Effects of Iodine Doping on Optoelectronic and Chemical Properties of Polyterpenol Thin Films
Abstract
:1. Introduction
2. Results and Discussion
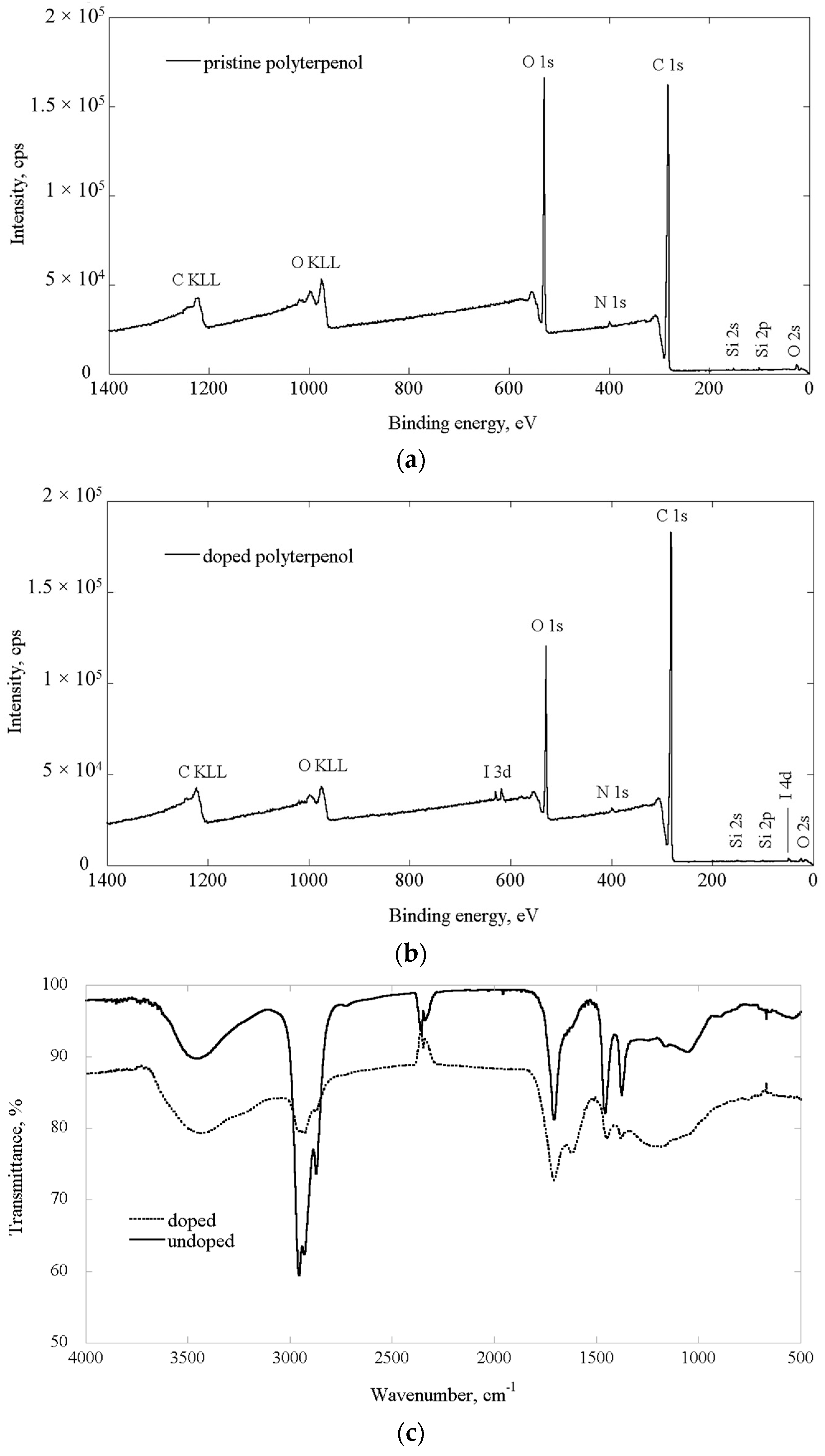
2.1. Chemical Properties
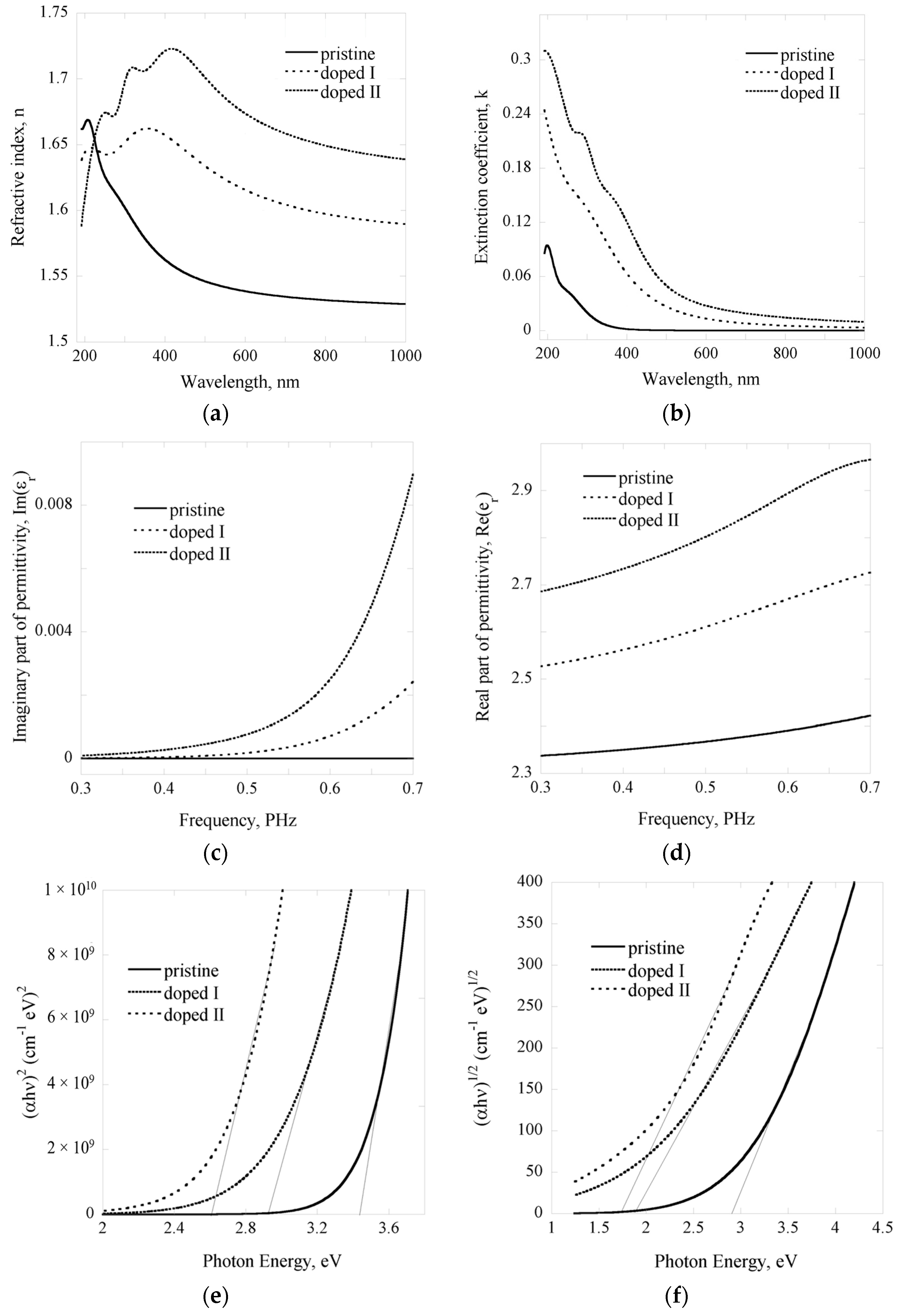
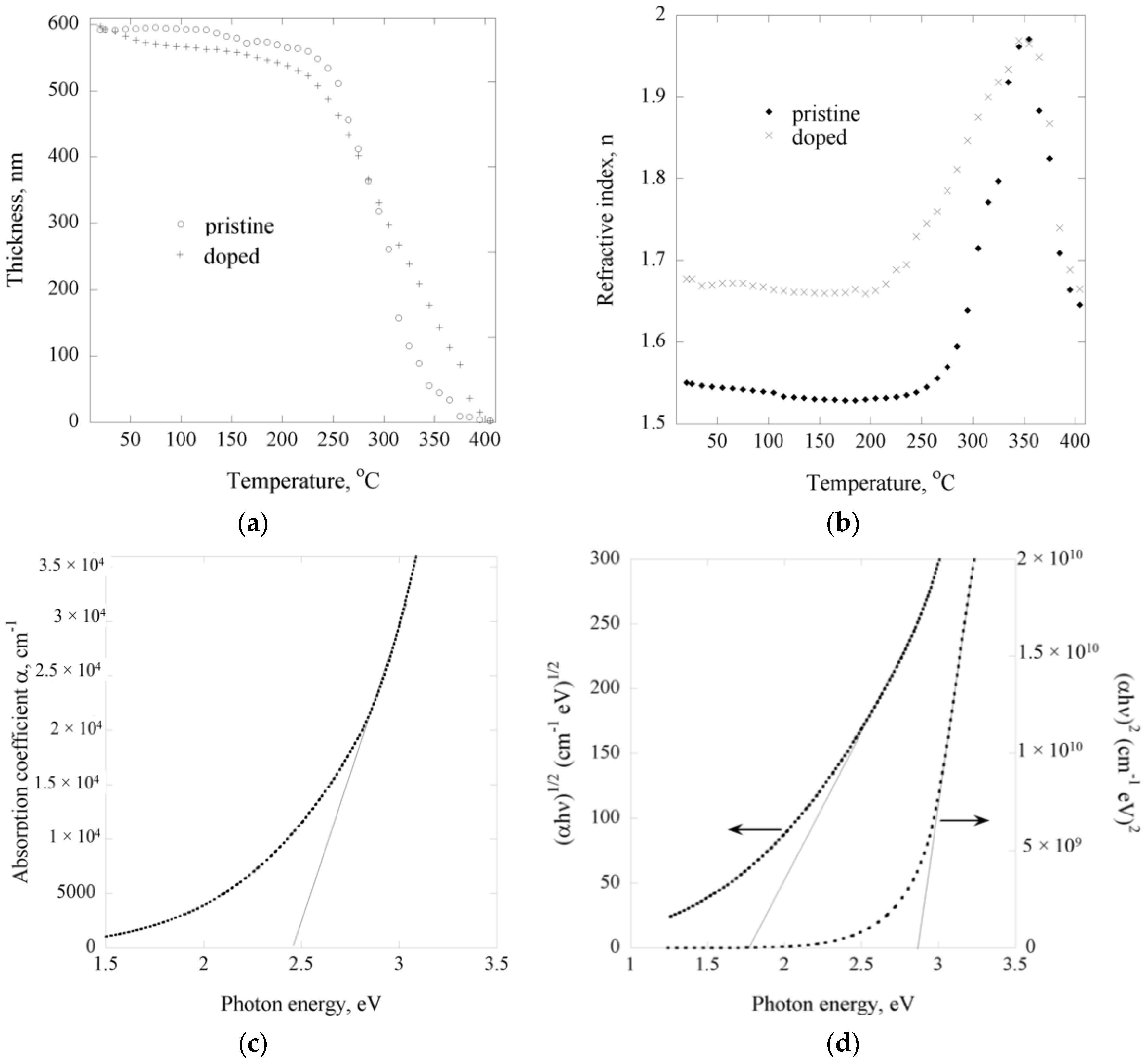
2.2. Optoelectrical Properties
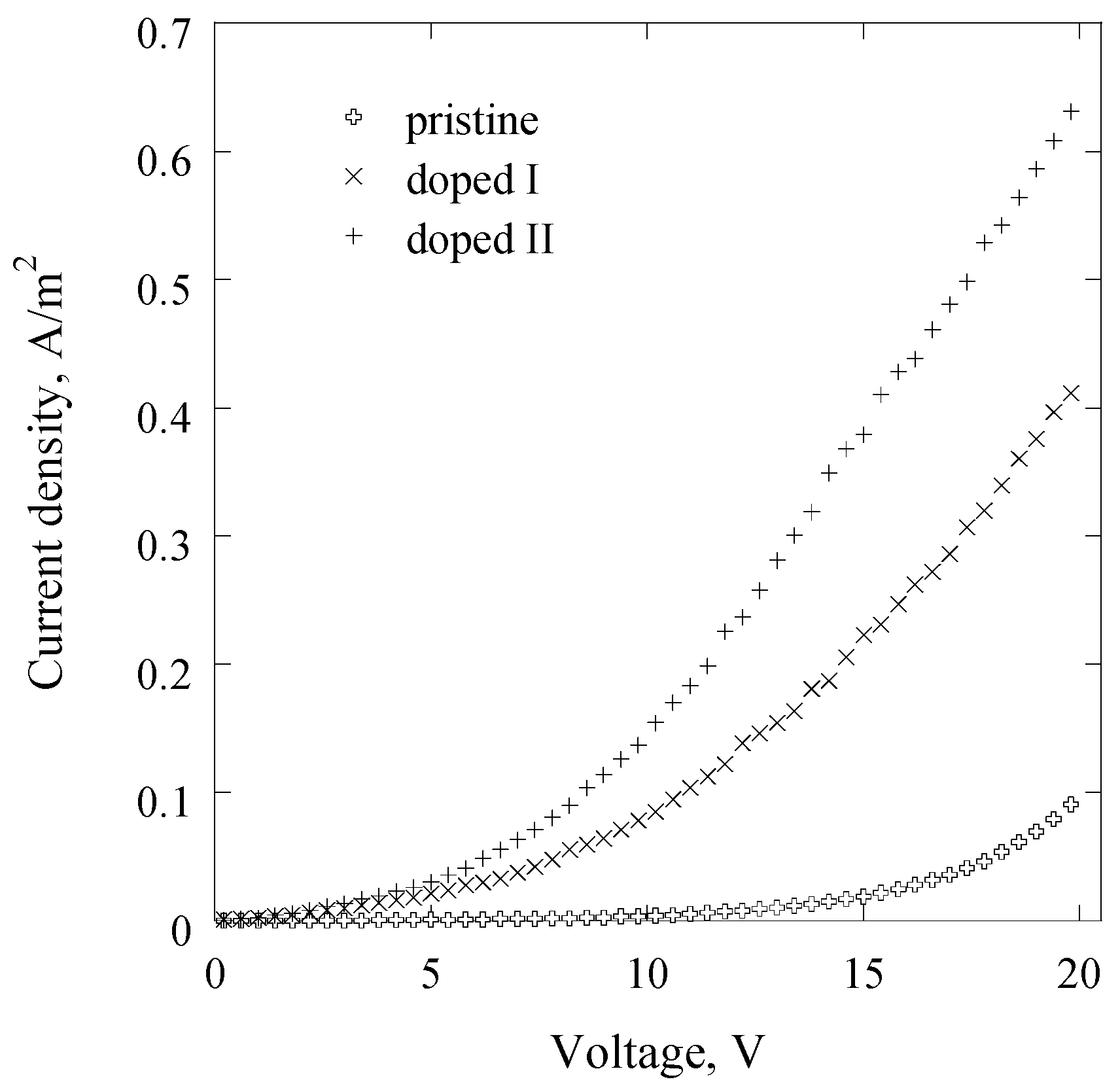
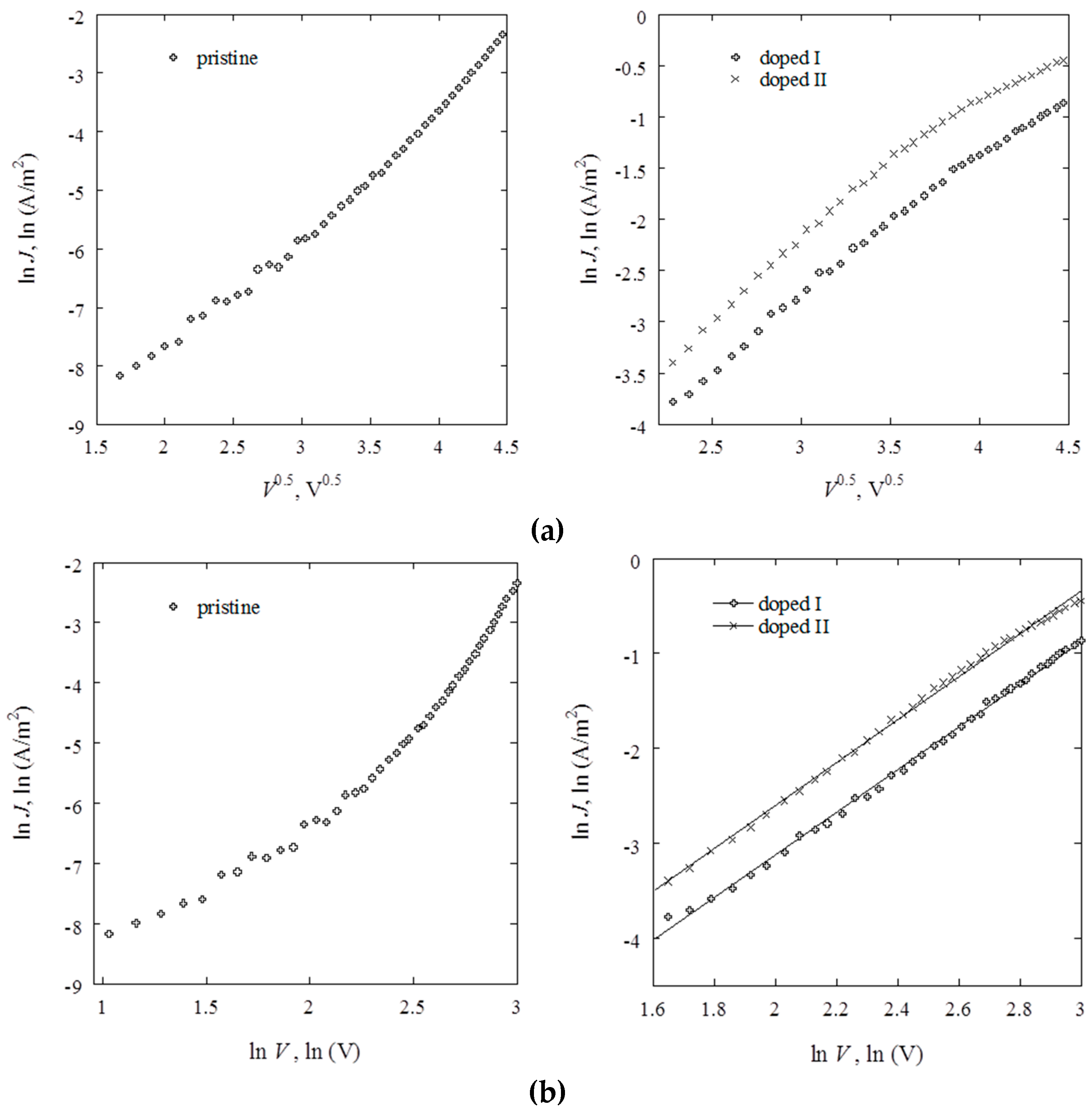
2.3. Electrical Studies
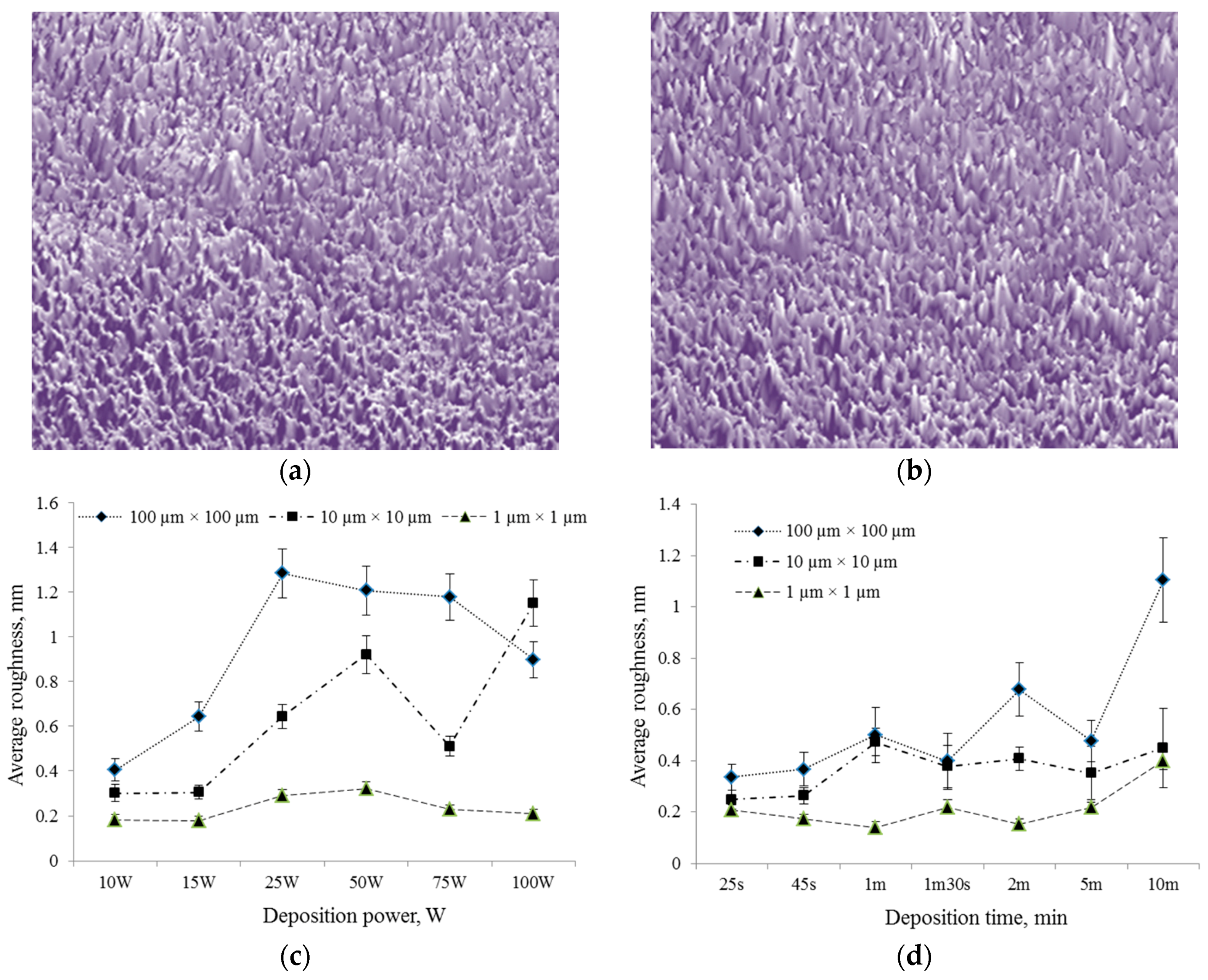
2.4. Surface Properties
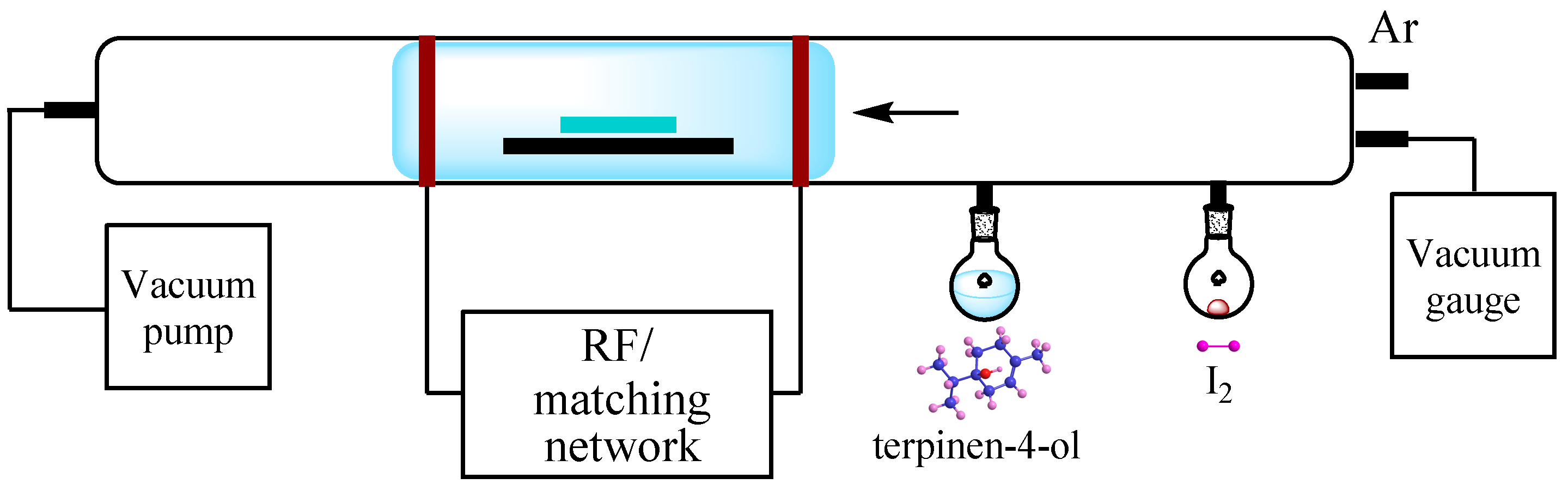
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bazaka, K.; Jacob, M.V.; Ostrikov, K. Sustainable life cycles of natural-precursor-derived nanocarbons. Chem. Rev. 2016, 116, 163–214. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Michelmore, A.; Martinek, P.; Chan, J.; Sah, V.; Griesser, H.J.; Short, R.D. Early stages of growth of plasma polymer coatings deposited from nitrogen-and oxygen-containing monomers. Plasma Process. Polym. 2010, 7, 824–835. [Google Scholar] [CrossRef]
- Michelmore, A.; Martinek, P.; Sah, V.; Short, R.D.; Vasilev, K. Surface morphology in the early stages of plasma polymer film growth from amine-containing monomers. Plasma Process. Polym. 2011, 8, 367–372. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Bowden, B.F. Optical and chemical properties of polyterpenol thin films deposited via plasma-enhanced chemical vapor deposition. J. Mater. Res. 2011, 26, 1018–1025. [Google Scholar] [CrossRef]
- Jacob, M.V.; Bazaka, K.; Taguchi, D.; Manaka, T.; Iwamoto, M. Electron-blocking hole-transport polyterpenol thin films. Chem. Phys. Lett. 2012, 528, 26–28. [Google Scholar] [CrossRef]
- Jacob, M.V.; Olsen, N.S.; Anderson, L.J.; Bazaka, K.; Shanks, R.A. Plasma polymerised thin films for flexible electronic applications. Thin Solid Films 2013, 546, 167–170. [Google Scholar] [CrossRef]
- Jacob, M.V.; Easton, C.D.; Anderson, L.J.; Bazaka, K. Rf plasma polymerised thin films from natural resources. Int. J. Mod. Phys. Conf. Ser. 2014, 32, 1460319. [Google Scholar] [CrossRef]
- Ahmad, J.; Bazaka, K.; Jacob, M.V. Optical and surface characterization of radio frequency plasma polymerized 1-isopropyl-4-methyl-1, 4-cyclohexadiene thin films. Electronics 2014, 3, 266–281. [Google Scholar] [CrossRef]
- Jacob, M.V.; Rawat, R.S.; Ouyang, B.; Bazaka, K.; Kumar, D.S.; Taguchi, D.; Iwamoto, M.; Neupane, R.; Varghese, O.K. Catalyst-free plasma enhanced growth of graphene from sustainable sources. Nano Lett. 2015, 15, 5702–5708. [Google Scholar] [CrossRef] [PubMed]
- Sakthi Kumar, D.; Nakamura, K.; Nishiyama, S.; Noguchi, H.; Ishii, S.; Kashiwagi, K.; Yoshida, Y. Electrical and optical properties of plasma polymerized eucalyptus oil films. J. Appl. Polym. Sci. 2003, 90, 1102–1107. [Google Scholar] [CrossRef]
- Brédas, J.L.; Thémans, B.; Fripiat, J.G.; André, J.M.; Chance, R.R. Highly conducting polyparaphenylene, polypyrrole, and polythiophene chains: An ab initio study of the geometry and electronic-structure modifications upon doping. Phys. Rev. B 1984, 29, 6761. [Google Scholar] [CrossRef]
- Chand, S.; Kumar, N. Effect of iodine doping on electrical conduction in PVB films. J. Mater. Sci. Lett. 1989, 8, 1009–1010. [Google Scholar] [CrossRef]
- Jacob, M.V.; Bazaka, K.; Weis, M.; Taguchi, D.; Manaka, T.; Iwamoto, M. Fabrication and characterization of polyterpenol as an insulating layer and incorporated organic field effect transistor. Thin Solid Films 2010, 518, 6123–6129. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Taguchi, D.; Manaka, T.; Iwamoto, M. Investigation of interfacial charging and discharging in double-layer pentacene-based metal-insulator-metal device with polyterpenol blocking layer using electric field induced second harmonic generation. Chem. Phys. Lett. 2011, 503, 105–111. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Truong, V.K.; Wang, F.; Pushpamali, W.A.A.; Wang, J.Y.; Ellis, A.V.; Berndt, C.C.; Crawford, R.J.; Ivanova, E.P. Plasma-enhanced synthesis of bioactive polymeric coatings from monoterpene alcohols: A combined experimental and theoretical study. Biomacromolecules 2010, 11, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley and Sons: Chichester, UK, 2000; pp. 1–23. [Google Scholar]
- González-Torres, M.; Cruz, G.J.; Olayo, M.G.; Gómez, L.M.; López, O.G.; Sánchez-Mendieta, V.; De Jesús, C. Plasma copolymerization of pyrrole and ethylenglycol to obtain porous polymers. Superficies y Vacío 2012, 25, 179–182. [Google Scholar]
- Deepak, A.S.; Kumar, S.; Katiyar, M.; Saxena, A.K.; Ranjan, A.; Tiwari, R.K. Optical characterization of polysilane thin films. Synth. Met. 2003, 139, 835–837. [Google Scholar]
- Campoy-Quiles, M.; Etchegoin, P.G.; Bradley, D.D.C. Exploring the potential of ellipsometry for the characterisation of electronic, optical, morphologic and thermodynamic properties of polyfluorene thin films. Synth. Met. 2005, 155, 279–282. [Google Scholar] [CrossRef]
- Jaglarz, J.; Wagner, T.; Cisowski, J.; Sanetra, J. Ellipsometric studies of carbazole-containing polymer layers. Opt. Mater. 2007, 29, 908–912. [Google Scholar] [CrossRef]
- Jellison, J.G.E.; Modine, F.A. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371–373. [Google Scholar] [CrossRef]
- J.A. Woollam Co. Inc. Guide to Using Wvase32; J.A. Woollam Co. Inc.: Lincoln, NE, USA, 2001. [Google Scholar]
- Anderson, L.J.; Jacob, M.V. Electrical characterisations of plasma polymerised linalyl acetate. Mater. Sci. Eng. B 2012, 177, 311–315. [Google Scholar] [CrossRef]
- Jiang, H.; Hong, L.; Venkatasubramanian, N.; Grant, J.T.; Eyink, K.; Wiacek, K.; Fries-Carr, S.; Enlow, J.; Bunning, T.J. The relationship between chemical structure and dielectric properties of plasma-enhanced chemical vapor deposited polymer thin films. Thin Solid Films 2007, 515, 3513–3520. [Google Scholar] [CrossRef]
- Ahmad, J.; Bazaka, K.; Vasilev, K.; Jacob, M.V. Electrical conduction in plasma polymerized thin films of γ-terpinene. J. Appl. Polym. Sci. 2015, 132, 42318. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob Mohan, V. Complex permittivity measurements of RF plasma polymerized polyterpenol organic thin films employing split post dielectric resonator. J. Polym. Eng. 2011, 31, 73. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A.; Wood, D.L. Optical and magnetic investigations of the localized states in semiconducting glasses. Phys. Rev. Lett. 1970, 25, 749. [Google Scholar] [CrossRef]
- Sajeev, U.; Mathai, C.; Saravanan, S.; Ashokan, R.; Venkatachalam, S.; Anantharaman, M. On the optical and electrical properties of rf and a.c. Plasma polymerized aniline thin films. Bull. Mater. Sci. 2006, 29, 159–163. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Ledzion, R. Electronic band structure of insulating hydrogenated carbon-germanium films. J. Appl. Phys. 1999, 86, 4412–4418. [Google Scholar] [CrossRef]
- Mujahid, M.; Srivastava, D.S.; Gupta, S.; Avasthi, D.K. Estimation of optical band gap and carbon cluster sizes formed in heavy ion irradiated polycarbonate. Radiat. Phys. Chem. 2005, 74, 118–122. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems v. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 903–922. [Google Scholar] [CrossRef]
- Basa, D.K. Correlation between the opto-electronic and structural parameters of amorphous semiconductors. Thin Solid Films 2002, 406, 75–78. [Google Scholar] [CrossRef]
- Bässler, H. Charge transport in disordered organic photoconductors a monte carlo simulation study. Phys. Status Solidi (b) 1993, 175, 15–56. [Google Scholar] [CrossRef]
- Tyczkowski, J. Electrical and optical properties of plasma polymers. In Plasma Polymer Films; Biederman, H., Ed.; Imperial College Press: London, UK, 2004; pp. 143–216. [Google Scholar]
- Arkhipov, V.I.; Heremans, P.; Emelianova, E.V.; Adriaenssens, G.J.; Bässler, H. Charge carrier mobility in doped disordered organic semiconductors. J. Non-Cryst. Solids 2004, 338–340, 603–606. [Google Scholar] [CrossRef]
- Mathai, C.J.; Saravanan, S.; Anantharaman, M.R.; Venkitachalam, S.; Jayalekshmi, S. Effect of iodine doping on the bandgap of plasma polymerized aniline thin films. J. Phys. D Appl. Phys. 2002, 35, 2206–2210. [Google Scholar] [CrossRef]
- Hollander, A.; Thome, J. Plasma Polymer Films: Degradation and Stability of Plasma Polymers; Biederman, H., Ed.; Imperial Collage Press: London, UK, 2004; p. 252. [Google Scholar]
- Dakhel, A.A. Mechanisms of dc-current transfer in tris(acetylacetonato)iron(III) films. J. Non-Cryst. Solids 2007, 353, 1529–1533. [Google Scholar] [CrossRef]
- Matin, R.; Bhuiyan, A.H. Electrical transport mechanism in plasma polymerized 2, 6, diethylaniline thin films. Thin Solid Films 2011, 519, 3462–3467. [Google Scholar] [CrossRef]
- Majumder, S.; Bhuiyan, A. DC conduction mechanism in plasma polymerized vinylene carbonate thin films prepared by glow discharge technique. Polym. Sci. Ser. A 2011, 53, 85–91. [Google Scholar] [CrossRef]
- Chenari, H.M.; Sedghi, H.; Talebian, M.; Golzan, M.M.; Hassanzadeh, A. Poole-frenkel conduction in Cu/Nano-SnO2/Cu arrangement. J. Nanomater. 2011, 2011, 190391. [Google Scholar] [CrossRef]
- Bazaka, K.; Destefani, R.; Jacob, M.V. Plant-derived cis-β-ocimene as a precursor for biocompatible, transparent, thermally-stable dielectric and encapsulating layers for organic electronics. Sci. Rep. 2016, 6, 38571. [Google Scholar] [CrossRef] [PubMed]
- Sakthi Kumar, D.; Krishna Pillai, M.G. Conduction mechanism in plasma polymerized lemongrass oil films. Thin Solid Films 1999, 353, 249–253. [Google Scholar] [CrossRef]
- Kiesow, A.; Heilmann, A. Deposition and properties of plasma polymer films made from thiophenes. Thin Solid Films 1999, 343, 338–341. [Google Scholar] [CrossRef]
- Cruz, G.J.; Morales, J.; Castillo-Ortega, M.M.; Olayo, R. Synthesis of polyaniline films by plasma polymerization. Synth. Met. 1997, 88, 213–218. [Google Scholar] [CrossRef]
- Groenewoud, L.M.H.; Engbers, G.H.M.; White, R.; Feijen, J. On the iodine doping process of plasma polymerised thiophene layers. Synth. Met. 2001, 125, 429–440. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V. Solubility and surface interactions of RF plasma polymerized polyterpenol thin films. Mater. Express 2012, 2, 285–293. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M. Synthesis of radio frequency plasma polymerized non-synthetic terpinen-4-ol thin films. Mater. Lett. 2009, 63, 1594–1597. [Google Scholar] [CrossRef]
| Constituent | Pristine | Doped |
|---|---|---|
| Atomic fractions 1, % | ||
| I | – | 0.1 |
| O | 22.8 | 15.2 |
| N | 0.9 | 0.6 |
| C | 76.0 | 83.9 |
| Si | 0.3 | 0.2 |
| Chemical structure | Group frequency 2, cm−1 | |
| Hydroxy group, H-bonded OH stretch | ~3460 | 3438 |
| Methyl asymmetric C–H stretch | 2955 | 2958 |
| Methylene asymmetric C–H stretch | 2930 | 2932 |
| Methyl symmetric C–H stretch | 2875 | 2874 |
| Ketone | 1708 | 1708 |
| Alkenyl C=C stretch | – | 1619 |
| Methylene C–H bend | 1457 | 1450 |
| Methyl symmetric C–H bend | 1377 | 1379 |
| Skeletal C–C vibrations | ~1020 | ~1180 |
| Aliphatic C–I stretch | – | 600–485 |
| Sample Type | Band Gap, eV | Direct Allowed Transitions Eg, eV | Indirect Allowed Transitions Eg, eV |
|---|---|---|---|
| pristine polyterpenol | 3.33 | 3.44 | 2.81 |
| iodine-doped (I) | 2.51 | 2.93 | 1.85 |
| iodine-doped (II) | 2.29 | 2.61 | 1.64 |
| iodine-doped (II) annealed 1 | 2.48 | 2.82 | 1.74 |
| Surface Parameters | Pristine | Doped I | Doped II |
|---|---|---|---|
| Surface roughness parameters 1 | |||
| Rmax, nm | 5.4 ± 1.6 | 6.1 ± 2.4 | 6.8 ± 2.7 |
| Rq, nm | 0.38 ± 0.02 | 0.42 ± 0.05 | 0.44 ± 0.05 |
| Ra, nm | 0.51 ± 0.06 | 0.56 ± 0.07 | 0.61 ± 0.08 |
| Rskw | 0.22 | 0.51 | 0.76 |
| Rkur | 1.65 | 2.13 | 2.51 |
| Contact angle, ° | |||
| water | 68.7 ± 1.6 | 27.8 ± 2.7 | 61.6 ± 2.0 |
| glycerol | 71.6 ± 1.8 | 44.4 ± 1.5 | 47.1 ± 1.7 |
| DIM | 38.1 ± 2.1 | 31.7 ± 1.9 | 28.4 ± 1.4 |
| Surface tension fractions, mJ·m−2 | |||
| γLW | 40.5 | 23.5 | 23.9 |
| γ+ | 0.3 | 2.35 | 5.6 |
| γ− | 19.8 | 57.3 | 14.2 |
| γS | 45.1 | 46.7 | 41.2 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaka, K.; Jacob, M.V. Effects of Iodine Doping on Optoelectronic and Chemical Properties of Polyterpenol Thin Films. Nanomaterials 2017, 7, 11. https://doi.org/10.3390/nano7010011
Bazaka K, Jacob MV. Effects of Iodine Doping on Optoelectronic and Chemical Properties of Polyterpenol Thin Films. Nanomaterials. 2017; 7(1):11. https://doi.org/10.3390/nano7010011
Chicago/Turabian StyleBazaka, Kateryna, and Mohan V. Jacob. 2017. "Effects of Iodine Doping on Optoelectronic and Chemical Properties of Polyterpenol Thin Films" Nanomaterials 7, no. 1: 11. https://doi.org/10.3390/nano7010011
APA StyleBazaka, K., & Jacob, M. V. (2017). Effects of Iodine Doping on Optoelectronic and Chemical Properties of Polyterpenol Thin Films. Nanomaterials, 7(1), 11. https://doi.org/10.3390/nano7010011






