Recent Advances in Research on the Synthetic Fiber Based Silica Aerogel Nanocomposites
Abstract
:1. Introduction
2. Synthesis of Silica Aerogels
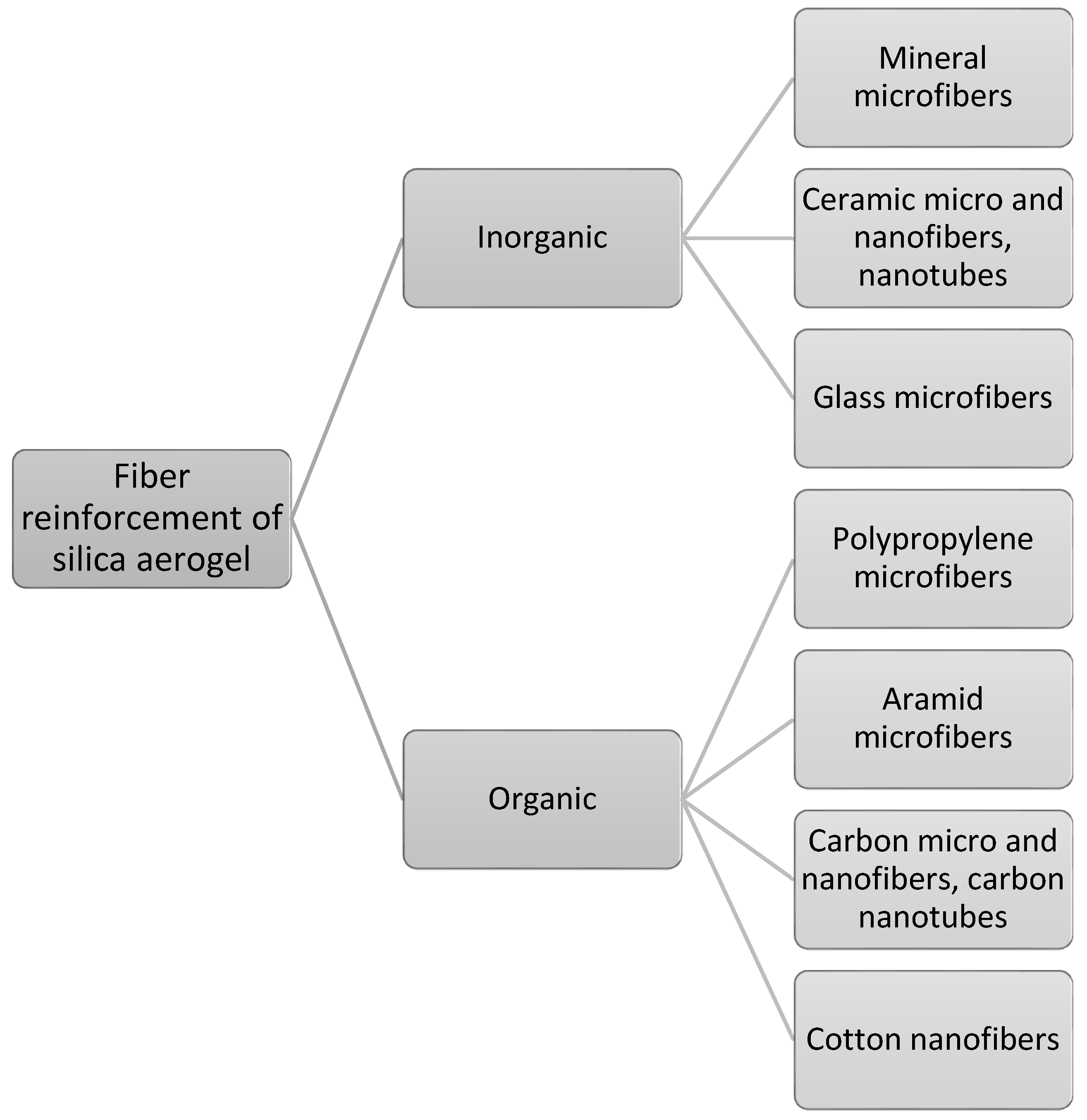
3. Fiber-Based Silica Aerogel Nanocomposites
3.1. Silica Aerogel Nanocomposites with Inorganic Microfibers
3.2. Silica Aerogel Nanocomposites with Organic Microfibers
3.3. Silica Aerogel Nanocomposites with Inorganic and Organic Nanofibers
3.4. Silica Aerogel Nanocomposites with Inorganic Nanotubes
4. Silica Aerogel Nanocomposites with Carbon-Based Fibers in Nano and Microscale
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef]
- Akimov, Y.K. Fields of application of aerogels (Review). Instrum. Exp. Tech. 2003, 46, 5–19. [Google Scholar] [CrossRef]
- Hrubesh, L.W.; Poco, J.F. Thin aerogel films for optical, thermal, acoustic and electronic applications. J. Non-Cryst. Solids 1995, 188, 46–53. [Google Scholar] [CrossRef]
- Aspen Aerogels. Available online: http://www.aerogel.com (accessed on 15 February 2017).
- Cabot Corporation. Available online: http://www.cabotcorp.com (accessed on 15 February 2017).
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings. A comprehensive review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Riffat, S.B.; Qiu, G. A review of state-of-the-art aerogel applications in buildings. Int. J. Low-Carbon Technol. Adv. Access 2012, 20, 1–6. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Zhang, X.; Yu, F.; Du, X. Thermal conductivities study on silica aerogel and its composite insulation materials. Int. J. Heat Mass Transf. 2011, 54, 2355–2366. [Google Scholar] [CrossRef]
- Cotana, F.; Pisselo, A.L.; Moretti, E.; Buratti, C. Multipurpose characterization of glazing systems with silica aerogel. In field experimental analysis of thermal-energy, lighting and acoustic performance. Build. Environ. 2014, 81, 92–102. [Google Scholar] [CrossRef]
- Gao, T.; Jelle, B.P.; Ihara, T.; Gustavsen, A. Insulating glazing units with silica aerogel granules: The impact of particle size. Appl. Energy 2014, 128, 27–34. [Google Scholar] [CrossRef]
- Luo, F.; Shao, Z.; Zhang, Y.; Cheng, X. Synthesis of paramagnetic iron incorporated silica aerogels by ambient pressure drying. Mater. Chem. Phys. 2013, 142, 113–118. [Google Scholar] [CrossRef]
- Balkis Ameen, K.; Rajasekar, K.; Rajasekharan, T. Silver nanoparticles in mesoporous aerogel exhibiting selective catalytic oxidation of benzene in CO2 free air. Catal. Lett. 2007, 119, 289–295. [Google Scholar] [CrossRef]
- Katagiri, N.; Ishikawa, M. Preparation and evaluation of Au nanoparticle-silica aerogel nanocomposite. J. Asian Ceram. Soc. 2015, 3, 151–155. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, L. In situ growth approach for preparation of Au nanoparticle-doped silica aerogel. Colloids Surf. A 2010, 372, 98–101. [Google Scholar] [CrossRef]
- Bozbag, S.E.; Kostenko, S.; Kurykin, M.A.; Khrustalev, V.N.; Khokhlov, A.R.; Zhang, L.; Aindow, M.; Erkey, C. Aerogel-copper nanocomposites prepared using the adsorption of a polyfluorinated complex from supercritical CO2. J. Nanopart. Res. 2012, 14, 973–985. [Google Scholar] [CrossRef]
- Cutrufello, M.G.; Rombi, E.; Ferino, I.; Loche, D.; Corrias, A.; Casula, M.F. Ni-based xero- and aerogels as catalysts for nitroxidation processes. J. Sol-Gel Sci. Technol. 2011, 60, 323–332. [Google Scholar] [CrossRef]
- Casas, L.; Roig, A.; Mollins, E.; Grenèche, J.M.; Asenjo, J.; Tejada, J. Iron oxide nanoparticles hosted in silica aerogels. Appl. Phys. A 2002, 74, 591–597. [Google Scholar] [CrossRef]
- Masoudian, S.; Monfared, H.H.; Aghaei, A. Silica aerogel-iron oxide nanocomposites: Recoverable catalysts for the oxidation of alcohols with hydrogen peroxide. Transit. Met. Chem. 2011, 36, 521–530. [Google Scholar] [CrossRef]
- Casula, M.F.; Corrias, A.; Paschina, G. Iron oxide-silica aerogel and xerogel nanocomposite materials. J. Non-Cryst. Solids 2001, 293–295, 25–31. [Google Scholar] [CrossRef]
- Heiligtag, F.J.; Kränzlin, N.; Süess, M.J.; Niederberger, M. Anatase-silica composite aerogels: A nanoparticle-based approach. J. Sol-Gel Sci. Technol. 2014, 70, 300–306. [Google Scholar] [CrossRef]
- Wei, T.-Y.; Kuo, C.-Y.; Hsu, Y.-J.; Lu, S.-Y.; Chang, Y.-C. Tin oxide nanocrystals embedded in silica aerogel: Photoluminescence and photocatalysis. Microporous Mesoporous Mater. 2008, 112, 580–588. [Google Scholar] [CrossRef]
- Marras, C.; Loche, D.; Corrias, A.; Konya, Z.; Casula, M.F. Bimetallic Fe/Mo-SiO2 aerogel catalyst for catalytic carbon vapour deposition production of carbon nanotubes. J. Sol-Gel Sci. Technol. 2015, 73, 379–388. [Google Scholar] [CrossRef]
- Loche, D.; Casula, M.F.; Corrias, A.; Marras, S.; Moggi, P. Bimetallic FeCo nanocrystals supported on highly porous silica aerogels as Fischer-Tropsch catalysts. Catal. Lett. 2012, 142, 1061–1066. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Ko, C.-C.; Chen, C.-H.; Tung, K.-L.; Chang, K.-S.; Chung, T.-W. Sol-gel preparation of polymethylsilsesquioxane aerogel membranes for CO2 absorption fluxes in membrane contactors. Appl. Energy 2014, 129, 25–31. [Google Scholar] [CrossRef]
- Tsou, P. Silica aerogel captures cosmic dust intact. J. Non-Cryst. Solids 1995, 186, 415–427. [Google Scholar] [CrossRef]
- Liu, H.; Sha, W.; Cooper, A.; Fan, M. Preparation and characterization of a novel silica aerogel as adsorbent for toxic organic compounds. Colloids Surf. A 2009, 347, 38–44. [Google Scholar] [CrossRef]
- Guenther, U.; Smirnova, I.; Neubert, R.H.H. Hydrophilic silica aerogels as dermal drug delivery systems—Dithranol as a model drug. Eur. J. Pharm. Biopharm. 2008, 69, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Suttiruengwong, S.; Arlt, W. Feasibility study of hydrophilic and hydrophobic silica aerogels as drug delivery systems. J. Non-Cryst. Solids 2004, 350, 54–60. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Nilsen, E.; Einarsrud, M.-A. Effect of precursors, methylation agents and solvents on the physicochemical properties of silica aerogels prepared by atmospheric pressure drying method. J. Non-Cryst. Solids 2001, 296, 165–171. [Google Scholar] [CrossRef]
- Tamon, H.; Kitamura, T.; Okazaki, M. Preparation of silica aerogel from TEOS. J. Colloid Interface Sci. 1998, 195, 353–358. [Google Scholar] [CrossRef]
- Patel, R.; Purohit, N.; Suthar, A. An overview of silica aerogels. Int. J. ChemTech Res. 2009, 1, 1052–1057. [Google Scholar]
- Lee, C.J.; Kim, G.S.; Hyun, S.H. Synthesis of silica aerogels from water glass via new modified ambient drying. J. Mater. Sci. 2002, 37, 2237–2241. [Google Scholar]
- Hwang, S.-W.; Kim, T.-Y.; Hyun, S.-H. Effect of surface modification conditions on the synthesis of mesoporous crack-free silica aerogel monoliths from water glass via ambient-drying. Microporous Mesoporous Mater. 2010, 130, 295–302. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, T. Preparation of silica aerogel from rice hull ash by supercritical carbon dioxide drying. J. Supercrit. Fluids 2005, 35, 91–94. [Google Scholar] [CrossRef]
- Setyawan, N.; Winardi, S. Synthesis of silica aerogel from bagasse ash by ambient pressure drying. In Proceedings of the 4th Nanoscience and Nanotechnology Symposium, Bali, Indonesia, 23–25 September 2011; Volume 1415, pp. 114–121.
- Shi, F.; Liu, J.-X.; Song, K.; Wang, Z.-Y. Cost-effective synthesis of silica aerogels from fly ash via ambient pressure drying. J. Non-Cryst. Solids 2010, 356, 2241–2246. [Google Scholar] [CrossRef]
- Gao, G.-M.; Xu, X.-C.; Zou, H.-F.; Ji, G.-J.; Gan, S.-C. Microstructural and physical properties of silica aerogel based on oil shale ash. Powder Technol. 2010, 202, 137–142. [Google Scholar] [CrossRef]
- Gao, G.-M.; Miao, L.-N.; Ji, G.-J.; Zou, H.-F.; Gan, S.-C. Preparation and characterization of silica aerogels from oil shale ash. Mater. Lett. 2009, 63, 2721–2724. [Google Scholar] [CrossRef]
- Hæreid, S.; Anderson, J.; Einarsrud, M.A.; Hua, D.W.; Smith, D.M. Thermal and temporal aging of TMOS-based aerogel precursors in water. J. Non-Cryst. Solids 1995, 185, 221–226. [Google Scholar] [CrossRef]
- Strøm, R.A.; Masmoudi, Y.; Rigacci, A.; Petermann, G.; Gullberg, L.; Chevalier, B.; Einarsrud, M.A. Strengthening and aging of wet silica gels for up-scaling of aerogel preparation. J. Sol-Gel Sci. Technol. 2007, 41, 291–298. [Google Scholar] [CrossRef]
- Yun, D.; Kim, H.; Yoo, J. Preparation of silica nanospheres: Effect of silicon alkoxide and alcohol on silica nanospheres. Bull. Korean Chem. Soc. 2005, 26, 1927–1934. [Google Scholar]
- González-García, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar]
- Novak, Z.; Želijko, K. Diffusion of methanol-liquid CO2 and methanol-supercritical CO2 in silica aerogels. J. Non-Cryst. Solids 1997, 221, 163–169. [Google Scholar] [CrossRef]
- Błaszczyński, T.; Ślosarczyk, A.; Morawski, M. Synthesis of silica aerogel by supercritical drying method. Proceedia Eng. 2013, 57, 200–206. [Google Scholar] [CrossRef]
- Roig, A.; Mata, I.; Molins, E.; Miravitlles, C.; Torras, J.; Llibre, J. Silica aerogels by supercritical extraction. J. Eur. Ceram. Soc. 1998, 18, 1141–1143. [Google Scholar] [CrossRef]
- Tajiri, K.; Igarashi, K.; Nishio, T. Effect of supercritical drying media on structure and properties of silica aerogel. J. Non-Cryst. Solids 1995, 186, 83–87. [Google Scholar] [CrossRef]
- Zhou, X.C.; Zhong, L.P.; Xu, Y.P. Surface modification of silica aerogels with trimethylchlorosilane in the ambient pressure drying. Inorg. Mater. 2008, 44, 976–979. [Google Scholar] [CrossRef]
- Parvathy Rao, A.; Venkateswara Rao, A. Modifying the surface energy and hydrophobicity of the low-density silica aerogels through the use of combinations of surface-modification agents. J. Mater. Sci. 2010, 45, 51–63. [Google Scholar] [CrossRef]
- Cui, S.; Liu, Y.; Fan, M.; Cooper, A.T.; Lin, B.; Liu, X.; Han, G.; Shen, X. Temperature dependent microstructure of MTES modified hydrophobic silica aerogels. Mater. Lett. 2011, 65, 606–609. [Google Scholar] [CrossRef]
- Nogami, M.; Hotta, S.; Kugimiya, K.; Matsubara, H. Synthesis and characterization of transparent silica-based aerogels using methyltrimethoxysilane precursor. J. Sol-Gel Sci. Technol. 2010, 56, 107–113. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Huo, L.; He, X. One step synthesis of hydrophobic silica aerogel via in situ surface modification. Mater. Lett. 2012, 87, 146–149. [Google Scholar] [CrossRef]
- Parmenter, K.E.; Milstein, F. Mechanical properties of silica aerogels. J. Non-Cryst. Solids 1998, 223, 179–189. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, J.; Wu, A.; Shen, J.; Zhou, B. High strength SiO2 aerogel insulation. J. Non-Cryst. Solids 1998, 225, 101–104. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Y.; Shi, D. Experimental investigation and modelling of the creep behavior of ceramic fiber-reinforced SiO2 aerogel. J. Non-Cryst. Solids 2012, 358, 519–524. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Y.; Shi, D.; Liu, J. Experimental investigation on mechanical properties of a fiber-reinforced silica aerogel composite. Mater. Sci. Eng. A 2011, 528, 4830–4836. [Google Scholar] [CrossRef]
- Wang, X. Base Research on the Application of Nanoporous SiO2 Aerogel Based Thermal Insulation Composites. Master’s Thesis, National University of Defense Technology, Chansha, China, 2006. [Google Scholar]
- He, J.; Li, X.; Su, D.; Ji, H.; Qiao, Y. High-strength mullite fibers reinforced ZrO2-SiO2 aerogels fabricated by rapid gel method. J. Mater. Sci. 2015, 50, 7488–7494. [Google Scholar] [CrossRef]
- Ryu, J. Flexible aerogel superinsulation and its manufacture. US6068882 A, 30 May 2000. [Google Scholar]
- Kim, C.-Y.; Lee, J.-K.; Kim, B.-I. Synthesis and pore analysis of aerogel-glass fiber composites by ambient drying method. Colloids Surf. A 2008, 313–314, 179–182. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L.; Cheng, X.; He, S.; Li, C.; Zhang, H. Flexible silica aerogel composites strengthened with aramid fibers and their thermal behavior. Mater. Des. 2016, 99, 329–355. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; He, S.; Shi, G.; Gong, L.; Zhang, H. Aramid fibers reinforced silica aerogel composites with low thermal conductivity and improved mechanical performance. Compos. A 2016, 84, 316–325. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, J.; Ni, X.; Wu, G.; Zhou, B.; Yang, M.; Gu, X.; Qian, M.; Wu, Y. Hydrophobic silica aerogels strengthened with nonwoven fibers. J. Macromol. Sci. A 2006, 43, 1663–1670. [Google Scholar] [CrossRef]
- Razaei, E.; Moghaddas, J. Thermal conductivities of silica aerogel composite insulating material. Adv. Mater. Lett. 2016, 7, 296–301. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Li, H.; Ji, H.; Sun, X.; He, J. Effect of sepiolite fiber on the structure and properties of the sepiolite/silica aerogel composite. J. Sol-Gel Sci. Technol. 2013, 67, 646–653. [Google Scholar] [CrossRef]
- Boday, D.J.; Muriithi, B.; Stover, R.J.; Loy, D.A. Polyaniline nanofiber-silica composite aerogels. J. Non-Cryst. Solids 2012, 358, 1575–1580. [Google Scholar] [CrossRef]
- Tang, X.; Sun, A.; Chu, C.; Yu, M.; Ma, S.; Cheng, Y.; Guo, J.; Xu, G. A novel silica nanowire-silica composite aerogels dried at ambient pressure. Mater. Des. 2017, 115, 415–421. [Google Scholar] [CrossRef]
- Sedova, A.; Bar, G.; Goldbart, O.; Ron, R.; Achrai, B.; Kaplan-Ashiri, I.; Brumfeld, V.; Zak, A.; Gvishi, R.; Wagner, H.D.; et al. Reinforcing silica aerogels with tungsten disulfide nanotubes. J. Supercrit. Fluids 2015, 106, 9–15. [Google Scholar] [CrossRef]
- Liu, H.; Chu, P.; Li, H.; Zhang, H.; Li, J. Novel three-dimensional halloysite nanotubes/silica composite aerogels with enhanced mechanical strength and low thermal conductivity prepared at ambient pressure. J. Sol-Gel Sci. Technol. 2016, 80, 651–659. [Google Scholar] [CrossRef]
- Wei, T.-Y.; Lu, S.-Y.; Chang, Y.-C. A new class of opacified monolithic aerogels of ultralow high-temperature thermal conductivities. J. Phys. Chem. C 2009, 113, 7424–7428. [Google Scholar] [CrossRef]
- Donnet, B.; Wang, T.K.; Peng, J.C.M.; Rebouillat, S. Carbon Fibers; Marcel Dekker Inc.: New York, NY, USA, 1998. [Google Scholar]
- Chung, D.D.L. Carbon Fiber Composites; Butterworth-Heinemann: Newton, MA, USA, 1994. [Google Scholar]
- Li, L.; Yalcin, B.; Nguyen, B.; Meador, M.A.; Cakmak, M. Flexible nanofiber-reinforced aerogel (xerogel) synthesis, manufacture, and characterization. Appl. Mater. Interfaces 2009, 11, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.A.; Vivod, S.; McCorkle, L.; Quade, D.; Sullivan, R.M.; Ghosn, L.J.; Clark, N.; Capadonna, L.A. Reinforcing polymer cross-linked aerogels with carbon nanofibers. J. Mater. Chem. 2008, 18, 1843–1852. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Strauchmann, W.; Ziółkowski, P.; Jakubowska, P. Synthesis and characterization of carbon fiber/silica aerogel nanocomposite. J. Non-Cryst. Solids 2015, 416, 1–3. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Barałkowski, M.; Niemier, S.; Jakubowska, P. Synthesis and characterization of silica aerogel/carbon microfibers nanocomposites dried in supercritical and ambient pressure conditions. J. Sol-Gel Sci. Technol. 2015, 76, 227–232. [Google Scholar] [CrossRef]
- Sarawade, P.; Kim, J.; Hilonga, A.; Quang, D.; Jeon, S.; Kim, H. Synthesis of sodium silicate-based hydrophilic silica aerogel beads with superior properties: Effect of heat-treatment. J. Non-Cryst. Solids 2011, 357, 2156–2162. [Google Scholar] [CrossRef]
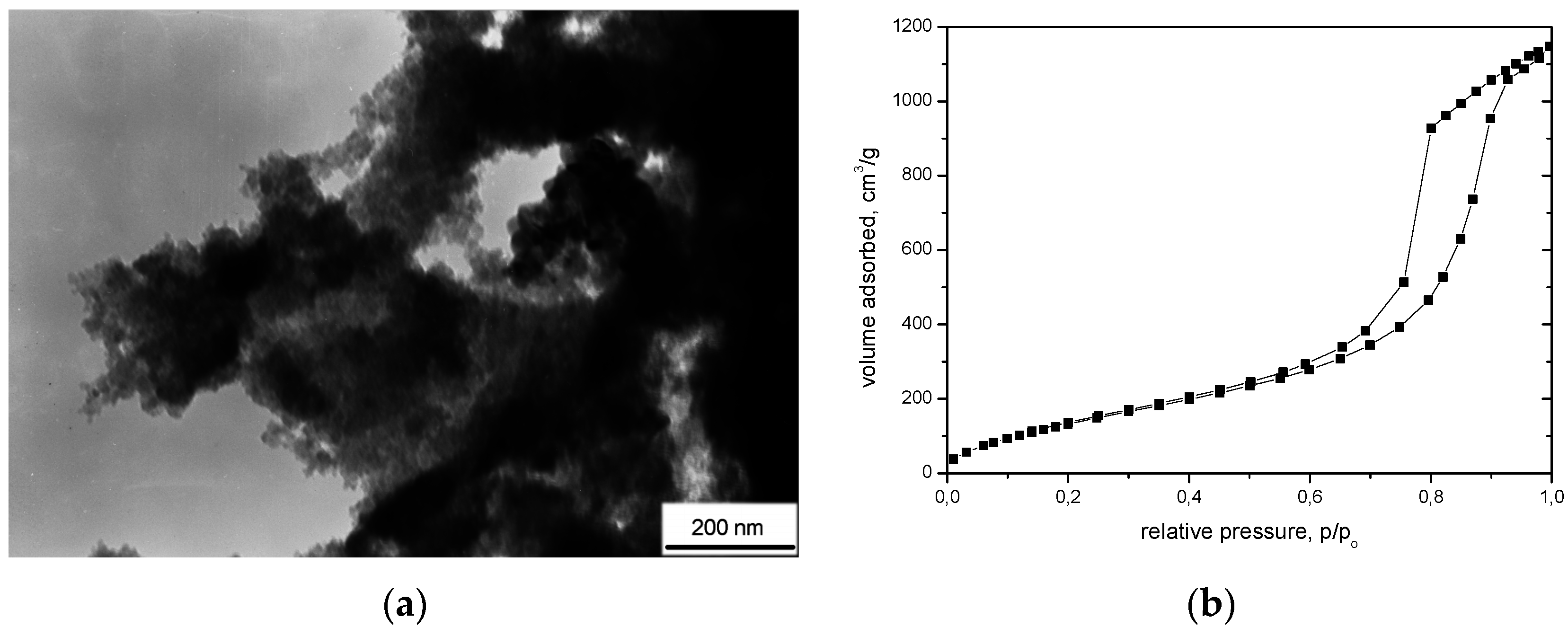
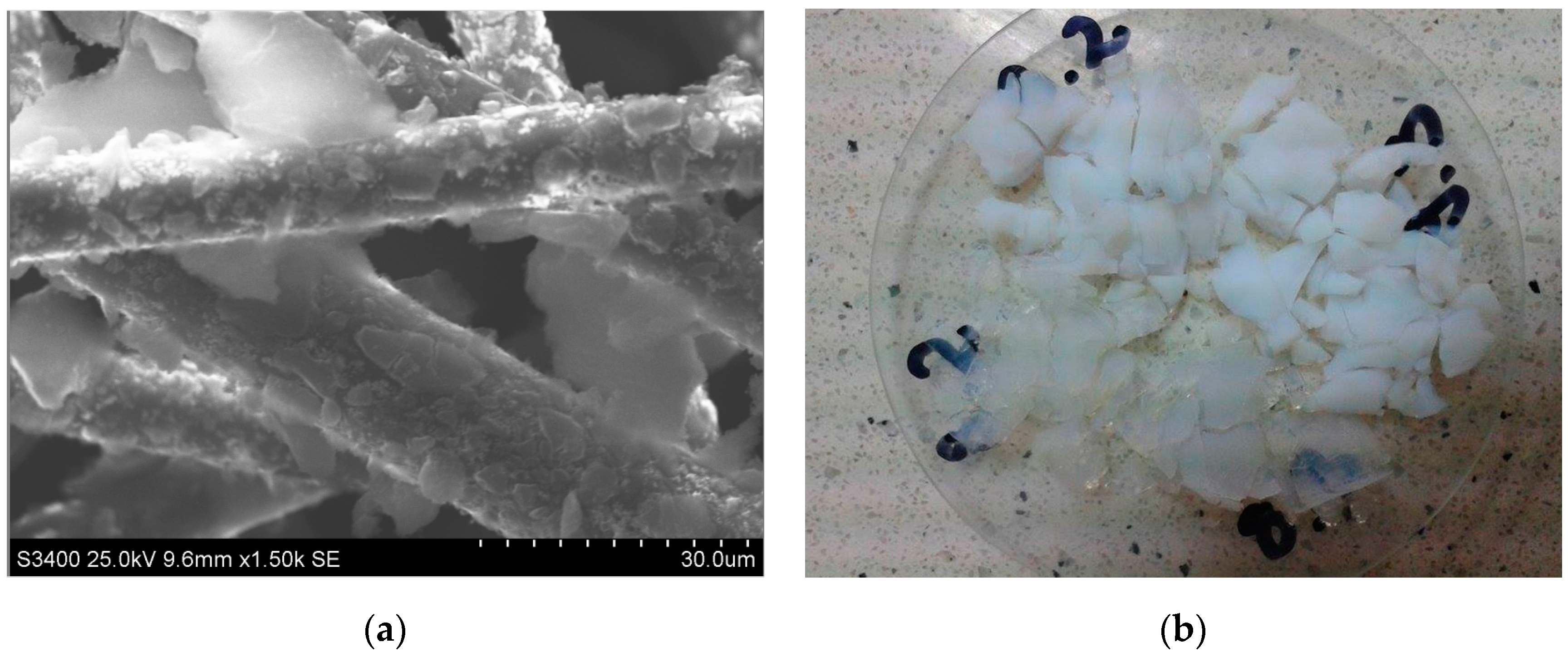
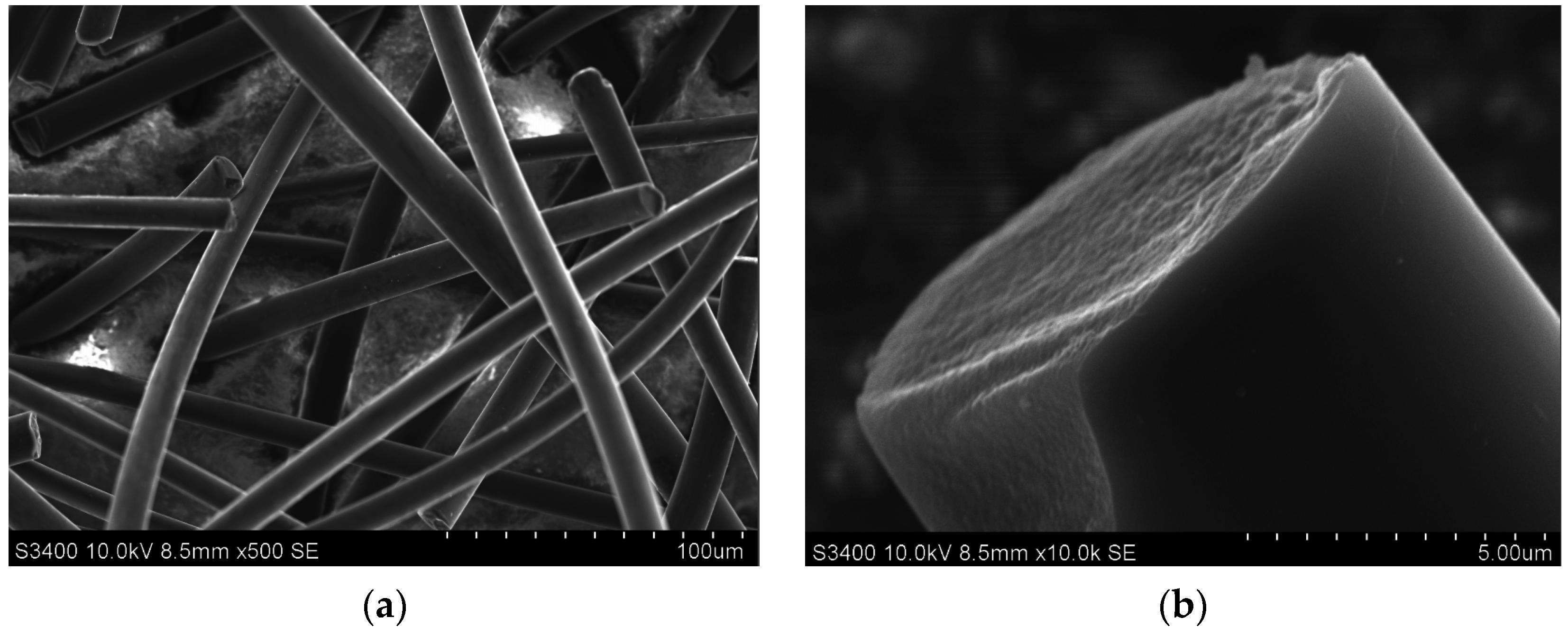
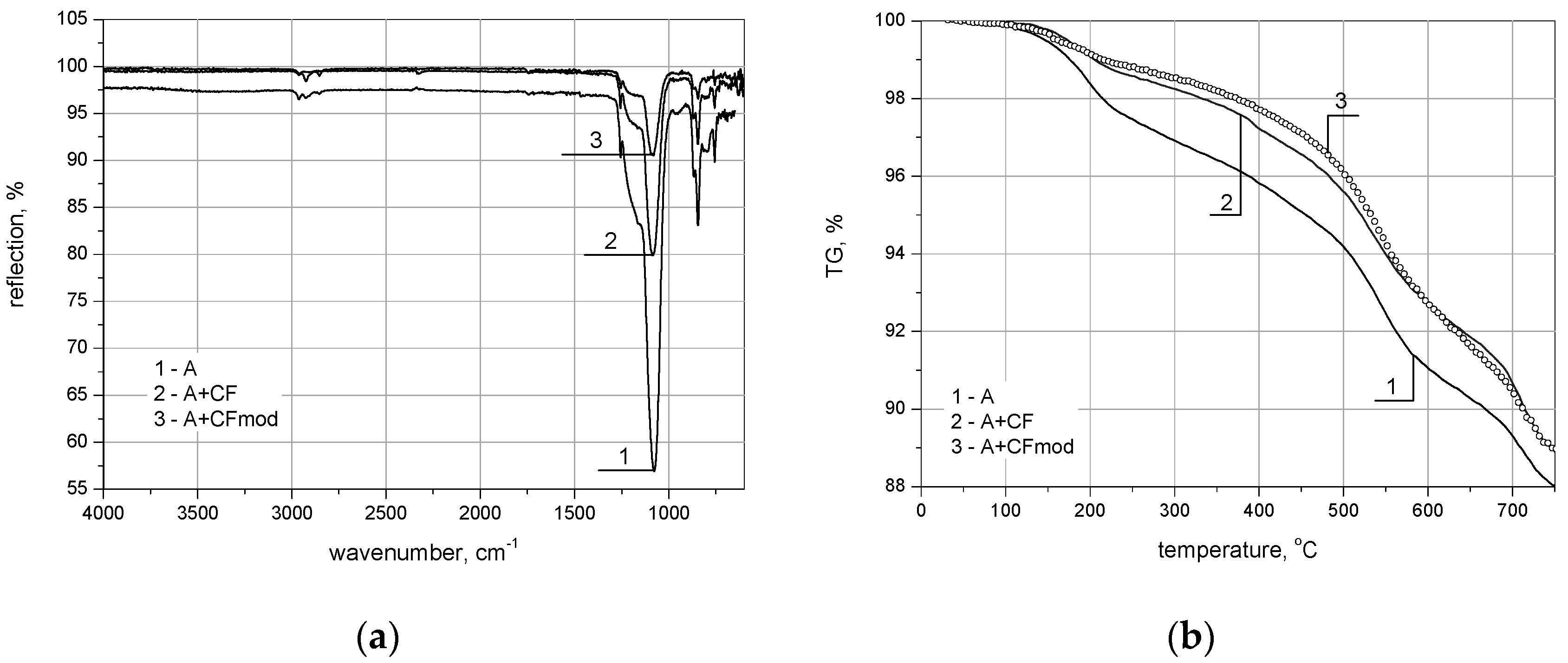

| Marked in Text | 1 (A) | 2 (A + CF) | 3 (A + CFmod) |
|---|---|---|---|
| Density, g/cm3 | 0.20 | 0.13 | 0.12 |
| Surface area by Braunauer-Emmet-Teller (BET), m2/g | 863.9 | 820.9 | 775.1 |
| Average pore diameter, nm | 12.2 | 10.9 | 10.7 |
| Volume shrinkage, % | 27.7 | 10.7 | 8.8 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ślosarczyk, A. Recent Advances in Research on the Synthetic Fiber Based Silica Aerogel Nanocomposites. Nanomaterials 2017, 7, 44. https://doi.org/10.3390/nano7020044
Ślosarczyk A. Recent Advances in Research on the Synthetic Fiber Based Silica Aerogel Nanocomposites. Nanomaterials. 2017; 7(2):44. https://doi.org/10.3390/nano7020044
Chicago/Turabian StyleŚlosarczyk, Agnieszka. 2017. "Recent Advances in Research on the Synthetic Fiber Based Silica Aerogel Nanocomposites" Nanomaterials 7, no. 2: 44. https://doi.org/10.3390/nano7020044
APA StyleŚlosarczyk, A. (2017). Recent Advances in Research on the Synthetic Fiber Based Silica Aerogel Nanocomposites. Nanomaterials, 7(2), 44. https://doi.org/10.3390/nano7020044





