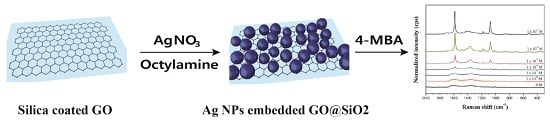
Silver Nanoparticle-Embedded Thin Silica-Coated Graphene Oxide as an SERS Substrate
Abstract
:1. Introduction
2. Results and Discussion
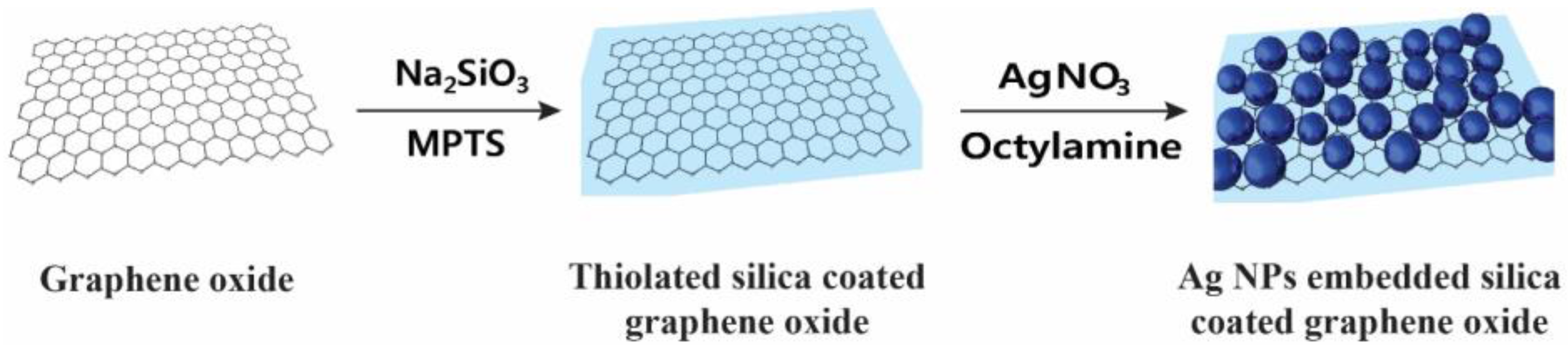
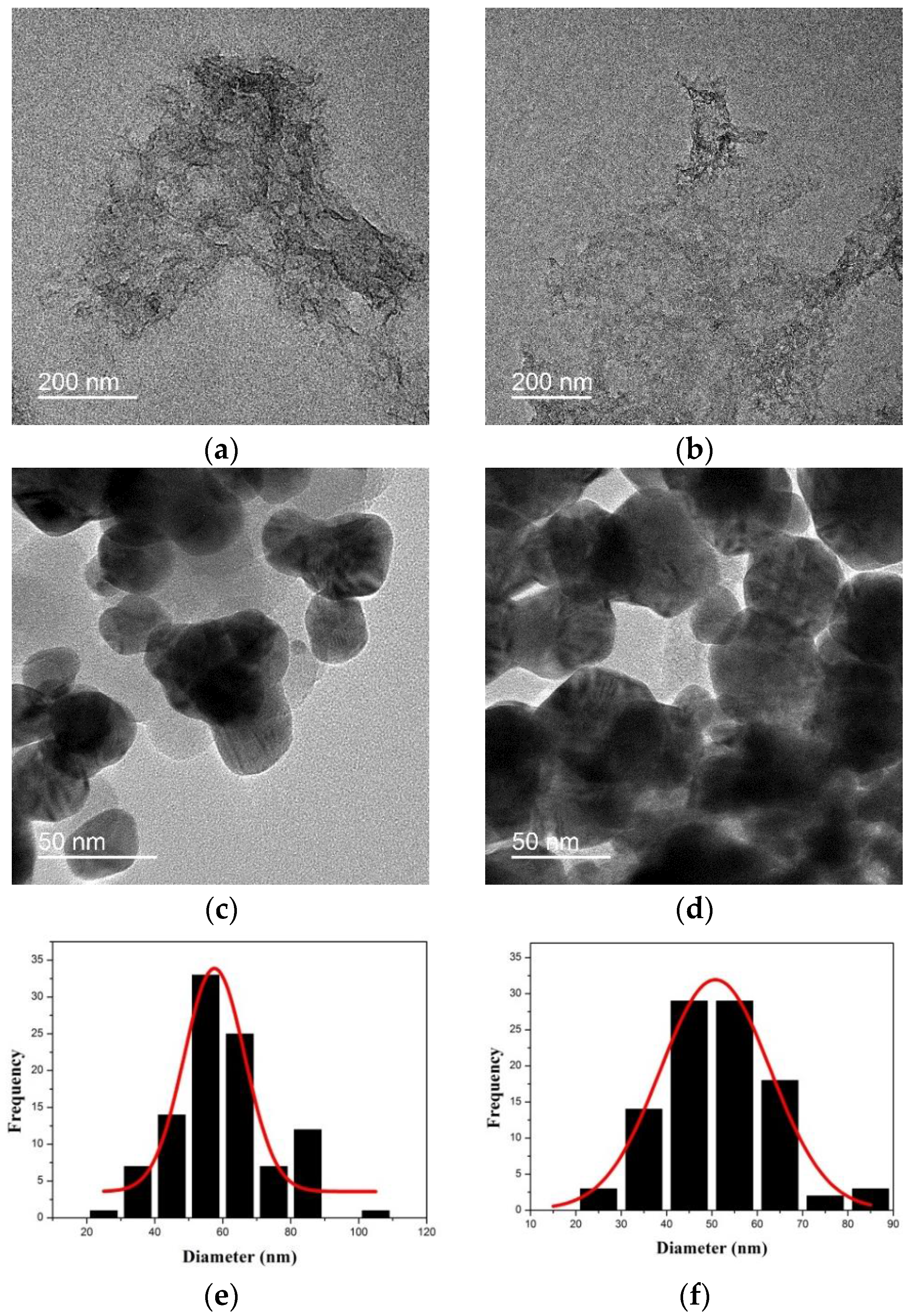
2.1. Preparation of Ag NP-Embedded Silica-Coated GO
2.2. SERS Activity of GO@SiO2@Ag NPs
3. Materials and Methods
3.1. Materials
3.2. Method
3.2.1. Thiolated Silica Coating on Graphene Oxide (GO@SiO2)
3.2.2. Preparation of Silver Nanoparticle-Embedded Silica-Coated Graphene Oxide (GO@SiO2@Ag NPs)
3.2.3. Incorporation of 4-mercaptobenzoic Acid (4-MBA) into GO@SiO2@Ag NPs
3.3. Instrument
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| SERS | Surface-enhanced Raman scattering |
| TEM | Transmission electron microscope |
| LOD | Limit of detection |
| AgNPs | Silver nanoparticles |
| 3-MPTS | 3-mercaptopropyl trimethoxysilane |
| TEOS | Tetraethylorthosilicate |
| 4-MBA | 4-mercaptobenzoic acid |
| GO | Graphene oxide |
| GO@SiO2 | Thiolated silica-coated graphene oxide |
| GO@Ag NPs | Silver-embedded graphene oxide |
| GO@SiO2@Ag NPs | Silver-embedded thin silica-coated graphene oxide |
References
- Schlücker, S. Surface-enhanced raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, B.; Chen, L. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef] [PubMed]
- Culha, M.; Cullum, B.; Lavrik, N.; Klutse, C.K. Surface-enhanced Raman scattering as an emerging characterization and detection technique. Nanotechnology 2012, 2012. [Google Scholar] [CrossRef]
- Jun, B.-H.; Kim, G.; Jeong, S.; Noh, M.S.; Pham, X.-H.; Kang, H.; Cho, M.-H.; Kim, J.-H.; Lee, Y.-S.; Jeong, D.H. Silica core-based surface-enhanced Raman scattering (SERS) tag: Advances in multifunctional SERS nanoprobes for bioimaging and targeting of biomarkers. Bull. Korean Chem. Soc. 2015, 36, 963–978. [Google Scholar]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.; Jeong, D.; Cha, M.G.; Choi, J.M.; Pham, X.-H.; Kim, H.-M.; Kim, H.; Lee, Y.-S.; Jeong, D.H.; Jung, S.; et al. β-CD dimer-immobilized Ag assembly embedded silica nanoparticles for sensitive detection of polycyclic aromatic hydrocarbons. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Pham, X.-H.; Cha, M.G.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Size effect of gold on Ag-coated Au nanoparticle-embedded silica nanospheres. RSC Adv. 2016, 6, 48644–48650. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Liao, J.-D.; Liu, B.H.; Yao, C.-K.; Luo, S.-C. Ag nanoclusters on Zno nanodome array as hybrid SERS-active substrate for trace detection of malachite green. Sens. Actuators B 2015, 207, 430–436. [Google Scholar] [CrossRef]
- Hu, Y.; Liao, J.; Wang, D.; Li, G. Fabrication of gold nanoparticle-embedded metal-organic framework for highly sensitive surface-enhanced Raman scattering detection. Anal. Chem. 2014, 86, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Osberg, K.D.; Macfarlane, R.J.; Langille, M.R.; Mirkin, C.A. Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem. Rev. 2011, 111, 3736–3827. [Google Scholar] [CrossRef] [PubMed]
- Banholzer, M.J.; Millstone, J.E.; Qin, L.; Mirkin, C.A. Rationally designed nanostructures for surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2008, 37, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Talley, C.E.; Jackson, J.B.; Oubre, C.; Grady, N.K.; Hollars, C.W.; Lane, S.M.; Huser, T.R.; Nordlander, P.; Halas, N.J. Surface-enhanced Raman scattering from individual Au nanoparticles and nanoparticle dimer substrates. Nano Lett. 2005, 5, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.B.; Halas, N.J. Surface-enhanced Raman scattering on tunable plasmonic nanoparticle substrates. Proc. Natl. Acad. Sci. USA 2004, 101, 17930–17935. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.B.; Halas, N.J. Silver nanoshells: Variations in morphologies and optical properties. J. Phys. Chem. B 2001, 105, 2743–2746. [Google Scholar] [CrossRef]
- Jun, B.H.; Kim, G.; Noh, M.S.; Kang, H.; Kim, Y.K.; Cho, M.H.; Jeong, D.H.; Lee, Y.S. Surface-enhanced Raman scattering-active nanostructures and strategies for bioassays. Nanomedicine 2011, 6, 1463–1480. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mao, N.; Zhang, J. Graphene: A platform for surface-enhanced Raman spectroscopy. Small 2013, 9, 1206–1224. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Xie, L.; Fang, Y.; Xu, H.; Zhang, H.; Kong, J.; Dresselhaus, M.S.; Zhang, J.; Liu, Z. Can graphene be used as a substrate for Raman enhancement? Nano Lett. 2010, 10, 553–561. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, K.-K.; Su, S.; Yan, J.; Mao, X.; Wang, D.; He, Y.; Li, L.-J.; Song, S.; Fan, C. Graphene-based high-efficiency surface-enhanced Raman scattering-active platform for sensitive and multiplex DNA detection. Anal. Chem. 2012, 84, 4622–4627. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Park, J.; Kim, Y.S.; Kim, K.S.; Hong, B.H.; Nam, J.-M. UV/Ozone-Oxidized Large-Scale Graphene Platform with Large Chemical Enhancement in Surface-Enhanced Raman Scattering. ACS Nano 2011, 5, 9799–9806. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cai, H.; Zhang, W.; Li, X.; Pan, N.; Luo, Y.; Wang, X.; Hou, J.G. Tuning chemical enhancement of SERS by controlling the chemical reduction of graphene oxide nanosheets. ACS Nano 2011, 5, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Zhao, Y.; Chang, L.; Qi, J. Graphene oxide shell-isolated Ag nanoparticles for surface-enhanced Raman scattering. Carbon 2015, 81, 767–772. [Google Scholar] [CrossRef]
- Murphy, S.; Huang, L.; Kamat, P.V. Reduced graphene oxide-silver nanoparticle composite as an active SERS material. J. Phys. Chem. C 2013, 117, 4740–4747. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.; Niu, L.; Wang, H.; Qian, H.; Yao, W. A novel surface-enhanced Raman scattering sensor to detect prohibited colorants in food by graphene/silver nanocomposite. Talanta 2012, 100, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Fu, H.; Zhao, T.; Wang, L.; Tan, T. Fabrication of small-sized silver NPs/graphene sheets for high-quality surface-enhanced Raman scattering. J. Colloid Interface Sci. 2012, 375, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Kim, E.; Kwon, D.-N.; Park, J.-K.; Kim, J.-H. Enhanced green fluorescent protein-mediated synthesis of biocompatible graphene. J. Nanobiotechnol. 2014, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Koh, J. Physiochemical and optical properties of chitosan based graphene oxide bionanocomposite. Int. J. Biol. Macromol. 2014, 70, 559–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Goo, N.I.; Kim, D.E. Mechanism of DNA adsorption and desorption on graphene oxide. Langmuir 2014, 30, 12587–12595. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Na, H.K.; Min, D.H.; Kim, D.E. Desorption of single-stranded nucleic acids from graphene oxide by disruption of hydrogen bonding. Analyst 2013, 138, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.S.; Mahapatra, S.S.; Yoo, H.J.; Kim, Y.A.; Cho, J.W. Soluble conducting polymer-functionalized graphene oxide for air-operable actuator fabrication. J. Mater. Chem. A 2014, 2, 4788–4794. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.H. Green synthesis of graphene and its cytotoxic effects in human breast cancer cells. Int. J. Nanomed. 2013, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Yoo, H.J.; Cho, J.W. Click coupled graphene for fabrication of high-performance polymer nanocomposites. J. Polym. Sci. Part B 2013, 51, 39–47. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kim, J.H. Green chemistry approach for the synthesis of biocompatible graphene. Int. J. Nanomed. 2013, 8, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.H. Microbial reduction of graphene oxide by Escherichia coli: A green chemistry approach. Colloid Surf. B 2013, 102, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.H. Biocompatibility of microbially reduced graphene oxide in primary mouse embryonic fibroblast cells. Colloids Surf. B 2013, 105, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Dayem, A.A.; Kwon, D.N.; Kim, J.H. Biocompatibility effects of biologically synthesized graphene in primary mouse embryonic fibroblast cells. Nanoscale Res. Lett 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.; Kim, J.H. Humanin: A novel functional molecule for the green synthesis of graphene. Colloid Surf. B 2013, 111, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Shon, Y.; Lee, J.; Byun, Y.; Choi, B.S.; Kim, Y.B.; Oh, Y.K. Double stranded aptamer-anchored reduced graphene oxide as target-specific nano detector. Biomaterials 2014, 35, 2999–3004. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, L.; Song, W.; Ai, K.; Lu, L. Functionalizing metal nanostructured film with graphene oxide for ultrasensitive detection of aromatic molecules by surface-enhanced Raman spectroscopy. ACS Appl. Mater. Interfaces 2011, 3, 2944–2952. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ling, X.; Xiao, J.; Dresselhaus, M.S.; Kong, J.; Xu, H.; Liu, Z.; Zhang, J. Surface enhanced Raman spectroscopy on a flat graphene surface. Proc. Natl. Acad. Sci. USA 2012, 109, 9281–9286. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xiao, J.; Chen, Y.; Chen, Y.; Ling, X.; Zhang, J. Graphene-veiled gold substrate for surface-enhanced Raman spectroscopy. Adv. Mater. 2013, 25, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Han, S.W.; Min, D.-H. Graphene oxide sheath on Ag nanoparticle/graphene hybrid films as an antioxidative coating and enhancer of surface-enhanced Raman scattering. ACS Appl. Mater. Interfaces 2012, 4, 6545–6551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, F.; Yang, W.; Guo, M.; Wang, X.; Zhang, B.; Tang, J. A facile one-pot method to high-quality Ag-graphene composite nanosheets for efficient surface-enhanced Raman scattering. Chem. Commun. 2011, 47, 6440–6442. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Li, H.; Liusman, C.; Yin, Z.; Wu, S.; Zhang, H. Surface enhanced Raman scattering of Ag or Au nanoparticle-decorated reduced graphene oxide for detection of aromatic molecules. Chem. Sci. 2011, 2, 1817–1821. [Google Scholar] [CrossRef]
- Fan, W.; Lee, Y.H.; Pedireddy, S.; Zhang, Q.; Liu, T.; Ling, X.Y. Graphene oxide and shape-controlled silver nanoparticle hybrids for ultrasensitive single-particle surface-enhanced Raman scattering (SERS) sensing. Nanoscale 2014, 6, 4843–4851. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Yang, T.; Liu, T.; Zou, Y.; Jiang, J. Graphene oxide wrapped individual silver nanocomposites with improved stability for surface-enhanced Raman scattering. RSC Adv. 2015, 5, 55801–55807. [Google Scholar] [CrossRef]
- Hui, K.S.; Hui, K.N.; Dinh, D.A.; Tsang, C.H.; Cho, Y.R.; Zhou, W.; Hong, X.; Chun, H.-H. Green synthesis of dimension-controlled silver nanoparticle-graphene oxide with in situ ultrasonication. Acta Mater. 2014, 64, 326–332. [Google Scholar] [CrossRef]
- Shen, J.; Li, T.; Shi, M.; Li, N.; Ye, M. Polyelectrolyte-assisted one-step hydrothermal synthesis of Ag-reduced graphene oxide composite and its antibacterial properties. Mater. Sci. Eng. C 2012, 32, 2042–2047. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. Competitive surface-enhanced Raman scattering effects in noble metal nanoparticle-decorated graphene sheets. Phys. Chem. Chem. Phys. 2011, 13, 21116–21120. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Xie, S.; Liu, Y.; Wang, L.; Xu, F. Graphene oxide-silver nanocomposite as SERS substrate for dye detection: Effects of silver loading amount and composite dosage. Appl. Surf. Sci. 2015, 345, 310–318. [Google Scholar] [CrossRef]
- Kou, L.; Gao, C. Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Kuila, T.; Kim, N.H.; Lee, J.H. Effects of surface-modified silica nanoparticles attached graphene oxide using isocyanate-terminated flexible polymer chains on the mechanical properties of epoxy composites. J. Mater. Chem. A 2014, 2, 10557–10567. [Google Scholar] [CrossRef]
- Choi, S.; Kim, K.; Nam, J.; Shim, S.E. Synthesis of silica-coated graphite by enolization of polyvinylpyrrolidone and its thermal and electrical conductivity in polymer composites. Carbon 2013, 60, 254–265. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Ma, C.-C.M.; Chiang, J.-C.; Ho, K.-K.; Chou, T.-Y.; Xie, X.; Tsai, C.-H.; Chang, L.-H.; Hsieh, C.-K. Thermally conductive and electrically insulating epoxy nanocomposites with thermally reduced graphene oxide-silica hybrid nanosheets. Nanoscale 2013, 5, 5863–5871. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Zhang, H.-B.; Li, X.; Gui, C.; Yu, Z.-Z. Thermally conductive and electrically insulating epoxy nanocomposites with silica-coated graphene. RSC Adv. 2014, 4, 15297–15303. [Google Scholar] [CrossRef]
- Kim, H.-M.; Jeong, S.; Hahm, E.; Kim, J.; Cha, M.G.; Kim, K.-M.; Kang, H.; Kyeong, S.; Pham, X.-H.; Lee, Y.-S.; et al. Large scale synthesis of surface-enhanced Raman scattering nanoprobes with high reproducibility and long-term stability. J. Ind. Eng. Chem. 2016, 33, 22–27. [Google Scholar] [CrossRef]
- Zhang, B.-Q.; Li, S.-B.; Xiao, Q.; Li, J.; Sun, J.-J. Rapid synthesis and characterization of ultra-thin shell Au@SiO2 nanorods with tunable SPR for shell-isolated nanoparticle-enhanced Raman spectroscopy (shiners). J. Raman Spectrosc. 2013, 44, 1120–1125. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mungse, H.P.; Kumar, N.; Choudhary, S.; Jain, S.L.; Sain, B.; Khatri, O.P. Graphene oxide: An efficient and reusable carbocatalyst for aza-Michael addition of amines to activated alkenes. Chem. Commun. 2011, 47, 12673–12675. [Google Scholar] [CrossRef] [PubMed]
- Burg, B.R.; Schneider, J.; Maurer, S.; Schirmer, N.C.; Poulikakos, D. Dielectrophoretic integration of single- and few-layer graphenes. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef]
- Kang, H.; Yang, J.-K.; Noh, M.S.; Jo, A.; Jeong, S.; Lee, M.; Lee, S.; Chang, H.; Lee, H.; Jeon, S.-J.; et al. One-step synthesis of silver nanoshells with bumps for highly sensitive near-IR SERS nanoprobes. J. Mater. Chem. B 2014, 2, 4415–4421. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, C.; Sarkar, S.; Pradhan, M.; Negishi, Y.; Pal, T. Silver nanoparticle decorated reduced graphene oxide (rGO) nanosheet: A platform for SERS based low-level detection of uranyl ion. ACS Appl. Mater. Interfaces 2013, 5, 8724–8732. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chen, L.; Chen, Y. Optical engineering of uniformly decorated graphene oxide nanoflakes via in situ growth of silver nanoparticles with enhanced plasmonic resonance. ACS Appl. Mater. Interfaces 2014, 6, 21069–21077. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Zhu, S.; Luo, X.; Zou, M.; Huang, S. Ultraviolet-visible spectroscopy of graphene oxides. AIP Adv. 2012, 2. [Google Scholar] [CrossRef]
- Genov, D.A.; Sarychev, A.K.; Shalaev, V.M. Metal-dielectric composite filters with controlled spectral windows of transparency. J. Nonlinear Opt. Phys. 2003, 12, 419–440. [Google Scholar] [CrossRef]
- Biswas, A.; Eilers, H.; Hidden, F.; Aktas, O.C.; Kiran, C.V.S. Large broadband visible to infrared plasmonic absorption from Ag nanoparticles with a fractal structure embedded in a teflon AF® matrix. Appl. Phys. Letts. 2006, 88. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Su, C.-Y.; Xu, Y.; Zhang, W.; Zhao, J.; Tang, X.; Tsai, C.-H.; Li, L.-J. Electrical and spectroscopic characterizations of ultra-large reduced graphene oxide monolayers. Chem. Mater. 2009, 21, 5674–5680. [Google Scholar] [CrossRef]
- Michota, A.; Bukowska, J. Surface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J. Raman Spectrosc. 2003, 34, 21–25. [Google Scholar] [CrossRef]
- Wang, F.; Widejko, R.G.; Yang, Z.; Nguyen, K.T.; Chen, H.; Fernando, L.P.; Christensen, K.A.; Anker, J.N. Surface-enhanced Raman scattering detection of pH with silica-encapsulated 4-mercaptobenzoic acid-functionalized silver nanoparticles. Anal. Chem. 2012, 84, 8013–8019. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Li, X.; Ji, Y.; Lu, T.; Gu, R. Surface-enhanced Raman scattering of 4-aminothiophenol in assemblies of nanosized particles and the macroscopic surface of silver. Langmuir 2003, 19, 632–636. [Google Scholar] [CrossRef]
- Anema, J.R.; Li, J.-F.; Yang, Z.-L.; Ren, B.; Tian, Z.-Q. Shell-isolated nanoparticle-enhanced Raman spectroscopy: Expanding the versatility of surface-enhanced Raman scattering. Annu. Rev. Anal. Chem 2011, 4, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface plasmon subwavelength optics. Nature 2003, 424, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, W. Graphene nanosheets-supported Ag nanoparticles for ultrasensitive detection of TNT by surface-enhanced raman spectroscopy. Biosens. Bioelectron. 2013, 46, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Cheng, Y.; Zhou, X.; Wu, J.; Xu, G. Fabrication of graphene oxide/Ag hybrids and their surface-enhanced Raman scattering characteristics. J. Colloid Interface Sci. 2013, 397, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Lee, H.K.; You, J.-M.; Jeong, H.; Jeon, S. Electrochemical biosensor for simultaneous determination of dopamine and serotonin based on electrochemically reduced GO-porphyrin. Sens. Actuators B 2014, 190, 886–895. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, X.-H.; Hahm, E.; Kim, H.-M.; Shim, S.; Kim, T.H.; Jeong, D.H.; Lee, Y.-S.; Jun, B.-H. Silver Nanoparticle-Embedded Thin Silica-Coated Graphene Oxide as an SERS Substrate. Nanomaterials 2016, 6, 176. https://doi.org/10.3390/nano6100176
Pham X-H, Hahm E, Kim H-M, Shim S, Kim TH, Jeong DH, Lee Y-S, Jun B-H. Silver Nanoparticle-Embedded Thin Silica-Coated Graphene Oxide as an SERS Substrate. Nanomaterials. 2016; 6(10):176. https://doi.org/10.3390/nano6100176
Chicago/Turabian StylePham, Xuan-Hung, Eunil Hahm, Hyung-Mo Kim, Seongbo Shim, Tae Han Kim, Dae Hong Jeong, Yoon-Sik Lee, and Bong-Hyun Jun. 2016. "Silver Nanoparticle-Embedded Thin Silica-Coated Graphene Oxide as an SERS Substrate" Nanomaterials 6, no. 10: 176. https://doi.org/10.3390/nano6100176
APA StylePham, X.-H., Hahm, E., Kim, H.-M., Shim, S., Kim, T. H., Jeong, D. H., Lee, Y.-S., & Jun, B.-H. (2016). Silver Nanoparticle-Embedded Thin Silica-Coated Graphene Oxide as an SERS Substrate. Nanomaterials, 6(10), 176. https://doi.org/10.3390/nano6100176