Optoelectronic and Electrochemical Properties of Vanadium Pentoxide Nanowires Synthesized by Vapor-Solid Process
Abstract
:1. Introduction
- The MgO substrates are etched in 0.5 M NiCl2 solutions for 1 to 30 min to create nanoscale pits/projections, then the depositions of MgO nanowires have been grown on the nanoscale pits/projections of MgO (100) substrates by VS method. In other words, the nanoscale pits/projections are the nucleation sites of this VS method.
- The MgO nanowires are directly grown on the MgO substrates without etching process. At the beginning of this reaction, the MgO nanoscale particles are deposited on the surface of MgO substrates, which include plenty of defects, i.e., steps, kinks and so on. These defects are the nucleation and the oriented growth of MgO nanowires.
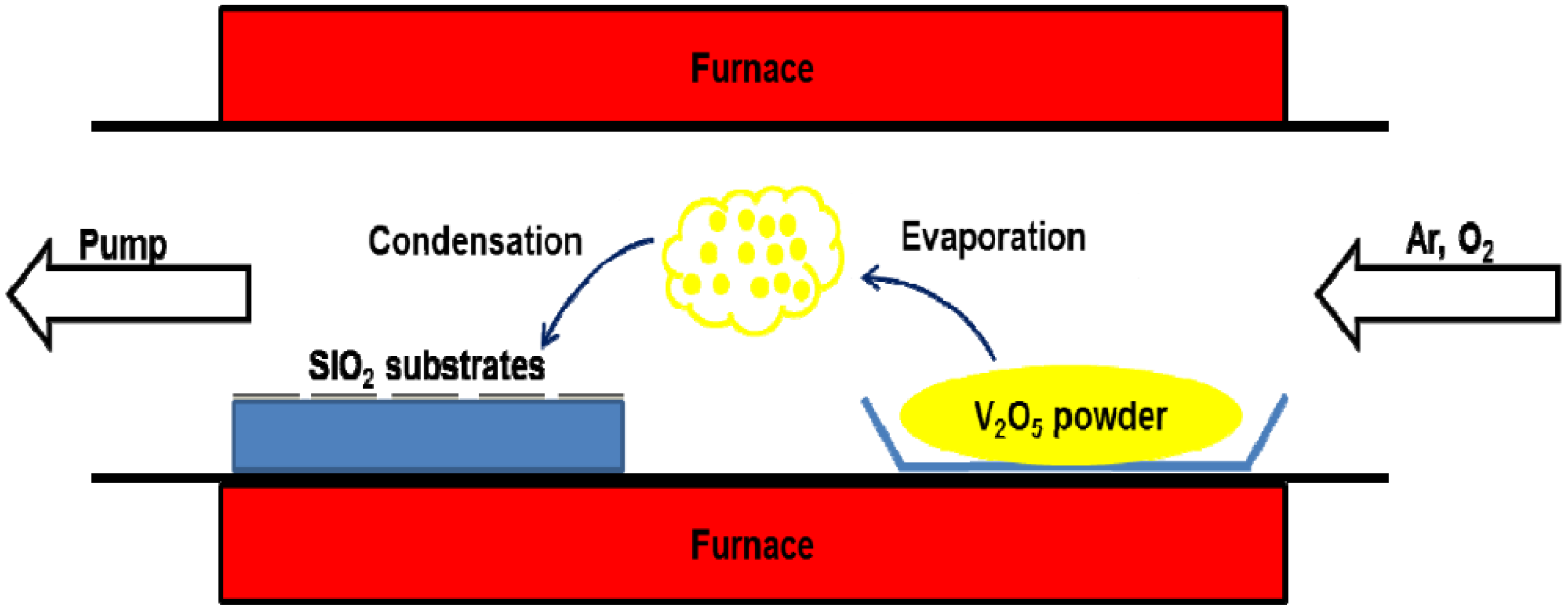
2. Experimental Method
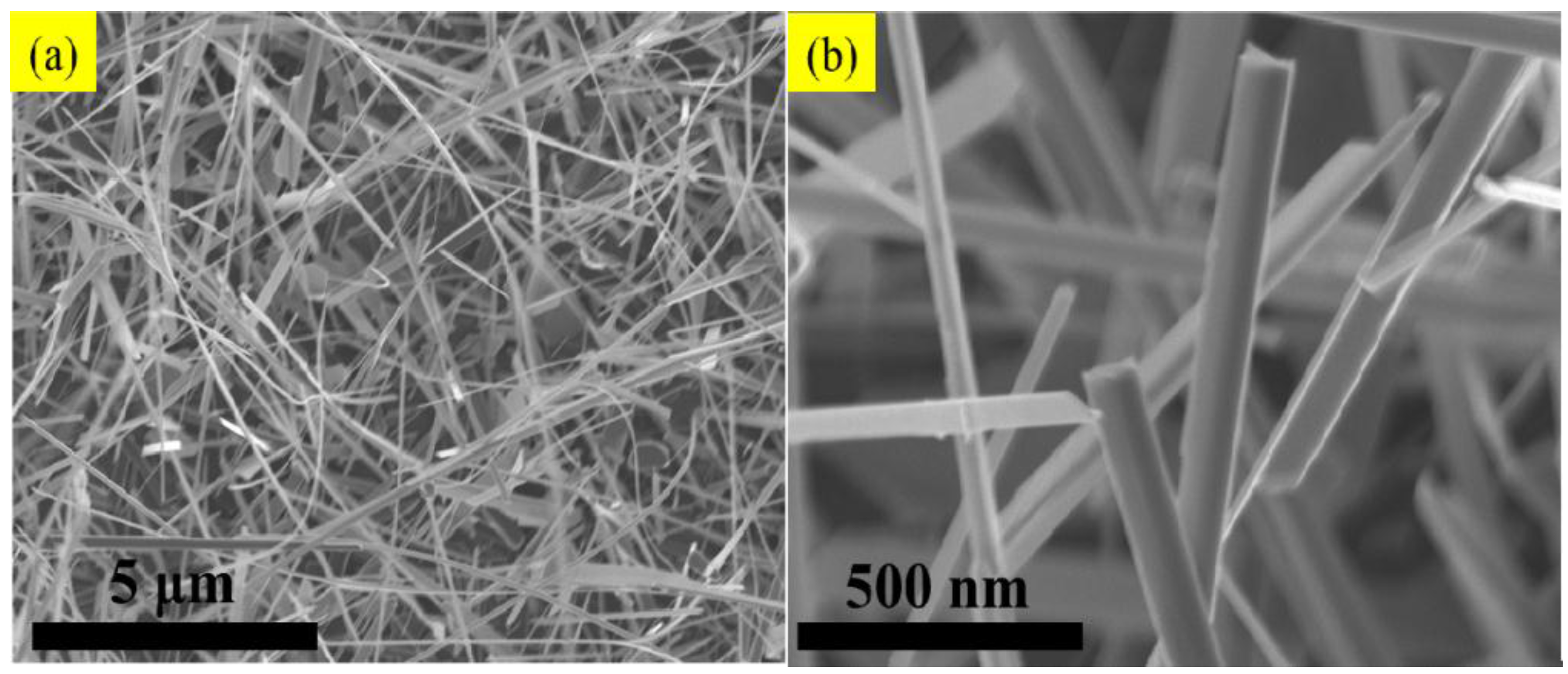
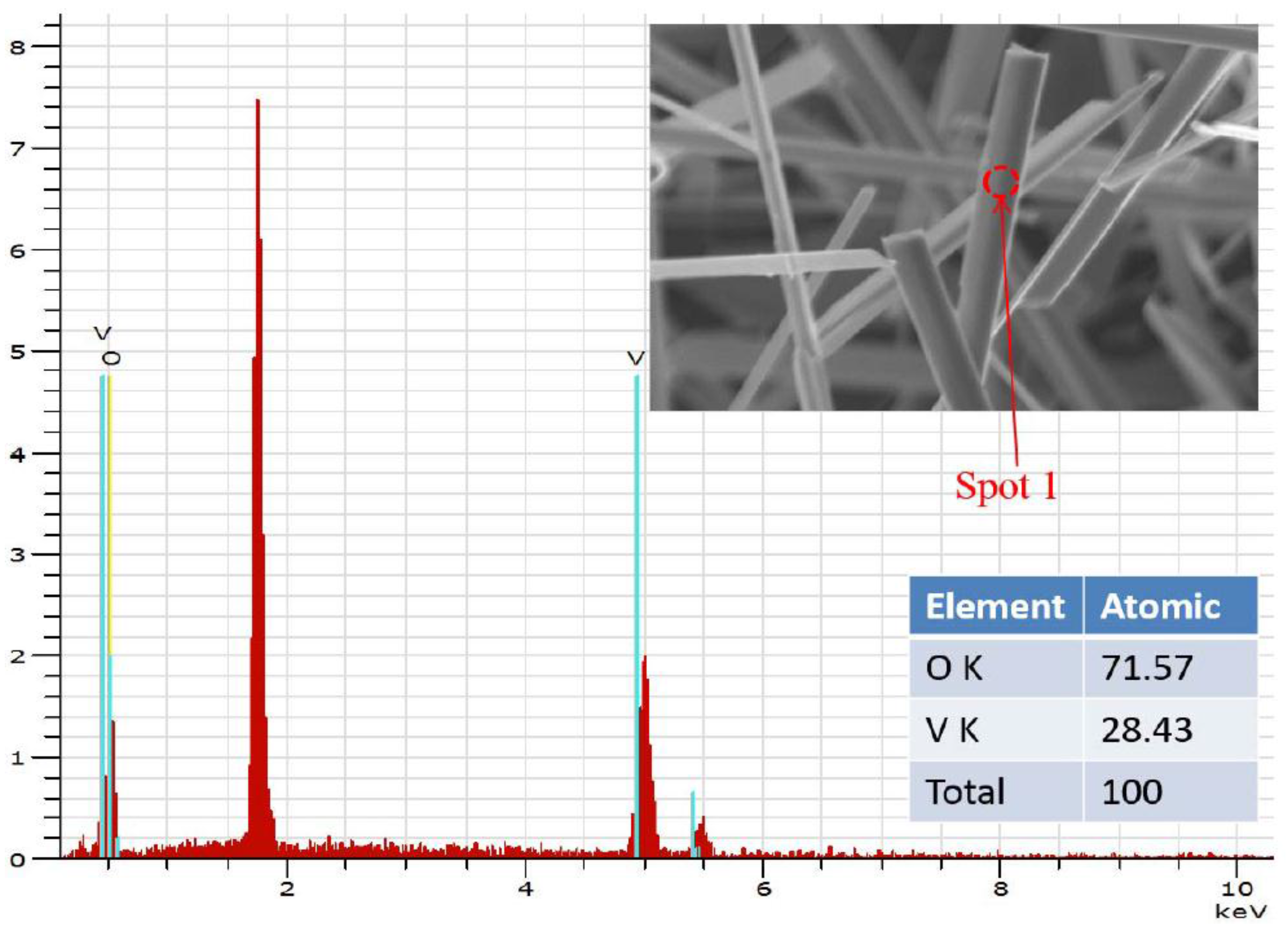
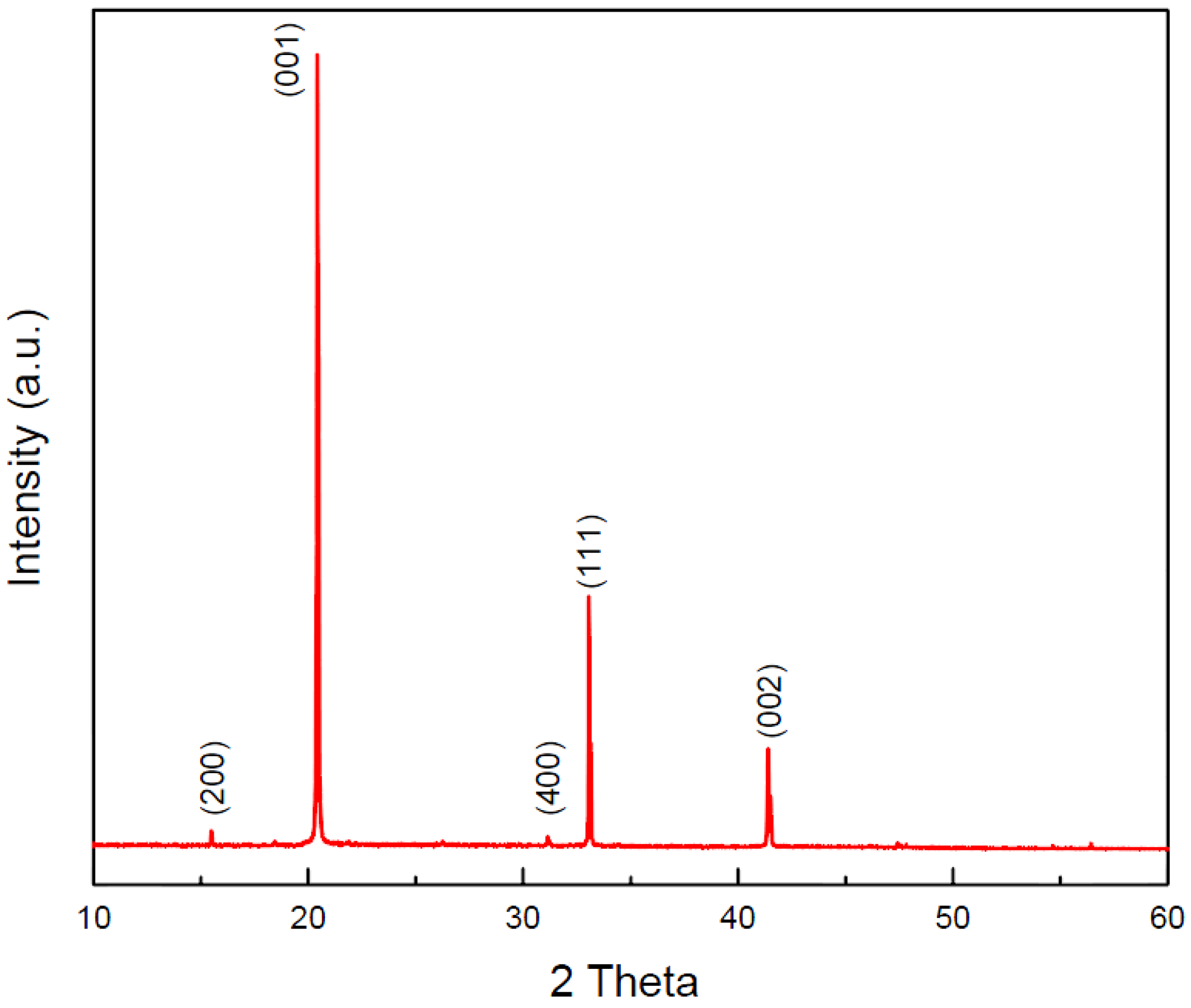
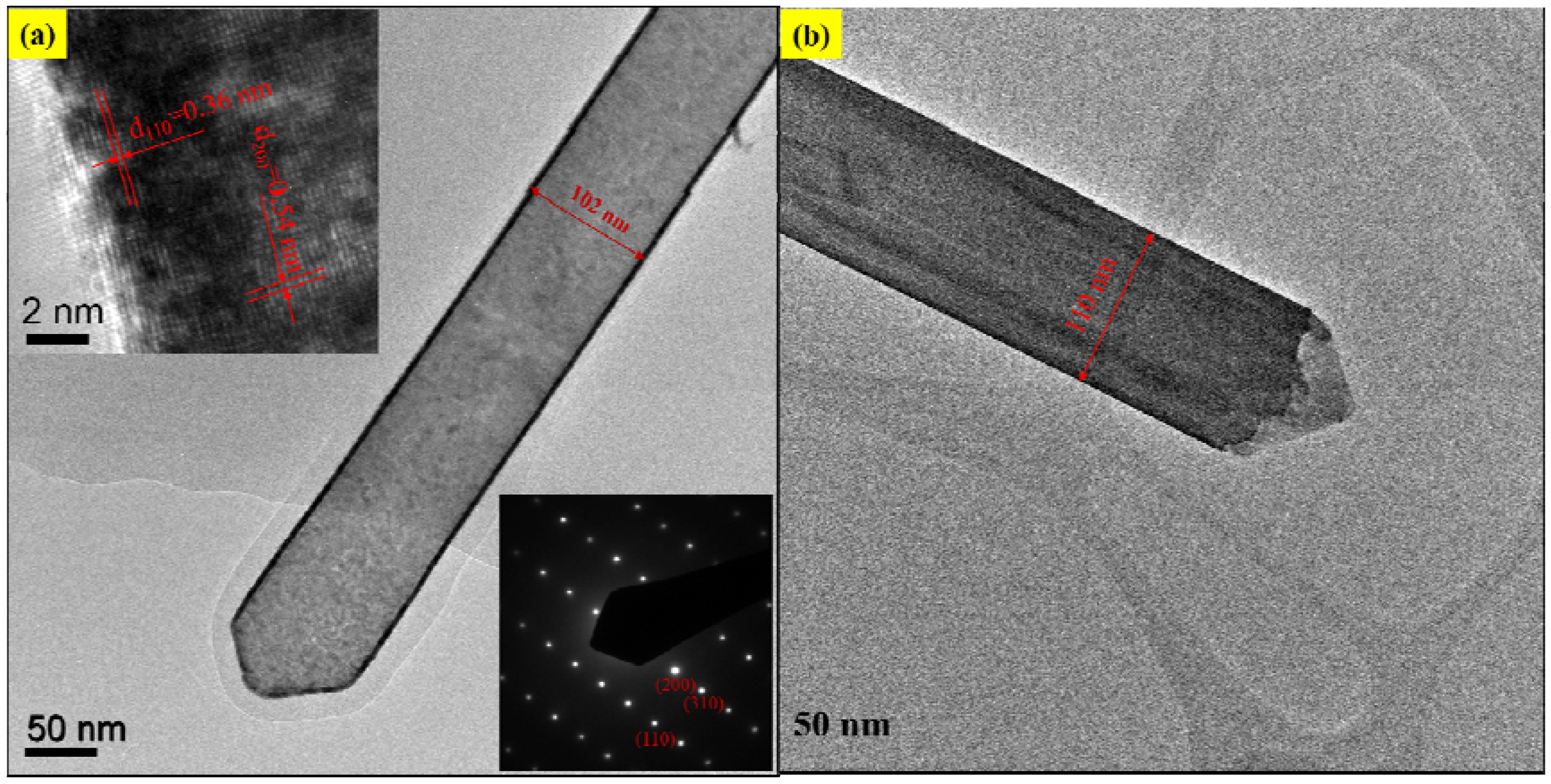
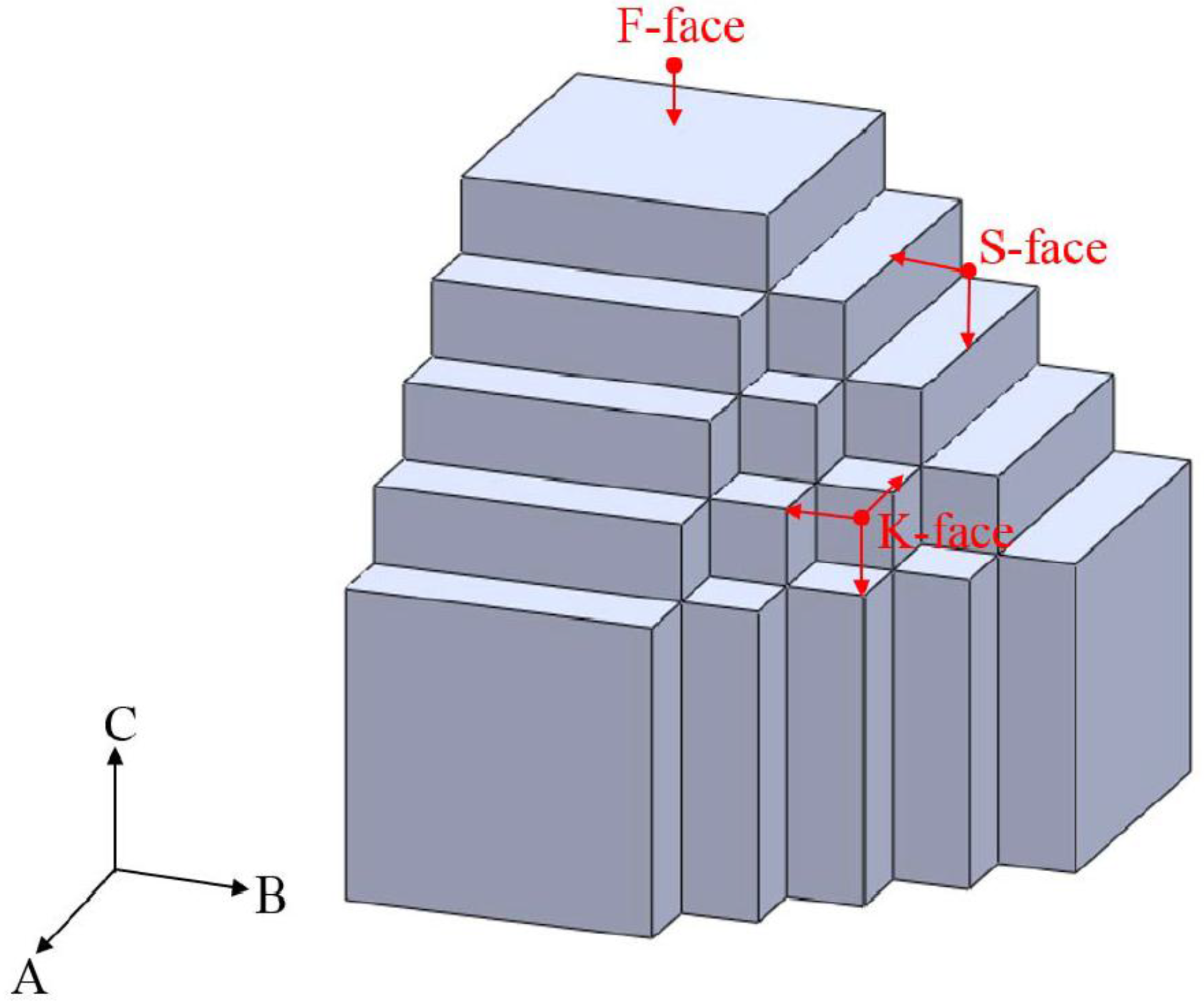
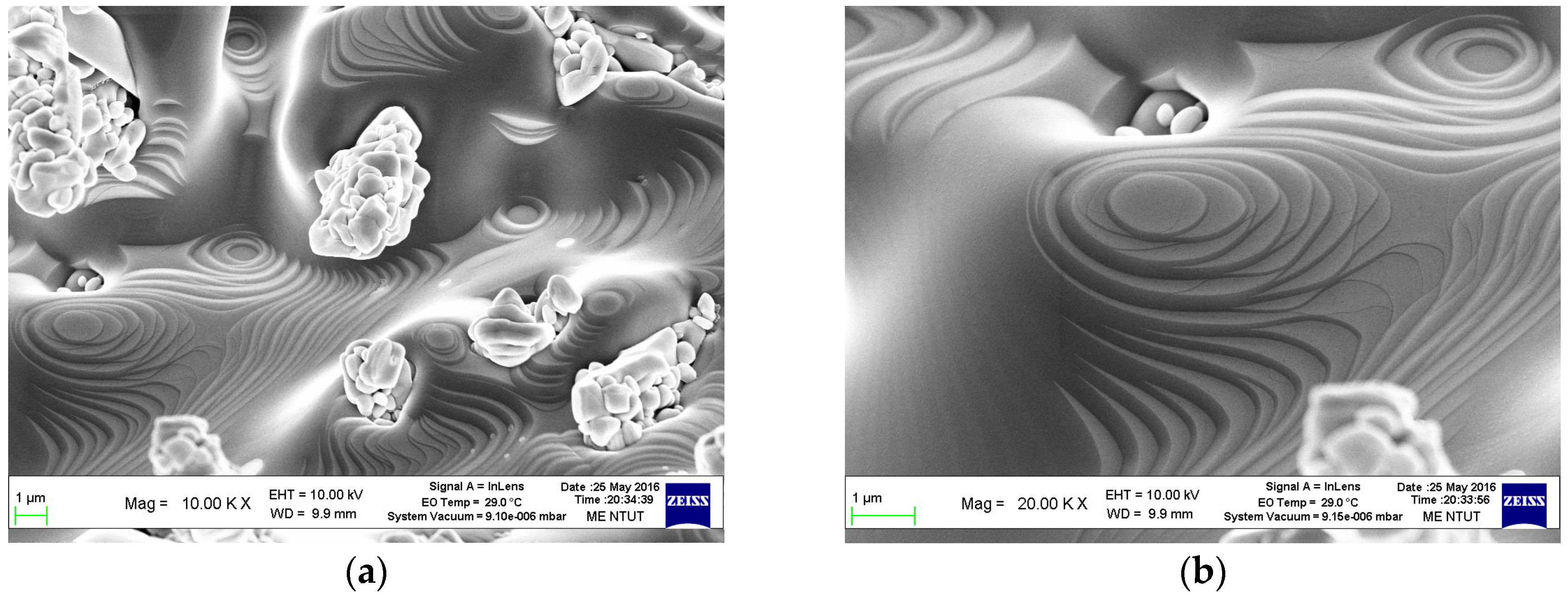
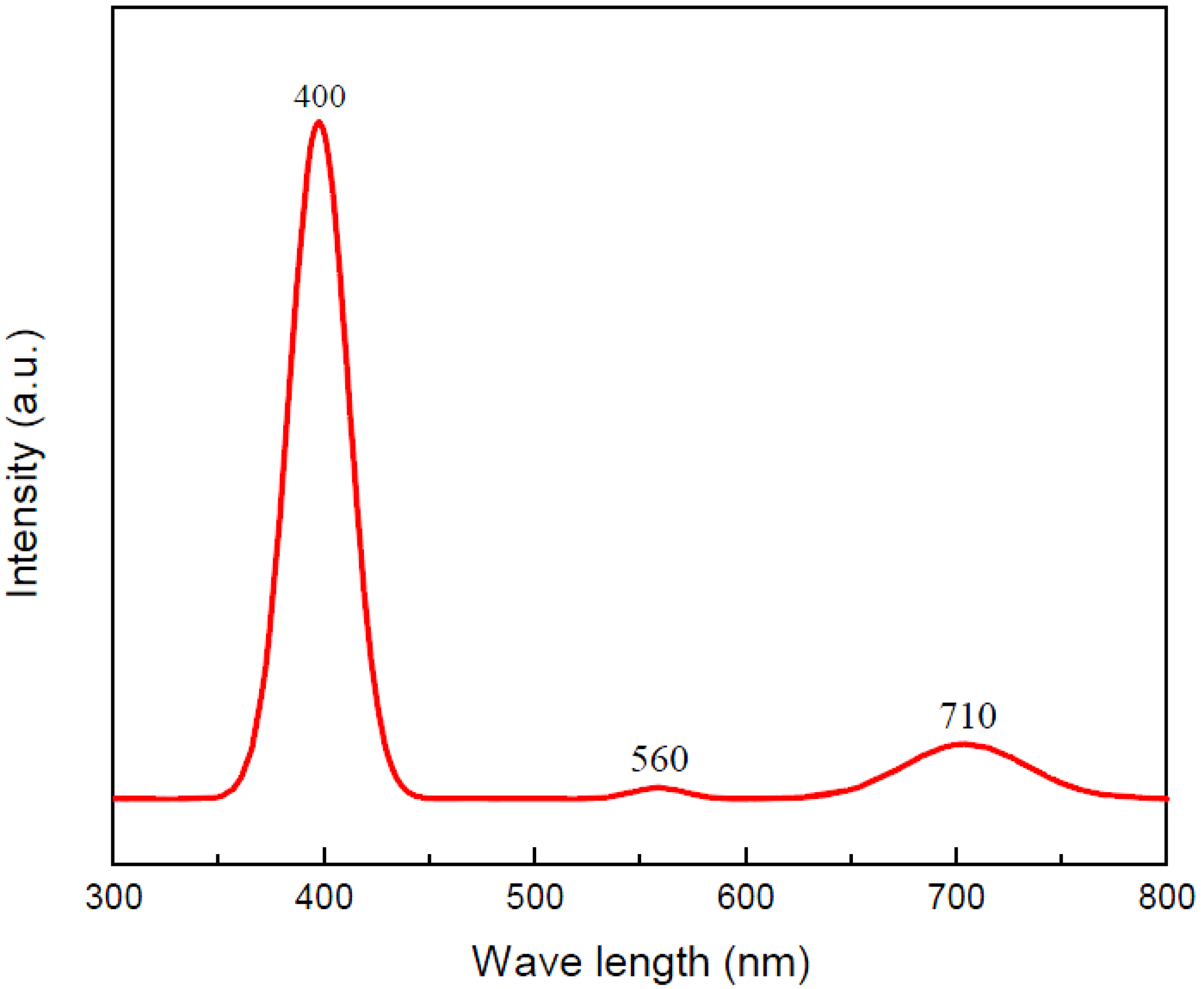
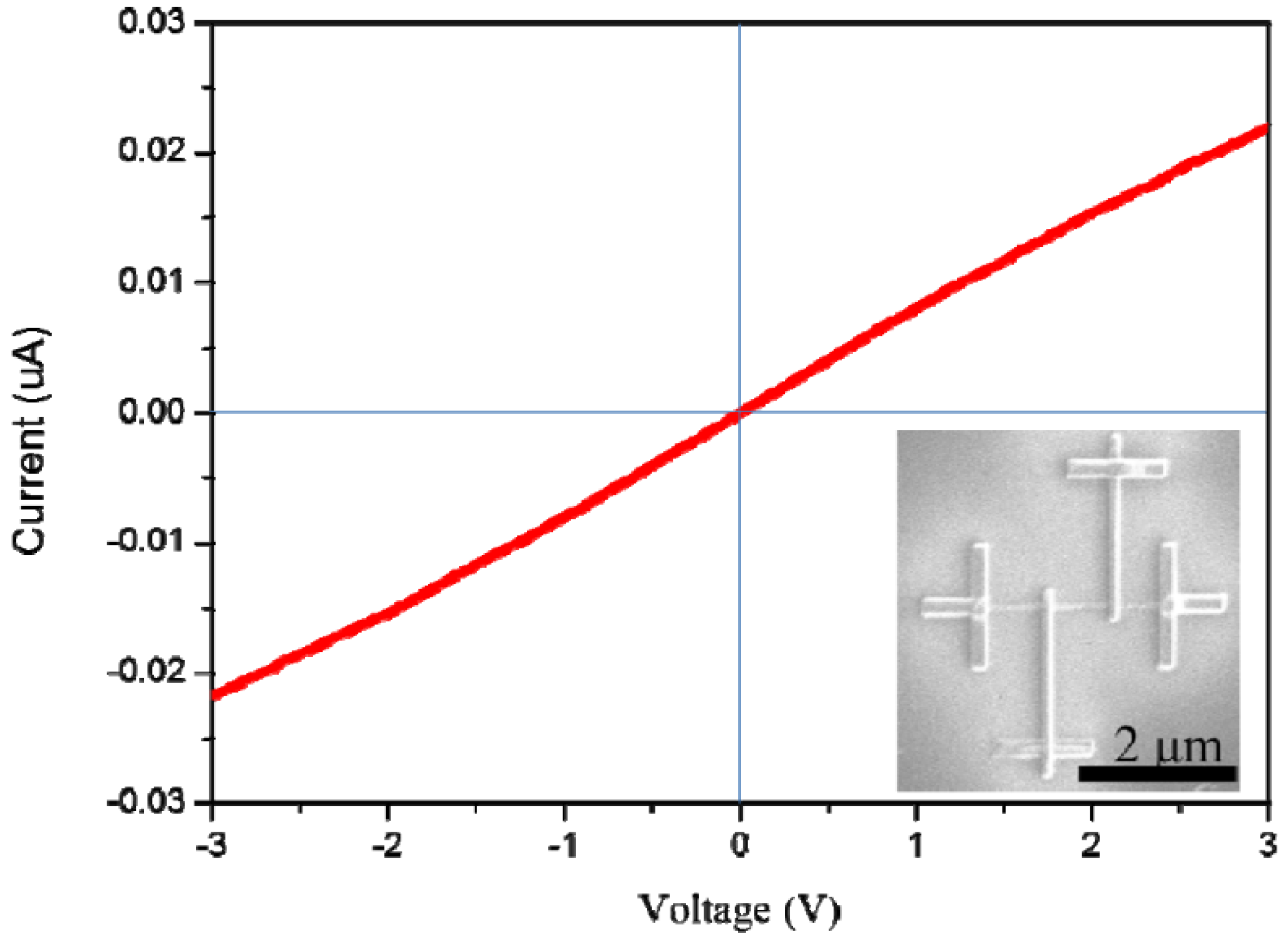
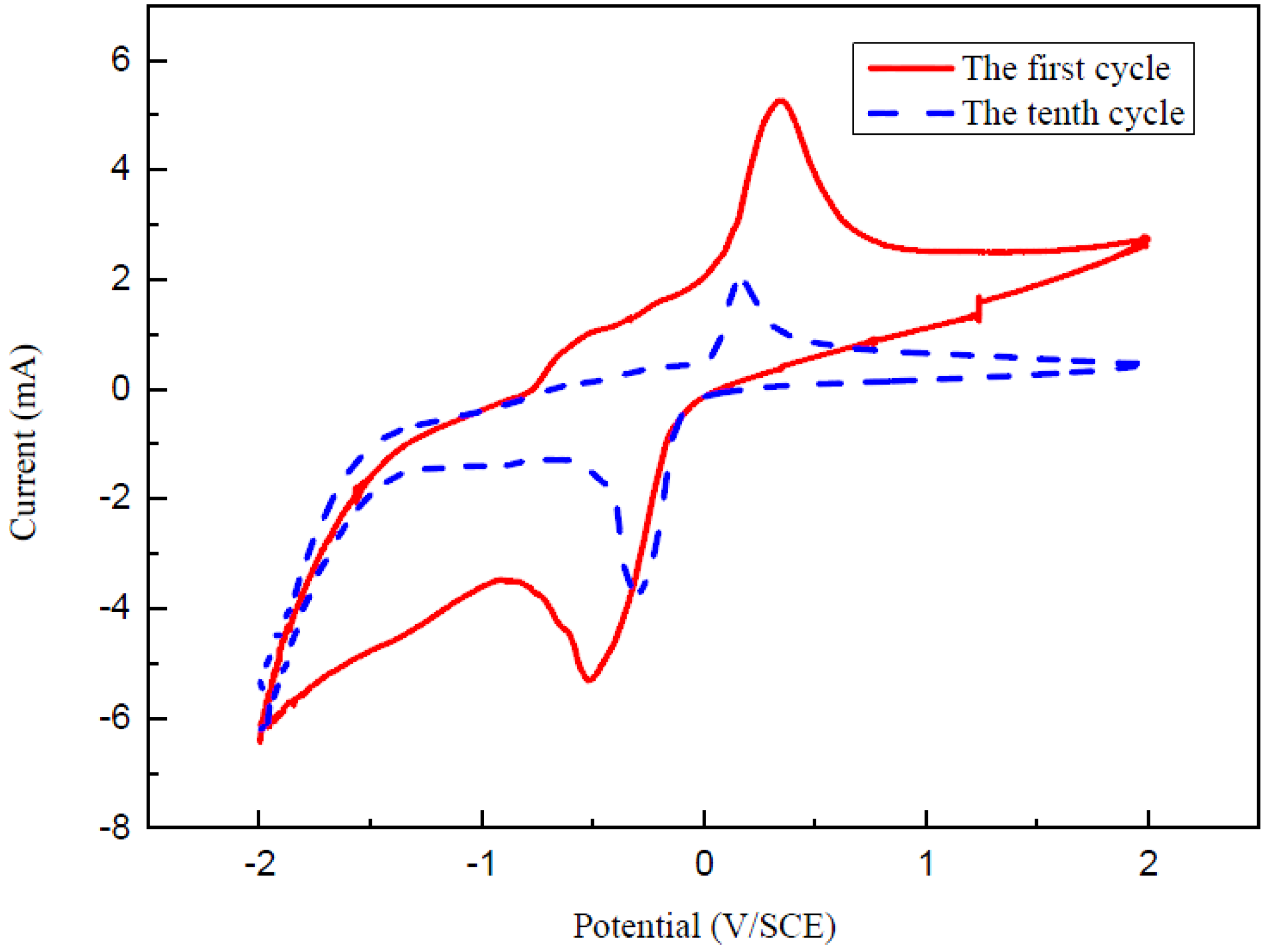
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, K.C.; Chen, F.R.; Kai, J.J. V2O5 Nanowires as a Functional Material for Electrochromic Device. Sol. Energy Mater. Sol. Cells 2006, 90, 1156–1165. [Google Scholar] [CrossRef]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. CAS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Wachs, I.E. The Generality of Surface Vanadium Oxide Phases in Mixed Oxide Catalysts. Appl. Catal. A Gen. 2011, 391, 36–42. [Google Scholar] [CrossRef]
- Lu, Y.R.; Wu, T.Z.; Chen, C.L.; Wei, D.H.; Chen, J.L.; Chou, W.C.; Dong, C.L. Mechanism of Electrochemical Deposition and Coloration of Electrochromic V2O5 Nano Thin Films: An In Situ X-Ray Spectroscopy Study. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.K.; Chang, C.C.; Wei, D.H.; Dong, C.L.; Chen, C.L.; Chen, J.L.; Jang, W.L.; Hsu, C.C.; Chan, T.S.; Kumar, K.; et al. Characterization of Gasochromic Vanadium Oxides Films by X-ray Absorption Spectroscopy. Thin Solid Films 2013, 544, 461–465. [Google Scholar] [CrossRef]
- Joseph, B.; Iadecola, A.; Maugeri, L.; Bendele, M.; Okubo, M.; Li, H.; Zhou, H.; Mizokawa, T.; Saini, N.L. Distinct Local Structure of Nanoparticles and Nanowires of V2O5 Probed by X-Ray Absorption Spectroscopy. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef]
- Chen, J.L.; Chang, C.C.; Ho, Y.K.; Chen, C.L.; Hsu, C.C.; Jang, W.L.; Wei, D.H.; Dong, C.L.; Pao, C.W.; Lee, J.F.; et al. Behind the color switching in gasochromic VO2. Phys. Chem. Chem. Phys. 2015, 17, 3482–2489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Takahashi, K.; Lee, L.; Cao, G. Nanostructured Vanadium Oxide Electrodes for Enhanced Lithium-Ion Intercalation. Adv. Funct. Mater. 2006, 16, 1133–1144. [Google Scholar] [CrossRef]
- Pyun, S.I.; Bae, J.S. The AC Impedance Study of Electrochemical Lithium Intercalation into Porous Vanadium Oxide Electrode. Electrochim. Acta 1996, 41, 919–925. [Google Scholar] [CrossRef]
- Portion, E.; Salle, A.L.G.A.; Verbaere, A.; Piffard, Y.; Guyomard, D. Electrochemically synthesized vanadium oxides as lithium insertion hosts. Electrochim. Acta 1999, 45, 197–214. [Google Scholar]
- Le Van, K.; Groult, H.; Mantoux, A.; Perrigaud, L.; Lantelme, F.; Lindstrom, R.; Badour-Hadjean, R.; Zanna, S.; Lincot, D. Amorphous vanadium oxide films synthesised by ALCVD for lithium rechargeable batteries. J. Power Sources 2006, 160, 592–601. [Google Scholar] [CrossRef]
- Ramana, C.V.; Smith, R.J.; Hussain, O.M.; Massot, M.; Julien, C.M. Surface analysis of pulsed laser-deposited V2O5 thin films and their lithium intercalated products studied by Raman spectroscopy. Surf. Interface Anal. 2005, 37, 406–411. [Google Scholar] [CrossRef]
- Baik, J.M.; Kim, M.H.; Larson, C.; Yavuz, C.T.; Stucky, G.D.; Wodtke, A.M.; Moskovits, M. Pd-Sensitized Single Vanadium Oxide Nanowires: Highly Responsive Hydrogen Sensing Based on the Metal–Insulator Transition. Nano Lett. 2009, 9, 3980–3984. [Google Scholar] [CrossRef] [PubMed]
- Byon, J.W.; Kim, M.B.; Kim, M.H.; Kim, S.Y.; Lee, S.H.; Lee, B.C.; Baik, J.M. Electrothermally Induced Highly Responsive and Highly Selective Vanadium Oxide Hydrogen Sensor Based on Metal–Insulator Transition. J. Phys. Chem. C 2012, 116, 226–230. [Google Scholar] [CrossRef]
- Chirayil, T.; Zavalij, P.Y.; Whittingham, M.S. Hydrothermal Synthesis of Vanadium Oxides. Chem. Mater. 1998, 10, 2629–2640. [Google Scholar] [CrossRef]
- Chandrappa, G.T.; Steunou, N.; Cassaignon, S.; Bauvais, C.; Livage, J. Hydrothermal Synthesis of Vanadium Oxide Nanotubes. Catal. Today 2003, 78, 85–89. [Google Scholar] [CrossRef]
- El Mandouh, Z.S.; Selim, M.S. Physical Properties of Vanadium Pentoxide Sol Gel Films. Thin Solid Films 2000, 371, 259–263. [Google Scholar] [CrossRef]
- Speck, K.R.; Hu, H.S.-W.; Sherwin, M.E.; Potember, R.S. Vanadium Dioxide Films Grown from Vanadium Tetraisopropoxide by the Sol-Gel Process. Thin Solid Films 1988, 165, 317–322. [Google Scholar] [CrossRef]
- Díaz-Guerra, C.; Piqueras, J. Thermal Deposition Growth and Luminescence Properties of Single-Crystalline V2O5 Elongated Nanostructures. Cryst. Growth Des. 2008, 8, 1031–1034. [Google Scholar] [CrossRef]
- Wu, J.M.; Chang, W.E. Ultrahigh Responsivity and External Quantum Efficiency of an Ultraviolet-Light Photodetector Based on a Single VO2 Microwire. ACS Appl. Mater. Interfaces 2014, 6, 14286–14292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Meng, G.W.; Peng, X.S.; Zhang, X.Y.; Zhang, L.D. Large-Scale Synthesis of GaN Nanorods and their Photoluminescence. Appl. Phys. A 2001, 74, 587–590. [Google Scholar] [CrossRef]
- Yang, P.; Lieber, C.M. Nanostructured high-temperature superconductors: Creation of Strong-Pinning Columnar Defects in Nanorods/Superconductor Composites. J. Mater. Res. 1997, 12, 2981–2986. [Google Scholar] [CrossRef]
- Zhao, L.X.; Meng, G.W.; Peng, X.S.; Zhang, X.Y.; Zhang, L.D. Synthesis, Raman Scattering, and Infrared Spectra of Large-Scale GaN Nanorods. J. Cryst. Growth 2002, 235, 124–128. [Google Scholar] [CrossRef]
- Burton, W.K.; Cabrera, N.; Frank, F.C. The Growth of Crystals and the Equilibrium Structure of their Surfaces. Phil. Trans. R. Soc. A 1951, 243, 299–358. [Google Scholar] [CrossRef]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials Synthesis, Properties, and Applications, 2nd ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2011. [Google Scholar]
- Hartman, P.; Perdok, W.G. On the Relation between Structure and Morphology of Crystals. I. Acta Cryst. 1955, 8, 49–52. [Google Scholar] [CrossRef]
- Smereka, P. Spiral Crystal Growth. Phys. D 2000, 138, 282–301. [Google Scholar] [CrossRef]
- Nishio, S.; Kakihana, M. Evidence for Light Photochromism of V2O5. Chem. Mater. 2002, 14, 3730–3733. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Synthesis and Enhanced Intercalation Properties of Nanostructured Vanadium Oxides. Chem. Mater. 2006, 18, 2787–2804. [Google Scholar] [CrossRef]
- Atzkern, S.; Borisenko, S.V.; Knupfer, M.; Golden, M.S.; Fink, J.; Yaresko, A.N.; Antonov, V.N.; Klemm, M.; Horn, S. Valence band excitations in V2O5. Phys. Rev. B 2000, 61, 12792–12798. [Google Scholar] [CrossRef]
- Feng, C.Q.; Wang, S.Y.; Zeng, R.; Guo, Z.P.; Konstantinov, K.; Liu, H.K. Synthesis of spherical porous vanadium pentoxide and its electrochemical properties. J. Power Sources 2008, 184, 485–488. [Google Scholar] [CrossRef]
- Xiong, C.; Aliev, A.E.; Gnade, B.; Balkus, K.J., Jr. Fabrication of silver vanadium oxide and V2O5 nanowires for electrochromics. ACS Nano 2008, 2, 293–301. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, K.-Y.; Wei, D.-H. Optoelectronic and Electrochemical Properties of Vanadium Pentoxide Nanowires Synthesized by Vapor-Solid Process. Nanomaterials 2016, 6, 140. https://doi.org/10.3390/nano6080140
Pan K-Y, Wei D-H. Optoelectronic and Electrochemical Properties of Vanadium Pentoxide Nanowires Synthesized by Vapor-Solid Process. Nanomaterials. 2016; 6(8):140. https://doi.org/10.3390/nano6080140
Chicago/Turabian StylePan, Ko-Ying, and Da-Hua Wei. 2016. "Optoelectronic and Electrochemical Properties of Vanadium Pentoxide Nanowires Synthesized by Vapor-Solid Process" Nanomaterials 6, no. 8: 140. https://doi.org/10.3390/nano6080140
APA StylePan, K.-Y., & Wei, D.-H. (2016). Optoelectronic and Electrochemical Properties of Vanadium Pentoxide Nanowires Synthesized by Vapor-Solid Process. Nanomaterials, 6(8), 140. https://doi.org/10.3390/nano6080140





