Formation and Controlled Drug Release Using a Three-Component Supramolecular Hydrogel for Anti-Schistosoma Japonicum Cercariae
Abstract
:1. Introduction
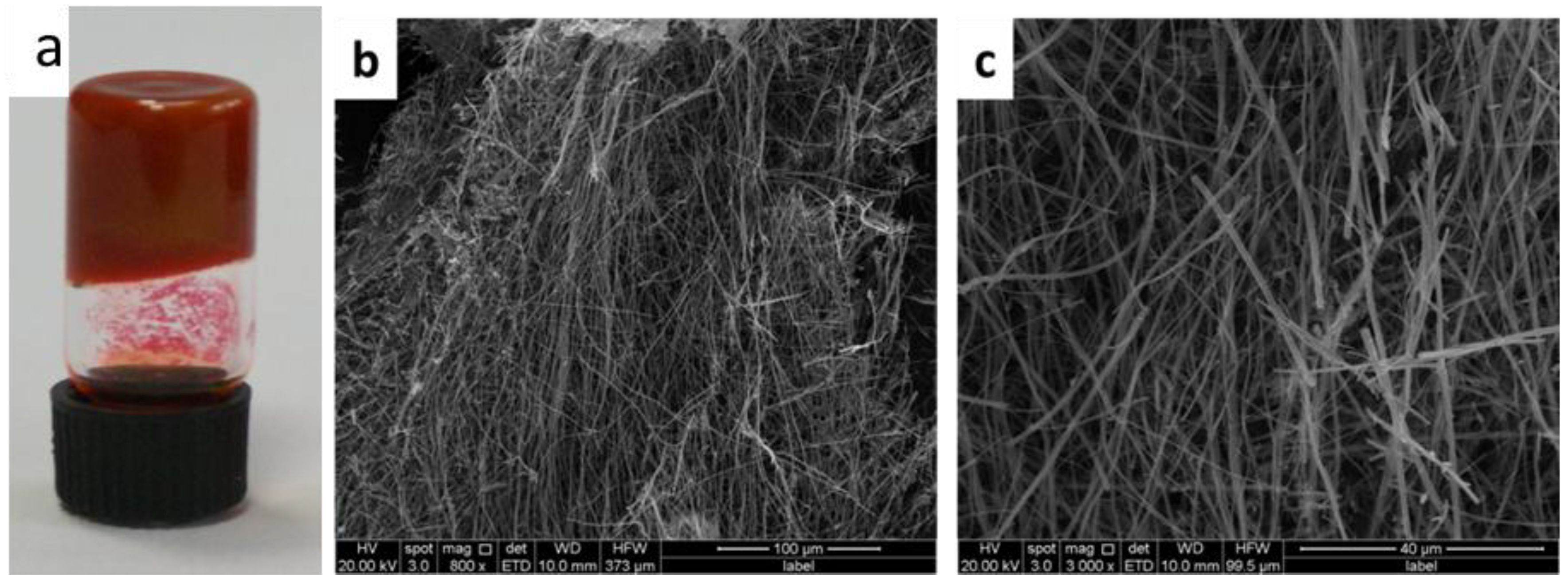
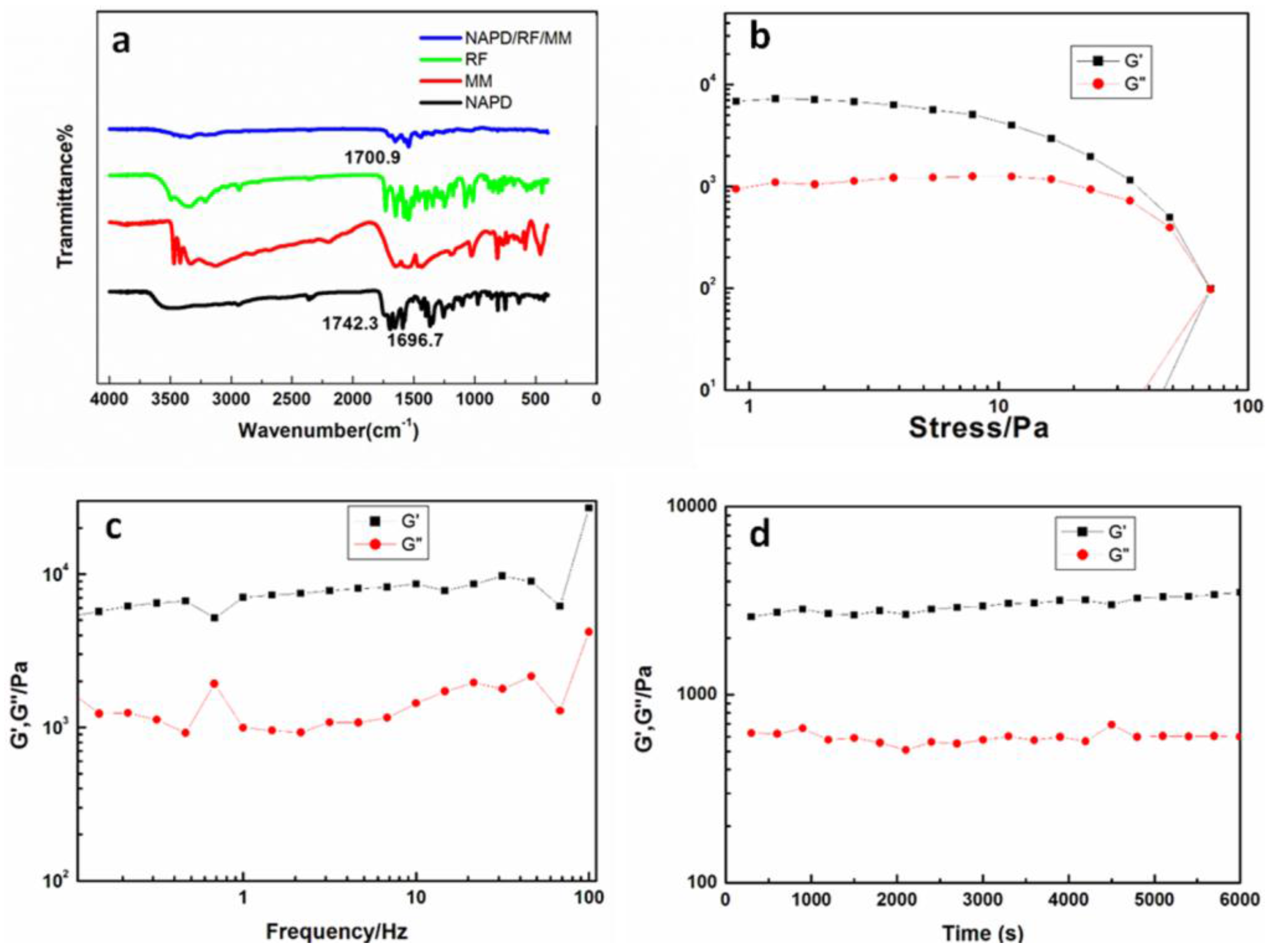
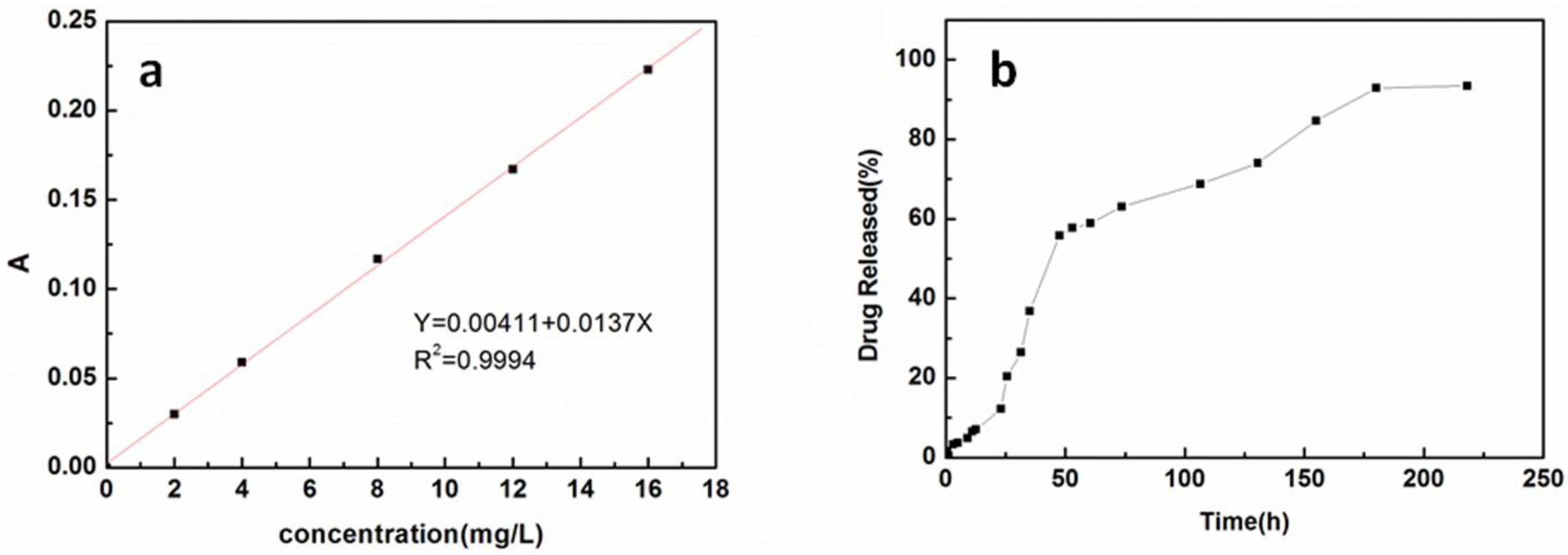
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of NAPD
3.3. Characterization
3.4. Gelation Test
3.5. Drug Loading Test
3.6. In Vitro Release
3.7. Anti-Cercarial Activity Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NAPD | N,N′-dialanine-3,4,9,10-tetracarboxylic diimide |
| RF | riboflavin |
| MM | melamine |
| NMD | Niclosamide derivatives |
References
- Xing, B.; Yu, C.W.; Chow, K.H.; Ho, P.L.; Fu, D.; Xu, B. Hydrophobic Interaction and Hydrogen Bonding Cooperatively Confer a Vancomycin Hydrogel: A Potential Candidate for Biomaterials. J. Am. Chem. Soc. 2002, 124, 14846–14847. [Google Scholar] [CrossRef] [PubMed]
- Sada, K.; Takeuchi, M.; Fujita, N.; Numata, M.; Shinkai, S. Post-polymerization of preorganized assemblies for creating shape-controlled functional materials. Chem. Soc. Rev. 2007, 36, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, C.J.; Nilson, B.L. A Reductive Trigger for Peptide Self-Assembly and Hydrogelation. J. Am. Chem. Soc. 2010, 132, 9526–9527. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.L.; Chuang, J.C.; Tran, T.; Aung, A.; Arya, G.; Varghese, S. Dynamic Electromechanical Hydrogel Matrices for Stem Cell Culture. Adv. Funct. Mater. 2011, 21, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Zhang, H.Q.; Yashima, E. Chiral Stimuli-Responsive Gels: Helicity Induction in Poly(phenylacetylene) Gels Bearing a Carboxyl Group with Chiral Amines. J. Am. Chem. Soc. 2003, 125, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Kiyonaka, S.; Sugiyasu, K.; Shinkai, S.; Hamachi, I. First Thermally Responsive Supramolecular Polymer Based on Glycosylated Amino Acid. J. Am. Chem. Soc. 2002, 124, 10954–10955. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water Gelation by Small Organic Molecules. Chem. Rev. 2003, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Saha, A.; Esterrani, A.; Nandi, A.K. Time sensitive, temperature and pH responsive photoluminescence behaviour of a melamine containing bicomponent hydrogel. Soft Matter 2010, 6, 3337–3345. [Google Scholar] [CrossRef]
- Yang, Z.M.; Xu, B. Supramolecular hydrogels based on biofunctional nanofibers of self-assembled small molecules. J. Mater. Chem. 2007, 17, 2385–2393. [Google Scholar] [CrossRef]
- Pal, A.; Basit, H.; Sen, S.; Aswalb, V.K.; Bhattacharya, S. Structure and properties of two component hydrogels comprising lithocholic acid and organic amines. J. Mater. Chem. 2009, 19, 4325–4334. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric Systems for Controlled Drug Release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Keller, A. Introductory lecture. Aspects of polymer gels. Faraday Discuss 1995, 101, 1–49. [Google Scholar] [CrossRef]
- Wang, R.; Geiger, C.; Chen, L.; Swanson, B.; Whitten, D.G. Direct Observation of Sol-Gel Conversion: The Role of the Solvent in Organogel Formation. J. Am. Chem. Soc. 2000, 122, 2399–2400. [Google Scholar] [CrossRef]
- Sakurai, K.; Jeong, Y.; Koumoto, K.; Friggeri, A.; Gronwald, O.; Sakurai, K.; Okamoto, S.; Inoue, K.; Shinkai, S. Supramolecular Structure of a Sugar-Appended Organogelator Explored with Synchrotron X-ray Small-Angle Scattering. Langmuir 2003, 19, 8211–8217. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maitra, U. A notable group of low molecular mass natural products that form gel are some of the bile acids. Curr. Sci. 2004, 87, 1666–1683. [Google Scholar]
- Zhang, J.W.; Ou, C.W.; Shi, Y.; Wang, L.; Chen, M.; Yang, Z. Visualized detection of melamine in milk by supramolecular hydrogelations. Chem. Commun. 2014, 50, 12873–12876. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, D.C.; Taskinen, K.A. Self-assembly in synthetic macromolecular systems via multiple hydrogen bonding interactions. Chem. Soc. Rev. 2001, 30, 83–93. [Google Scholar] [CrossRef]
- Saha, A.; Manna, S.; Nandi, A.K. Hierarchical tuning of 1-D macro morphology by changing the composition of a binary hydrogel and its influence on the photoluminescence property. Chem. Commun. 2008, 32, 3732–3734. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.P.; Seto, C.T.; Simanek, E.E.; Whitesides, G.M. Self-Assembly through Hydrogen Bonding: Preparation and Characterization of Three New Types of Supramolecular Aggregates Based on Parallel Cyclic CA3.cntdot.M3 “Rosettes”. J. Am. Chem. Soc. 1994, 116, 1725–1736. [Google Scholar] [CrossRef]
- Manna, S.; Saha, A.; Nandi, A.K. A two component thermoreversible hydrogel of riboflavin and melamine: Enhancement of photoluminescence in the gel form. Chem. Commun. 2006, 41, 4285–4287. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, M.R. Energy, Charge, and Spin Transport in Molecules and Self-Assembled Nanostructures Inspired by Photosynthesis. J. Org. Chem. 2006, 71, 5051–5066. [Google Scholar] [CrossRef] [PubMed]
- Langhals, H. Control of the Interactions in Multichromophores: Novel Concepts. Perylene Bis-imides as Components for Larger Functional Units. Chim. Acta 2005, 88, 1309–1343. [Google Scholar] [CrossRef]
- Li, C.; Wonneberger, H. Perylene Imides for Organic Photovoltaics: Yesterday, Today, and Tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, L.Z.; Zhong, H.Z.; Li, Y.F.; Yang, S.H. Platinum nanoparticle clusters immobilized on multiwalled carbon nanotubes: Electrodeposition and enhanced electrocatalytic activity for methanol oxidation. Adv. Funct. Mater. 2007, 17, 1537–1541. [Google Scholar] [CrossRef]
- Sato, N.; Yoshikawa, M. Valence electronic structure at the interface of organic thin films. J. Electron Spectrosc. Relat. Phenom. 1996, 78, 387–390. [Google Scholar] [CrossRef]
- Cui, H.; Webber, M.J.; Stupp, S.I. Self-Assembly of Peptide Amphiphiles: From Molecules to Nanostructures to Biomaterials. Pept. Sci. 2010, 94, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Kuang, Y.; Shi, J.F.; Gao, Y.; Lin, H.C.; Xu, B. Multifunctional, biocompatible supramolecular hydrogelators consist only of nucleobase, amino acid, and glycoside. J. Am. Chem. Soc. 2011, 133, 17513–17518. [Google Scholar] [CrossRef] [PubMed]
- Salick, D.A.; Kretsinger, J.K.; Pochan, D.J.; Schneider, J.P. Inherent antibacterial activity of a peptide-based beta-hairpin hydrogel. J. Am. Chem. Soc. 2007, 129, 14793–14799. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Tanida, T.; Yoshii, T.; Hamachi, I. Rational Molecular Design of Stimulus-Responsive Supramolecular Hydrogels Based on Dipeptides. Adv. Mater. 2011, 23, 2819–2822. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of neglected tropical diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, M.J.; de Vlas, S.; Brookerc, S.; Loomana, C.W.N.; Nagelkerkea, N.J.D.; Habbemaa, J.D.F.; Engelsd, D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003, 86, 125–139. [Google Scholar] [CrossRef]
- King, C.H.; Dickman, K.; Tisch, D.J. Reassessment of the cost of chronic helminthic infection: A meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 2005, 365, 1561–1569. [Google Scholar] [CrossRef]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Cherfas, J. New weapon in the war against schistosomiasis. Science 1989, 246, 1242–1243. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Yang, T.S.; Li, X.; Wu, J.C.; Yi, T.; Li, F.Y.; Huang, C.H.; Fan, X.L. Novel derivatives of Niclosamide synthesis: Its bioactivity and interaction with Schistosoma japonicum cercariae. Dyes Pigments 2011, 88, 326–332. [Google Scholar] [CrossRef]
- Devarakonda, B.; Hill, R.A.; Liebenberg, W.; Brits, M.; Villiers, M.M. Comparison of the aqueous solubilization of practically insoluble Niclosamide by polyamidoamine (PAMAM) dendrimers and cyclodextrins. Int. J. Pharm. 2005, 304, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Xie, Y.Z.; Liu, C.H.; Guo, W.; Li, X.K.; Li, X.; Zeng, Q.D.; Fan, X.L. Nano-film pesticide for Schistosoma japonicum cercariae: Synthesis, characterization, toxicity and insecticidal effect. RSC Adv. 2013, 3, 19956–19960. [Google Scholar] [CrossRef]
- Lowe, D.; Xi, J.Y.; Meng, X.H.; Wu, Z.S.; Qiu, D.C.; Spear, R. Transport of Schistosoma japonicum cercariae and the feasibility of Niclosamide for cercariae control. Parasitol. Int. 2005, 54, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Meng, L.; Cao, X.; Jiang, M.; Yi, T. Morphological transformation between three-dimensional gel network and spherical vesicles via sonication. Soft Matter 2012, 8, 4494–4498. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Wen, Y.; Liu, K.; Chen, L.; Mao, Y.; Yang, S.; Yi, T. (−)-Menthol based thixotropic hydrogel and its application as a universal antibacterial carrier. Soft Matter 2014, 10, 3077–3085. [Google Scholar] [CrossRef] [PubMed]


| Release Time (h) | Mortality (%) | |||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | |
| 1 | 0 | 23.5 | 41.2 | 82.4 |
| 2 | 6.67 | 20 | 80 | 100 |
| 5 | 85.7 | 100 | 100 | 100 |
| 24 | 100 | 100 | 100 | 100 |
| 200 | 100 | 100 | 100 | 100 |
| black | 0 | 0 | 0 | 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhu, L.; Fan, Y.; Li, Y.; Cheng, L.; Liu, W.; Li, X.; Fan, X. Formation and Controlled Drug Release Using a Three-Component Supramolecular Hydrogel for Anti-Schistosoma Japonicum Cercariae. Nanomaterials 2016, 6, 46. https://doi.org/10.3390/nano6030046
Li Y, Zhu L, Fan Y, Li Y, Cheng L, Liu W, Li X, Fan X. Formation and Controlled Drug Release Using a Three-Component Supramolecular Hydrogel for Anti-Schistosoma Japonicum Cercariae. Nanomaterials. 2016; 6(3):46. https://doi.org/10.3390/nano6030046
Chicago/Turabian StyleLi, Yibao, Lei Zhu, Yulan Fan, Yayun Li, Linxiu Cheng, Wei Liu, Xun Li, and Xiaolin Fan. 2016. "Formation and Controlled Drug Release Using a Three-Component Supramolecular Hydrogel for Anti-Schistosoma Japonicum Cercariae" Nanomaterials 6, no. 3: 46. https://doi.org/10.3390/nano6030046
APA StyleLi, Y., Zhu, L., Fan, Y., Li, Y., Cheng, L., Liu, W., Li, X., & Fan, X. (2016). Formation and Controlled Drug Release Using a Three-Component Supramolecular Hydrogel for Anti-Schistosoma Japonicum Cercariae. Nanomaterials, 6(3), 46. https://doi.org/10.3390/nano6030046





