Copper Nanowires and Their Applications for Flexible, Transparent Conducting Films: A Review
Abstract
:1. Introduction
2. Relationship of Optoelectronic Properties and Nanowire Dimensions
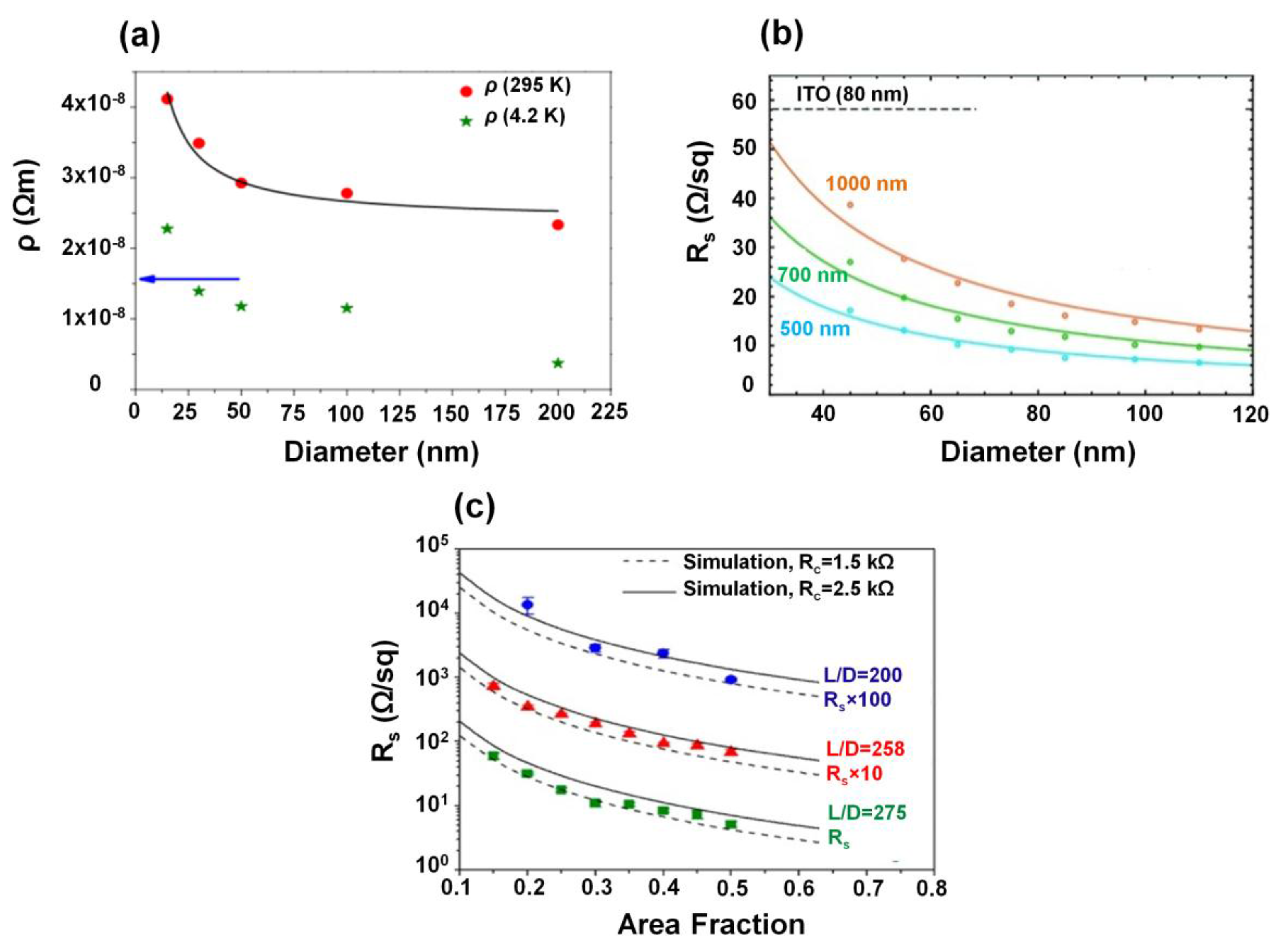
2.1. Electrical Properties of Nanowire Films
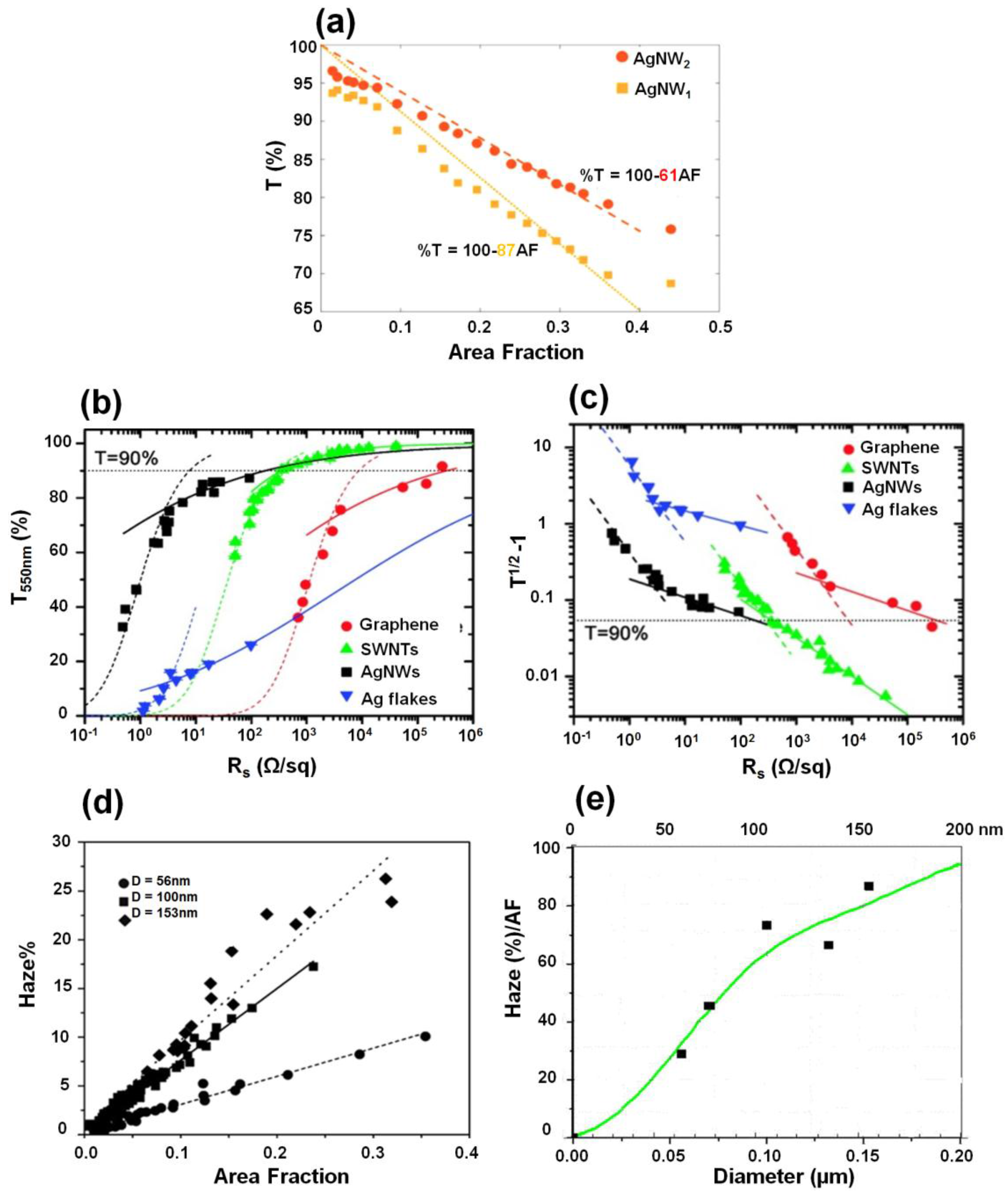
2.2. Optical Properties of Nanowire Films
3. Synthesis of Cu NWs and Fabrication of Cu NW Thin Films
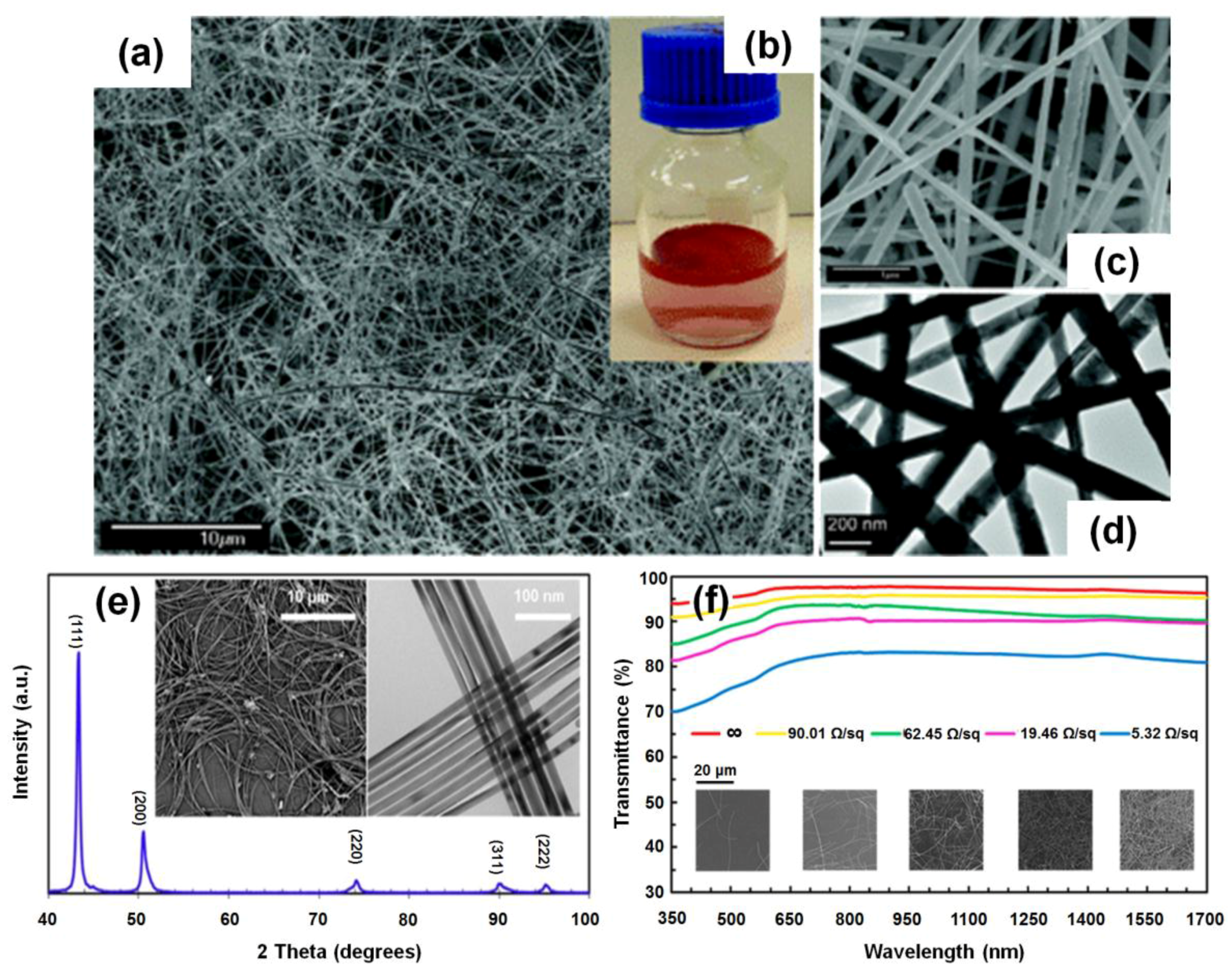
3.1. Synthesis of Cu NWs
3.1.1. Amine-Assisted Synthesis
3.1.2. Catalyst-Assisted Synthesis
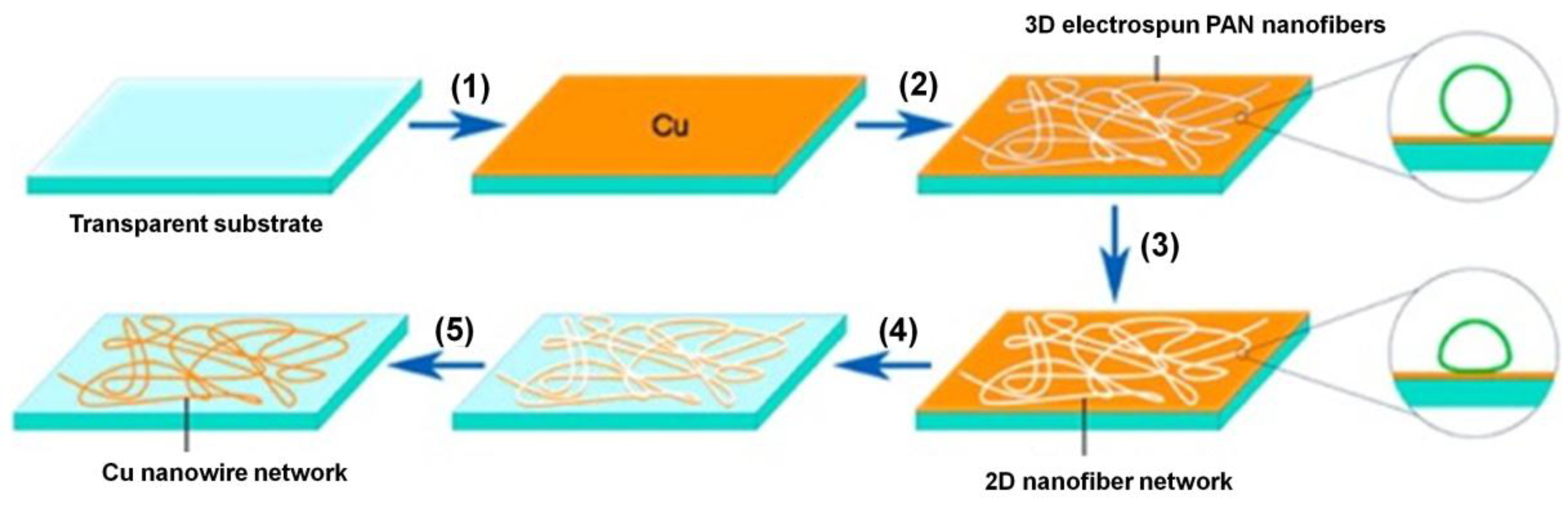
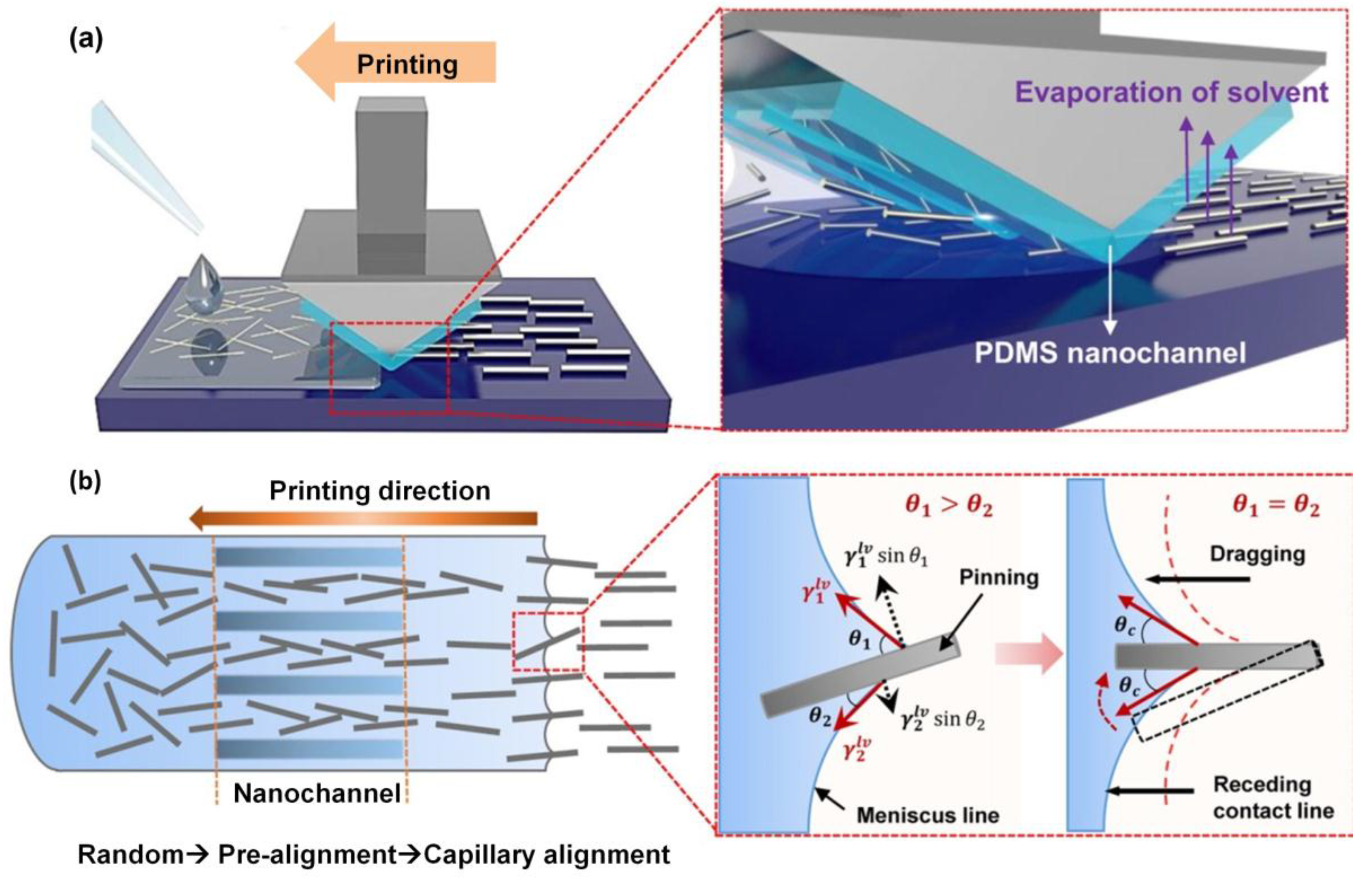
3.2. Fabrication of Cu NW Thin Films
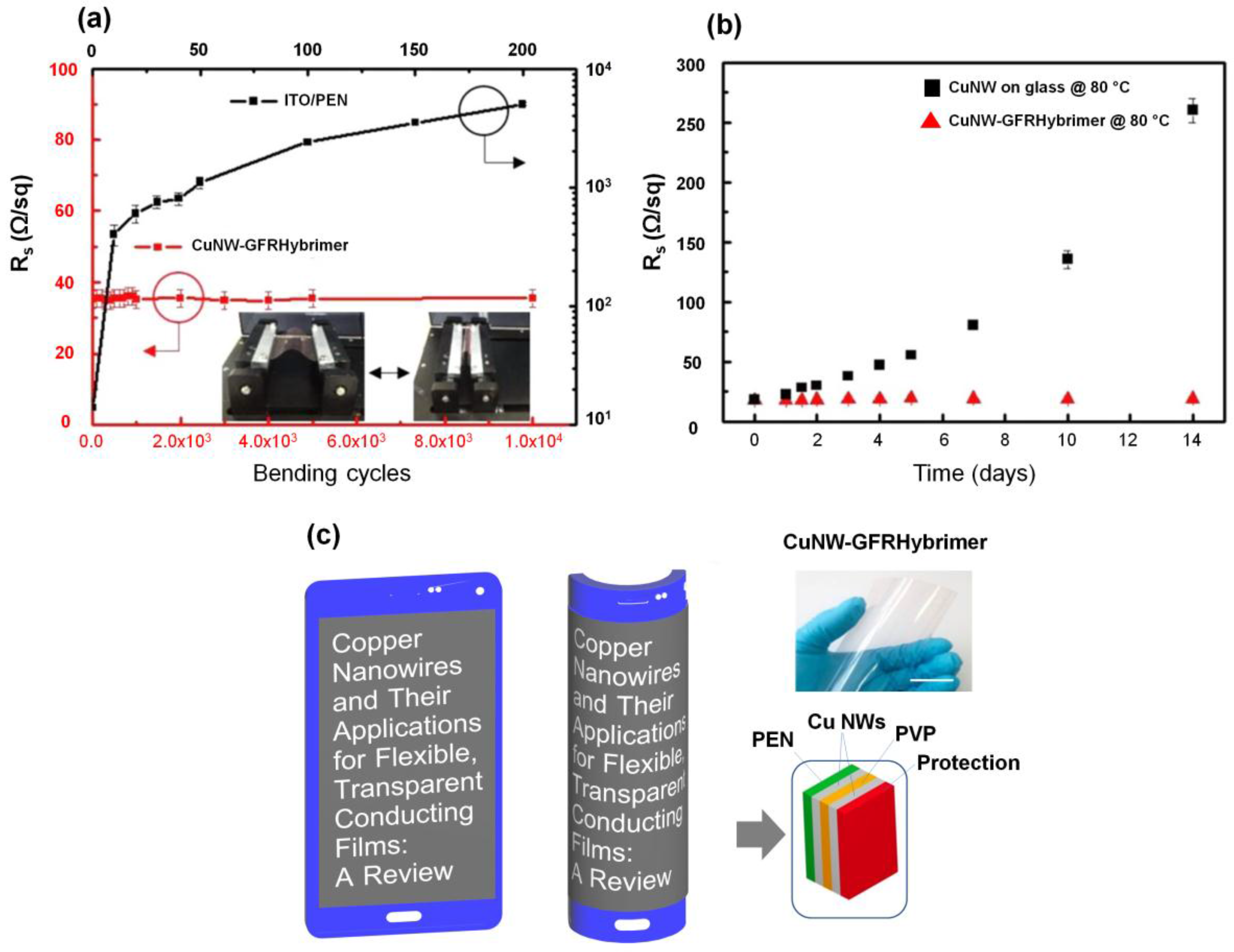
4. Protection of Cu NWs Films against Oxidation
5. Applications and Perspective
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kang, M.G.; Kim, M.S.; Kim, J.; Guo, L.J. Organic solar cells using nanoimprinted transparent metal electrodes. Adv. Mater. 2008, 20, 4408–4413. [Google Scholar] [CrossRef]
- Lee, D.; Rho, Y.; Allen, F.I.; Minor, A.M.; Ko, S.H.; Grigoropoulos, C.P. Synthesis of hierarchical TiO2 nanowires with densely-packed and omnidirectional branches. Nanoscale 2013, 5, 11147–11152. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Agrawal, M.; Becerril, H.A.; Bao, Z.; Liu, Z.; Chen, Y.; Peumans, P. Organic light-emitting diodes on solution-processed graphene transparent electrodes. ACS Nano 2009, 4, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.-H.; Kim, K.-B.; Park, H.-G.; Lee, K.-H.; Lee, J.-R. Criteria for ITO (indium–tin-oxide) thin film as the bottom electrode of an organic light emitting diode. Thin Solid Films 2002, 411, 12–16. [Google Scholar] [CrossRef]
- Loureiro, J.; Neves, N.; Barros, R.; Mateus, T.; Santos, R.; Filonovich, S.; Reparaz, S.; Sotomayor-Torres, C.M.; Wyczisk, F.; Divay, L.; et al. Transparent aluminium zinc oxide thin films with enhanced thermoelectric properties. J. Mater. Chem. A 2014, 2, 6649–6655. [Google Scholar] [CrossRef]
- Bai, S.L.; Sun, C.Z.; Wan, P.B.; Wang, C.; Luo, R.X.; Li, Y.P.; Liu, J.F.; Sun, X.M. Transparent conducting films of hierarchically nanostructured polyaniline networks on flexible substrates for high-performance gas sensors. Small 2015, 11, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.G. Criteria for choosing transparent conductors. MRS Bull. 2000, 25, 52–57. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Bergin, S.M.; Hua, Y.L.; Li, Z.Y.; Wiley, B.J. The growth mechanism of copper nanowires and their properties in flexible, transparent conducting films. Adv. Mater. 2010, 22, 3558–3563. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.Q.; Zhu, Y.P.; Branford-White, C.; Sun, K.; Um-I-Zahra, S.; Quan, J.; Nie, H.L.; Zhu, L.M. Self-assembled transparent conductive composite films of carboxylated multi-walled carbon nanotubes/poly(vinyl alcohol) electrospun nanofiber mats. Mater. Lett. 2014, 128, 310–313. [Google Scholar] [CrossRef]
- McCarthy, M.A.; Liu, B.; Donoghue, E.P.; Kravchenko, I.; Kim, D.Y.; So, F.; Rinzler, A.G. Low-voltage, low-power, organic light-emitting transistors for active matrix displays. Science 2011, 332, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Sagar, R.U.R.; Zhang, X.Z.; Xiong, C.Y.; Yu, Y. Semiconducting amorphous carbon thin films for transparent conducting electrodes. Carbon 2014, 76, 64–70. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Girtan, M.; Vlad, A.; Mallet, R.; Bodea, M.A.; Pedarnig, J.D.; Stanculescu, A.; Mardare, D.; Leontie, L.; Antohe, S. On the properties of aluminium doped zinc oxide thin films deposited on plastic substrates from ceramic targets. Appl. Surf. Sci. 2013, 274, 306–313. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chen, T.-Y. Effects of annealing temperature on properties of Ti-Ga-doped ZnO films deposited on flexible substrates. Nanomaterials 2015, 5, 1831–1839. [Google Scholar] [CrossRef]
- Lee, D.; Pan, H.; Ko, S.H.; Park, H.K.; Kim, E.; Grigoropoulos, C.P. Non-vacuum, single-step conductive transparent ZnO patterning by ultra-short pulsed laser annealing of solution-deposited nanoparticles. Appl. Phys. A 2012, 107, 161–171. [Google Scholar] [CrossRef]
- De, S.; Higgins, T.M.; Lyons, P.E.; Doherty, E.M.; Nirmalraj, P.N.; Blau, W.J.; Boland, J.J.; Coleman, J.N. Silver nanowire networks as flexible, transparent, conducting films: Extremely high DC to optical conductivity ratios. ACS Nano 2009, 3, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Ronning, F.; Gofryk, K.; Mara, N.A.; Haberkorn, N.; Zou, G.F.; Wang, H.Y.; Lee, J.H.; Bauer, E.; McCleskey, T.M.; et al. Aligned carbon nanotubes sandwiched in epitaxial NbC film for enhanced superconductivity. Nanoscale 2012, 4, 2268–2271. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Paeng, D.; Park, H.K.; Grigoropoulos, C.P. Vacuum-free, maskless patterning of Ni electrodes by laser reductive sintering of NiO nanoparticle ink and its application to transparent conductors. ACS Nano 2014, 8, 9807–9814. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Sie, Y.Y.; Liu, C.A.; Lee, M.T. A novel laser direct writing system integrated with A & F XXY alignment platform for rapid fabrication of flexible electronics. Smart Sci. 2015, 3, 87–91. [Google Scholar]
- Ge, Z.B.; Wu, S.T. Nanowire grid polarizer for energy efficient and wide-view liquid crystal displays. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, J.D.; Lee, K.D. Fabrication of a nano-wire grid polarizer for brightness enhancement in liquid crystal display. Nanotechnology 2006, 17, 4436–4438. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, K.; Kim, S.Y.; Lee, H.; Park, J.; Choi, K.H.; Kim, H.K.; Kim, D.G.; Lee, D.Y.; Nam, S.; et al. High-performance, transparent, and stretchable electrodes using graphene-metal nanowire hybrid structures. Nano Lett. 2013, 13, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.S.; Stupp, S.I.; Hersam, M.C. Enrichment of single-walled carbon nanotubes by diameter in density gradients. Nano Lett. 2005, 5, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Song, M.; You, D.S.; Lim, K.; Park, S.; Jung, S.; Kim, C.S.; Kim, D.H.; Kim, D.G.; Kim, J.K.; Park, J.; et al. Highly efficient and bendable organic solar cells with solution-processed silver nanowire electrodes. Adv. Funct. Mater. 2013, 23, 4177–4184. [Google Scholar] [CrossRef]
- Yao, H.F.; Sun, J.L.; Liu, W.; Sun, H.S. Effect of a magnetic field on the preparation of silver nanowires using solid electrolyte thin films. J. Mater. Sci. Technol. 2007, 23, 39–42. [Google Scholar]
- Eom, H.; Lee, J.; Pichitpajongkit, A.; Amjadi, M.; Jeong, J.H.; Lee, E.; Lee, J.Y.; Park, I. Ag@Ni core-shell nanowire network for robust transparent electrodes against oxidation and sulfurization. Small 2014, 10, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Jewell, S.; Kimball, S. Mineral Commodity Summaries 2014; US Geological Survey: Reston, VA, USA, 2014. [Google Scholar]
- Cui, F.; Yu, Y.; Dou, L.T.; Sun, J.W.; Yang, Q.; Schildknecht, C.; Schierle-Arndt, K.; Yang, P.D. Synthesis of ultrathin copper nanowires using tris(trimethylsilyl)silane for high-performance and low-haze transparent conductors. Nano Lett. 2015, 15, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.R.; Rathmell, A.R.; Stewart, I.E.; Ha, Y.C.; Wilson, A.R.; Chen, Z.F.; Wiley, B.J. A rapid synthesis of high aspect ratio copper nanowires for high-performance transparent conducting films. Chem. Commun. 2014, 50, 2562–2564. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hong, S.; Ham, J.; Yeo, J.; Lee, J.; Kang, B.; Lee, P.; Kwon, J.; Lee, S.S.; Yang, M.Y.; et al. Fast plasmonic laser nanowelding for a Cu-nanowire percolation network for flexible transparent conductors and stretchable electronics. Adv. Mater. 2014, 26, 5808–5814. [Google Scholar] [CrossRef] [PubMed]
- Hecht, D.S.; Hu, L.B.; Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.; Haughn, C.; An, K.H.; Pipe, K.P.; Shtein, M. Transparent and conductive electrodes based on unpatterned, thin metal films. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef]
- Fuchs, K. The conductivity of thin metallic films according to the electron theory of metals. Math. Proc. Camb. Philos. Soc. 1938, 34, 100–108. [Google Scholar] [CrossRef]
- Dingle, R. The electrical conductivity of thin wires. Proc. R. Soc. Lond. A 1950, 201, 545–560. [Google Scholar] [CrossRef]
- Bid, A.; Bora, A.; Raychaudhuri, A.K. Temperature dependence of the resistance of metallic nanowires of diameter ≥ 15 nm: Applicability of bloch-gruneisen theorem. Phys. Rev. B 2006, 74. [Google Scholar] [CrossRef]
- De, S.; King, P.J.; Lyons, P.E.; Khan, U.; Coleman, J.N. Size effects and the problem with percolation in nanostructured transparent conductors. ACS Nano 2010, 4, 7064–7072. [Google Scholar] [CrossRef] [PubMed]
- Van de Groep, J.; Spinelli, P.; Polman, A. Transparent conducting silver nanowire networks. Nano Lett. 2012, 12, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.-L. Finite-size scaling in stick percolation. Phys. Rev. E 2009, 80. [Google Scholar] [CrossRef] [PubMed]
- Bergin, S.M.; Chen, Y.H.; Rathmell, A.R.; Charbonneau, P.; Li, Z.Y.; Wiley, B.J. The effect of nanowire length and diameter on the properties of transparent, conducting nanowire films. Nanoscale 2012, 4, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Borchert, J.W.; Stewart, I.E.; Ye, S.R.; Rathmell, A.R.; Wiley, B.J.; Winey, K.I. Effects of length dispersity and film fabrication on the sheet resistance of copper nanowire transparent conductors. Nanoscale 2015, 7, 14496–14504. [Google Scholar] [CrossRef] [PubMed]
- Mutiso, R.M.; Sherrott, M.C.; Rathmell, A.R.; Wiley, B.J.; Winey, K.I. Integrating simulations and experiments to predict sheet resistance and optical transmittance in nanowire films for transparent conductors. ACS Nano 2013, 7, 7654–7663. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Kim, D.; Kim, O.; Nam, J. Analysis of transparent conductive silver nanowire films from dip coating flow. J. Coat. Technol. Res. 2015, 12, 855–862. [Google Scholar] [CrossRef]
- Kim, T.; Canlier, A.; Cho, C.; Rozyyev, V.; Lee, J.Y.; Han, S.M. Highly transparent Au-coated ag nanowire transparent electrode with reduction in haze. ACS Appl. Mater. Interfaces 2014, 6, 13527–13534. [Google Scholar] [CrossRef] [PubMed]
- Khanarian, G.; Joo, J.; Liu, X.Q.; Eastman, P.; Werner, D.; O’Connell, K.; Trefonas, P. The optical and electrical properties of silver nanowire mesh films. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Preston, C.; Xu, Y.L.; Han, X.G.; Munday, J.N.; Hu, L.B. Optical haze of transparent and conductive silver nanowire films. Nano Res. 2013, 6, 461–468. [Google Scholar] [CrossRef]
- Preston, C.; Fang, Z.Q.; Murray, J.; Zhu, H.L.; Dai, J.Q.; Munday, J.N.; Hu, L.B. Silver nanowire transparent conducting paper-based electrode with high optical haze. J. Mater. Chem. C 2014, 2, 1248–1254. [Google Scholar] [CrossRef]
- Araki, T.; Jiu, J.T.; Nogi, M.; Koga, H.; Nagao, S.; Sugahara, T.; Suganuma, K. Low haze transparent electrodes and highly conducting air dried films with ultra-long silver nanowires synthesized by one-step polyol method. Nano Res. 2014, 7, 236–245. [Google Scholar] [CrossRef]
- Choi, H.; Park, S.H. Seedless growth of free-standing copper nanowires by chemical vapor deposition. J. Am. Chem. Soc. 2004, 126, 6248–6249. [Google Scholar] [CrossRef] [PubMed]
- Haase, D.; Hampel, S.; Leonhardt, A.; Thomas, J.; Mattern, N.; Buchner, B. Facile one-step-synthesis of carbon wrapped copper nanowires by thermal decomposition of copper(II)-acetylacetonate. Surf. Coat. Technol. 2007, 201, 9184–9188. [Google Scholar] [CrossRef]
- Khalil, A.; Hashaikeh, R.; Jouiad, M. Synthesis and morphology analysis of electrospun copper nanowires. J. Mater. Sci. 2014, 49, 3052–3065. [Google Scholar] [CrossRef]
- Zang, W.L.; Li, P.; Fu, Y.M.; Xing, L.L.; Xue, X.Y. Hydrothermal synthesis of Co-ZnO nanowire array and its application as piezo-driven self-powered humidity sensor with high sensitivity and repeatability. RSC Adv. 2015, 5, 84343–84349. [Google Scholar] [CrossRef]
- Xu, P.; Chen, W.Z.; Wang, Q.; Zhu, T.S.; Wu, M.J.; Qiao, J.L.; Chen, Z.W.; Zhang, J.J. Effects of transition metal precursors (Co, Fe, Cu, Mn, or Ni) on pyrolyzed carbon supported metal-aminopyrine electrocatalysts for oxygen reduction reaction. RSC Adv. 2015, 5, 6195–6206. [Google Scholar] [CrossRef]
- Panciera, F.; Chou, Y.C.; Reuter, M.C.; Zakharov, D.; Stach, E.A.; Hofmann, S.; Ross, F.M. Synthesis of nanostructures in nanowires using sequential catalyst reactions. Nat. Mater. 2015, 14, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lye, M.L.; Zeng, H.C. Large-scale synthesis of high-quality ultralong copper nanowires. Langmuir 2005, 21, 3746–3748. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, H.; Chen, L.Q.; Huang, X.J. Obtaining ultra-long copper nanowires via a hydrothermal process. Sci. Technol. Adv. Mater. 2005, 6, 761–765. [Google Scholar] [CrossRef]
- Jin, M.S.; He, G.N.; Zhang, H.; Zeng, J.; Xie, Z.X.; Xia, Y.N. Shape-controlled synthesis of copper nanocrystals in an aqueous solution with glucose as a reducing agent and hexadecylamine as a capping agent. Angew. Chem. Int. Ed. 2011, 50, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Z.; Lin, N.; Chen, Y.Z.; Wang, Z.W.; Xie, Q.S.; Zheng, T.C.; Gao, N.; Li, S.P.; Kang, J.Y.; Cai, D.J.; et al. Copper nanowires as fully transparent conductive electrodes. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Wang, R.R.; Wen, M.C.; Weng, D.; Cui, X.; Sun, J.; Li, H.X.; Lu, Y.F. Synthesis of ultralong copper nanowires for high-performance transparent electrodes. J. Am. Chem. Soc. 2012, 134, 14283–14286. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Jeong, Y.; Lee, Y. Improved thermal oxidation stability of solution-processable silver nanowire transparent electrode by reduced graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 6410–6414. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.G.; Huang, B.Y.; Liu, H.W.; Huang, Y.Y.; Pan, H.T.; Meng, H.F.; Yu, P.C. Flexible silver nanowire meshes for high-efficiency microtextured organic-silicon hybrid photovoltaics. ACS Appl. Mater. Interfaces 2012, 4, 6856–6863. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Song, T.B.; Bob, B.; Zhu, R.; Duan, H.S.; Yang, Y. Silver nanowire composite window layers for fully solution-deposited thin-film photovoltaic devices. Adv. Mater. 2012, 24, 5499–5504. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.E.; Rathmell, A.R.; Yan, L.; Ye, S.R.; Flowers, P.F.; You, W.; Wiley, B.J. Solution-processed copper-nickel nanowire anodes for organic solar cells. Nanoscale 2014, 6, 5980–5988. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Chen, Y.Y.; Huang, L.J.; Pan, D.C. Large-scale synthesis of well-dispersed copper nanowires in an electric pressure cooker and their application in transparent and conductive networks. Inorg. Chem. 2014, 53, 4440–4444. [Google Scholar] [CrossRef] [PubMed]
- Akter, T.; Kim, W.S. Reversibly stretchable transparent conductive coatings of spray-deposited silver nanowires. ACS Appl. Mater. Interfaces 2012, 4, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Celle, C.; Mayousse, C.; Moreau, E.; Basti, H.; Carella, A.; Simonato, J.P. Highly flexible transparent film heaters based on random networks of silver nanowires. Nano Res. 2012, 5, 427–433. [Google Scholar] [CrossRef]
- Deng, B.; Hsu, P.C.; Chen, G.C.; Chandrashekar, B.N.; Liao, L.; Ayitimuda, Z.; Wu, J.X.; Guo, Y.F.; Lin, L.; Zhou, Y.; et al. Roll-to-roll encapsulation of metal nanowires between graphene and plastic substrate for high-performance flexible transparent electrodes. Nano Lett. 2015, 15, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, T.; Cho, S.; Lee, Y.; Choe, A.; Walker, B.; Ko, S.J.; Kim, J.Y.; Ko, H. Capillary printing of highly aligned silver nanowire transparent electrodes for high-performance optoelectronic devices. Nano Lett. 2015, 15, 7933–7942. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.B.; Kim, H.S.; Lee, J.Y.; Peumans, P.; Cui, Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Tokuno, T.; Nogi, M.; Karakawa, M.; Jiu, J.T.; Nge, T.T.; Aso, Y.; Suganuma, K. Fabrication of silver nanowire transparent electrodes at room temperature. Nano Res. 2011, 4, 1215–1222. [Google Scholar] [CrossRef]
- Lee, J.Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-processed metal nanowire mesh transparent electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Garnett, E.C.; Cai, W.S.; Cha, J.J.; Mahmood, F.; Connor, S.T.; Christoforo, M.G.; Cui, Y.; McGehee, M.D.; Brongersma, M.L. Self-limited plasmonic welding of silver nanowire junctions. Nat. Mater. 2012, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Xie, A.; Reneker, D.H.; Zhu, Y. A tough and high-performance transparent electrode from a scalable and transfer-free method. ACS Nano 2014, 8, 4782–4789. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, W.; Burkhard, G.F.; McGehee, M.D.; Peumans, P. Smooth nanowire/polymer composite transparent electrodes. Adv. Mater. 2011, 23, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.B.; Zhang, Q.W.; Li, L.; Chen, Q.; Niu, X.F.; Liu, J.; Pei, Q.B. Highly flexible silver nanowire electrodes for shape-memory polymer light-emitting diodes. Adv. Mater. 2011, 23, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.S.; O’Kane, J.C.; Niec, A.; Carmichael, R.S.; Carmichael, T.B. Silver nanowire/optical adhesive coatings as transparent electrodes for flexible electronics. ACS Appl. Mater. Interfaces 2013, 5, 10165–10172. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Wu, H.; Carney, T.J.; McDowell, M.T.; Yang, Y.; Garnett, E.C.; Li, M.; Hu, L.B.; Cui, Y. Passivation coating on electrospun copper nanofibers for stable transparent electrodes. ACS Nano 2012, 6, 5150–5156. [Google Scholar] [CrossRef] [PubMed]
- Rathmell, A.R.; Nguyen, M.; Chi, M.F.; Wiley, B.J. Synthesis of oxidation-resistant cupronickel nanowires for transparent conducting nanowire networks. Nano Lett. 2012, 12, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Ye, S.R.; Stewart, I.E.; Wiley, B.J. Copper nanowire networks with transparent oxide shells that prevent oxidation without reducing transmittance. ACS Nano 2014, 8, 9673–9679. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Chugh, S.; Chen, Z. Enhanced electrical and thermal conduction in graphene-encapsulated copper nanowires. Nano Lett. 2015, 15, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Z.; Li, J.H.; Xu, J.Y.; Zeng, H.B. Superstable transparent conductive Cu@Cu4Ni nanowire elastomer composites against oxidation, bending, stretching, and twisting for flexible and stretchable optoelectronics. Nano Lett. 2014, 14, 6298–6305. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hong, S.; Yeo, J.; Kim, D.; Kang, B.; Yang, M.Y.; Ko, S.H. Nanorecycling: Monolithic integration of copper and copper oxide nanowire network electrode through selective reversible photothermochemical reduction. Adv. Mater. 2015, 27. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.X.; Yang, J.; Gao, H.; Li, F.M.; Yao, Y.F.; Yang, B.; Fu, G.; Zhou, X.X.; Yu, T.; Qin, Y.Q.; et al. In situ fabrication of highly conductive metal nanowire networks with high transmittance from deep-ultraviolet to near-infrared. ACS Nano 2015, 9, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Jeong, Y.; Lee, D.; Lee, Y. Copper nanowire-graphene core-shell nanostructure for highly stable transparent conducting electrodes. ACS Nano 2015, 9, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.; Kim, A.; Lee, D.; Yang, W.; Woo, K.; Jeong, S.; Moon, J. Annealing-free fabrication of highly oxidation-resistive copper nanowire composite conductors for photovoltaics. NPG Asia Mater. 2014, 6. [Google Scholar] [CrossRef]
- Ko, S.H. Review of the multi-scale nano-structure approach to the development of high efficiency solar cells. Smart Sci. 2014, 2, 54–62. [Google Scholar]
- Won, Y.; Kim, A.; Yang, W.; Jeong, S.; Moon, J. A highly stretchable, helical copper nanowire conductor exhibiting a stretchability of 700%. NPG Asia Mater. 2014, 6. [Google Scholar] [CrossRef]
- Im, H.G.; Jung, S.H.; Jin, J.; Lee, D.; Lee, J.; Lee, D.; Lee, J.Y.; Kim, I.D.; Bae, B.S. Flexible transparent conducting hybrid film using a surface-embedded copper nanowire network: A highly oxidation-resistant copper nanowire electrode for flexible optoelectronics. ACS Nano 2014, 8, 10973–10979. [Google Scholar] [CrossRef] [PubMed]
- Stortini, A.M.; Moretto, L.M.; Mardegan, A.; Ongaro, M.; Ugo, P. Arrays of copper nanowire electrodes: Preparation, characterization and application as nitrate sensor. Sens. Actuators B 2015, 207, 186–192. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Fan, L.L.; Zhang, Y.; Zhao, H.; Li, X.J.; Li, Y.P.; Wen, L.; Yan, Z.F.; Huo, Z.Y. Hyper-branched Cu@Cu2O coaxial nanowires mesh electrode for ultra-sensitive glucose detection. ACS Appl. Mater. Interfaces 2015, 7, 16802–16812. [Google Scholar] [CrossRef] [PubMed]
- Rowell, M.W.; McGehee, M.D. Transparent electrode requirements for thin film solar cell modules. Energ Environ. Sci. 2011, 4, 131–134. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, V.B.; Lee, D. Copper Nanowires and Their Applications for Flexible, Transparent Conducting Films: A Review. Nanomaterials 2016, 6, 47. https://doi.org/10.3390/nano6030047
Nam VB, Lee D. Copper Nanowires and Their Applications for Flexible, Transparent Conducting Films: A Review. Nanomaterials. 2016; 6(3):47. https://doi.org/10.3390/nano6030047
Chicago/Turabian StyleNam, Vu Binh, and Daeho Lee. 2016. "Copper Nanowires and Their Applications for Flexible, Transparent Conducting Films: A Review" Nanomaterials 6, no. 3: 47. https://doi.org/10.3390/nano6030047
APA StyleNam, V. B., & Lee, D. (2016). Copper Nanowires and Their Applications for Flexible, Transparent Conducting Films: A Review. Nanomaterials, 6(3), 47. https://doi.org/10.3390/nano6030047