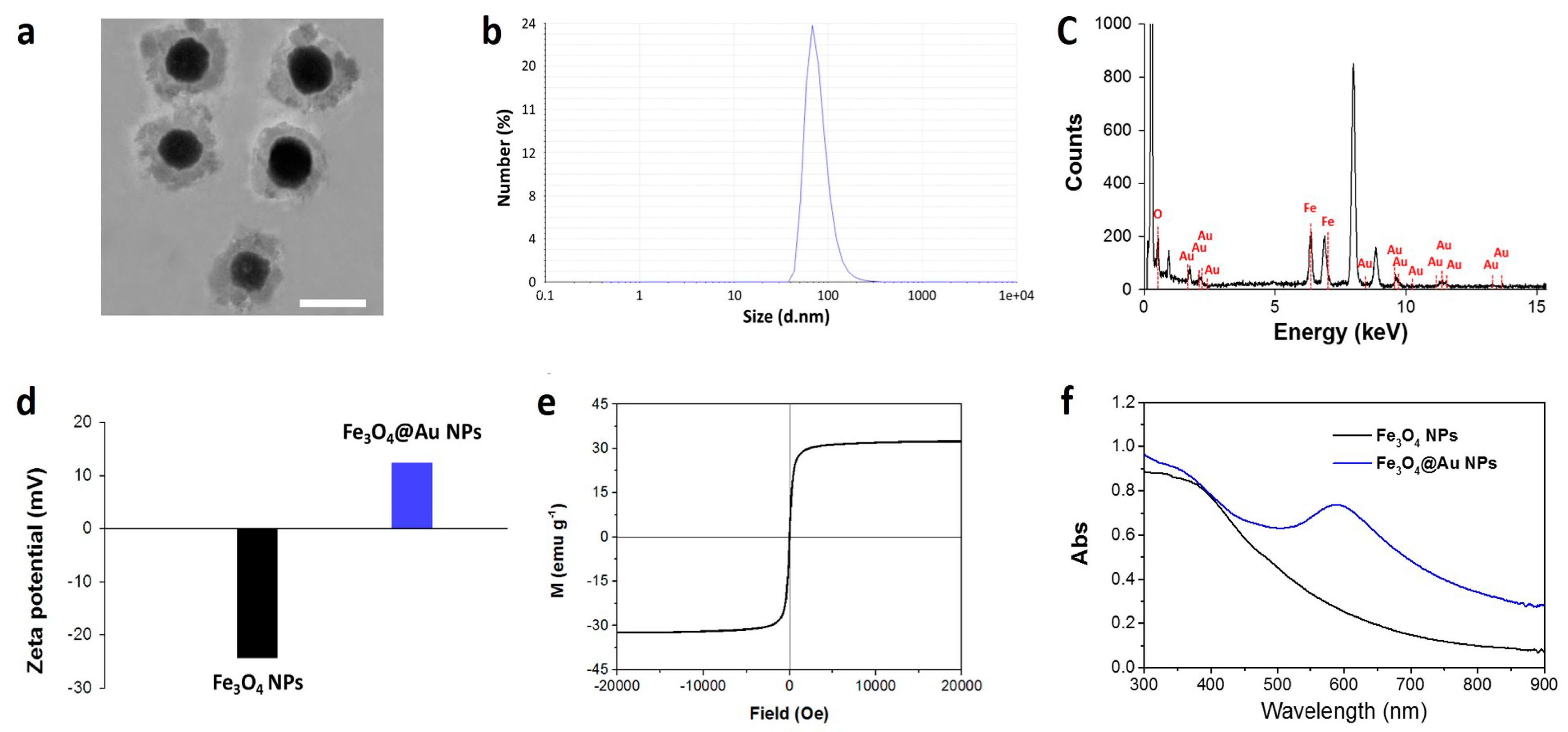
2.1. Synthesis and Characterization
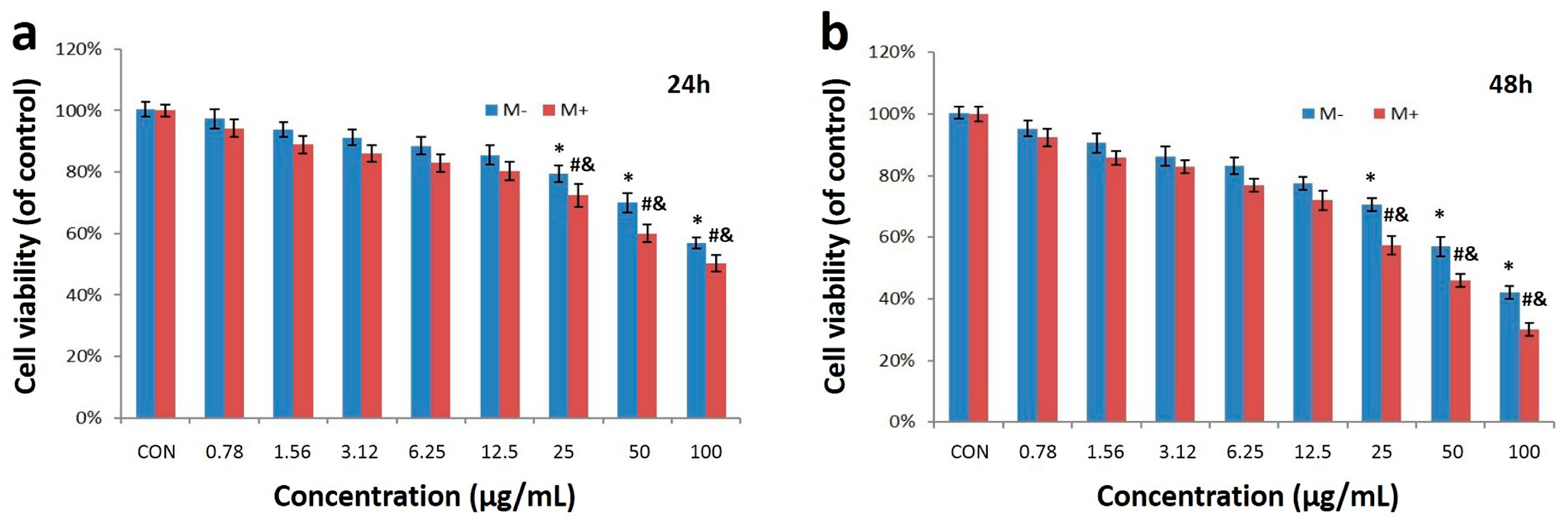
2.2. Cell Uptake and Cytotoxicity of Fe3O4@Au NPs
2.3. Photothermal Effect
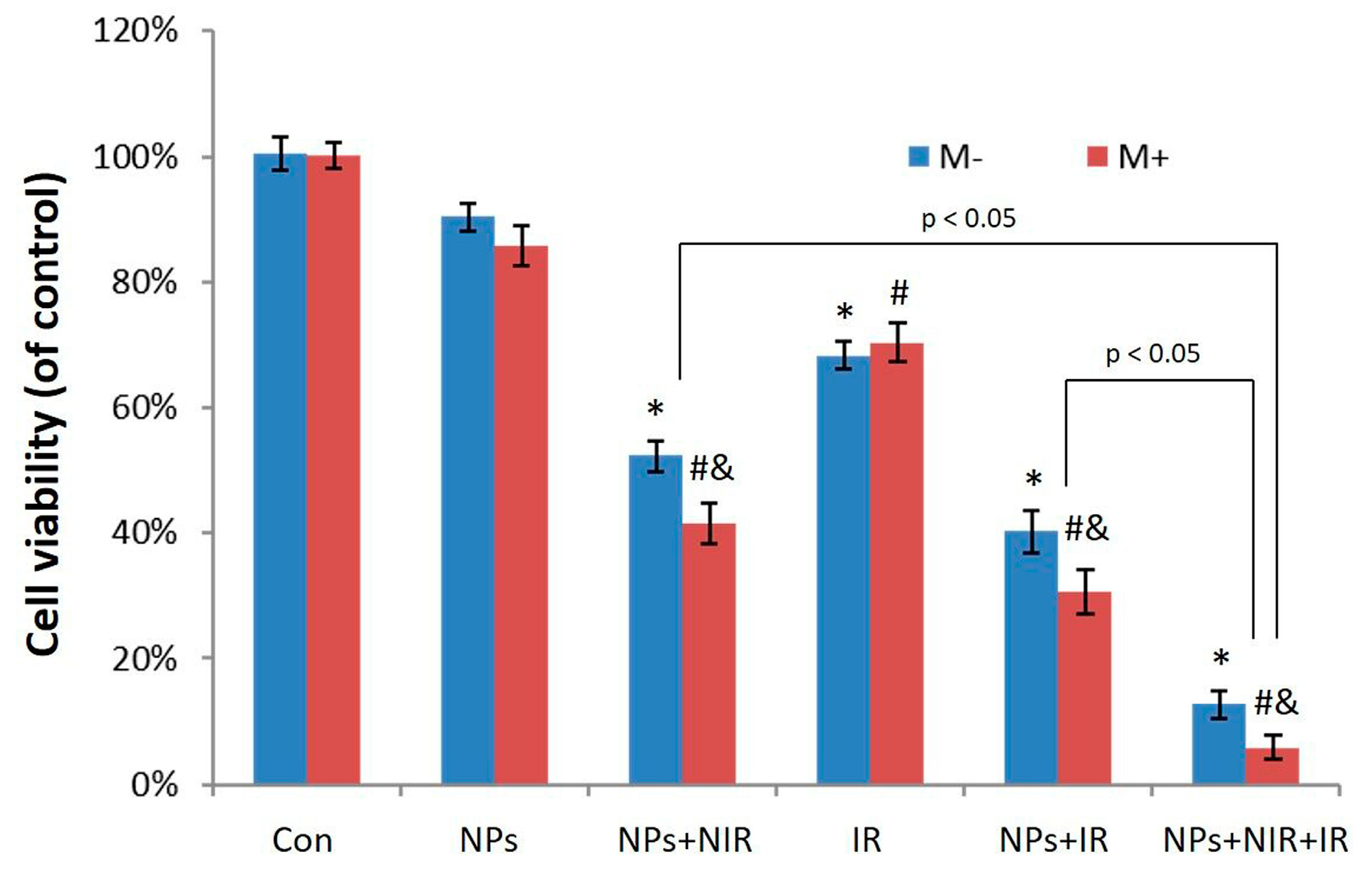
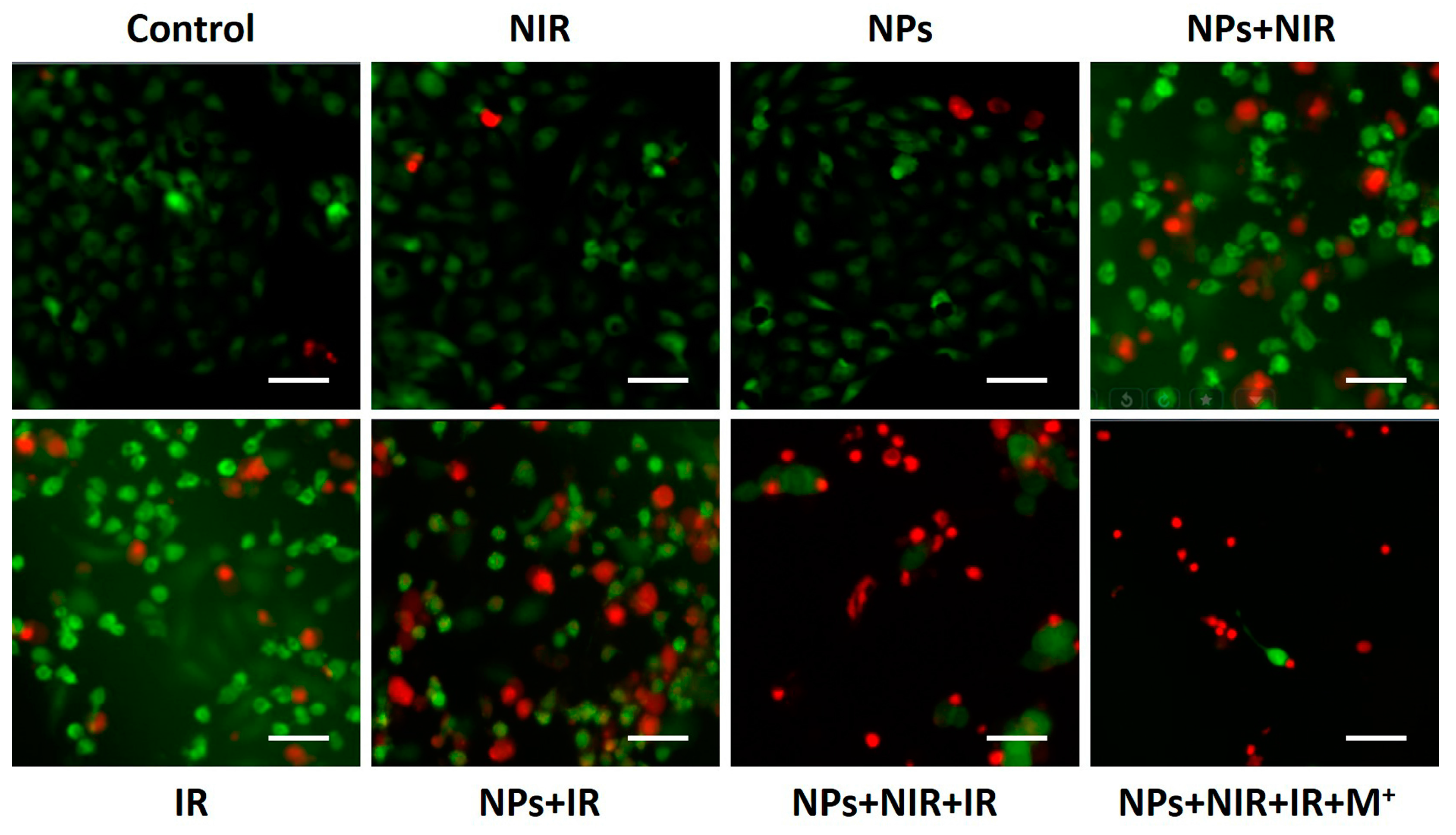
2.4. Photothermal-Radiotherapeutic Effect In Vitro
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Fe3O4 Nanoparticles and Fe3O4@Au Nanoparticles
3.3. Characterization of Core-Shell Fe3O4@Au NPs
3.4. Cell Uptake Test
3.5. In Vitro Cytotoxicity Assay
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maluccio, M.; Covey, A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. Cancer J. Clin. 2012, 62, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; De Sanjosé, S. Human papillomavirus and cervical cancer--burden and assessment of causality. J. Natl. Cancer Inst. Monogr. 2002, 31, 3–13. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Tewari, K.S.; Koh, W.J. Multimodality therapy for locally advanced cervical carcinoma: State of the art and future directions. J. Clin. Oncol. 2007, 25, 2952–2965. [Google Scholar] [CrossRef] [PubMed]
- Rose, P. Chemoradiotherapy for cervical cancer. Eur. J. Cancer 2002, 38, 270–278. [Google Scholar] [CrossRef]
- Small, W.; Winter, K.; Levenback, C. Extended-field irradiation and intracavitary brachytherapy combined with cisplatin chemotherapy for cervical cancer with positive para-aortic or high common iliac lymph nodes: Results of ARM 1 of RTOG 0116. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- RYU, H.S.; Kang, S.; Kim, K.T.; Chang, K.H.; Kim, J.; Kim, J.H. Efficacy of different types of treatment in FIGO stage IB2 cervical cancer in Korea: Results of a multicenter retrospective Korean study (KGOG-1005). Int. J. Gynecol. Cancer 2007, 17, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Chien, T.Y.; Huang, S.H.; Wu, C.J.; Shih, B.Y.; Chang, S.C. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol. Oncol. 2004, 93, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Dobashi, K.; Fujimoto, S. A late phase II study of CPT-11 on uterine cervical cancer and ovarian cancer. Research Groups of CPT-11 in Gynecologic Cancers. Gan Kagaku Ryoho 1991, 18, 1681–1689. [Google Scholar]
- Takeda, N.; Sakuragi, N.; Takeda, M. Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet. Gynecol. Scand. 2002, 81, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Ungár, L.; Pálfalvi, L.; Hogg, R. Abdominal radical trachelectomy: A fertility-preserving option for women with early cervical cancer. BJOG 2005, 112, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.R.; Bogart, J.A. Radiation Therapy-Related Toxicity (including Pneumonitis and Fibrosis). Clin. Oncol. 2010, 24, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Greimel, E.R.; Winter, R.; Kapp, K.S.; Haas, J. Quality of life and sexual functioning after cervical cancer treatment: A long-term follow-up study. Psycho-Oncology 2009, 18, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Prehydrated Electron and Its Role in Ionizing Radiation Induced DNA Damage and Molecular Mechanisms of Action of Halogenated Sensitizers for Radiotherapy of Cancer; UWSpace: Waterloo, ON, Canada, 2012. [Google Scholar]
- Cole, J.R.; Mirin, N.A.; Knight, M.W.; Goodrich, G.P.; Halas, N.J. Photothermal efficiencies of nanoshells and nanorods for clinical therapeutic applications. J. Phys. Chem. C 2009, 113, 12090–12094. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Detappe, A.; Kumar, R. Nanoparticle mediated tumor vascular disruption: A novel strategy in radiation therapy. Nano Lett. 2015, 15, 7488–7496. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Dilmanian, F.A.; Vadas, T.M.; Smilowitz, H.M. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomedicine 2014, 10, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Diagaradjane, P.; Shetty, A.; Wang, J.C. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: Characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Lett. 2008, 8, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Chang, Z. Berberine-loaded Janus Nanocarriers for Magnetic Field-Enhanced Therapy against Hepatocellular Carcinoma. Chem. Biol. Drug Des. 2016, 89, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Li, J.; Zheng, X. Janus “nano-bullets” for Magnetic Targeting Liver Cancer Chemotherapy. Biomaterials 2016, 100, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Wang, Z.; Dong, W.F. Facile Synthesis of Core–shell Magnetic Mesoporous Silica Nanoparticles for pH-sensitive Anticancer Drug Delivery. Chem. Biol. Drug Des. 2015, 86, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Lu, M. Janus Au–mesoporous silica nanocarriers for chemo-photothermal treatment of liver cancer cells. RSC Adv. 2016, 6, 44498–44505. [Google Scholar] [CrossRef]
- Mieszawska, A.J.; Mulder, W.J.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Shao, D.; Zhang, L. Gold nanorods-silica Janus nanoparticles for theranostics. Appl. Phys. Lett. 2015, 106, 173705. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Franchi, P.; Pedulli, G.F.; Pengo, P.; Scrimin, P.; Pasquato, L. EPR study of dialkyl nitroxides as probes to investigate the exchange of solutes between the ligand shell of monolayers of protected gold nanoparticles and aqueous solutions. J. Am. Chem. Soc. 2004, 126, 9326–9329. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Peng, X.H.; Qian, X.; Mao, H. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int. J. Nanomed. 2008, 3, 311–321. [Google Scholar]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhuang, Y.; Hu, H. Silica-coated manganese oxide nanoparticles as a platform for targeted magnetic resonance and fluorescence imaging of cancer cells. Adv. Funct. Mater. 2010, 20, 1733–1741. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Park, H.-Y.; Stephanie, I.; Lim, I.; Schadt, M.J.; Mott, D.; Luo, J.; Wang, X.; Zhong, C.-J. Core@shell nanomaterials: Gold-coated magnetic oxide nanoparticles. J. Mater. Chem. 2008, 18, 2629–2635. [Google Scholar] [CrossRef]
- Ahmadi, A.; Shirazi, H.; Pourbagher, N.; Akbarzadeh, A.; Omidfar, K. An electrochemical immunosensor for digoxin using core–shell gold coated magnetic nanoparticles as labels. Mol. Boil. Rep. 2014, 41, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Tamer, U.; Gündoğdu, Y.; Boyacı, İ.H.; Pekmez, K. Synthesis of magnetic core–shell Fe3O4–Au nanoparticle for biomolecule immobilization and detection. J. Nanopart. Res. 2010, 12, 1187–1196. [Google Scholar] [CrossRef]
- Park, H.-Y.; Schadt, M.J.; Wang, L.; Lim, I.-I.S.; Njoki, P.N.; Kim, S.H.; Jang, M.-Y.; Luo, J.; Zhong, C.-J. Fabrication of Magnetic Core@Shell Fe Oxide@Au Nanoparticles for Interfacial Bioactivity and Bio-separation. Langmuir 2007, 23, 9050–9056. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Tung, L.D.; Maenosono, S.; Wälti, C.; Thanh, N.T. Synthesis of core-shell gold coated magnetic nanoparticles and their interaction with thiolated DNA. Nanoscale 2010, 2, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic Cu9S5 Nanocrystals: A Photothermal Agent with a 25.7% Heat Conversion Efficiency for Photothermal Ablation of Cancer Cells In Vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef] [PubMed]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Zheng, M.; Wu, J.; Li, C.; Shen, D.; Yang, D.; Li, L.; Ge, M.; Chang, Z.; Dong, W. Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials 2017, 7, 111. https://doi.org/10.3390/nano7050111
Hu R, Zheng M, Wu J, Li C, Shen D, Yang D, Li L, Ge M, Chang Z, Dong W. Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials. 2017; 7(5):111. https://doi.org/10.3390/nano7050111
Chicago/Turabian StyleHu, Rui, Minxue Zheng, Jinchang Wu, Cheng Li, Danqing Shen, Dian Yang, Li Li, Mingfeng Ge, Zhimin Chang, and Wenfei Dong. 2017. "Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer" Nanomaterials 7, no. 5: 111. https://doi.org/10.3390/nano7050111
APA StyleHu, R., Zheng, M., Wu, J., Li, C., Shen, D., Yang, D., Li, L., Ge, M., Chang, Z., & Dong, W. (2017). Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials, 7(5), 111. https://doi.org/10.3390/nano7050111




