Recent Advance on Mesoporous Silica Nanoparticles-Based Controlled Release System: Intelligent Switches Open up New Horizon
Abstract
:1. Introduction
2. Non-Functionalized MSNs-Based Controlled Release Systems
3. Functionalized MSN-Based Controlled Release Systems
3.1. Redox-Responsive Controlled Release Systems

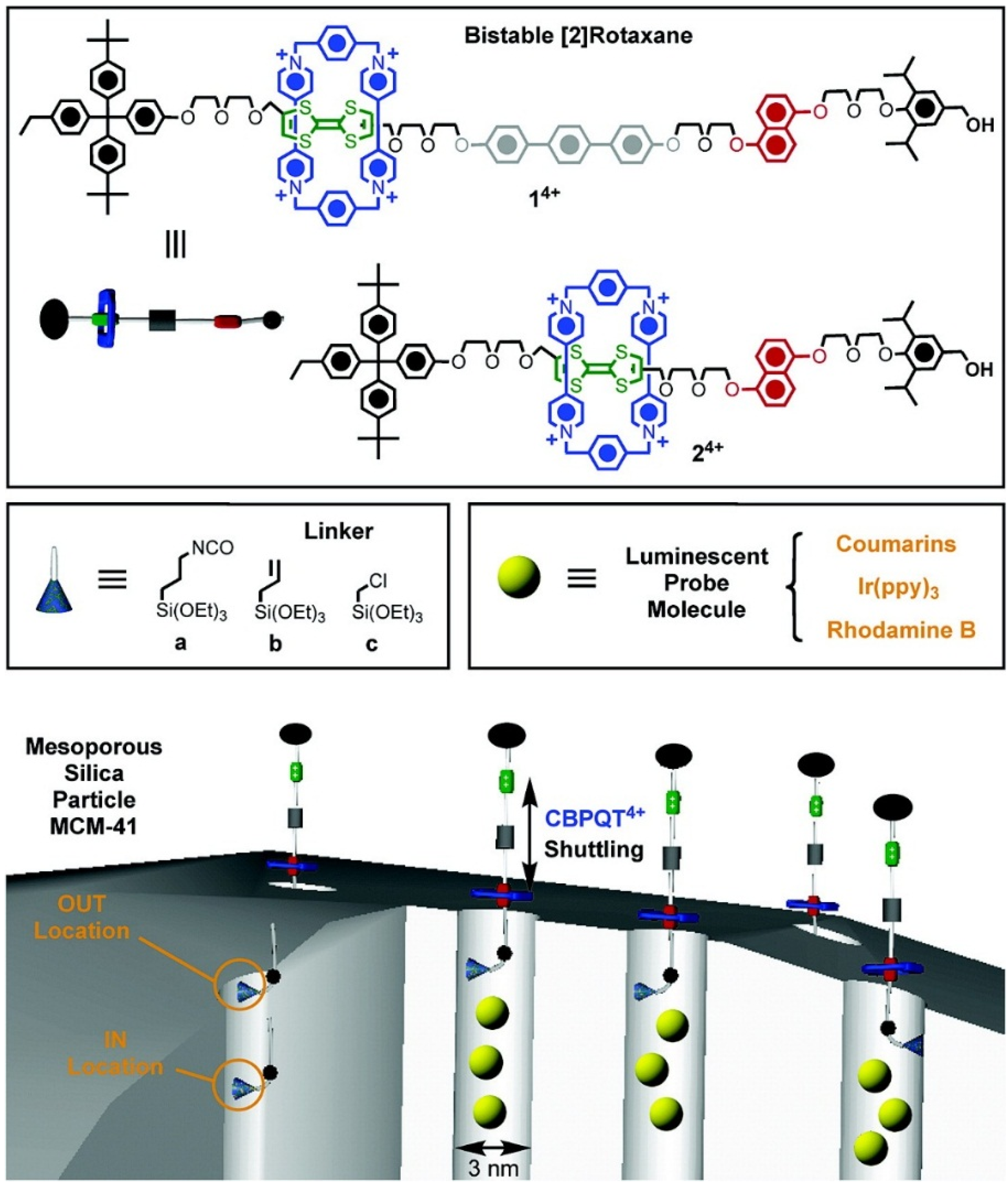
3.2. pH-Responsive Controlled Release Systems
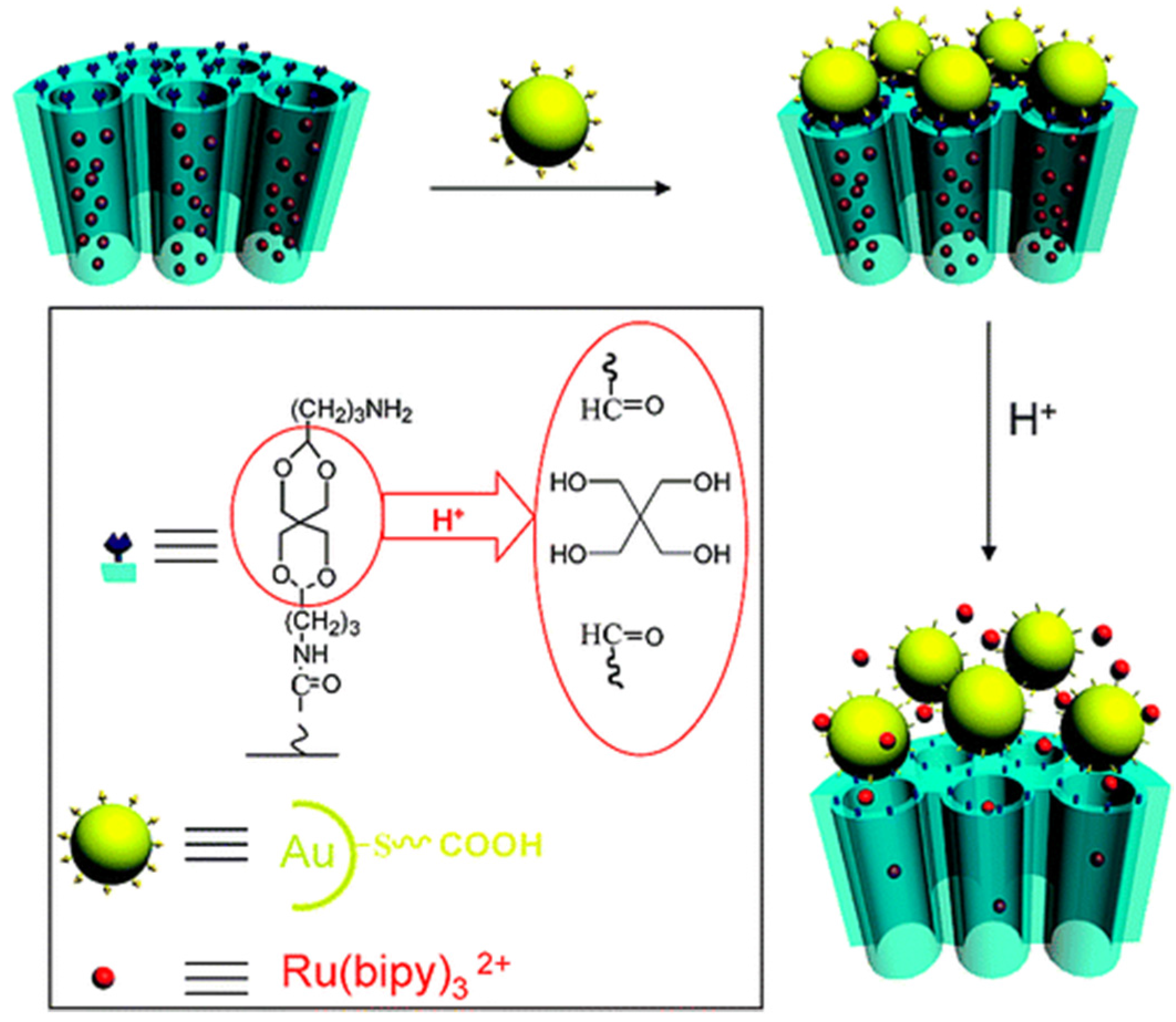
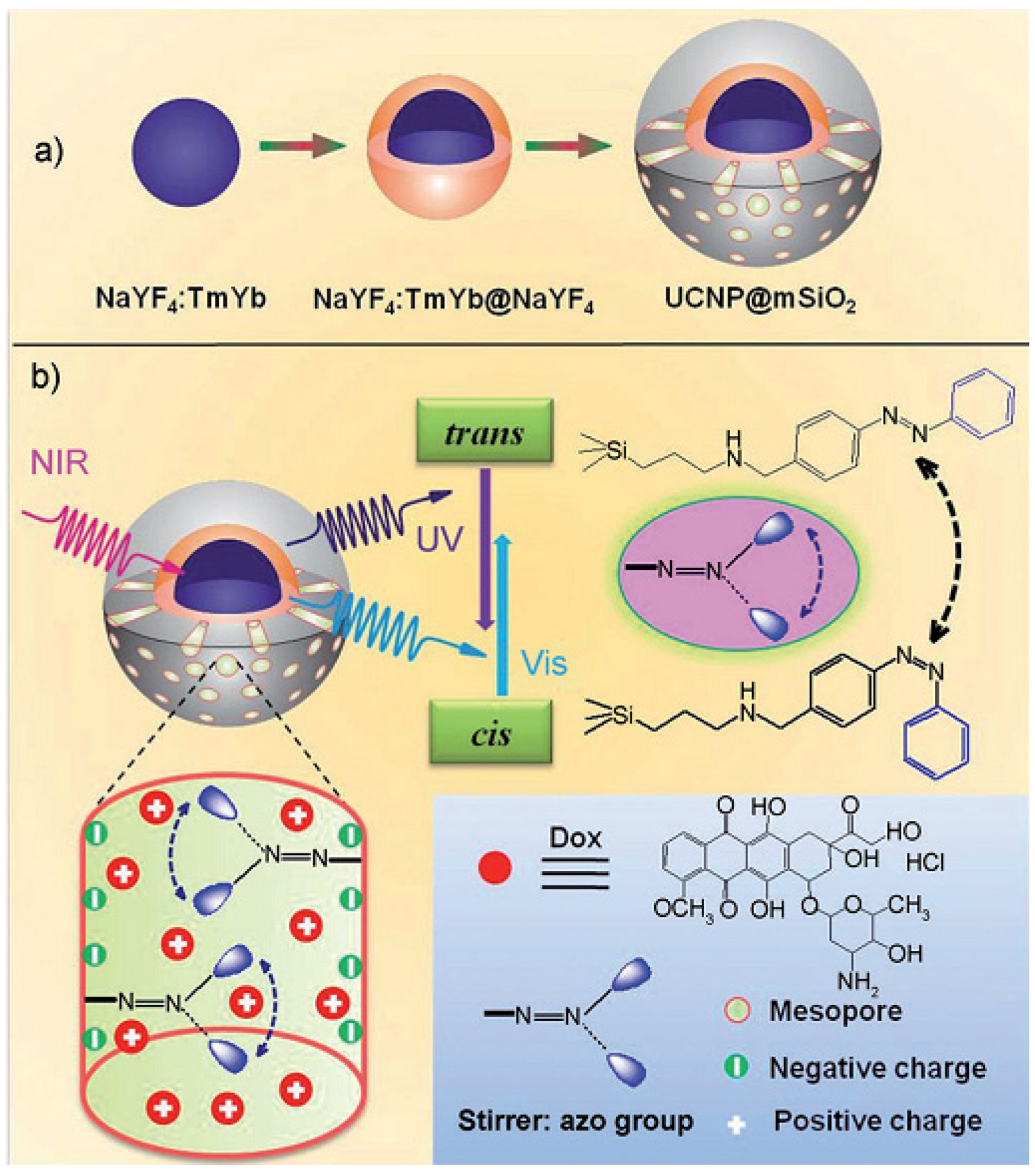
3.3. Light-Responsive Controlled Release Systems
3.4. Temperature-Responsive Controlled Release Systems
3.5. Magnet-Responsive Controlled Release Systems

3.6. Biomolecule-Related Controlled Release Systems
3.6.1. Enzyme-Responsive Controlled Release Systems
3.6.2. Glucose-Responsive Controlled Release Systems
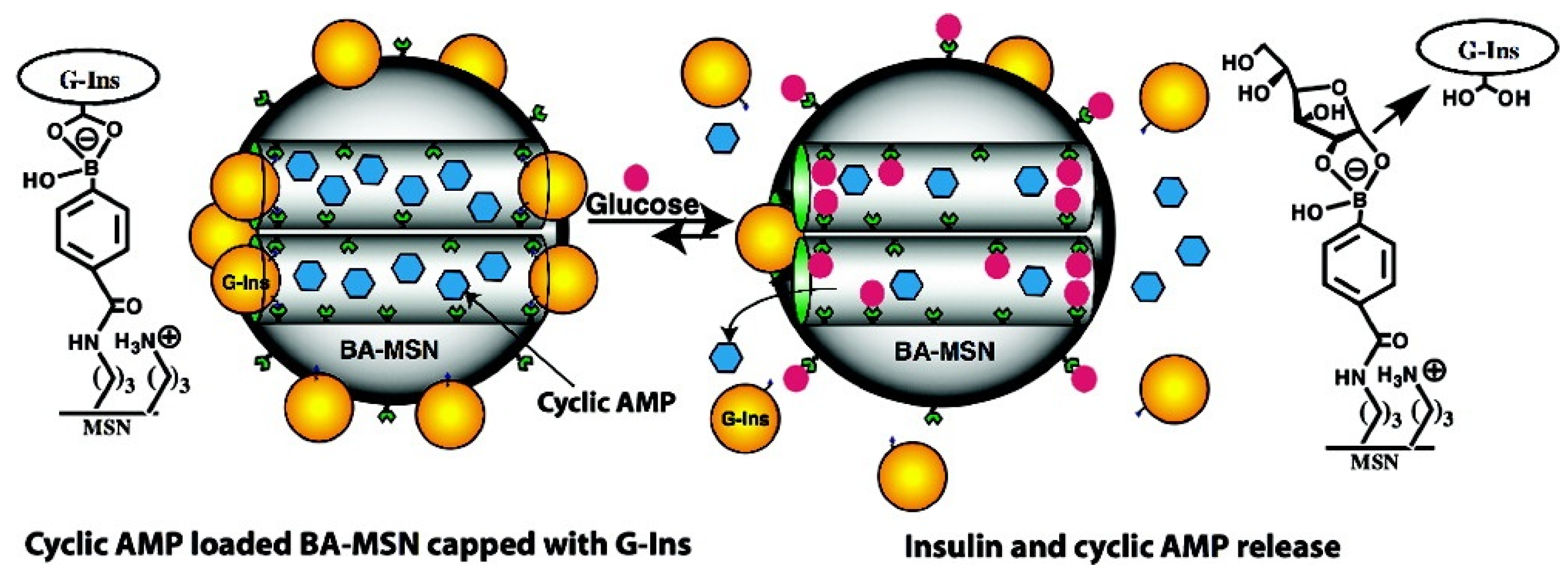
3.6.3. DNA-Based Controlled Release Systems
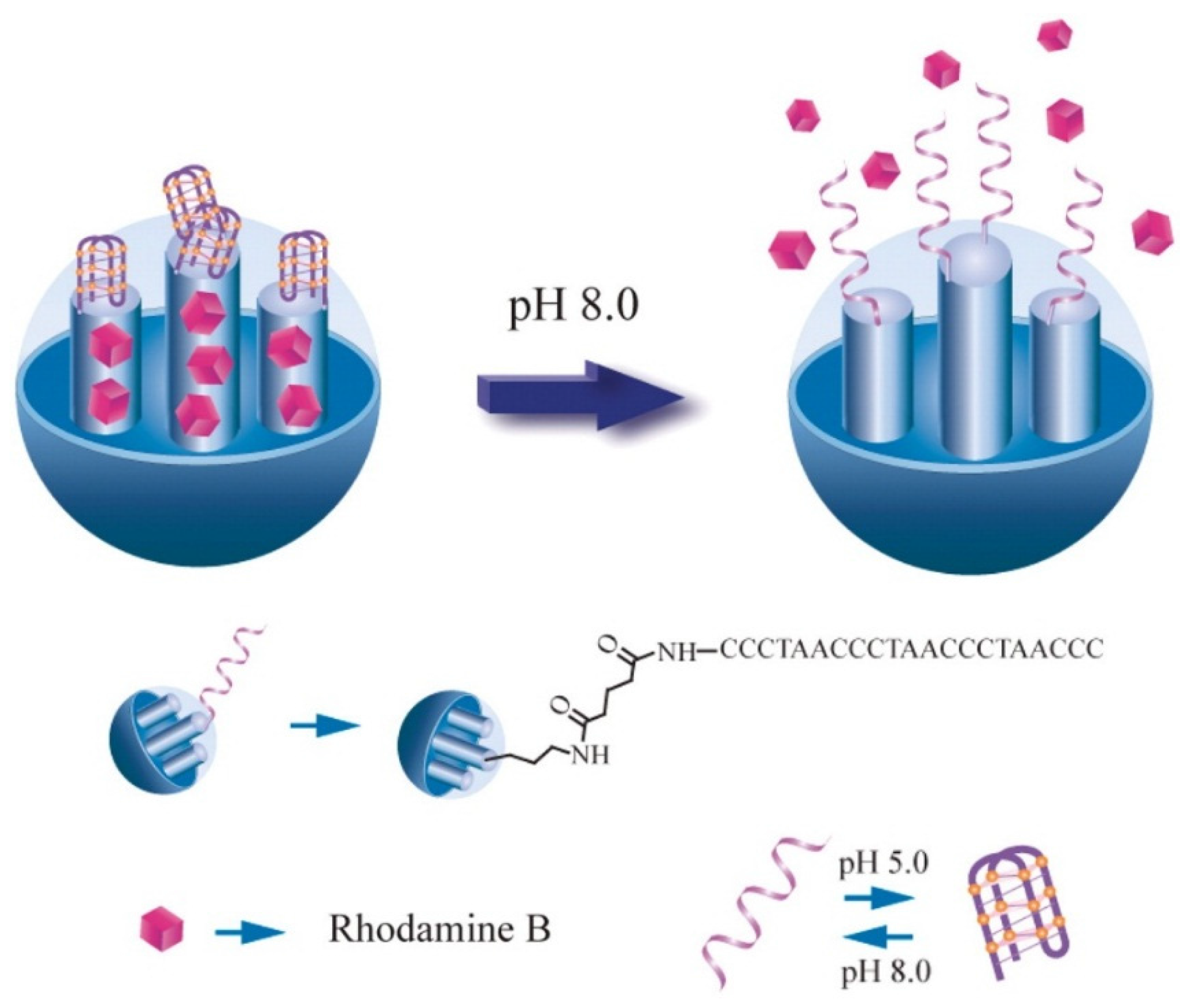
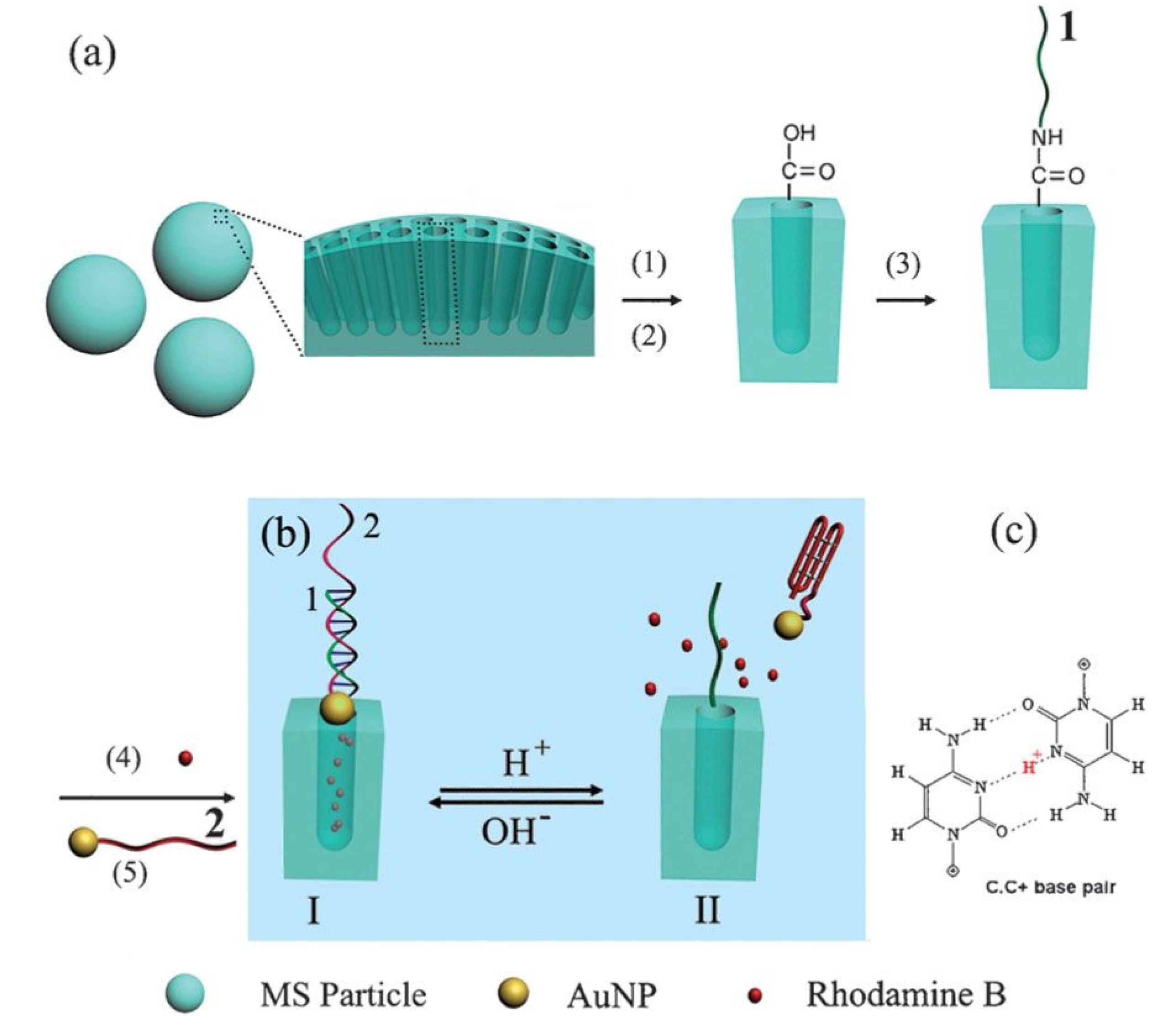
3.6.4. Targeted Delivery Controlled Release Systems
3.7. Multiple-Responsive Controlled Release Systems
3.7.1. pH- and Cation-Responsive Controlled Release Systems
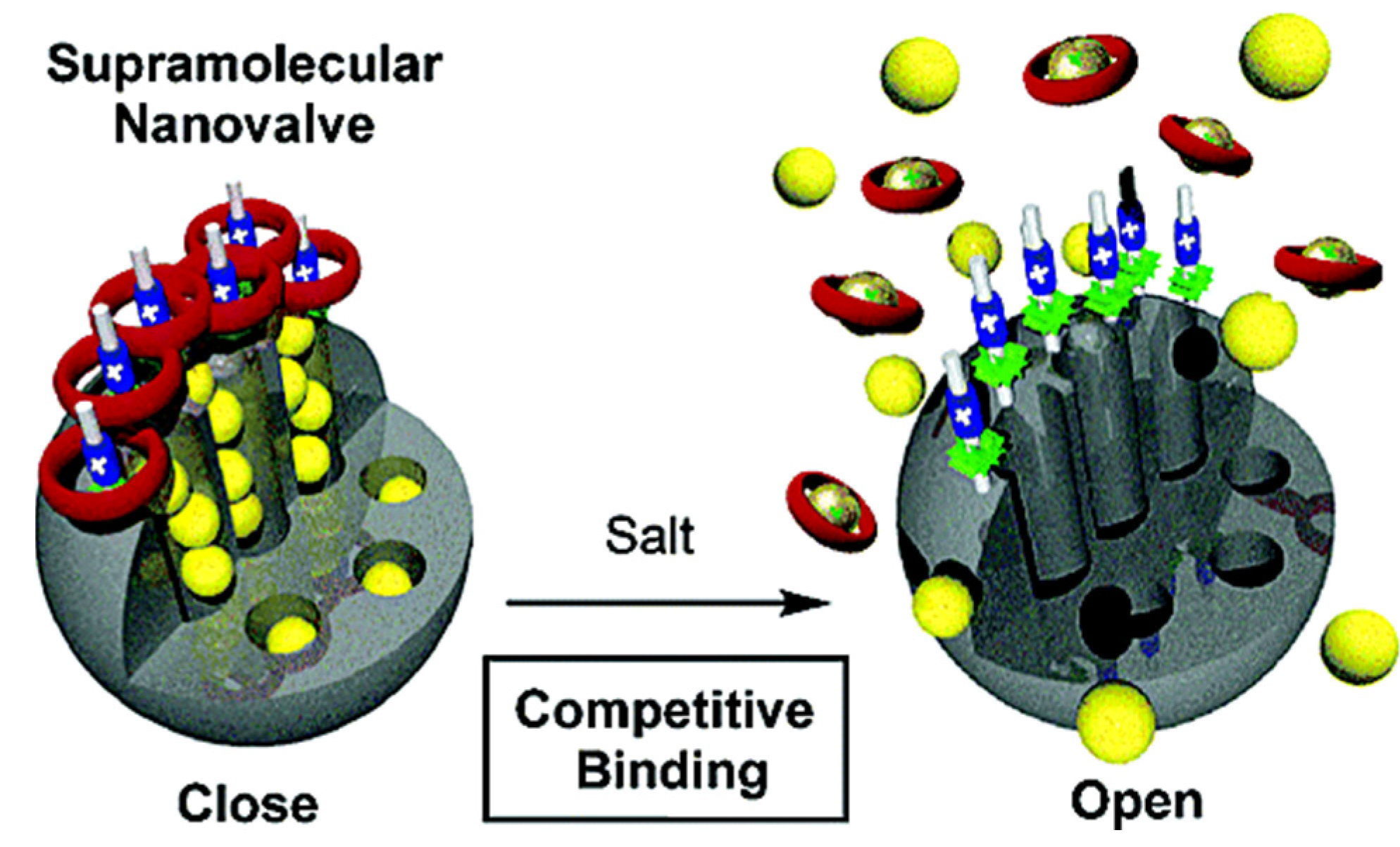
3.7.2. pH- and Anion-Responsive Controlled Release Systems
3.7.3. pH- and Light-Responsive Controlled Release Systems
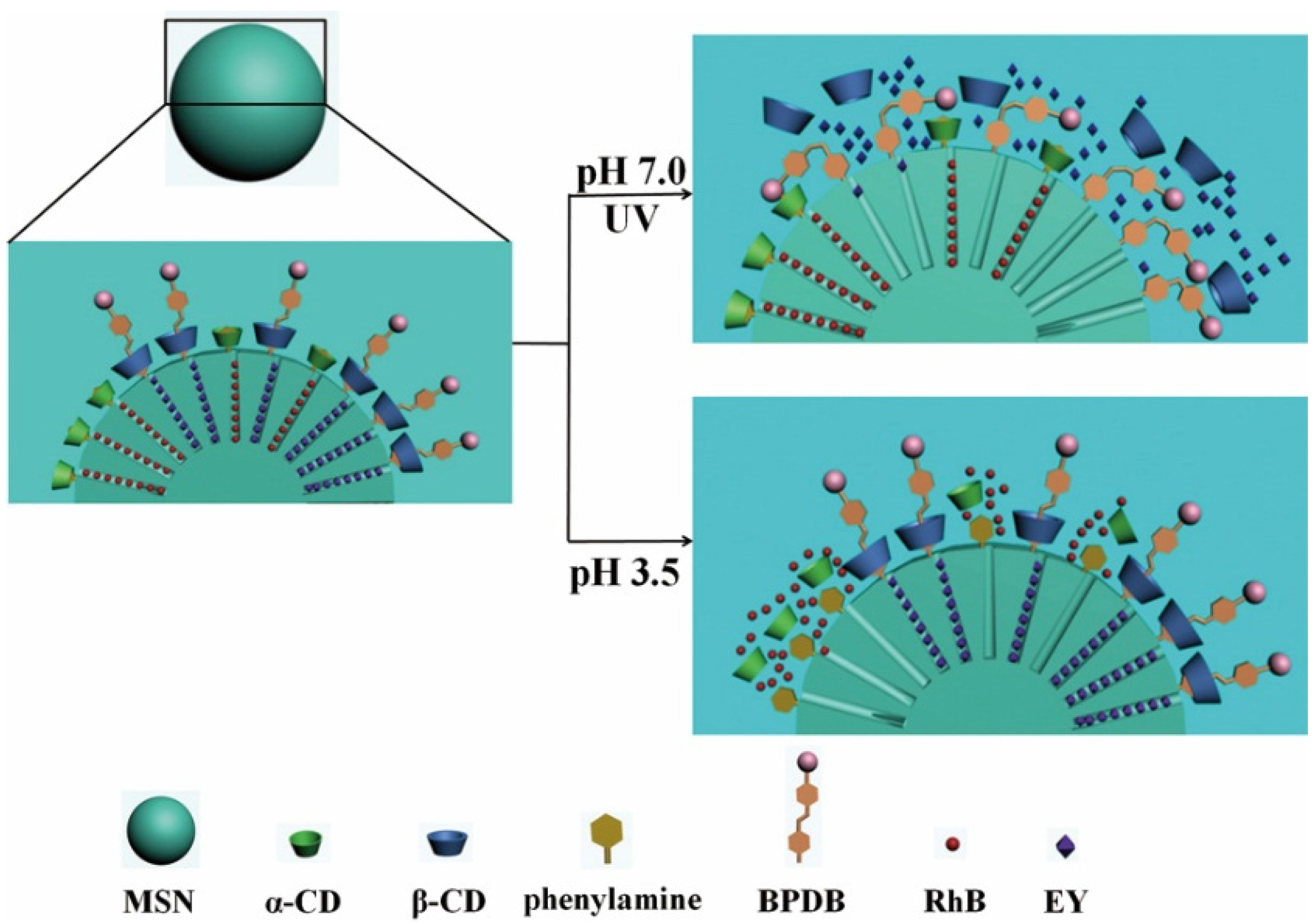
3.7.4. pH- and Temperature-Responsive Controlled Release Systems
3.7.5. pH- and Enzyme-Responsive Controlled Release Systems
3.7.6. pH- and Electrical-Responsive Controlled Release Systems
3.7.7. Light- and Redox-Responsive Controlled Release Systems
3.7.8. Light- and Temperature-Responsive Controlled Release Systems
3.7.9. Magnet- and Temperature-Responsive Controlled Release Systems
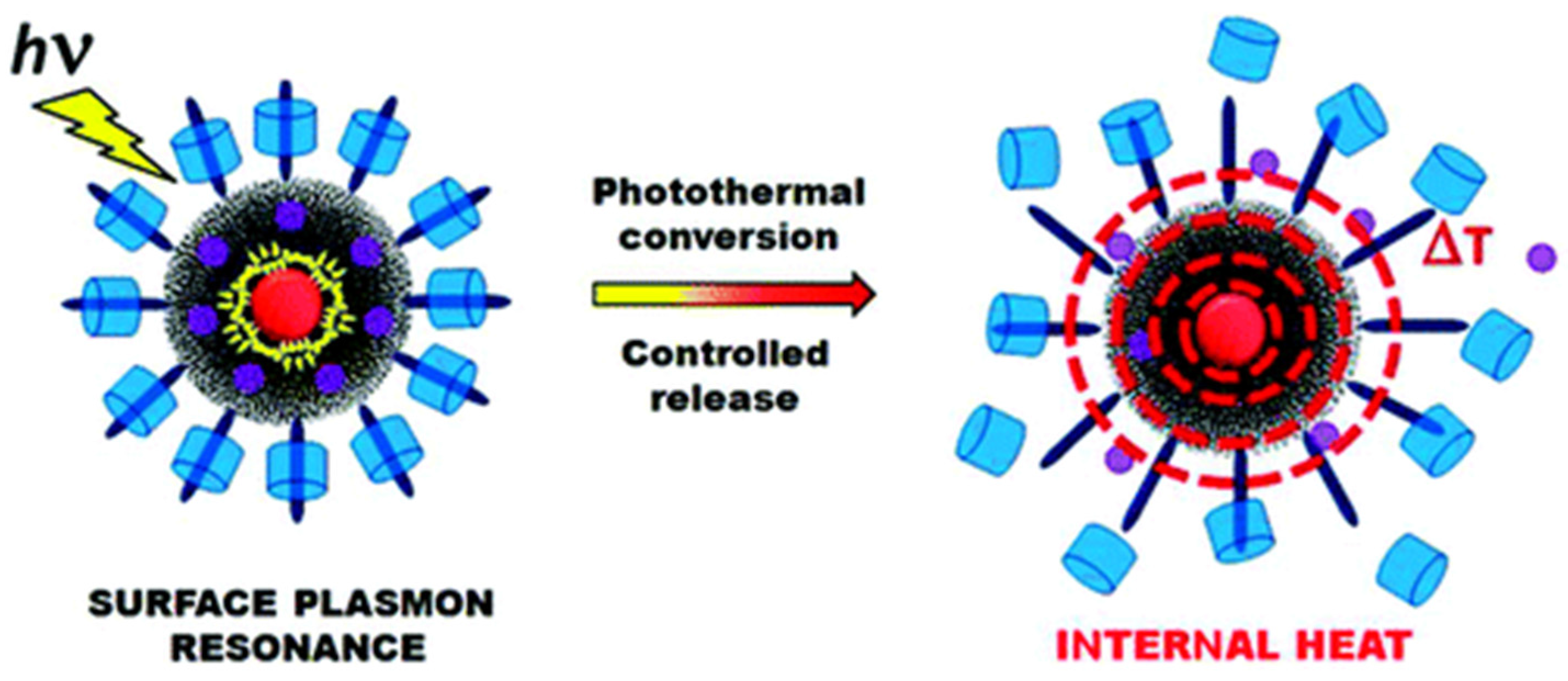
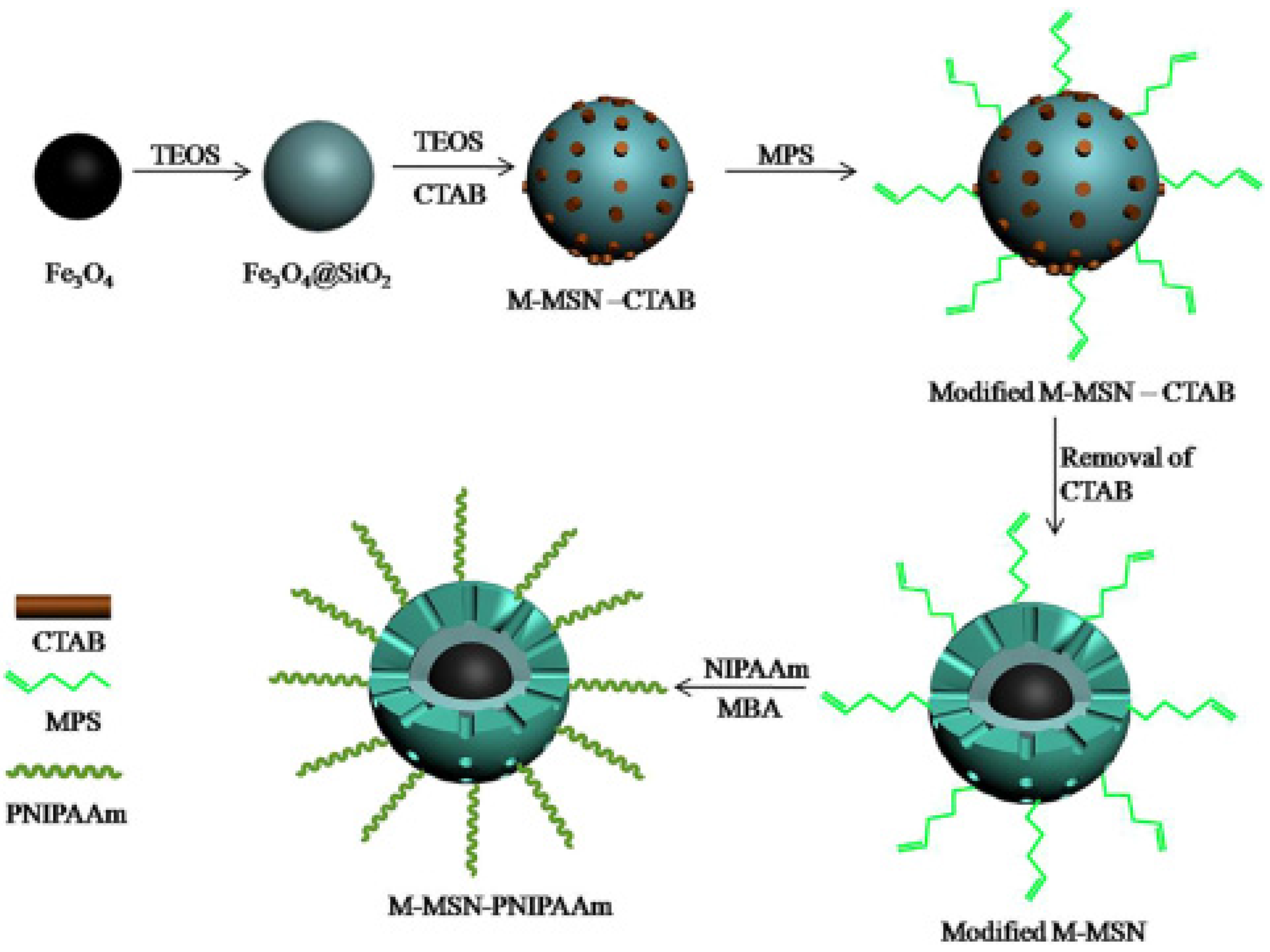
3.8. Free-Blockage Controlled Release Systems
3.9. MSN-Based Multifunctional Controlled Release Systems
4. Conclusions
| Category | Mechanism of Action | Components | Main Characteristics | Reference |
|---|---|---|---|---|
| Redox | disulfide linkages cleaved by oxidation-reduction reaction | CdS, Fe3O4, Au nanoparticles (AuNPs) | (1) easier to design and operate (2) the change of pH and redox homeostasis are internal signals of many serious diseases in human body | [21,22,23,24,25,26,27,28,29,30,31,32,33] |
| polymer | ||||
| different affinity between oxidized and reduced | pseudorotaxane | |||
| pH | protonation | amine group contained compounds | [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] | |
| pseudorotaxane | ||||
| acid hydrolysis | ZnO quantum dots | |||
| acetal group | ||||
| oppositely charged ionic interaction | the negative group and the positive group | |||
| Light | photodimerization | coumarin | remote responsiveness, non-invasiveness, highly controllable, low toxicity, convenient operation | [16,57,58,59,60,61,62,63,64,65] |
| photocleavage | cyclobutane dimer | |||
| photoisomerization | azobenzene, spiropyrane | |||
| Enzyme | catalyze the hydrolysis of complex | biotin-avidin complex | better biocompatible, specificity, accurate responsive | [75] |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.A.; Prouzet, E.; Pinnavaia, T.J. Templating of mesoporous molecular sieves by nonionic polyethylene oxide surfactants. Science 1995, 269, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Fukushima, Y.; Kuroda, K. Synthesis of highly ordered mesoporous materials from a layered polysilicate. Chem. Commun. 1993, 8, 680–682. [Google Scholar] [CrossRef]
- Wu, S.H.; Mou, C.Y.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; Tao, Z. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z. Mesoporous silica-based nanodevices for biological applications. RSC Adv. 2014, 4, 18961–18980. [Google Scholar] [CrossRef]
- Song, N.; Yang, Y.W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem. Soc. 2015, 44, 3474–3504. [Google Scholar] [CrossRef] [PubMed]
- Radin, S.; Ducheyne, P.; Kamplain, T.; Tan, B.H. Silica sol-gel for the controlled release of antibiotics. I. Synthesis, characterization, and in vitro release. J. Biomed. Mater. Res. 2001, 57, 313–320. [Google Scholar] [CrossRef]
- Jin, S.; Ye, K. Nanoparticle-mediated drug delivery and gene therapy. Biotechnol. Prog. 2007, 23, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.A. Nanostructure-mediated drug delivery. Nanomedicine 2005, 1, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrinand caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell. Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Ramila, A.; Del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Mal, N.K.; Fujiwara, M.; Tanaka, Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature 2003, 421, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Trewyn, B.G.; Whitman, C.M.; Lin, V.S.Y. Morphological control of room-temperature ionic liquid templated mesoporous silica nanoparticles for controlled release of antibacterial agents. Nano Lett. 2004, 4, 2139–2143. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Giri, S.; Slowing, I.I.; Lin, V.S.Y. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commun. 2007, 31, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, X.; He, H.; Ren, Z.; Liu, Y.; Wang, J.; Li, Z.; Shen, G.; Han, G. Mesoporous silica nanoparticles with manipulated microstructures for drug delivery. Biointerfaces 2012, 95, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.M.; Yang, J.Y.; Qiu, Y.; Zhou, Y.; Guan, C.X.; Hou, Q.; Lin, W.G.; Zhu, J.H. Sustained release of heparin on enlarged-pore and functionalized MCM-41. ACS Appl. Mater. Interfaces 2012, 4, 4113–4122. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Simard, J.M.; Worrall, J.W.; Rotello, V.M. Tunable reactivation of nanoparticle-inhibited β-galactosidase by glutathione at intracellular concentrations. J. Am. Chem. Soc. 2004, 126, 13987–13991. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.Y. A Mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug mlecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Trewyn, B.G.; Stellmaker, M.P.; Lin, V.S. Stimuli-responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Torney, F.; Trewyn, B.G.; Lin, V.S.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, Y.; Lin, V.S.; Slowing, I.I.; Trewyn, B.G. Luciferase and luciferin co-immobilized mesoporous silica nanoparticle materials for intracellular biocatalysis. J. Am. Chem. Soc. 2011, 133, 18554–18557. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Tseng, H.R.; Wong, J.W.; Stoddart, J.F.; Zink, J.I. An operational supramolecular nanovalve. J. Am. Chem. Soc. 2004, 126, 3370–3371. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Tseng, H.R.; Celestre, P.C.; Flood, A.H.; Liu, Y.; Stoddart, J.F.; Zink, J.I. A reversible molecular valve. Proc. Natl. Acad. Sci. USA 2005, 102, 10029–10034. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Liu, Y.; Saha, S.; Leung, K.C.F.; Stoddart, J.F.; Zink, J.I. Design and optimization of molecular nanovalves based on redox-switchable bistable rotaxanes. J. Am. Chem. Soc. 2007, 129, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, X.; Wu, T.; Feng, P. Tunable redox-responsive hybrid nanogated ensembles. J. Am. Chem. Soc. 2008, 130, 14418–14419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.L.; Song, X.Y.; Zhou, W.H.; Yang, H.H.; Wen, Y.H.; Wang, X.R. An efficient cell-targeting and intracellular controlled-release drug delivery system based on MSN-PEM-aptamer conjugates. J. Mater. Chem. 2009, 19, 7765–7770. [Google Scholar]
- Kim, H.; Kim, S.; Park, C.; Lee, H.; Park, H.J.; Kim, C. Glutathione-induced intracellular release of guests from mesoporous silica nanocontainers with cyclodextrin gatekeepers. Adv. Mater. 2010, 22, 4280–4283. [Google Scholar] [CrossRef] [PubMed]
- Nadrah, P.; Maver, U.; Jemec, A.; Tišler, T.; Bele, M.; Dražić, G.; Benčina, M.; Pintar, A.; Planinšek, O.; Gaberšček, M. Hindered disulfide bonds to regulate release rate of model drug from mesoporous silica. ACS Appl. Mater. Interface 2013, 5, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Dong, H.; Cai, X.; Wang, D.; Li, Y. Mesoporous silica nanoparticles capped with disulfide-linked PEG gatekeepers for glutathione-mediated controlled release. ACS Appl. Mater. Interface 2012, 4, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Nadrah, P.; Porta, F.; Planinšek, O.; Kros, A.; Gaberšček, M. Poly(propylene imine) dendrimer caps on mesoporous silica nanoparticles for redox-responsive release. Smaller is better. Phys. Chem. Chem. Phys. 2013, 15, 10740–10748. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [PubMed]
- Gethin, G.T.; Cowman, S.; Conroy, R.M. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int. Wound. J. 2008, 5, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Zhao, X.; Agarwal, A.; Mueller, L.J.; Feng, P. pH-responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker. J. Am. Chem. Soc. 2010, 132, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.; Guo, M.; Qi, W.; Sun, F.; Wang, A.; Guo, Y.; Zhu, G. pH-Triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids. J. Am. Chem. Soc. 2011, 133, 8778–8781. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Hashidzume, A.; Harada, A. Contrast viscosity changes upon photoirradiation for mixtures of poly(acrylic acid)-based α-cyclodextrin and azobenzene polymers. J. Am. Chem. Soc. 2006, 128, 2226–2227. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Oh, K.; Lee, S.C.; Kim, C. Controlled release of guest molecules from mesoporous silica particles based on a pH-responsive polypseudorotaxane motif. Angew. Chem. Int. Ed. 2007, 46, 1455–1457. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liao, S.; Khatib, H.A.; Stoddart, J.F.; Zink, J.I. Controlled-access hollow mechanized silica nanocontainers. J. Am. Chem. Soc. 2009, 131, 15136–15142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Li, Z.; Kabehie, S.; Botros, Y.Y.; Stoddart, J.F.; Zink, J.I. pH-operated nanopistons on the surfaces of mesoporous silica nanoparticles. J. Am. Chem. Soc. 2010, 132, 13016–13025. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xue, M.; Xia, T.; Zhao, Y.L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.I.; Nel, A.E. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 2010, 132, 12690–12697. [Google Scholar] [CrossRef] [PubMed]
- Angelos, S.; Yang, Y.W.; Patel, K.; Stoddart, J.F.; Zink, J.I. pH-responsive supramolecular nanovalves based on cucurbit[6]uril pseudorotaxanes. Angew. Chem. Int. Ed. 2008, 120, 2254–2258. [Google Scholar] [CrossRef]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The cucurbit[n]uril family. Angew. Chem. Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef] [PubMed]
- Angelos, S.; Khashab, N.M.; Yang, Y.W.; Trabolsi, A.; Khatib, H.A.; Stoddart, J.F.; Zink, J.I. pH clock-operated mechanized nanoparticles. J. Am. Chem. Soc. 2009, 131, 12912–12914. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, N.; Fu, J. Controlled release of cargo molecules from hollow mesoporous silica nanoparticles based on acid and base dual-responsive cucurbit[7]uril pseudorotaxanes. Chem. Commun. 2013, 49, 6555–6557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Q.; Wang, S.; Fan, P.; Wang, L.; Di, Y.; Lin, K.; Xiao, F.S. pH-responsive carrier system based on carboxylic acid modified mesoporous silica and polyelectrolyte for drug delivery. Chem. Mater. 2005, 17, 5999–6003. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, Q.; Yang, D.; Zhang, J.Z.; Zhang, F.; Hu, J. Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery. J. Phys. Chem. C 2011, 115, 9926–9932. [Google Scholar] [CrossRef]
- Xing, R.; Lin, H.; Jiang, P.; Qu, F. Biofunctional mesoporous silica nanoparticles for magnetically oriented target and pH-responsive controlled release of ibuprofen. Colloids Surf. A 2012, 403, 7–14. [Google Scholar] [CrossRef]
- Samart, C.; Prawingwong, P.; Amnuaypanich, S.; Zhang, H.; Kajiyoshi, K.; Reubroycharoen, P. Preparation of poly acrylic acid grafted-mesoporous silica as pH responsive releasing material. J. Ind. Eng. Chem. 2014, 20, 2153–2158. [Google Scholar] [CrossRef]
- Liu, R.; Liao, P.; Liu, J.; Feng, P. Responsive polymer-coated mesoporous silica as a pH-sensitive nanocarrier for controlled release. Langmuir 2011, 27, 3095–3099. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, Y. Chitosan enclosed mesoporous silica nanoparticles as drug nano-carriers: Sensitive response to the narrow pH range. Micropor. Mesopor. Mater. 2012, 150, 83–89. [Google Scholar] [CrossRef]
- Mei, X.; Chen, D.; Li, N.; Xu, Q.; Ge, J.; Li, H.; Lu, J. Hollow mesoporous silica nanoparticles conjugated with pH-sensitive amphiphilic diblock polymer for controlled drug release. Micropor. Mesopor. Mater. 2012, 152, 16–24. [Google Scholar] [CrossRef]
- He, Q.; Gao, Y.; Zhang, L.; Zhang, Z.; Gao, F.; Ji, X.; Li, Y.; Shi, J. A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials 2011, 32, 7711–7720. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, J.; Shen, W.; Dong, X.; Feng, J.; Ruan, M.; Li, Y. Stimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core-shell structure. Angew. Chem. Int. Ed. 2005, 117, 5213–5217. [Google Scholar] [CrossRef]
- Yu, F.; Tang, X.; Pei, M. Facile synthesis of PDMAEMA-coated hollow mesoporous silica nanoparticles and their pH-responsive controlled release. Micropor. Mesopor. Mater. 2013, 173, 64–69. [Google Scholar] [CrossRef]
- He, D.; He, X.; Wang, K.; Cao, J.; Zhao, Y. A light-responsive reversible molecule-gated system using thymine-modified mesoporous silica nanoparticles. Langmuir 2012, 28, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Angelos, S.; Choi, E.; Vögtle, F.; de Cola, L.; Zink, J.I. Photo-driven expulsion of molecules from mesostructured silica nanoparticles. J. Phys. Chem. C 2007, 111, 6589–6592. [Google Scholar] [CrossRef]
- Lu, J.; Choi, E.; Tamanoi, F.; Zink, J.I. Light-activated nanoimpeller-controlled drug release in cancer cells. Small 2008, 4, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.P.; Zhao, Y.L.; Khashab, N.M.; Khatib, H.A.; Stoddart, J.F.; Zink, J.I. Light-operated mechanized nanoparticles. J. Am. Chem. Soc. 2009, 131, 1686–1688. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bu, W.; Pan, L.; Shi, J.; Vivero-Escoto, J.L.; Slowing, I.I.; Wu, C.W.; Lin, V.S. Nir-triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica. Angew. Chem. Int. Ed. 2013, 52, 4375–4379. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Slowing, II; Wu, C.W.; Lin, V.S.Y. Photoinduced intracellular controlled release drug delivery in human cells by gold-capped mesoporous silica nanosphere. J. Am. Chem. Soc. 2009, 131, 3462–3463. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, F.; Sundararaju, B.; Surkus, A.E.; Boddien, A.; Loges, B.; Junge, H.; Dixneuf, P.H.; Beller, M. Light-driven hydrogen generation: Efficient iron-based water reduction catalysts. Angew. Chem. Int. Ed. 2009, 48, 9962–9965. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.Ž.; Lin, V.S.Y. A magnetic mesoporous silica nanoparticle-based drug delivery system for photosensitive cooperative treatment of cancer with a mesopore-capping agent and mesopore-loaded drug. Nanoscale 2013, 5, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rao, G.R.; Ista, L.K.; Wu, Y.; Andrzejewski, B.P.; Sklar, L.A.; Ward, TL.; López, G.P. Control of molecular transport through stimuli-responsive ordered mesoporous materials. Adv. Mater. 2003, 15, 1262–1266. [Google Scholar] [CrossRef]
- You, Y.Z.; Kalebaila, K.K.; Brock, S.L. Temperature-controlled uptake and release in PNIPAM-modified porous silica nanoparticles. Chem. Mater. 2008, 20, 3354–3359. [Google Scholar] [CrossRef]
- Sun, J.T.; Yu, Z.Q.; Hong, C.Y.; Pan, C.Y. Biocompatible zwitterionic sulfobetaine copolymer-coated mesoporous silica nanoparticles for temperature-responsive drug release. Macromol. Rapid. Commun. 2012, 33, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Karesoja, M.; McKee, J.; Karjalainen, E.; Hietala, S.; Bergman, L.; Linden, M.; Tenhu, H. Mesoporous silica particles grafted with poly(ethyleneoxide-block-N-vinylcaprolactam). J. Polym. Sci. Pol. Chem. 2013, 51, 5012–5020. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Zhu, D.; Zong, S.; Yang, L.; Zhong, Y.; Cui, Y. pH and thermo dual-stimuli-responsive drug carrier based on mesoporous silica nanoparticles encapsulated in a copolymer-lipid bilayer. ACS Appl. Mater. Interfaces 2013, 5, 10895–10903. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Hu, S.H.; Hsiao, C.S.; Chen, Y.Y.; Liu, D.M.; Chen, S.Y. Multifunctional magnetically removable nanogated lids of Fe3O4-capped mesoporous silica nanoparticles for intracellular controlled release and MR imaging. J. Mater. Chem. 2011, 21, 2535–2543. [Google Scholar] [CrossRef]
- Thomas, C.R.; Ferris, D.P.; Lee, J.H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.S.; Cheon, J.; Zink, J.I. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernandez, E.; Baeza, A.; Vallet-Regí, M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Angelos, S.; Dichtel, W.R.; Coskun, A.; Yang, Y.W.; Zink, J.I.; Stoddart, J.F. Enzyme-responsive snap-top covered silica nanocontainers. J. Am. Chem. Soc. 2008, 130, 2382–2383. [Google Scholar] [CrossRef] [PubMed]
- Schlossbauer, A.; Kecht, J.; Bein, T. Biotin-avidin as a protease-responsive cap system for controlled guest release from colloidal mesoporous silica. Angew. Chem. Int. Ed. 2009, 48, 3092–3095. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, H.; Kim, S.; Kim, C. Enzyme responsive nanocontainers with cyclodextrin gatekeepers and synergistic effects in release of guests. J. Am. Chem. Soc. 2009, 131, 16614–16615. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, A.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Barat, J.M.; Amorós, P. Enzyme-responsive controlled release using mesoporous silica supports capped with lactose. Angew. Chem. Int. Ed. 2009, 48, 5884–5887. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Karambelkar, A.; Gu, L.; Lin, K.; Miller, J.S.; Chen, C.S.; Sailor, M.J.; Bhatia, S.N. Bioresponsive mesoporous silica nanoparticles for triggered drug release. J. Am. Chem. Soc. 2011, 133, 19582–19585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Trewyn, B.G.; Slowing, II; Lin, V.S.Y. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, C.; He, C.; Zhu, W.; Xu, Y.; Lu, Y. A glucose-responsive controlled release system using glucose oxidase-gated mesoporous silica nanocontainers. Chem. Commum. 2012, 48, 9522–9524. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Soto, J.; Maquieira, A.; Amorós, P. Controlled delivery using oligonucleotide-capped mesoporous silica nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7281–7283. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Geng, J.; Pu, F.; Yang, X.; Ren, J.; Qu, X. Polyvalent nucleic acid/mesoporous silica nanoparticle conjugates: Dual stimuli-responsive vehicles for intracellular drug delivery. Angew. Chem. Int. Ed. 2011, 50, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Schlossbauer, A.; Warncke, S.; Gramlich, P.M.; Kecht, J.; Manetto, A.; Carell, T.; Bein, T. A programmable DNA-based molecular valve for colloidal mesoporous silica. Angew. Chem. Int. Ed. 2010, 49, 4734–4737. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pu, F.; Huang, Z.; Liu, Z.; Ren, J.; Qu, X. Stimuli-responsive controlled-release system using quadruplex DNA-capped silica nanocontainers. Nucl. Acids. Res. 2011, 39, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Di, J.; Cao, C.; Zhao, Y.; Ma, Y.; Luo, J.; Wen, Y.; Song, W.; Song, Y.; Jiang, L. A pH-driven DNA nanoswitch for responsive controlled release. Chem. Commun. 2011, 47, 2850–2852. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xu, L.; Wang, W.; Wang, D.; Du, H.; Zhang, X. Highly efficient remote controlled release system based on light-driven DNA nanomachine functionalized mesoporous silica. Nanoscale 2012, 4, 4473–4476. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, L.; Geng, J.; Ren, J.; Qu, X. Photosensitizer-incorporated quadruplex DNA-gated nanovechicles for light-triggered, targeted dual drug delivery to cancer cells. Small 2013, 9, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wen, Y.; Su, B.; Di, J.; Song, Y.; Jiang, L. Programmable DNA switch for bioresponsive controlled release. J. Mater. Chem. 2011, 21, 13811–13816. [Google Scholar] [CrossRef]
- He, X.; Zhao, Y.; He, D.; Wang, K.; Xu, F.; Tang, J. ATP-responsive controlled release system using aptamer-functionalized mesoporous silica nanoparticles. Langmuir 2012, 28, 12909–12915. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xu, L.; Li, C.; Du, H.; Chen, L.; Su, B.; Zhang, Z.; Zhang, X.; Song, Y. DNA-based intelligent logic controlled release systems. Chem. Commun. 2012, 48, 8410–8412. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Cai, K.; Hu, Y.; Zhao, L.; Liu, P.; Duan, L.; Yang, W. Mesoporous silica nanoparticles end-capped with collagen: Redox-responsive nanoreservoirs for targeted drug delivery. Angew. Chem. Int. Ed. 2011, 50, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.L.; Lu, C.H.; Song, X.Y.; Yang, H.H.; Wang, X.R. Bioresponsive controlled release using mesoporous silica nanoparticles capped with aptamer-based molecular gate. J. Am. Chem. Soc. 2011, 133, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cheng, F.; Zhou, R.; Cao, J.; Li, J.; Burda, C.; Min, Q.; Zhu, J.J. DNA-hybrid-gated multifunctional mesoporous silica nanocarriers for dual-targeted and microRNA-responsive controlled drug delivery. Angew. Chem. Int. Ed. 2014, 53, 2371–2375. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.F.; Nguyen, T.D.; Stoddart, J.F.; Zink, J.I. Supramolecular nanovalves controlled by proton abstraction and competitive binding. Chem. Mater. 2006, 18, 5919–5928. [Google Scholar] [CrossRef]
- Casasús, R.; Climent, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Amorós, P.; Cano, J.; Ruiz, E. Dual aperture control on pH- and anion-driven supramolecular nanoscopic hybrid gate-like ensembles. J. Am. Chem. Soc. 2008, 130, 1903–1917. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.N.; Sancenón, F.L.; Soto, J.; Amorós, P.; Guillem, C. pH- and photo-switched release of guest molecules from mesoporous silica supports. J. Am. Chem. Soc. 2009, 131, 6833–6843. [Google Scholar] [CrossRef] [PubMed]
- Angelos, S.; Yang, Y.W.; Khashab, N.M.; Stoddart, J.F.; Zink, J.I. Dual-controlled nanoparticles exhibiting and logic. J. Am. Chem. Soc. 2009, 131, 11344–11346. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wen, Y.; Xu, L.; Du, H.; Zhou, Y.; Zhang, X. A selective release system based on dual-drug-loaded mesoporous silica for nanoparticle-assisted combination therapy. Chem. Eur. J. 2014, 20, 7796–7802. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hao, X.; Wu, Y.; Zhang, J.; Zhang, X.; Wang, P.C.; Zou, G.; Liang, X.J. Multifunctional hybrid silica nanoparticles for controlled doxorubicin loading and release with thermal and pH dual response. J. Mater. Chem. B. 2013, 1, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, D.; Jin, C.; Song, X.; Cheng, J.; Zhao, X.; Qia, X.; Zhang, G. A dual responsive targeted drug delivery system based on smart polymer coated mesoporous silica for laryngeal carcinoma treatment. New J. Chem. 2014, 38, 4830–4836. [Google Scholar] [CrossRef]
- Chen, X.; Soeriyadi, A.H.; Lu, X.; Sagnella, S.M.; Kavallaris, M.; Gooding, J.J. Dual bioresponsive mesoporous silica nanocarrier as an “And” logic gate for targeted drug delivery cancer cells. Adv. Funct. Mater. 2014, 24, 6999–7006. [Google Scholar] [CrossRef]
- Hakeem, A.; Duan, R.; Zahid, F.; Dong, C.; Wang, B.; Hong, F.; Ou, X.; Jia, Y.; Lou, X.; Xia, F. Dual stimuli-responsive nano-vehicles for controlled drug delivery: Mesoporous silica nanoparticles end-capped with natural chitosan. Chem. Commun. 2014, 50, 13268–13271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Feng, P. Multiresponsive supramolecular nanogated ensembles. J. Am. Chem. Soc. 2009, 131, 15128–15129. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.; Zink, J.I. Nanovalve-controlled cargo release activated by plasmonic heating. J. Am. Chem. Soc. 2012, 134, 7628–7631. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Teh, C.; Sreejith, S.; Zhu, L.; Kwok, A.; Fang, W.; Ma, X.; Nguyen, K.T.; Korzh, V.; Zhao, Y. Functional mesoporous silica nanoparticles for photothermal-controlled drug delivery in vivo. Angew. Chem. Int. Ed. 2012, 51, 8373–8377. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Guisasola, E.; Ruiz-Hernández, E.; Vallet-Regí, M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Dong, L.; Peng, H.; Wang, S.; Zhang, Z.; Li, J.; Ai, F.; Qjao, Z.; Luo, M.; Xiong, H.; Chen, L. Thermally and magnetically dual-responsive mesoporous silica nanospheres: Preparation, characterization, and properties for the controlled release of sophoridine. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Su, B.; Wen, Y.; Li, C.; Zhou, Y.; Li, M.; Shi, X.; Du, H.; Song, Y.; et al. A light-responsive release platform by controlling the wetting behavior of hydrophobic surface. ACS Nano 2014, 8, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, L.; Xu, L.P.; Du, H.; Wen, Y.; Song, Y.; Zhang, X. A free-blockage controlled release system based on the hydrophobic/hydrophilic conversion of mesoporous silica nanopores. Chem. Eur. J. 2015, 21, 2680–2685. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Liu, Z.; Pu, F.; Ren, J.; Qu, X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv. Mater. 2012, 24, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Wang, J.; Jiang, X.; Li, X.; Hu, Z.; Ji, Y.; Wu, X.; Chen, C. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv. Mater. 2012, 24, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Lai, N.C.; Chuang, Y.J.; Chou, F.I.; Yang, C.M.; Lin, C.C. Trivalent galactosyl-functionalized mesoporous silica nanoparticles as a target-specific delivery system for boron neutron capture therapy. Nanoscale 2013, 5, 9412–9418. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, M.; Zeng, Y.; Zhang, D.; Zheng, A.; Liu, X.; Liu, J. Multifunctional PEG modified Dox loaded mesoporous silica nanoparticle@CuS nanohybrids as photo-thermal agent and thermal-triggered drug release vehicle for hepatocellular carcinoma treatment. Nanotechnology 2015, 26, 025102. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Cheng, Z.; Yang, D.; Ma, P.A.; Shang, M.; Peng, C.; Dai, Y.; Lin, J. Design and synthesis of multifunctional drug carriers based on luminescent rattle-type mesoporous silica microspheres with a thermosensitive hydrogel as a controlled switch. Adv. Funct. Mater. 2012, 22, 1470–1481. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Q.; Ji, X.; Zhang, S.; Chen, H.; Zheng, Y.; Sun, Y.; Qu, H.; Wang, Z.; Li, Y.; et al. Manganese oxide-based multifunctionalized mesoporous silica nanoparticles for pH-responsive MRI, ultrasonography and circumvention of MDR in cancer cells. Biomaterials 2012, 33, 7126–7137. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, J.; Gong, J.; Zheng, N. Photo- and pH-triggered release of anticancer drugs from mesoporous silica-coated Pd@Ag nanoparticles. Adv. Funct. Mater. 2012, 22, 842–848. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Kim, S.; Lee, H.; Kim, J.; Kim, N.; Park, H.J.; Choi, E.K.; Lee, J.S.; Kim, C. A multifunctional mesoporous nanocontainer with an iron oxide core and a cyclodextrin gatekeeper for an efficient theranostic platform. J. Mater. Chem. 2012, 22, 14061–14067. [Google Scholar] [CrossRef]
- Wang, C.; Lv, P.; Wei, W.; Tao, S.; Hu, T.; Yang, J.; Meng, C. A smart multifunctional nanocomposite for intracellular targeted drug delivery and self-release. Nanotechnology 2011, 22, 415101–415108. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tang, L.; An, L.; Wang, X.; Zhang, H.; Shi, J.; Yang, S. pH-responsive magnetic mesoporous silica nanospheres for magnetic resonance imaging and drug delivery. React. Funct. Polym. 2012, 72, 329–336. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Slowing, II; Lin, V.S.Y. Tuning the release of anticancer drugs from magnetic iron oxide/mesoporous silica core/shell nanoparticles. ChemPlusChem 2012, 77, 48–55. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Wang, W.; Wen, Y.; Zhang, X. Recent Advance on Mesoporous Silica Nanoparticles-Based Controlled Release System: Intelligent Switches Open up New Horizon. Nanomaterials 2015, 5, 2019-2053. https://doi.org/10.3390/nano5042019
Sun R, Wang W, Wen Y, Zhang X. Recent Advance on Mesoporous Silica Nanoparticles-Based Controlled Release System: Intelligent Switches Open up New Horizon. Nanomaterials. 2015; 5(4):2019-2053. https://doi.org/10.3390/nano5042019
Chicago/Turabian StyleSun, Ruijuan, Wenqian Wang, Yongqiang Wen, and Xueji Zhang. 2015. "Recent Advance on Mesoporous Silica Nanoparticles-Based Controlled Release System: Intelligent Switches Open up New Horizon" Nanomaterials 5, no. 4: 2019-2053. https://doi.org/10.3390/nano5042019
APA StyleSun, R., Wang, W., Wen, Y., & Zhang, X. (2015). Recent Advance on Mesoporous Silica Nanoparticles-Based Controlled Release System: Intelligent Switches Open up New Horizon. Nanomaterials, 5(4), 2019-2053. https://doi.org/10.3390/nano5042019






