Comparison of Anionic, Cationic and Nonionic Surfactants as Dispersing Agents for Graphene Based on the Fluorescence of Riboflavin
Abstract
1. Introduction
2. Results and Discussion
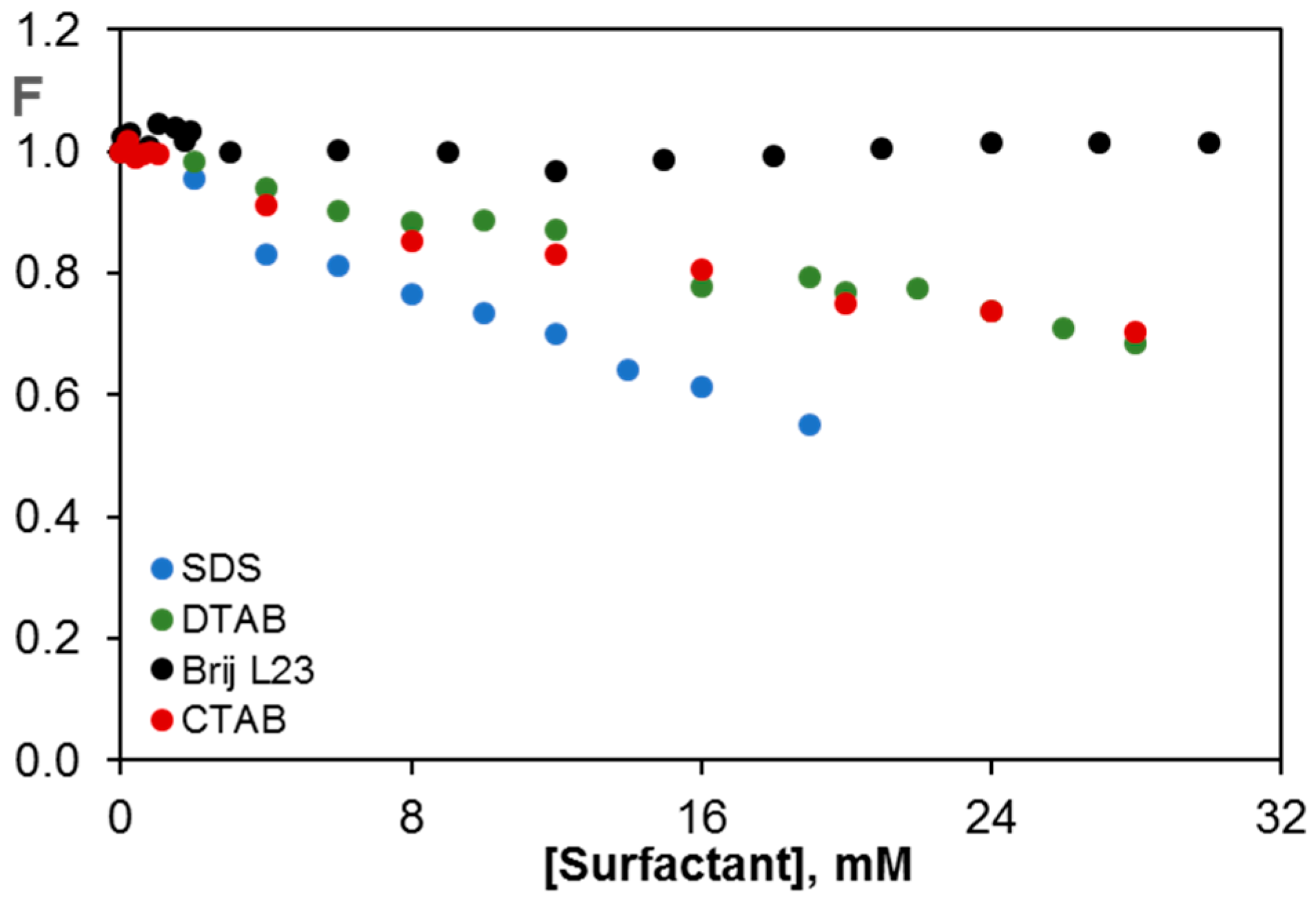
2.1. Fluorescence of Riboflavin in Aqueous Solutions of Surfactants of Different Nature
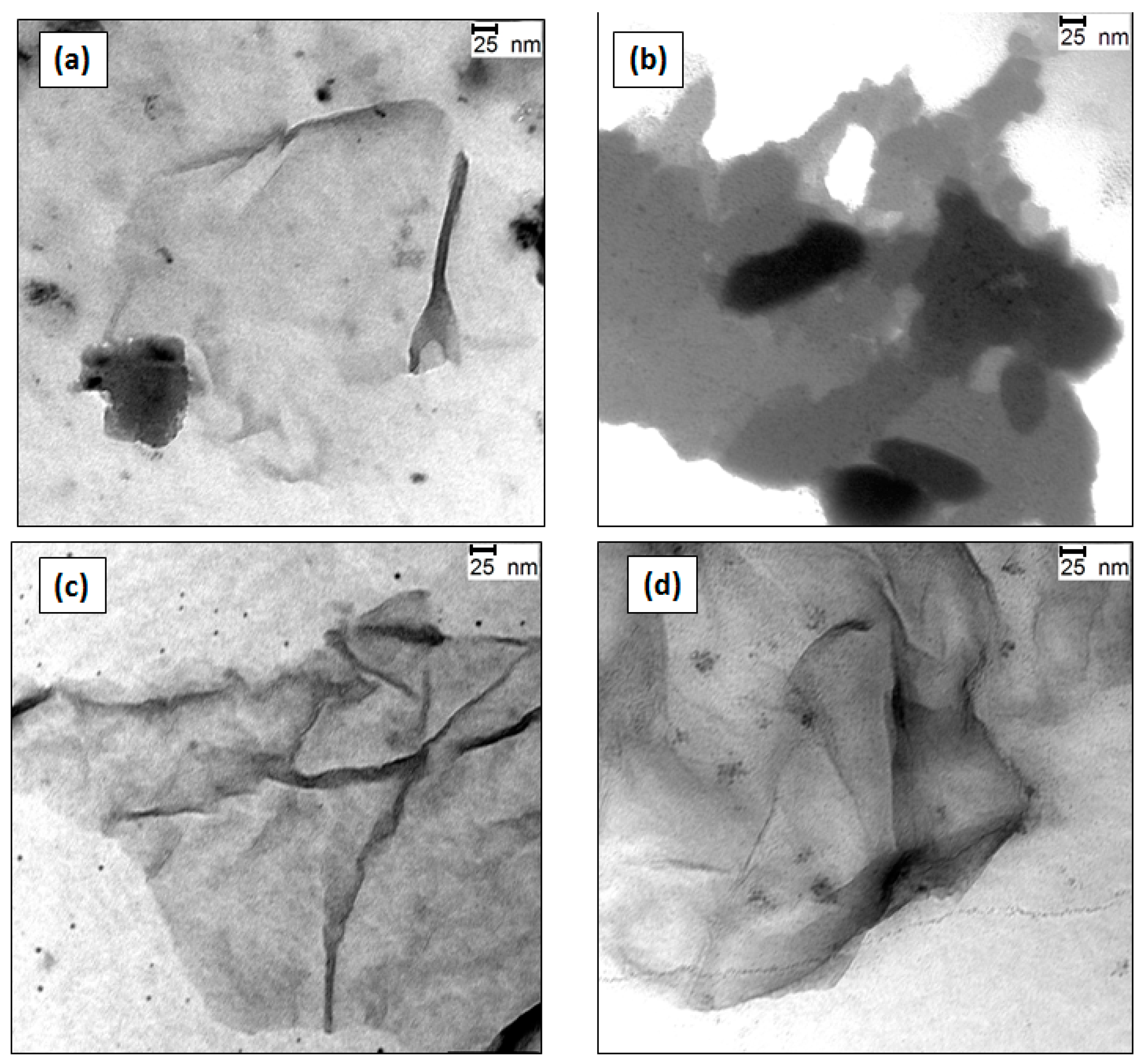
2.2. Transmission Electron Microscopy (TEM) Analysis of the G Dispersions
2.3. Fluorescence of Riboflavin in G Dispersions in Surfactants
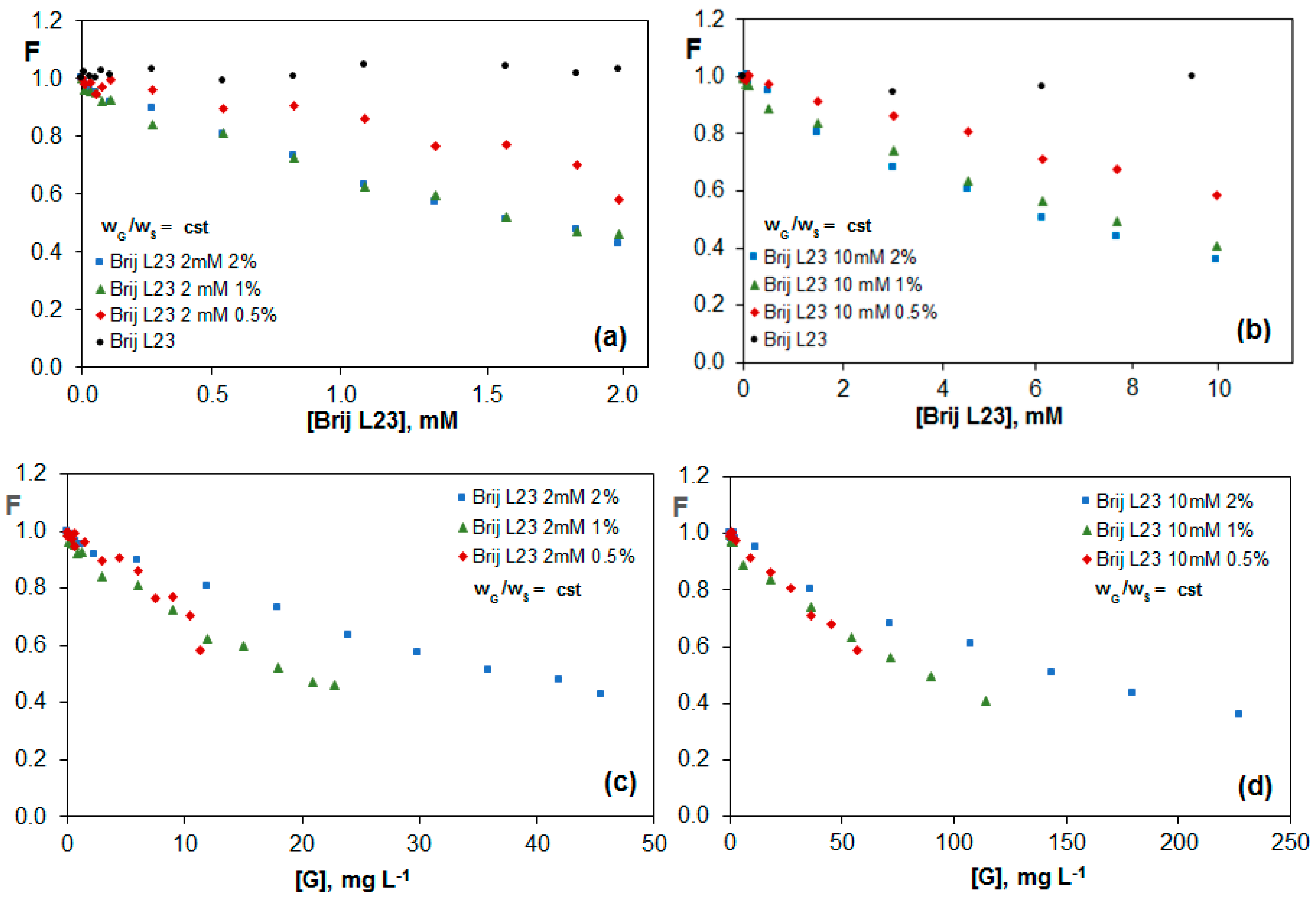
2.3.1. Fluorescence of Riboflavin in G Dispersions in Brij L23 Aqueous Solutions
2.3.2. Fluorescence of Riboflavin in G Dispersions in SDS Aqueous Solutions
2.3.3. Fluorescence of Riboflavin in G Dispersions in DTAB and CTAB Aqueous Solutions
2.3.4. Comparison of the Quenching Effect in G (2 wt %) Dispersions in the Different Surfactants
2.3.5. Study of the Quenching Process
3. Materials and Methods
3.1. Reagents
3.2. Instrumentation
3.3. Procedure
3.3.1. Preparation of the Vitamin Solutions and the Graphene Dispersions in the Surfactant Solutions
3.3.2. Riboflavin Fluorescence Spectra in the Different Media
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Founding
References
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Latil, S.; Henrard, L. Charge carriers in few-lawyer graphene films. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, S.; Tian, X.N.; Zhao, X.S. Layered graphene oxide nanostructures with sandwiched conducting polymers as supercapacitor electrodes. Langmuir 2010, 26, 16624–16628. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, B.; Merkoci, A. Carbon nanotubes and graphene in analytical sciences. Mikrochim. Acta 2012, 179, 1–16. [Google Scholar] [CrossRef]
- Ge, S.; Lan, F.; Yu, F.; Yu, J. Applications of graphene and related nanomaterials in analytical chemistry. New J. Chem. 2015, 39, 2380–2395. [Google Scholar] [CrossRef]
- Martín, A.; Escarpa, A. Graphene: The cutting-edge interaction between chemistry and electrochemistry. Trends Anal. Chem. 2014, 56, 13–26. [Google Scholar] [CrossRef]
- Deng, X.; Tang, H.; Jiang, J. Recent progress in graphene-material-based optical sensors. Anal. Bioanal. Chem. 2014, 406, 6903–6916. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.; Díez-Pascual, A.M.; Lázaro, E.; Vera, S.; Gomez-Fatou, M. Chemical sensors based on polymer composites with carbon nanotubes and grapheme: The role of the polymer. J. Mater. Chem. 2014, 2, 14289–14328. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Naffakh, M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. High-performance nanocomposites based on polyetherketones. Prog. Mater. Sci. 2012, 57, 1106–1190. [Google Scholar] [CrossRef]
- Qian, W.; Hao, R.; Hou, Y.; Tian, Y.; Shen, C.; Gao, H.; Liang, X. Solvothermal-assisted exfoliation process to produce graphene with high yield and high quality. Nano Res. 2009, 2, 2706–2712. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid exfoliation of defect-free graphene. Acc. Chem. Res. 2013, 46, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lotya, M.; Hernández, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, T.; et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Chih-Jen, S.; Strano, M.S.; Blankschtein, D. Molecular insights into the surface morphology, layering structure, and aggregation kinetics of surfactant stabilized graphene dispersions. J. Am. Chem. Soc. 2011, 133, 12810–12823. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Lotya, M.; Coleman, J.N. The importance of repulsive potential barriers for the dispersion of graphene using surfactants. New J. Phys. 2010, 12. [Google Scholar] [CrossRef]
- Guardia, L.; Fernández-Merino, M.J.; Paredes, J.I.; Solís-Fernández, P.; Villar-Rodil, A.; Martínez-Alonso, A.; Tascón, J.M.D. High-throughput production of pristine graphene in an aqueous dispersion assisted by non-ionic surfactants. Carbon 2011, 49, 1653–1662. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Paredes, J.I.; Villar-Rodil, S.; Guardia, L.; Solís-Fernández, P.; Salinas-Torres, D.; Cazorla-Amorós, D.; Morallón, E.; Martínez-Alonso, A.; Tascón, J.M.D. Investigating the influence of surfactants on the stabilization of aqueous reduced graphene oxide dispersions and the characteristics of their composite films. Carbon 2012, 50, 3184–3194. [Google Scholar] [CrossRef]
- San Andrés, M.P.; Díez-Pascual, A.M.; Palencia, S.; San Torcuato, J.; Valiente, M.; Vera, S. Fluorescence quenching of α-tocopherol by graphene dispersed in aqueous surfactant solutions. J. Lumin. 2017, 187, 169–180. [Google Scholar] [CrossRef]
- Narayan, R.; Kim, S.O. Surfactant mediated liquid phase exfoliation of graphene. Nano Converg. 2015, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef]
- Wang, S.; Yi, M.; Shen, Z. The effect of surfactants and their concentrations on the liquid-exfoliation of graphene. RSC Adv. 2016, 6, 56705–56710. [Google Scholar] [CrossRef]
- Tommaso, R.I.C.; Donatella, N.; Laura, S.; Antonio, S. Assay of riboflavin in sample wines by capillary zone electrophoresis and laser-induced fluorescence detection. J. Agric. Food Chem. 2002, 50, 6643–6647. [Google Scholar] [CrossRef]
- Rivlin, S.L. Riboflavin and Cancer: A Review. Cancer Res. 1973, 33, 1977–1986. [Google Scholar] [PubMed]
- Bassett, J.K.; Baglietto, L.; Hodge, A.M. Dietary intake of B vitamins and methionine and breast cancer risk. Cancer Causes Control 2013, 24, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, S.; Stojanovic, B.; Veljkovic, J.; Prazic-Arsic, L.; Rogli, G.; Manojlovi, D. Simultaneous determination of some water-soluble vitamins and preservatives in multivitamin syrup by validated stability-indicating high-performance liquid chromatography method. J. Chromatogr. A 2008, 1202, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, L.; Liu, M.; Lin, J.M. Determination of riboflavin by enhancing the chemiluminescence intensity of peroxomonosulfate-cobalt(II) system. Spectrochim. Acta Part A 2007, 66, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Chen, H.Y. A spectroelectrochemical study of the interaction of riboflavin with β-cyclodextrin. Spectrochim. Acta Part A 1996, 52, 599–605. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, F.; Gokavi, S.; Chow, A.; Sheng, Q.; Guo, M. Quantification of water-soluble vitamins in milk-based infant formulae using biosensor-based assays. Food Chem. 2008, 110, 769–776. [Google Scholar] [CrossRef]
- Hua, L.; Yang, X.; Wang, C.; Yuan, H.; Xiao, D. Determination of riboflavin in urine and beverages by capillary electrophoresis with in-column optical fiber laser-induced fluorescence detection. J. Chromatogr. B 2007, 856, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ghann, W.E. Studies of Surfactants Effect on Riboflavin Fluorescence and Its Determination in Commercial Food Products and Vitamin Tablets. Master’s Thesis, East Tennessee State University, Johnson City, TN, USA, 2008. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Kasry, A.; Ardakani, A.A.; Tulevski, G.S.; Menges, B.; Copel, M.; Vyklicky, L. Highly efficient fluorescence quenching with graphene. J. Phys. Chem. C 2012, 116, 2858–2862. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, S.P.; Shao, X.N. Noncovalent binding of xanthene and phthalocyanine dyes with graphene sheets: The effect of the molecular structure revealed by a photophysical study. Spectrochim. Acta Part A 2013, 113, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Cui, Y.; Ma, Y.; Qian, H.; Shen, X. Fluorescence quenching of cationic organic dye by graphene: Interaction and its mechanism. Micro Nano Lett. 2012, 7, 608–612. [Google Scholar] [CrossRef]
- Zhang, X.F.; Xi, Q. A Graphene sheet as an efficient electron acceptor and conductor for photoinduced charge separation. Carbon 2011, 49, 3842–3850. [Google Scholar] [CrossRef]
- Zhang, X.F.; Li, F. Interaction of graphene with excited and ground state rhodamine revealed by steady state and time resolved fluorescence. J. Photochem. Photobiol. A 2012, 246, 8–15. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shao, X. π-π binding ability of different carbon nano-materials with aromatic phthalocyanine molecules: Comparison between graphene, graphene oxide and carbon nanotubes. J. Photochem. Photobiol. A 2014, 278, 69–74. [Google Scholar] [CrossRef]
- Ratajczak, K.; Stobiecka, M. Ternary interactions and energy transfer between fluorescein isothiocyanate, adenosine triphosphate, and graphene oxide nanocarriers. J. Phys. Chem. B 2017, 121, 6822–6830. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Dworakowska, B.; Jakiela, S.; Lukasiak, A.; Chalupa, A.; Zembrzycki, K. Sensing of survivin mRNA in malign antastrocytes using graphene oxide nanocarrier-supported oligonucleotide molecular beacons. Sens. Actuators B 2016, 235, 136–145. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; García-García, D.; San Andrés, M.P.; Vera, S. Determination of riboflavin based on fluorescence quenching by graphene dispersions in polyethylene glycol. RSC Adv. 2016, 6, 19686–19699. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Hermosa, C.; San Andrés, M.P.; Valiente, M.; Vera, S. Effect of graphene and graphene oxide dispersions in poloxamer-407 on the fluorescence of riboflavin: A comparative study. J. Phys. Chem. C 2017, 121, 830–843. [Google Scholar] [CrossRef]
- Palencia, S.; Vera, S.; Díez-Pascual, A.M.; San Andrés, M.P. Quenching of fluorene fluorescence by single-walled carbon nanotube dispersions with surfactants: Application for fluorene quantification in wastewater. Anal. Bioanal. Chem. 2015, 407, 4671–4682. [Google Scholar] [CrossRef] [PubMed]
- White, B.; Banerjee, S.; O’Brien, S.; Turro, N.J.; Herman, I.P. Zeta-potential measurements of surfactant-wrapped individual single-walled carbon nanotubes. J. Phys. Chem. C 2007, 111, 13684–13690. [Google Scholar] [CrossRef]
- Hsieh, A.G.; Korkut, S.; Punckt, C.; Aksay, I.A. Dispersion stability of functionalized graphene in aqueous sodium dodecyl sulfate solutions. Langmuir 2013, 29, 14831–14838. [Google Scholar] [CrossRef] [PubMed]
- Bavali, A.; Parvin, P.; Mortazavi, S.Z.; Nourazar, S.S. Laser induced fluorescence spectroscopy of various carbon nanostructures (GO, G and nanodiamond) in Rd6G solution. Biomed. Opt. Express 2015, 6, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley: Weinheim, Germany, 2002. [Google Scholar]

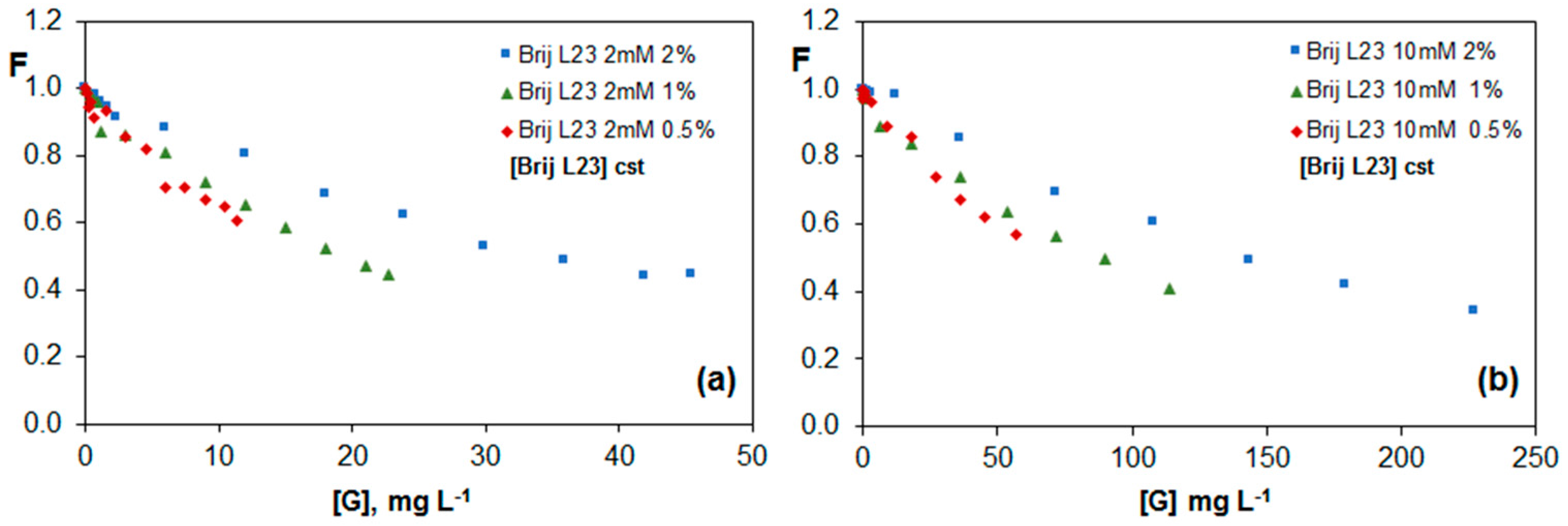

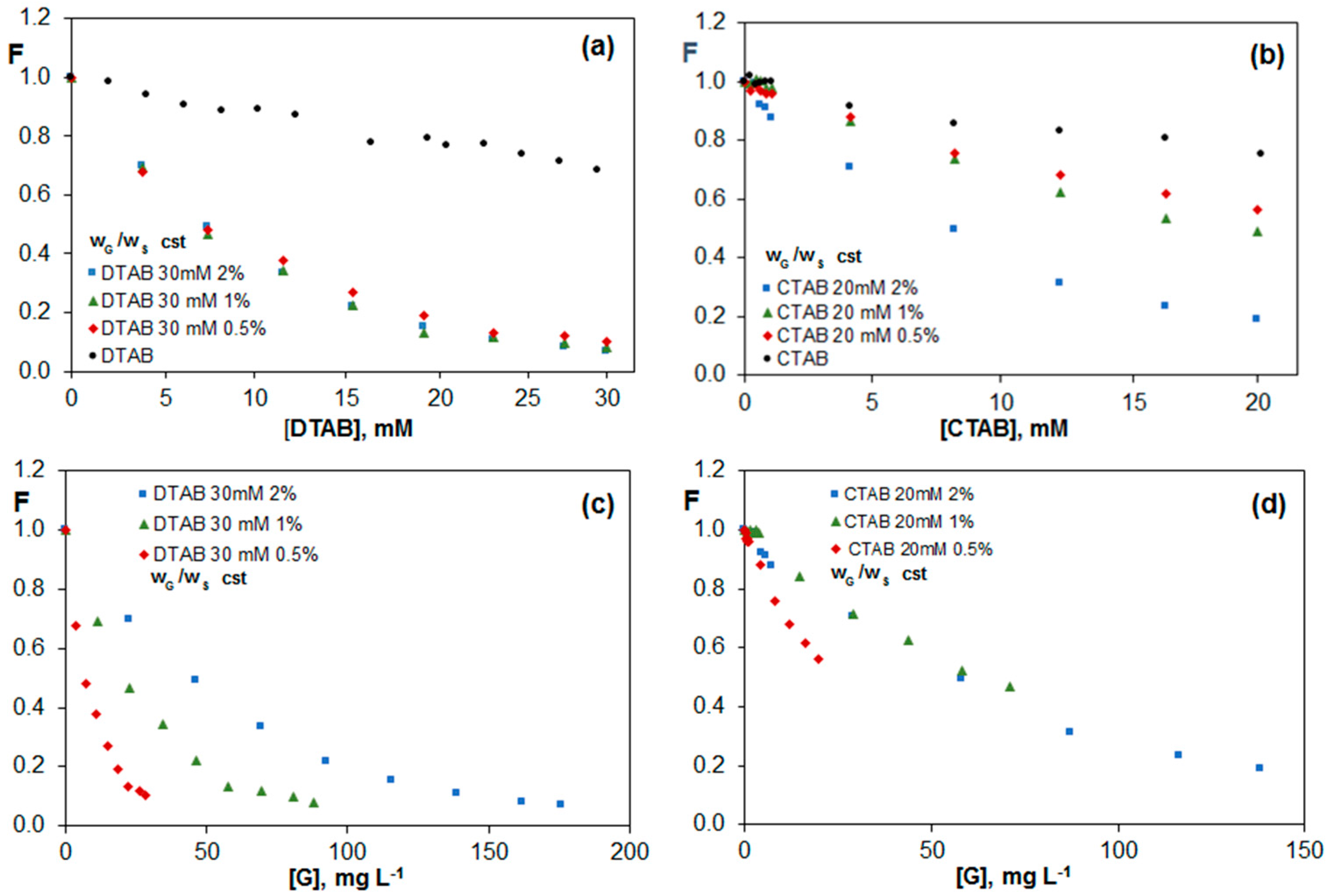
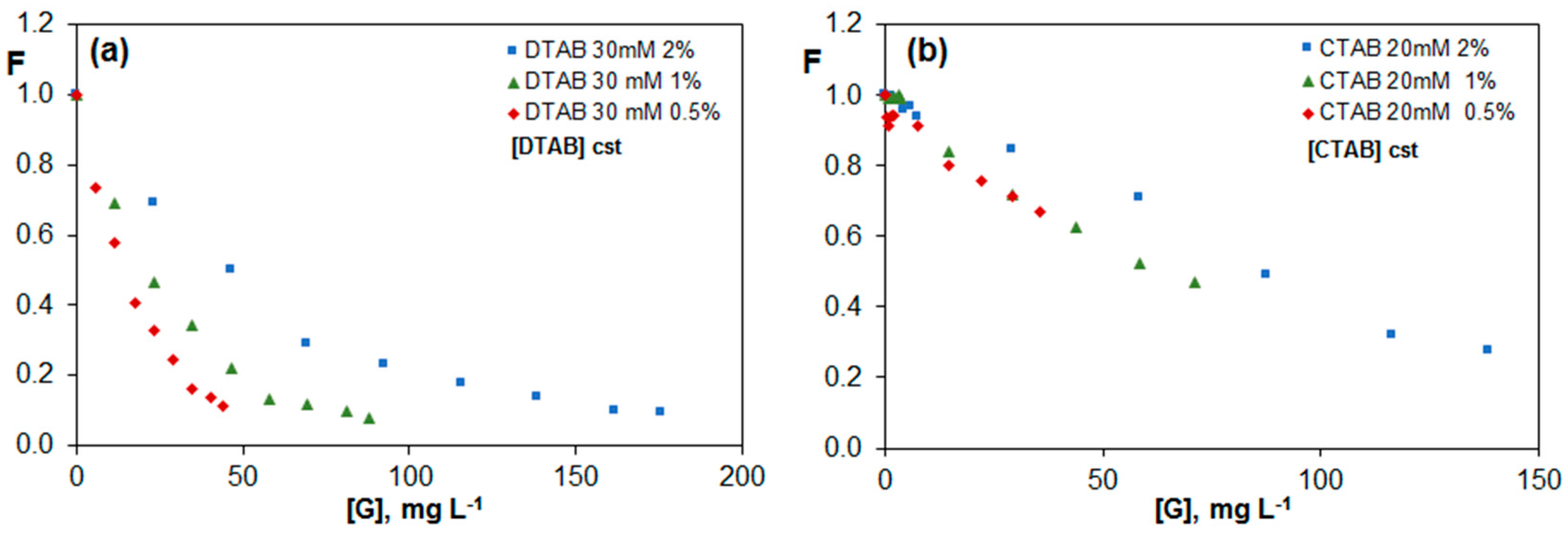
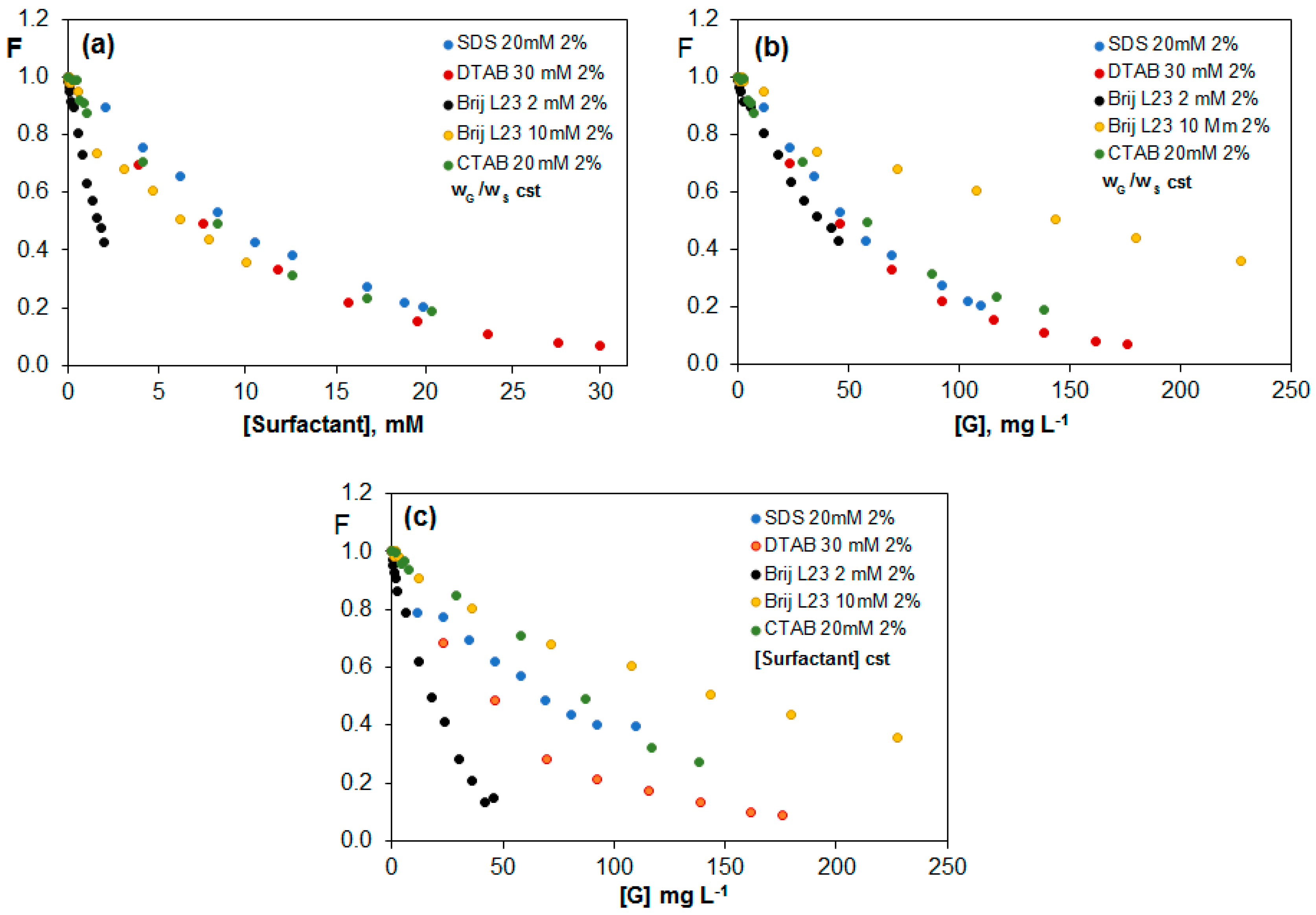
| Brij L23 2 mM | Brij L23 10 mM | |||
| G, wt % | F0/FW | F/F0 | F0/FW | F/F0 |
| 0 | 1.04 | 1.00 | ||
| 0.5 | 0.62 ± 0.03 | 0.58 ± 0.01 | ||
| 1.0 | 0.46 ± 0.01 | 0.41 ± 0.01 | ||
| 2.0 | 0.42 ± 0.03 | 0.36 ± 0.01 | ||
| SDS 20 mM | ||||
| G, wt % | F0/FW | F/F0 [SDS] = cte | ||
| 0 | 0.55 | |||
| 0.5 | 0.6 ± 0.1 | |||
| 1.0 | 0.36 ± 0.03 | |||
| 2.0 | 0.28 ± 0.06 | |||
| DTAB 30 mM | CTAB 20 mM | |||
| G, wt % | F0/FW | F/F0 | F0/FW | F/F0 |
| 0 | 0.68 | 0.75 | ||
| 0.5 | 0.10 ± 0.01 | 0.61 ±0.06 | ||
| 1.0 | 0.08 ± 0.01 | 0.49 ± 0.02 | ||
| 2.0 | 0.08 ± 0.01 | 0.23 ± 0.04 | ||
| DISPERSION | [Surfactant], mM | G, mg L−1 | K × 103, L mg−1 | K, L mg−1 |
|---|---|---|---|---|
| SDS 20 mM | 0–20 | 0 | 0.135 ± 0.003 | ––– |
| SDS 20 mM/G 0.5% | 0–5 | 0–10 | G/SDS wt % constant | 0.018 ± 0.006 |
| SDS 20 mM/G 1% | 0–6 | 0–20 | 0.02 ± 0.01 | |
| SDS 20 mM/G 2% | 0–8 | 0–35 | 0.021 ± 0.003 | |
| SDS 20 mM/G 0.5% | 20 | 0–10 | [SDS] constant | 0.017 ± 0.002 |
| SDS 20 mM/G 1% | 20 | 0–55 | 0.024 ± 0.005 | |
| SDS 20 mM/G 2% | 20 | 0–110 | 0.018 ± 0.002 | |
| Brij L23 2 mM | 0–2 | 0 | 0 | ––– |
| Brij L23 2 mM/G 0.5% | 0–2 | 0–12 | G/Brij L23 wt % constant | 0.049 ± 0.004 |
| Brij L23 2 mM /G 1% | 0–2 | 0–23 | 0.048 ± 0.003 | |
| Brij L23 2 mM/G 2% | 0–2 | 0–42 | 0.04 ± 0.02 | |
| Brij L23 2 mM/G 0.5% | 2 | 0–12 | [Brij L23] constant | 0.049 ± 0.004 |
| Brij L23 2 mM/G 1% | 2 | 0–23 | 0.049 ± 0.002 | |
| Brij L23 2 mM/G 2% | 2 | 0–42 | 0.032 ± 0.002 | |
| Brij L23 10 mM | 0–10 | 0 | 0 | ––– |
| Brij L23 10 mM/G 0.5% | 0–10 | 0–60 | G/Brij L23 wt % constant | 0.012 ± 0.001 |
| Brij L23 10 mM/G 1% | 0–10 | 0–120 | 0.012 ± 0.001 | |
| Brij L23 10 mM/G 2% | 0–10 | 0–230 | 0.0069 ± 0.0005 | |
| Brij L23 10 mM/G 0.5% | 10 | 0–60 | [Brij L23] constant | 0.0133 ± 0.0008 |
| Brij L23 10 mM/G 1% | 10 | 0–120 | 0.010 ± 0.001 | |
| Brij L23 10 mM/G 2% | 10 | 0–230 | 0.0080 ± 0.0008 | |
| CTAB 20 mM | 0–20 | 0 | 0.041 ± 0.002 | ––– |
| CTAB 20 mM/G 0.5% | 0–20 | 0–35 | G/CTAB wt % constant | 0.022 ± 0.001 |
| CTAB 20 mM/G 1% | 0–20 | 0–70 | 0.0148 ± 0.0008 | |
| CTAB 20 mM/G 2% | 0–8 | 0–60 | 0.018 ± 0.003 | |
| CTAB 20 mM/G 0.5% | 20 | 0–35 | [CTAB] constant | 0.0075 ± 0.0009 |
| CTAB 20 mM/G 1% | 20 | 0–70 | 0.0066 ± 0.0007 | |
| CTAB 20 mM/G 2% | 20 | 0–60 | 0.007 ± 0.001 | |
| DTAB 30 mM | 0–30 | 0 | 0.045 ± 0.006 | ––– |
| DTAB 30 mM/G 0.5% | 0–12 | 0–25 | G/DTAB wt % constant | 0.09 ± 0.03 |
| DTAB 30 mM/G 1% | 0–12 | 0–35 | 0.06 ± 0.03 | |
| DTAB 30 mM/G 2% | 0–12 | 0–92 | 0.03 ± 0.02 | |
| DTAB 30 mM/G 0.5% | 30 | 0–25 | [DTAB] constant | 0.09 ± 0.03 |
| DTAB 30 mM/G 1% | 30 | 0–35 | 0.06 ± 0.01 | |
| DTAB 30 mM/G 2% | 30 | 0–50 | 0.02 ± 0.03 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos, R.; Vera, S.; Valiente, M.; Díez-Pascual, A.M.; San Andrés, M.P. Comparison of Anionic, Cationic and Nonionic Surfactants as Dispersing Agents for Graphene Based on the Fluorescence of Riboflavin. Nanomaterials 2017, 7, 403. https://doi.org/10.3390/nano7110403
Mateos R, Vera S, Valiente M, Díez-Pascual AM, San Andrés MP. Comparison of Anionic, Cationic and Nonionic Surfactants as Dispersing Agents for Graphene Based on the Fluorescence of Riboflavin. Nanomaterials. 2017; 7(11):403. https://doi.org/10.3390/nano7110403
Chicago/Turabian StyleMateos, Rocío, Soledad Vera, Mercedes Valiente, Ana María Díez-Pascual, and María Paz San Andrés. 2017. "Comparison of Anionic, Cationic and Nonionic Surfactants as Dispersing Agents for Graphene Based on the Fluorescence of Riboflavin" Nanomaterials 7, no. 11: 403. https://doi.org/10.3390/nano7110403
APA StyleMateos, R., Vera, S., Valiente, M., Díez-Pascual, A. M., & San Andrés, M. P. (2017). Comparison of Anionic, Cationic and Nonionic Surfactants as Dispersing Agents for Graphene Based on the Fluorescence of Riboflavin. Nanomaterials, 7(11), 403. https://doi.org/10.3390/nano7110403