Automatic Echographic Detection of Halloysite Clay Nanotubes in a Low Concentration Range
Abstract
:1. Introduction
2. Results and Discussion
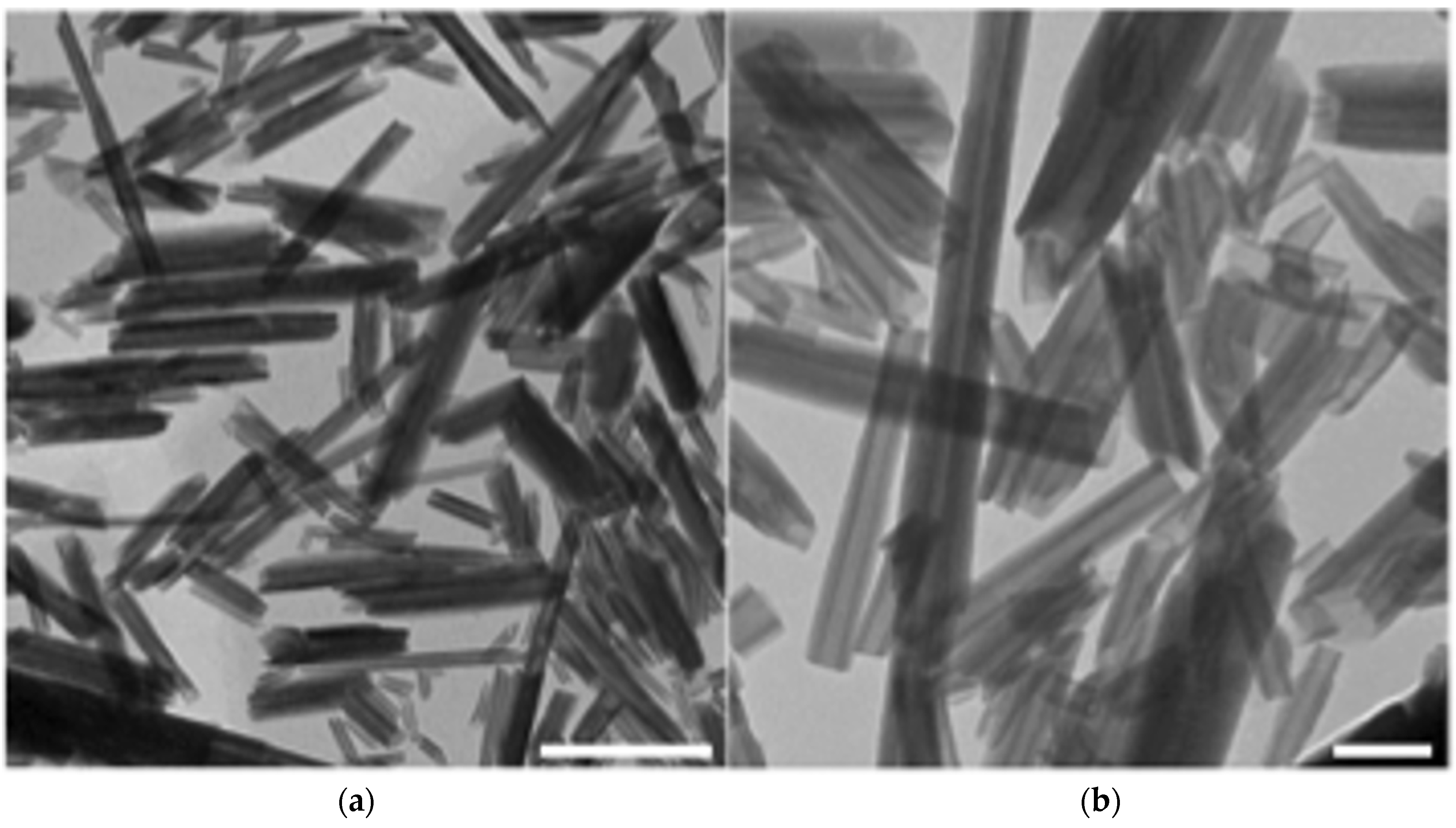
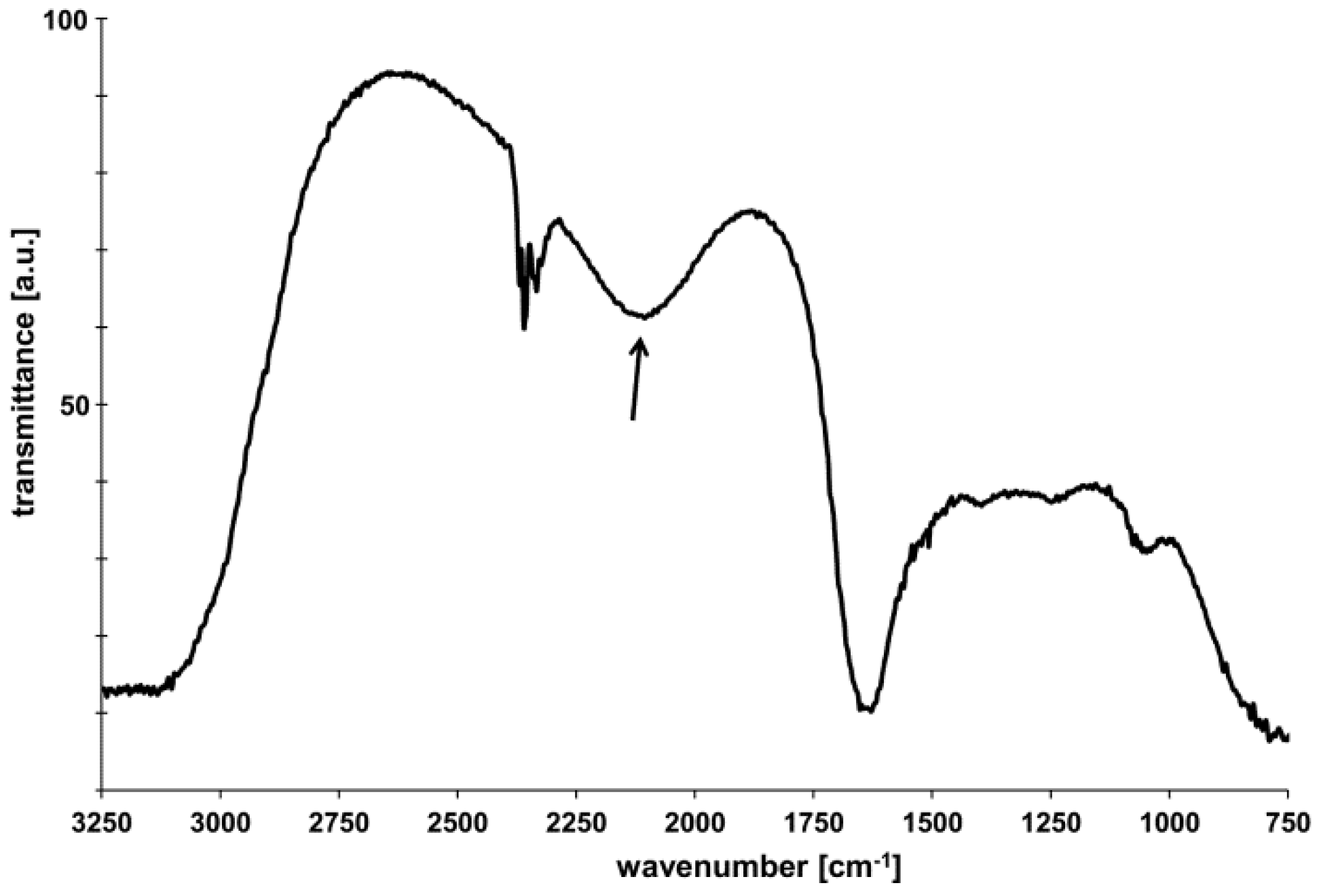
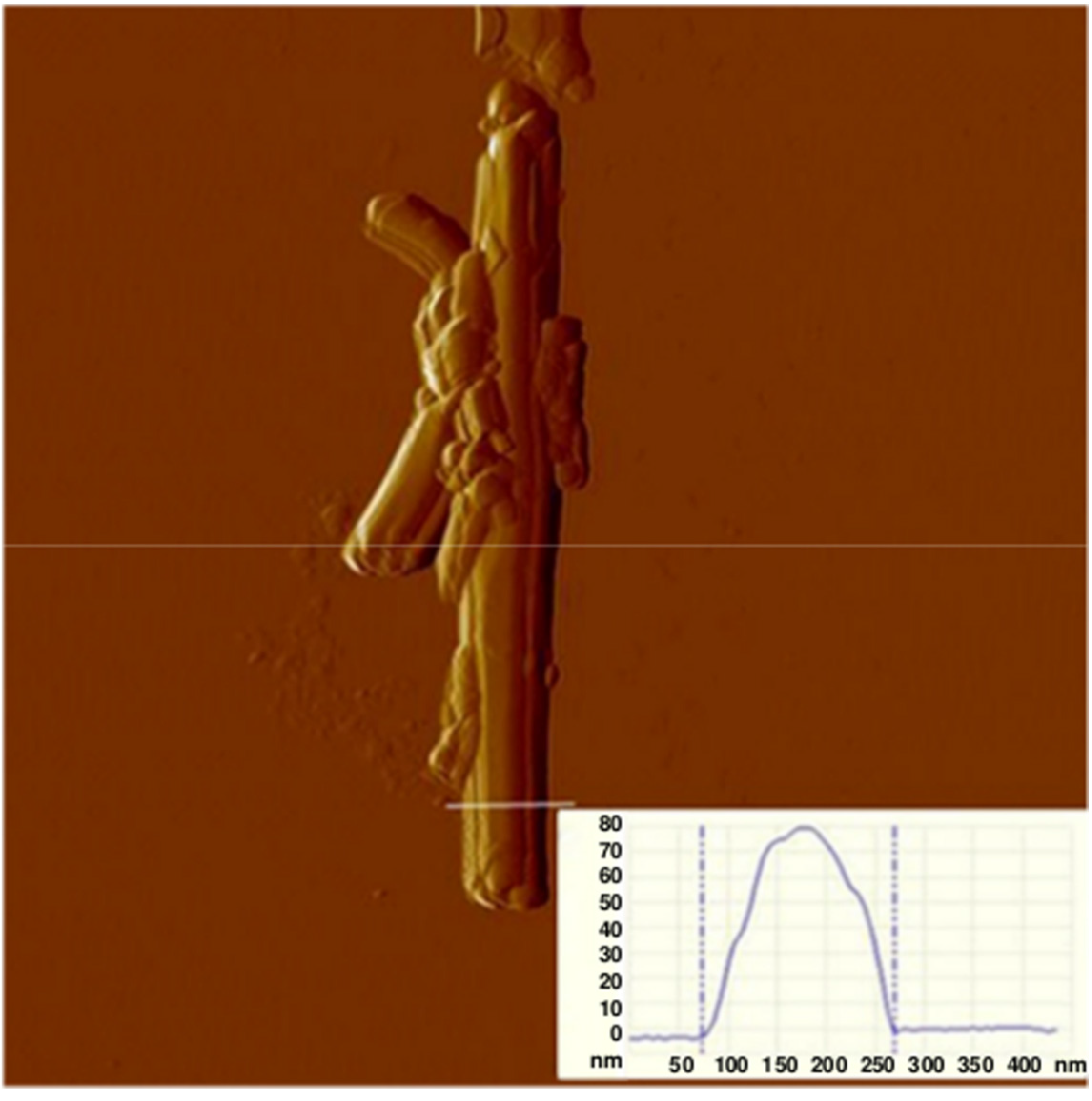
2.1. Physicochemical Characterization of HNTs
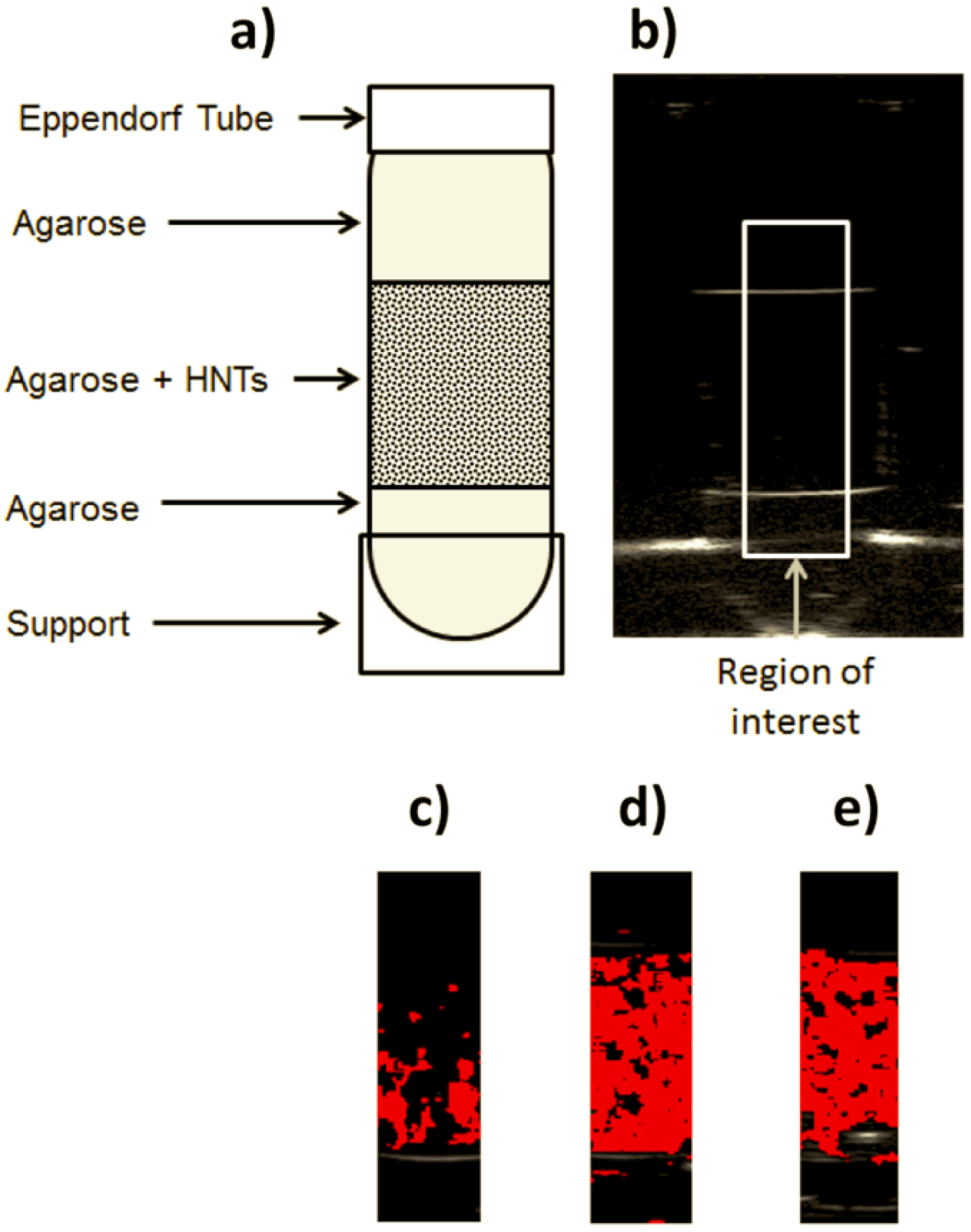
2.2. Automatic Echographic Detection of HNTs
2.3. Potential Applications of HNTs for Targeted Contrast Enhancement in Clinical Ultrasound Imaging
3. Experimental Section
3.1. Physicochemical Characterization of HNTs
3.1.1. Transmission Electron Microscopy
3.1.2. Z-Potential Measurements
3.1.3. FT-IR Spectroscopy
3.1.4. Scanning Force Microscopy
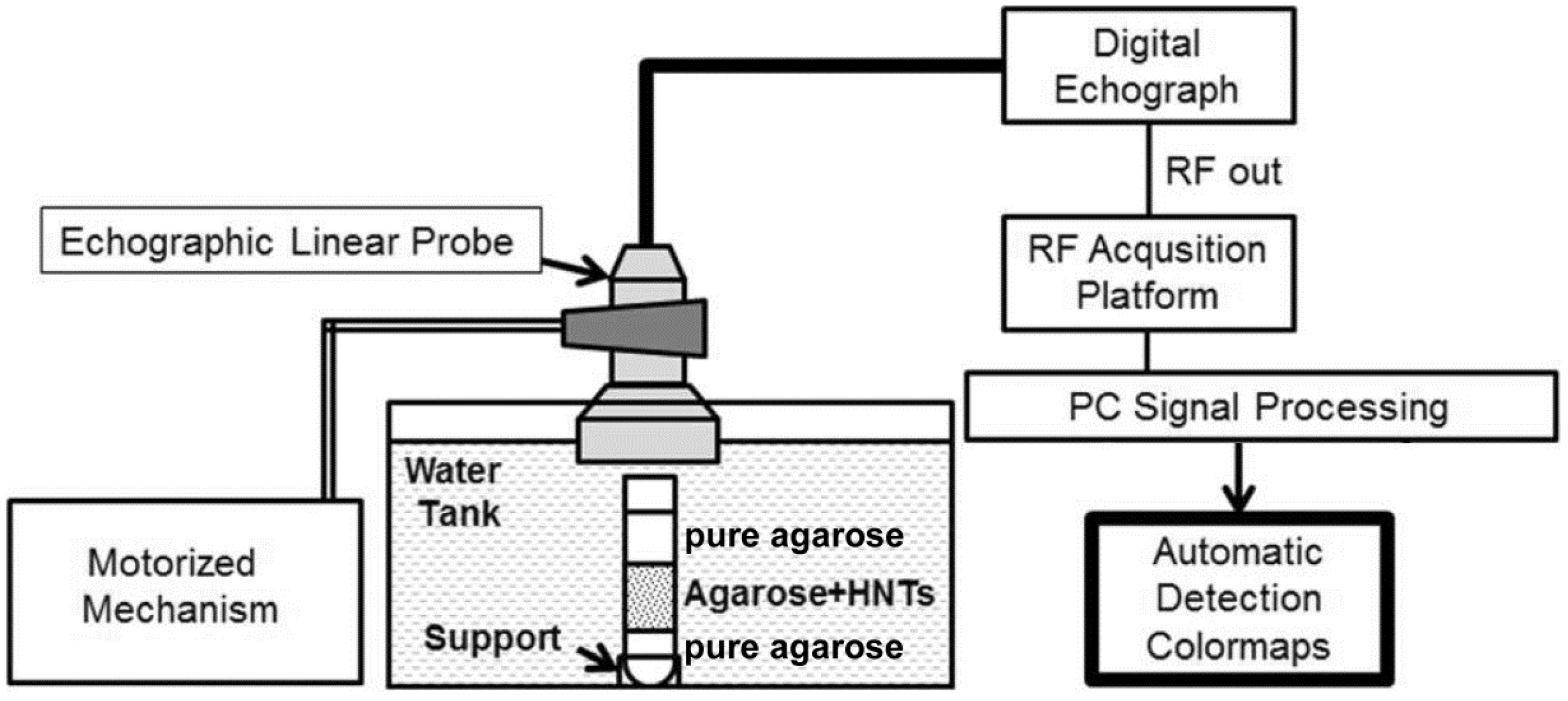
3.2. Echographic Acquisitions
3.2.1. Preparation of HNT-Containing Tissue-Mimicking Phantoms
3.2.2. Experimental Set-up
3.2.3. RF Signal Analysis for Automatic HNT Detection
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bose, T.; Latawiec, D.; Mondal, P.P.; Mandal, S. Overview of nano-drugs characteristics for clinical application: The journey from the entry to the exit point. J. Nanopart. Res. 2014, 16. [Google Scholar] [CrossRef]
- Prabhu, P.; Patravale, V. The upcoming field of theranostic nanomedicine: An overview. J. Biomed. Nanotechnol. 2012, 8, 859–882. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, F.A. Personalized nanomedicine: Future medicine for cancer treatment. Int. J. Nanomed. 2013, 8, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, Z.Y.; Lin, Y. Advancement of targeted ultrasound contrast agents and their applications in molecular imaging and targeted therapy. Curr. Pharm. Des. 2013, 19, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, F.; Fokong, S.; Koczera, P.; Lederle, W.; Lammers, T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J. Nucl. Med. 2012, 53, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, S.; Klibanov, A.L. Microbubbles as ultrasound contrast agents for molecular imaging: Preparation and application. Am. J. Roentgenol. 2012, 199, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, S. Theranostic applications: Non-ionizing cellular and molecular imaging through innovative nanosystems for early diagnosis and therapy. World J. Radiol. 2011, 3, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Chiriacò, F.; Conversano, F.; Soloperto, G.; Casciaro, E.; Ragusa, A.; Sbenaglia, E.A.; Dipaola, L.; Casciaro, S. Epithelial cells biocompatibility of silica nanospheres for contrast enhanced ultrasound molecular imaging. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Liu, J.; Levine, A.L.; Mattoon, J.S.; Yamaguchi, M.; Lee, R.J.; Pan, X.; Rosol, T.J. Nanoparticles as image enhancing agents for ultrasonography. Phys. Med. Biol. 2006, 51, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Pascali, G.; Conversano, F.; Casciaro, S.; Salvadori, P.A. Translational perspectives in molecular imaging: Methodological evolution and nanostructured materials. Recenti Prog. Med. 2012, 103, 142–153. [Google Scholar] [PubMed]
- Soloperto, G.; Casciaro, S. Progress in atherosclerotic plaque imaging. World J. Radiol. 2012, 4, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Postema, M.; Gilja, O.H. Contrast-enhanced and targeted ultrasound. World J. Gastroenterol. 2011, 17, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-S.; Tang, S.-L.; Chiang, C.-H.; Hosseinkhani, H.; Hong, P.-D.; Yeh, M.-K. Characterization and anti-tumor effects of chondroitin sulfate-chitosan nanoparticles delivery system. J. Nanopart. Res. 2014, 16. [Google Scholar] [CrossRef]
- Song, X.-X.; Wan, H.-P.; Zhang, J.-S.; Tang, Q. Paclitaxel-loaded KMnF3 nanoparticles for cancer imaging and therapy in vivo. J. Nanopart. Res. 2014, 16. [Google Scholar] [CrossRef]
- Patel, S.; Jammalamadaka, U.; Sun, L.; Tappa, K.; David, K.; Mills, D. Sustained release of antibacterial agents from doped halloysite nanotubes. Bioengineering 2016, 3. [Google Scholar] [CrossRef]
- Lvov, Y.; Shchukin, D.; Mohwald, H.; Price, R. Clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, V.; Abdullayev, E.; Lvov, Y.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, V.; Lvov, Y.M.; Leporatti, S. Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol. Biosci. 2012, 12, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals: A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Price, R.; Gaber, B.; Lvov, Y. Release characteristics of tetracycline, khellin and NAD from halloysite: A cylindrical mineral for delivery of biologically active agents. J. Microencapsul. 2001, 18, 713–722. [Google Scholar] [PubMed]
- Dzamukova, M.R.; Naumenko, E.A.; Lvov, Y.M.; Fakhrullin, R.F. Enzyme-activated intracellular drug delivery with tubule clay nanoformulation. Sci. Rep. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Price, R.; Shchukin, D.; Lvov, Y. Halloysite tubes as nanocontainers for anticorrosion coating with benzotriazole. ACS Appl. Mater. Interfaces 2009, 2, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.; Deasy, P.; Ziaka, E.; Claffey, N. Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int. J. Pharm. 2004, 274, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Kommireddy, D.; Ichinose, I.; Lvov, Y.; Mills, D. Nanoparticle multilayer: Surface modification for cell attachment and growth. J. Biomed. Nanotechnol. 2005, 1, 286–290. [Google Scholar] [CrossRef]
- Li, M.; Guo, B.; Zou, Q.; Du, M.; Jia, D. Interaction between halloysite nanotubes and 2,5-bis(2-benzoxazolyl) thiophene and their effect on reinforcement of polypropylene nanocomposites. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Veerabadran, N.G.; Price, R.R.; Lvov, Y.M. Clay nanotubes for encapsulation and sustained release of drugs. Nano 2007, 2, 115–120. [Google Scholar] [CrossRef]
- Veerabadran, N.; Mongayt, D.; Torchilin, V.; Price, R.; Lvov, Y. Organized shells on clay nanotubes for controlled release of macromolecules. Macromol. Rapid Commun. 2009, 30, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Viseras, M.T.; Aguzzi, C.; Cerezo, P.; Cultrone, G.; Viseras, C. Supramolecular structure of 5-aminosalycilic acid/halloysite composites. J. Microencapsul. 2009, 26, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Barna, B.P.; Judson, M.A.; Thomassen, M.J. Carbon nanotubes and chronic granulomatous disease. Nanomaterials 2014, 4, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Rasulev, B.; Leszczynski, J. Modeling the dispersibility of single walled carbon nanotubes in organic solvents by quantitative structure-activity relationship approach. Nanomaterials 2015, 5, 778–791. [Google Scholar] [CrossRef]
- Wu, H.; Shi, Y.; Huang, C.; Zhang, Y.; Wu, J.; Shen, H.; Jia, N. Multifunctional nanocarrier based on clay nanotubes for efficient intracellular siRNA delivery and gene silencing. J. Biomater. Appl. 2014, 28, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Agarwal, M.; Lvov, Y.M.; Pachpande, C.; Varahramyan, K.; Witzmann, F.A. Proteomic profiling of halloysite clay nanotube exposure in intestinal cell co-culture. J. Appl. Toxicol. 2013, 33, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D.; Mattison, J.; Western, L.T.; Powderly, J.D.; Greene, B.T.; King, M.R. Microtube device for selectin-mediated capture of viable circulating tumor cells from blood. Clin. Chem. 2012, 58, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, M. Silver nanoparticle supported on halloysite nanotubes catalyzed reduction of 4-nitrophenol. Appl. Surf. Sci. 2009, 255, 3989–3993. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.; Liu, Z.; Green, M.; Hook, J.; Antill, S.; Kepert, C. Functionalization of halloysite clay nanotubes by grafting with aminopropyltriethosilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, B.; Liu, M.; Liao, R.; Rabie, A.; Jia, D. Poly(vinylalcohol)/halloysite nanotubes bionanocomposite films. J. Biomed. Mater. Res. Part A 2009, 2, 1456–1463. [Google Scholar]
- Casciaro, S.; Conversano, F.; Ragusa, A.; Malvindi, M.A.; Franchini, R.; Greco, A.; Pellegrino, T.; Gigli, G. Optimal enhancement configuration of silica nanoparticles for ultrasound imaging and automatic detection at conventional diagnostic frequencies. Investig. Radiol. 2010, 45, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Delogu, L.G.; Vidili, G.; Venturelli, E.; Ménard-Moyon, C.; Zoroddu, M.A.; Pilo, G.; Nicolussi, P.; Ligios, C.; Bedognetti, D.; Sgarrella, F.; et al. Functionalized multiwalled carbon nanotubes as ultrasound contrast agents. Proc. Natl. Acad. Sci. USA 2012, 109, 16612–16617. [Google Scholar] [CrossRef] [PubMed]
- Conversano, F.; Greco, A.; Casciaro, E.; Ragusa, A.; Lay-ekuakille, A.; Casciaro, S. Harmonic ultrasound imaging of nanosized contrast agents for multimodal molecular diagnoses. IEEE Trans. Instrum. Meas. 2012, 61, 1848–1856. [Google Scholar] [CrossRef]
- Malvindi, M.A.; Greco, A.; Conversano, F.; Figuerola, A.; Corti, M.; Bonora, M.; Lascialfari, A.; Doumari, H.A.; Moscardini, M.; Cingolani, R.; et al. Magnetic/silica nanocomposites as dual-mode contrast agents for combined magnetic resonance imaging and ultrasonography. Adv. Funct. Mater. 2011, 21, 2548–2555. [Google Scholar] [CrossRef]
- Casciaro, S.; Pisani, P.; Soloperto, G.; Greco, A.; Lay-Ekuakille, A.; Conversano, F. An innovative ultrasound signal processing technique to selectively detect nanosized contrast agents in echographic images. IEEE Trans. Instrum. Meas. 2015, 64, 2136–2145. [Google Scholar] [CrossRef]
- Casciaro, S.; Conversano, F.; Pisani, P.; Greco, A.; Casciaro, E.; Di Paola, M.; Franchini, R.; Lay-Ekuakille, A.; Leporatti, S.; Gigli, G. Ultrasound signal enhancement of halloysite clay nanotubes at medical diagnostic frequencies. Nanotechnol. Instrum. Meas. Workshop Proc. 2015, 1, 129–132. [Google Scholar]
- Soloperto, G.; Conversano, F.; Greco, A.; Casciaro, E.; Ragusa, A.; Leporatti, S.; Lay-Ekuakille, A.; Casciaro, S. Multiparametric evaluation of the acoustic behaviour of halloysite nanotubes for medical echographic image enhancement. IEEE Trans. Instrum. Meas. 2014, 63, 1423–1430. [Google Scholar] [CrossRef]
- Liang, H.D.; Blomley, M.J. The role of ultrasound in molecular imaging. Br. J. Radiol. 2003, 76, S140–S150. [Google Scholar] [CrossRef] [PubMed]
- Pochon, S.; Tardy, I.; Bussat, P. BR55: A lipopeptide-based VEGFR2 targeted ultrasound contrast agent for molecular imaging of angiogenesis. Investig. Radiol. 2010, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Arap, W.; McDonald, D.M. Probing the structural and molecular diversity of tumor vasculature. Trends Mol. Med. 2002, 8, 563–571. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Di Paola, M.; Quarta, A.; Pisani, P.; Conversano, F.; Sbenaglia, E.A.; Leporatti, S.; Gigli, G.; Casciaro, S. Surface coating highly improves cytocompatibility of halloysite nanotubes: A metabolic and ultrastructural study. IEEE Trans. Nanotechnol. 2016, in press. [Google Scholar] [CrossRef]
- Shi, Y.F.; Tian, Z.; Zhang, Y.; Shen, H.B.; Jia, N.Q. Functionalized halloysite nanotube-based carrier for intracellular delivery of antisense oligonucleotides. Nanoscale Res. Lett. 2011, 6, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, S.; Demitri, C.; Conversano, F.; Casciaro, E.; Distante, A. Experimental investigation and theoretical modelling of the nonlinear acoustical behaviour of a liver tissue and comparison with a tissue mimicking hydrogel. J. Mater. Sci. Mater. Med. 2008, 19, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, S.; Conversano, F.; Musio, S.; Casciaro, E.; Demitri, C.; Sannino, A. Full experimental modelling of a liver tissue mimicking phantom for medical ultrasound studies employing different hydrogels. J. Mater. Sci. Mater. Med. 2009, 20, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; Sannino, A.; Conversano, F.; Casciaro, S.; Distante, A.; Maffezzoli, A. Hydrogel based tissue mimicking phantom for in vitro ultrasound contrast agents studies. J. Biomed. Mater. Res. B 2008, 87B, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Masotti, L.; Biagi, E.; Scabia, M.; Acquafresca, A.; Facchini, R.; Ricci, A.; Bini, D. FEMMINA Real-time, radio-frequency echo-signal equipment for testing novel investigation methods. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1783–1795. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A.; Franceschelli, A.; Schiavina, R.; Marchiori, D.; Baccos, A.; Pernetti, R.; Granchi, S.; Biagi, E.; Masotti, L.; Martorana, G. Accuracy of a new echographic method (RULES, radiofrequency ultrasonic local estimators) in prostate cancer diagnosis. Anticancer Res. 2008, 28, 1883–1886. [Google Scholar] [PubMed]
- Soloperto, G.; Conversano, F.; Greco, A.; Casciaro, E.; Franchini, R.; Casciaro, S. Advanced spectral analyses for real-time automatic echographic tissue-typing of simulated tumor masses at different compression stages. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 2692–2701. [Google Scholar] [CrossRef] [PubMed]
- Conversano, F.; Franchini, R.; Demitri, C.; Massoptier, L.; Montagna, F.; Maffezzoli, A.; Malvasi, A.; Casciaro, S. Hepatic vessel segmentation for 3D planning of liver surgery: Experimental evaluation of a new fully automatic algorithm. Acad. Radiol. 2011, 18, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Massoptier, L.; Casciaro, S. A new fully automatic and robust algorithm for fast segmentation of liver tissue and tumors from CT scans. Eur. Radiol. 2008, 18, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
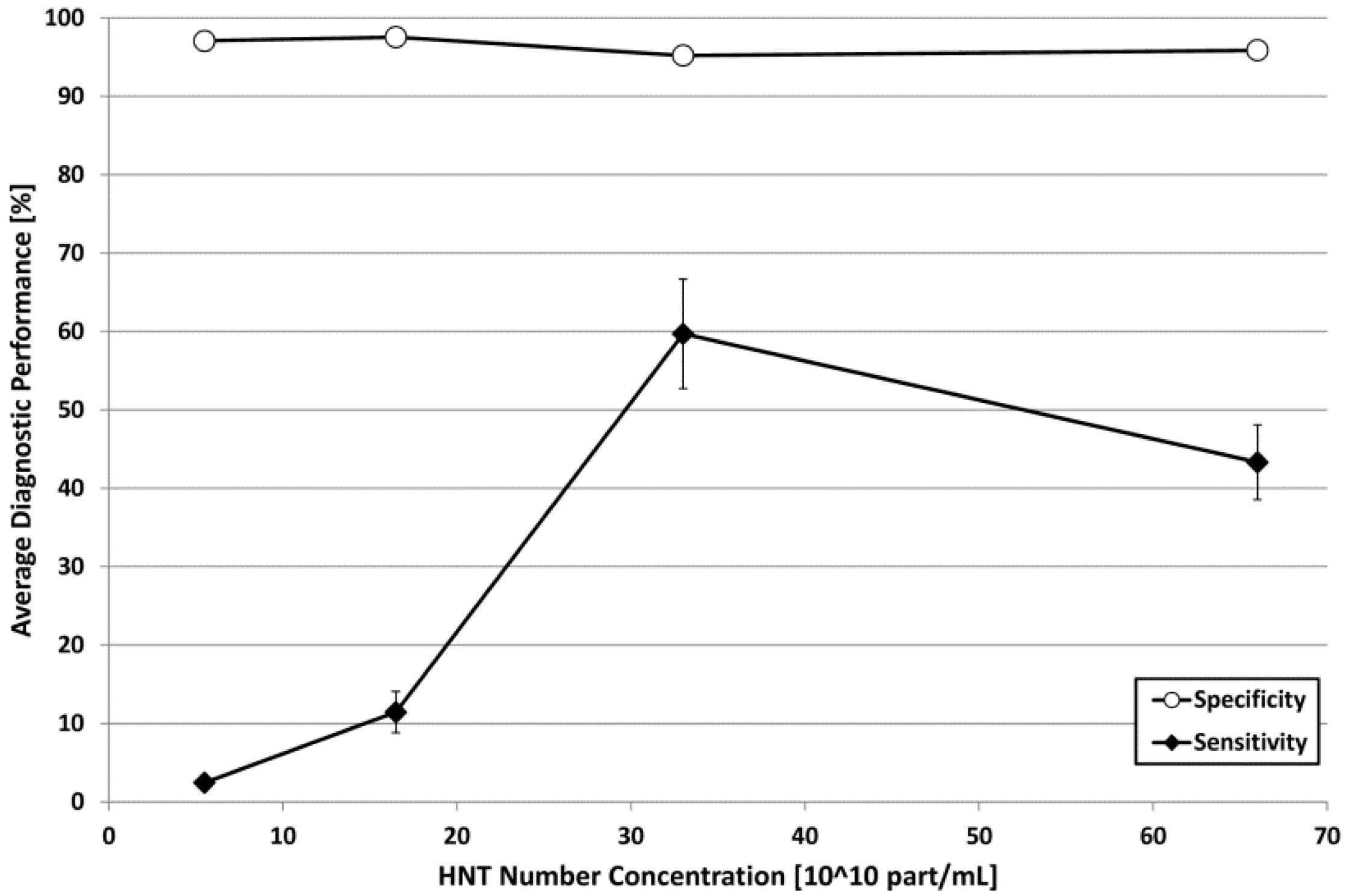
| HNT Concentration (1010 part/mL) | DSC (%) |
|---|---|
| 5.5 | 4.3 |
| 16.5 | 19.1 |
| 33 | 67.3 |
| 66 | 49.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conversano, F.; Pisani, P.; Casciaro, E.; Di Paola, M.; Leporatti, S.; Franchini, R.; Quarta, A.; Gigli, G.; Casciaro, S. Automatic Echographic Detection of Halloysite Clay Nanotubes in a Low Concentration Range. Nanomaterials 2016, 6, 66. https://doi.org/10.3390/nano6040066
Conversano F, Pisani P, Casciaro E, Di Paola M, Leporatti S, Franchini R, Quarta A, Gigli G, Casciaro S. Automatic Echographic Detection of Halloysite Clay Nanotubes in a Low Concentration Range. Nanomaterials. 2016; 6(4):66. https://doi.org/10.3390/nano6040066
Chicago/Turabian StyleConversano, Francesco, Paola Pisani, Ernesto Casciaro, Marco Di Paola, Stefano Leporatti, Roberto Franchini, Alessandra Quarta, Giuseppe Gigli, and Sergio Casciaro. 2016. "Automatic Echographic Detection of Halloysite Clay Nanotubes in a Low Concentration Range" Nanomaterials 6, no. 4: 66. https://doi.org/10.3390/nano6040066
APA StyleConversano, F., Pisani, P., Casciaro, E., Di Paola, M., Leporatti, S., Franchini, R., Quarta, A., Gigli, G., & Casciaro, S. (2016). Automatic Echographic Detection of Halloysite Clay Nanotubes in a Low Concentration Range. Nanomaterials, 6(4), 66. https://doi.org/10.3390/nano6040066









