Near-Infrared Fluorescent Nanoprobes for in Vivo Optical Imaging
Abstract
:1. Introduction
| Modality | Resolution | Depth | Optimal use | Signal | Training/ expertise required | Cost + |
|---|---|---|---|---|---|---|
| MRI | 10–100 μm | No limit | Anatomical assessment, investigation of physiological, metabolic, molecular and genetic events. | RF * waves (Nonionizing radiation) | Yes (Certified radiologists) | $$$ |
| PET | 0.8–1.4 mm | No limit | Investigation of physiological, metabolic, molecular and genetic events. | γ-rays (Ionizing radiation) | Yes (Certified radiologists) | $$$ |
| SPECT | 0.8–1.4 mm | No limit | Investigation of physiological, metabolic, molecular and genetic events. | γ-rays (Ionizing radiation) | Yes (Certified radiologists) | $$ |
| CT | 50 μm | No limit | Anatomical assessment. | X-ray (Ionizing radiation) | Yes (Certified radiologists) | $$ |
| Ultrasound | 50 μm | mm | Anatomical assessment, investigation of physiological, metabolic, molecular and genetic events. | Sound waves (Nonionizing radiation) | Yes (Certified sonographers) | $$ |
| Fluorescence optical imaging | 0.3 µm | <1 cm | Metabolic, molecular and genetic events. | Light waves (Nonionizing radiation) | No | $ |
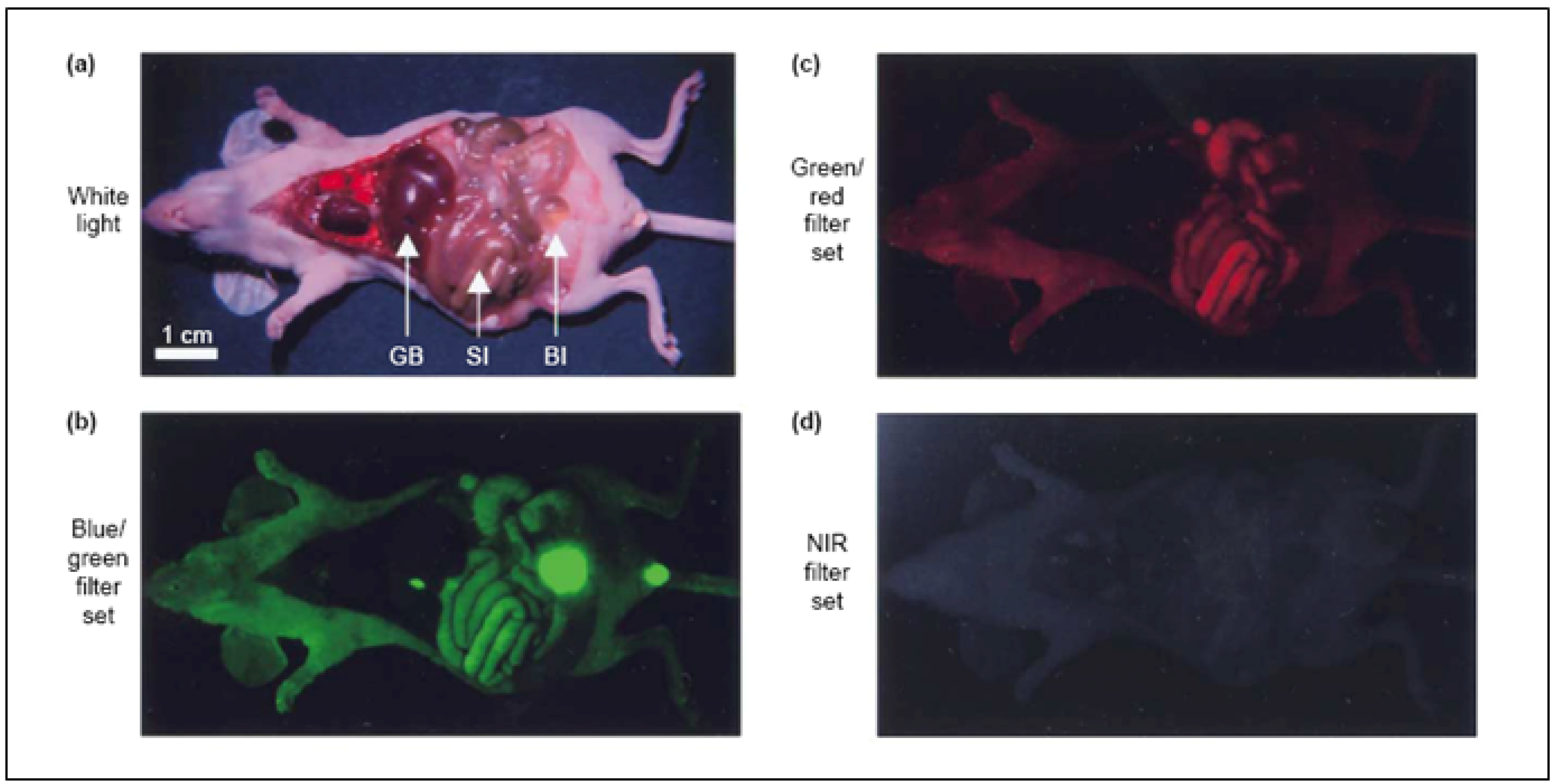
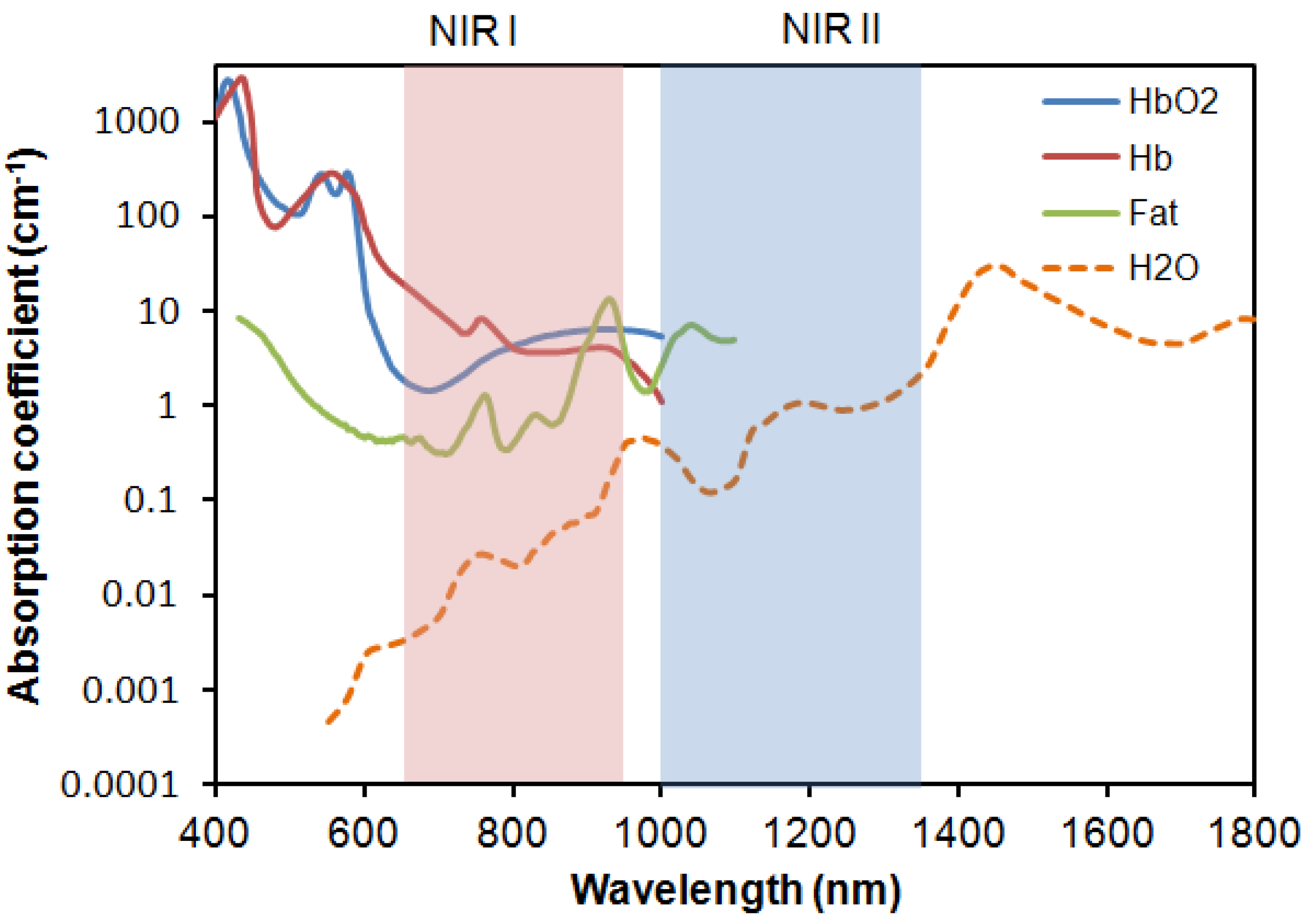
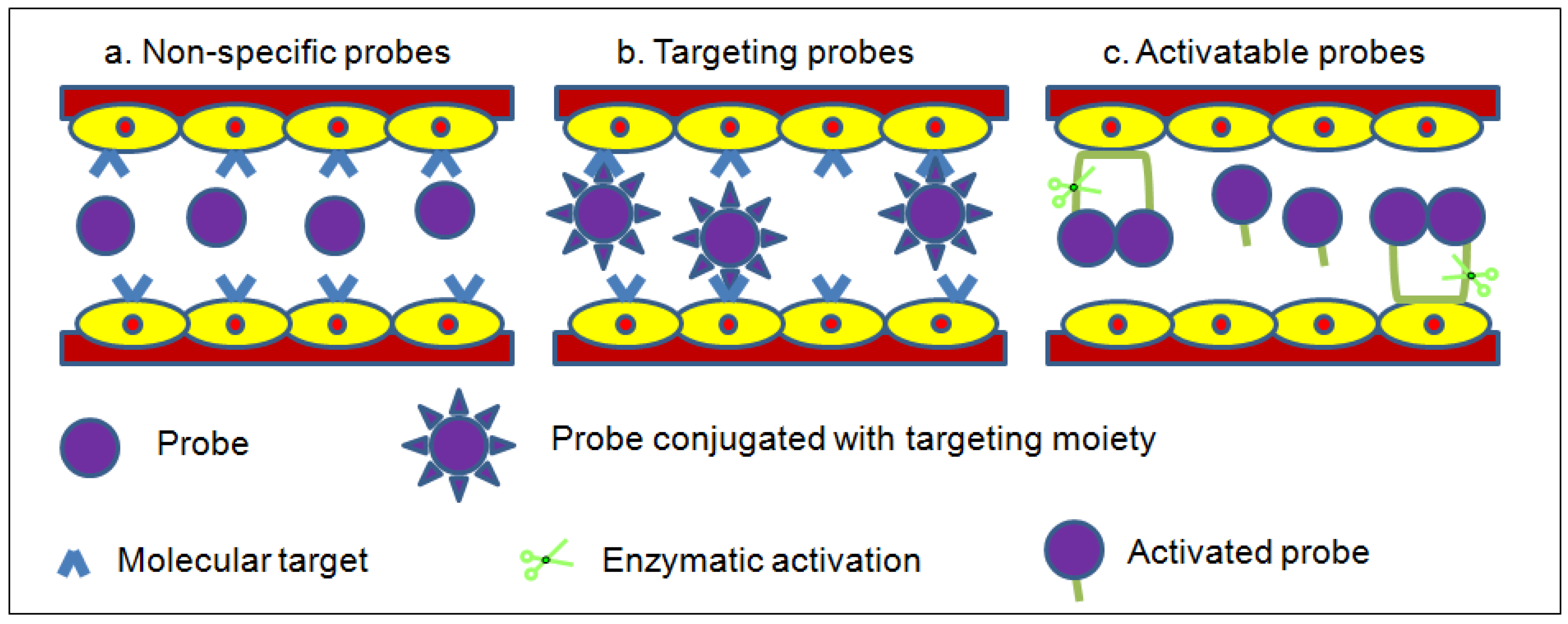
2. Labeling Mechanism of Fluorescent Probes
3. Small Organic Fluorophores
3.1. Non-Specific Organic-Dye Probes
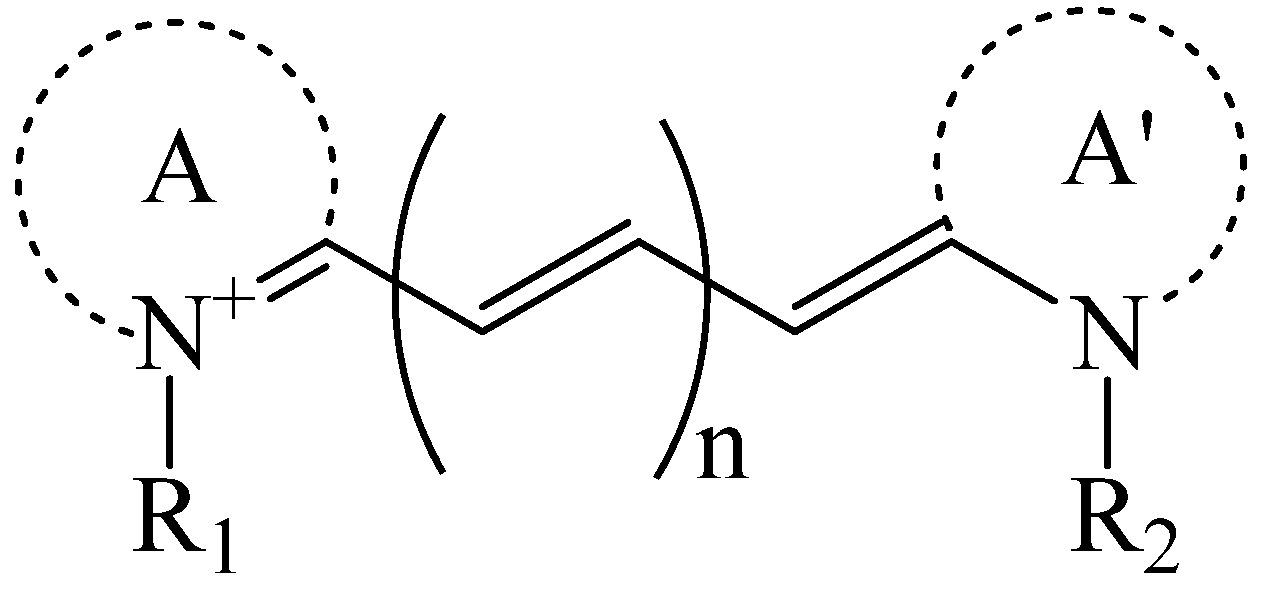
3.2. Targeting Organic-Dye Probes
| Dye | Structure | Abs/Em (nm) | Molar extinction coefficient (M−1 cm−1) | Quantum yield |
|---|---|---|---|---|
| Indo-Cyanine Green (ICG) [17] |  | 807/822 | 121,000 | 0.09 |
| Cy5.5 NHS ester ∗ | 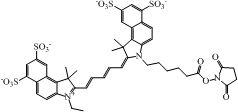 | 675/694 | 250,000 | 0.23 |
| Cy7 NHS ester ∗ | 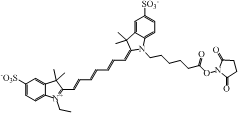 | 747/774 | 200,000 | 0.28 |
| Cy7.5 NHS ester § |  | 788/808 | 223,000 | N.A. |
3.3. Activatable Organic-Dye Probes
4. Nanomaterial-Based Fluorescent Probes
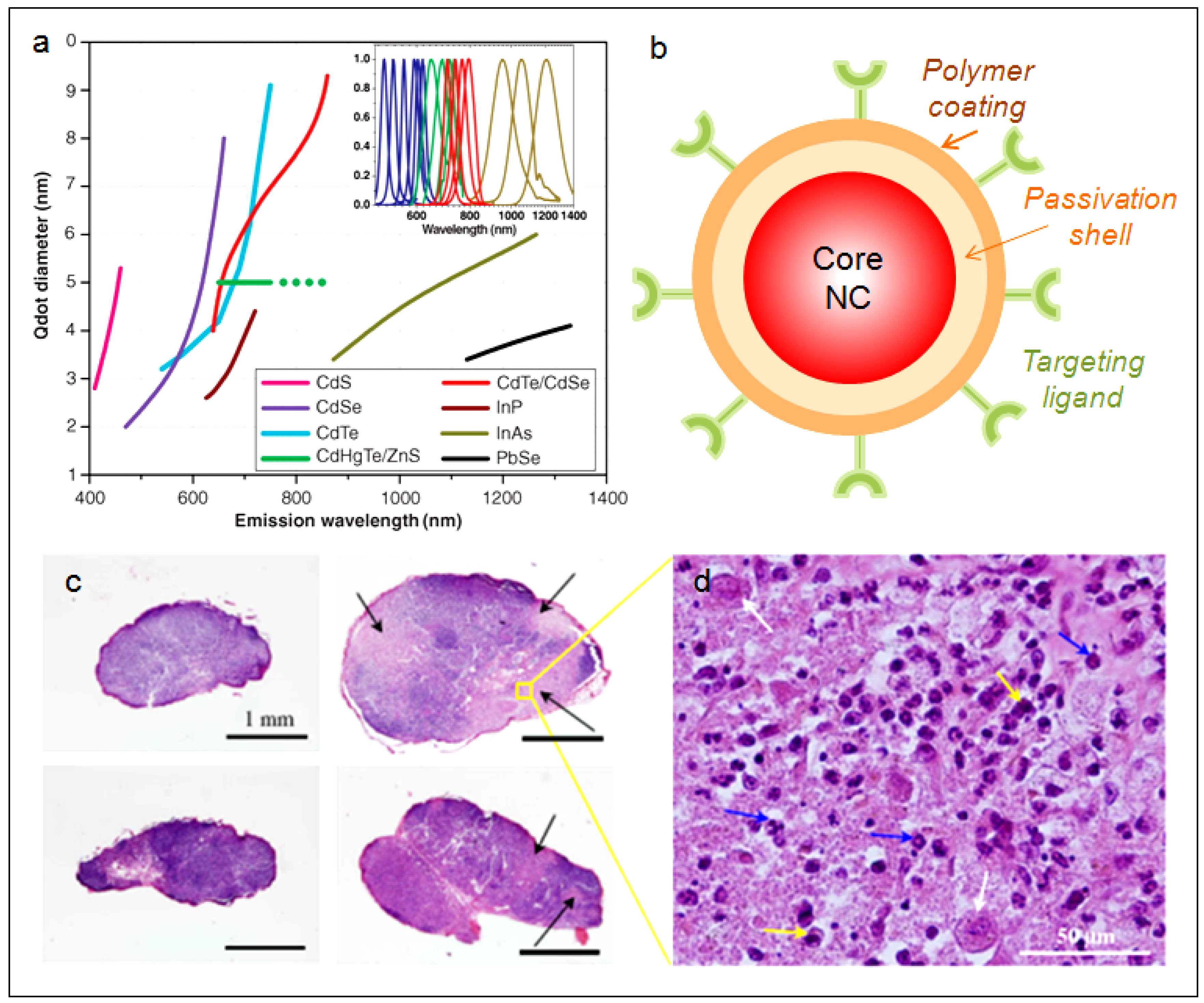
4.1. Quantum Dots
4.2. Colloidal Silicon Quantum Dots

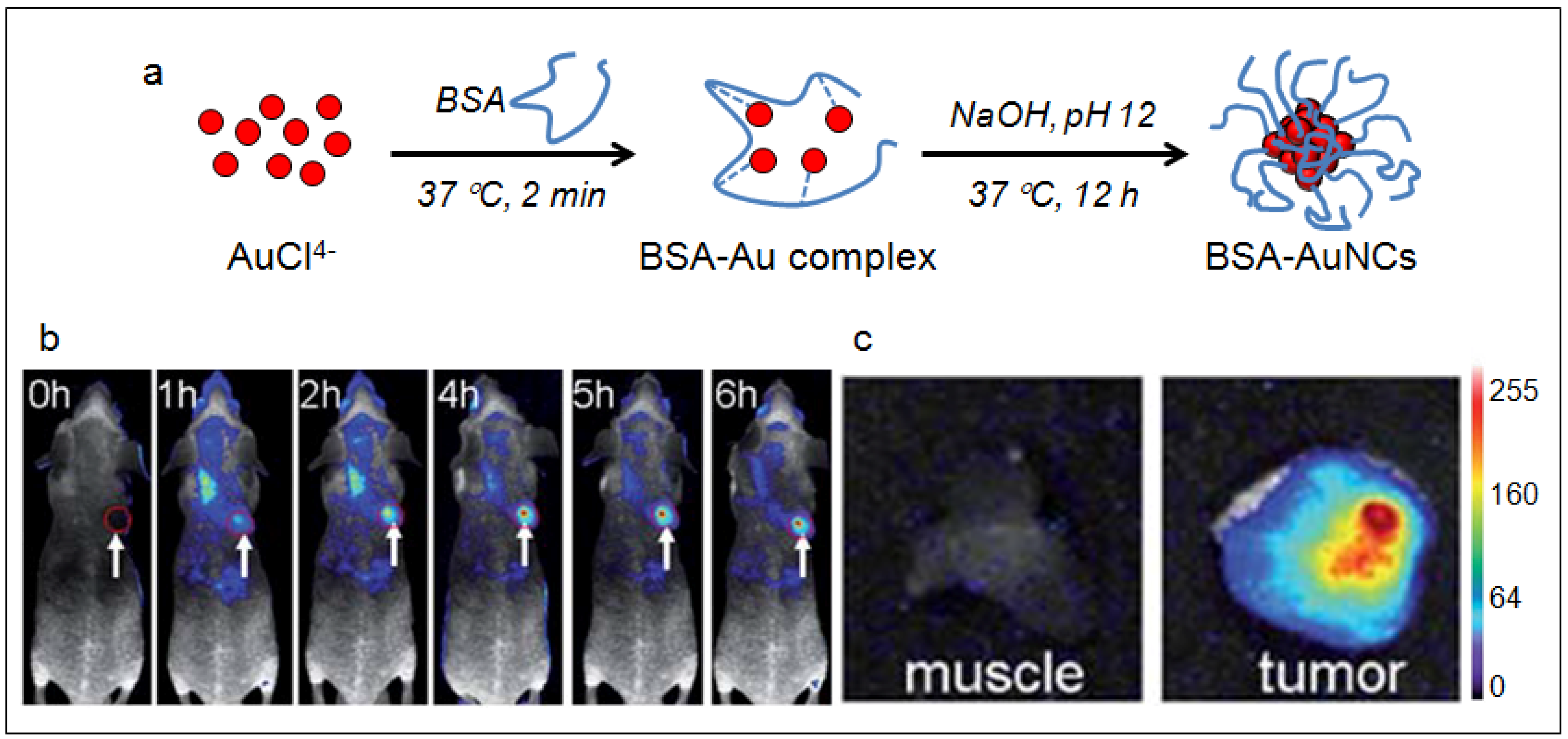
4.3. Gold Nanoclusters
5. Carbon Materials
5.1. Single-Walled Carbon Nanotubes
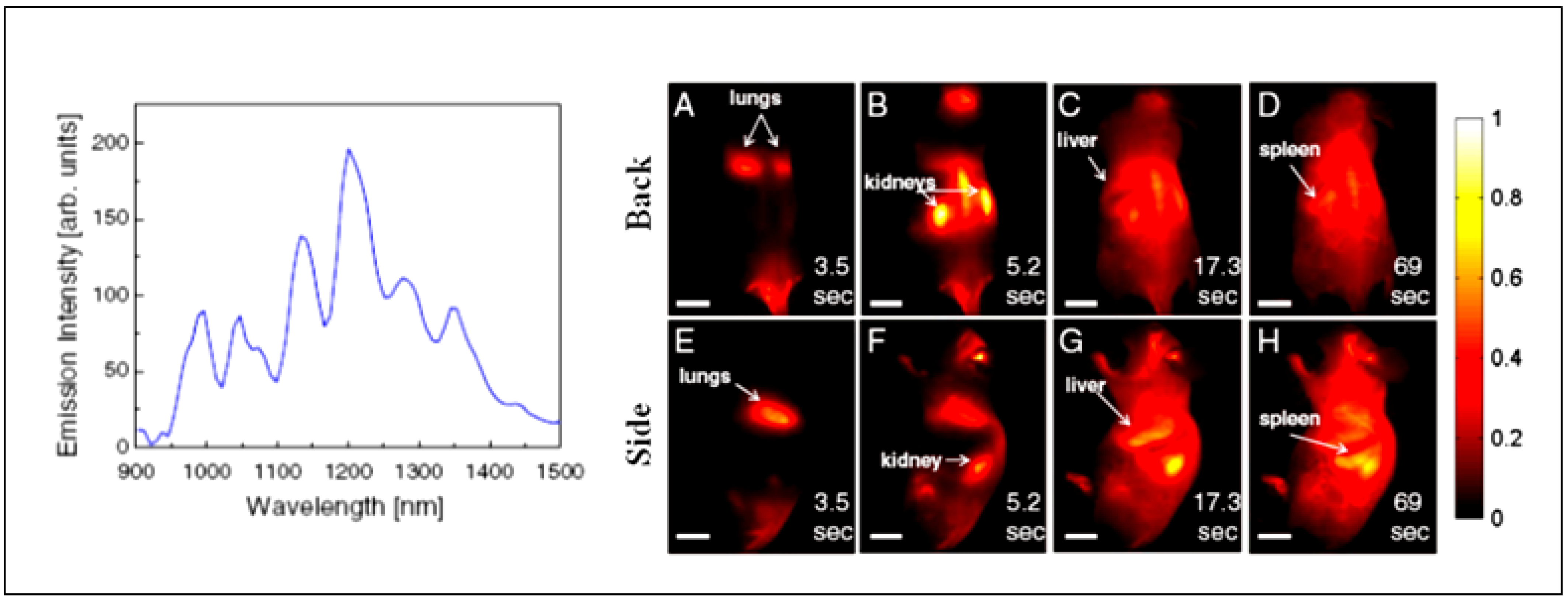
6. Conclusion and Outlook
Acknowledgment
References
- Kevles, B. Naked to the Bone: Medical Imaging in the Twentieth Century; Sloan Technology Series; Rutgers; Rutgers University Press: New Brunswick, NJ, Canada, 1997. [Google Scholar]
- Rudin, M.; Weissleder, R. Molecular imaging in drug discovery and development. Nat. Rev. Drug Discov. 2003, 2, 123–131. [Google Scholar] [CrossRef]
- Massoud, T.F.; Gambhir, S.S. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Gene. Dev. 2003, 17, 545–580. [Google Scholar] [CrossRef]
- Hargreaves, R.J. The role of molecular imaging in drug discovery and development. Clin. Pharmacol. Ther. 2008, 83, 349–353. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Weissleder, R. Scaling down imaging: Molecular mapping of cancer in mice. Nat. Rev. Cancer 2002, 2, 11–18. [Google Scholar] [CrossRef]
- Contag, P.R. Whole-animal cellular and molecular imaging to accelerate drug development. Drug Discov. Today 2002, 7, 555–562. [Google Scholar] [CrossRef]
- Gross, S.; Piwnica-Worms, D. Molecular imaging strategies for drug discovery and development. Curr. Opin. Chem. Biol. 2006, 10, 334–342. [Google Scholar] [CrossRef]
- Rudin, M. Noninvasive structural, functional, and molecular imaging in drug development. Curr. Opin. Chem. Biol. 2009, 13, 360–371. [Google Scholar] [CrossRef]
- Dufort, S.; Sancey, L.; Wenk, C.; Josserand, V.; Coll, J.L. Optical small animal imaging in the drug discovery process. BBA. Biomembranes 1798, 2266–2273. [Google Scholar]
- Sivaraman, D.; Biswas, P.; Cella, L.N.; Yates, M.V.; Chen, W. Detecting RNA viruses in living mammalian cells by fluorescence microscopy. Trends Biotech. 2011, 29, 307–313. [Google Scholar] [CrossRef]
- Koba, W.; Kim, K.; Lipton, M.L.; Jelicks, L.; Das, B.; Herbst, L.; Fine, E. Imaging devices for use in small animals. Semin. Nucl. Med. 2011, 41, 151–165. [Google Scholar] [CrossRef]
- Hickson, J. In vivo optical imaging: Preclinical applications and considerations. Urol. Oncol. Semin. Orig. Investi. 2009, 27, 295–297. [Google Scholar] [CrossRef]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S.M. BIOIMAGING: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef]
- Gioux, S.; Choi, H.S.; Frangioni, J.V. Image-guided surgery using invisible near-infrared light: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 237–255. [Google Scholar]
- Schaafsma, B.E.; Mieog, J.S.D.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.K.; Lowik, C.; Frangioni, J.V.; van de Velde, C.J.H.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A review. Chem.Rev. 2000, 100, 1973–2011. [Google Scholar]
- Licha, K. Contrast agents for optical imaging. In Contrast Agents Ii; Krause, W., Ed.; Springer-Verlag: Berlin, Germany, 2002; pp. 1–29. [Google Scholar]
- Kim, D.E.; Jaffer, F.A.; Weissleder, R.; Tung, C.H.; Schellingerhout, D. Near-infrared fluorescent imaging of cerebral thrombi and blood-brain barrier disruption in a mouse model of cerebral venous sinus thrombosis. J. Cerebr. Blood Flow Metabol. 2005, 25, 226–233. [Google Scholar] [CrossRef]
- Liu, X.; Guo, X.L.; Liu, F.; Zhang, Y.; Zhang, H.; Hu, G.S.; Bai, J. Imaging of indocyanine green perfusion in mouse liver with fluorescence diffuse optical tomography. IEEE Trans. Biomed. Eng. 2011, 58, 2139–2143. [Google Scholar] [CrossRef]
- Herbort, C.P.; LeHoang, P.; Guex-Crosier, Y. Schematic interpretation of indocyanine green angiography in posterior uveitis using a standard angiographic protocol. Ophthalmology 1998, 105, 432–440. [Google Scholar] [CrossRef]
- Achilefu, S.; Dorshow, R.B. Dynamic and continuous monitoring of renal and hepatic functions with exogenous markers. Contrast Agent. II 2002, 222, 31–72. [Google Scholar] [CrossRef]
- Liebert, A.; Wabnitz, H.; Obrig, H.; Erdmann, R.; Moller, M.; Macdonald, R.; Rinneberg, H.; Villringer, A.; Steinbrink, J. Non-invasive detection of fluorescence from exogenous chromophores in the adult human brain. Neuroimage 2006, 31, 600–608. [Google Scholar] [CrossRef]
- Liebert, A.; Sawosz, P.; Milej, D.; Kacprzak, M.; Weigl, W.; Botwicz, M.; Maczewska, J.; Fronczewska, K.; Mayzner-Zawadzka, E.; Krolicki, L.; Maniewski, R. Assessment of inflow and washout of indocyanine green in the adult human brain by monitoring of diffuse reflectance at large source-detector separation. J. Biomed. Opt. 2011, 16, 046011. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sasaguri, S.; Sato, T. Assessing intraoperative blood flow in cardiovascular surgery. Surg. Today 2011, 41, 1467–1474. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Gerlach, R.; Zimmermann, M.; Seifert, V. Near-infrared indocyanine green video angiography: A new method for intraoperative assessment of vascular flow. Neurosurgery 2003, 52, 132–139. [Google Scholar]
- Taggart, D.P.; Choudhary, B.; Anastasiadis, K.; Abu-Omar, Y.; Balacumaraswami, L.; Pigott, D.W. Preliminary experience with a novel intraoperative fluorescence imaging technique to evaluate the patency of bypass grafts in total arterial revascularization. Ann. Thorac. Surg. 2003, 75, 870–873. [Google Scholar] [CrossRef]
- Folli, S.; Westermann, P.; Braichotte, D.; Pelegrin, A.; Wagnieres, G.; Vandenbergh, H.; Mach, J.P. Antibody-indocyanin conjugates for immunophotodetection of human squamous-cell carcinoma in nude-mice. Cancer Res. 1994, 54, 2643–2649. [Google Scholar]
- Ballou, B.; Fisher, G.W.; Waggoner, A.S.; Farkas, D.L.; Reiland, J.M.; Jaffe, R.; Mujumdar, R.B.; Mujumdar, S.R.; Hakala, T.R. Tumor labeling in-vivo using cyanine-conjugated monoclonal-antibodies. Cancer Immunol. Immunother. 1995, 41, 257–263. [Google Scholar] [CrossRef]
- Ballou, B.; Fisher, G.W.; Hakala, T.R.; Farkas, D.L. Tumor detection and visualization using cyanine fluorochrome-labeled antibodies. Biotechnol. Progr. 1997, 13, 649–658. [Google Scholar] [CrossRef]
- Neri, D.; Carnemolla, B.; Nissim, A.; Leprini, A.; Querze, G.; Balza, E.; Pini, A.; Tarli, L.; Halin, C.; Neri, P.; Zardi, L.; Winter, G. Targeting by affinity-matured recombinant antibody fragments of an angiogenesis associated fibronectin isoform. Nat. Biotechnol. 1997, 15, 1271–1275. [Google Scholar]
- Birchler, M.; Neri, G.; Tarli, L.; Halin, C.; Viti, F.; Neri, D. Infrared photodetection for the in vivo localisation of phage-derived antibodies directed against angiogenic markers. J. Immunol.Method. 1999, 231, 239–248. [Google Scholar] [CrossRef]
- Bugaj, J.E.; Achilefu, S.; Dorshow, R.B.; Rajagopalan, R. Novel fluorescent contrast agents for optical imaging of in vivo tumors based on a receptor-targeted dye-peptide conjugate platform. J. Biomed.Opt. 2001, 6, 122–133. [Google Scholar] [CrossRef]
- Achilefu, S.; Jimenez, H.N.; Dorshow, R.B.; Bugaj, J.E.; Webb, E.G.; Wilhelm, R.R.; Rajagopalan, R.; Johler, J.; Erion, J.L. Synthesis, in vitro receptor binding, and in vivo evaluation of fluorescein and carbocyanine peptide-based optical contrast agents. J. Med. Chem. 2002, 45, 2003–2015. [Google Scholar] [CrossRef]
- Becker, A.; Hessenius, C.; Licha, K.; Ebert, B.; Sukowski, U.; Semmler, W.; Wiedenmann, B.; Grotzinger, C. Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat. Biotechnol. 2001, 19, 327–331. [Google Scholar] [CrossRef]
- Weissleder, R.; Tung, C.H.; Mahmood, U.; Bogdanov, A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999, 17, 375–378. [Google Scholar] [CrossRef]
- Bullok, K.E.; Maxwell, D.; Kesarwala, A.H.; Gammon, S.; Prior, J.L.; Snow, M.; Stanley, S.; Piwnica-Worms, D. Biochemical and in vivo characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. Biochemistry 2007, 46, 4055–4065. [Google Scholar] [CrossRef]
- Edgington, L.E.; Berger, A.B.; Blum, G.; Albrow, V.E.; Paulick, M.G.; Lineberry, N.; Bogyo, M. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nature Med. 2009, 15, 967–973. [Google Scholar]
- Bremer, C.; Ntziachristos, V.; Weissleder, R. Optical-based molecular imaging: Contrast agents and potential medical applications. European Radiol. 2003, 13, 231–243. [Google Scholar]
- Figueiredo, J.L.; Alencar, H.; Weissleder, R.; Mahmood, U. Near infrared thoracoscopy of tumoral protease activity for improved detection of peripheral lung cancer. Int. J. Cancer 2006, 118, 2672–2677. [Google Scholar] [CrossRef]
- Blum, G.; von Degenfeld, G.; Merchant, M.J.; Blau, H.M.; Bogyo, M. Noninvasive optical imaging of cysteine protease activity using fluorescently quenched activity-based probes. Nat. Chem. Biol. 2007, 3, 668–677. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nature Med. 2003, 9, 123–128. [Google Scholar]
- Marten, K.; Bremer, C.; Khazaie, K.; Sameni, M.; Sloane, B.; Tung, C.H.; Weissleder, R. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology 2002, 122, 406–414. [Google Scholar] [CrossRef]
- Escobedo, J.O.; Rusin, O.; Lim, S.; Strongin, R.M. NIR dyes for bioimaging applications. Curr. Opin. Chem. Biol. 2010, 14, 64–70. [Google Scholar] [CrossRef]
- Lordi, V.; Yao, N.; Wei, J. Method for supporting platinum on single-walled carbon nanotubes for a selective hydrogenation catalyst. Chem. Mater. 2001, 13, 733–737. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar]
- Fischer, J.E.; Dai, H.; Thess, A.; Lee, R.; Hanjani, N.M.; Dehaas, D.L.; Smalley, R.E. Metallic resistivity in crystalline ropes of single-wall carbon nanotubes. Phys. Rev. B 1997, 55, R4921–R4924. [Google Scholar]
- Porti, M.; Blasco, X.; Nafria, M.; Aymerich, X. Electrical characterization and fabrication of SiO2 based metal-oxide-semiconductor nanoelectronic devices with atomic force microscopy. Nanotechnology 2003, 14, 584–587. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar]
- Buhro, W.E.; Colvin, V.L. Semiconductor nanocrystals—Shape matters. Nat. Mater. 2003, 2, 138–139. [Google Scholar] [CrossRef]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar]
- Alivisatos, P. The use of nanocrystals in biological detection. Nat. Biotechnol. 2004, 22, 47–52. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Maxwell, D.J.; Gao, X.H.; Bailey, R.E.; Han, M.Y.; Nie, S.M. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar]
- Clarke, S.J.; Hollmann, C.A.; Zhang, Z.J.; Suffern, D.; Bradforth, S.E.; Dimitrijevic, N.M.; Minarik, W.G.; Nadeau, J.L. Photophysics of dopamine-modified quantumdots and effects on biological systems. Nat. Mater. 2006, 5, 409–417. [Google Scholar]
- Li, J.B.; Wu, D.D.; Miao, Z.R.; Zhang, Y. Preparation of quantum dot bioconjugates and their applications in bio-imaging. Curr. Pharm. Biotechnol. 2010, 11, 662–671. [Google Scholar] [CrossRef]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762. [Google Scholar]
- Gao, X.H.; Cui, Y.Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S.M. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chen, A.A.; Min, D.H.; Ruoslahti, E.; Bhatia, S.N. Targeted quantum dot conjugates for siRNA delivery. Bioconjug. Chem. 2007, 18, 1391–1396. [Google Scholar] [CrossRef]
- Tan, W.B.; Jiang, S.; Zhang, Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials 2007, 28, 1565–1571. [Google Scholar] [CrossRef]
- Lee, H.; Kim, I.K.; Park, T.G. Intracellular trafficking and unpacking of siRNA/quantum dot-PEI complexes modified with and without cell penetrating peptide: Confocal and flow cytometric FRET analysis. Bioconjug. Chem. 2010, 21, 289–295. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Cai, W.B. In vivo imaging of RNA interference. J. Nucl. Med. 2010, 51, 169–172. [Google Scholar] [CrossRef]
- Clapp, A.R.; Medintz, I.L.; Mattoussi, H. Forster resonance energy transfer investigations using quantum-dot fluorophores. ChemPhysChem 2006, 7, 47–57. [Google Scholar] [CrossRef]
- Chen, H.H.; Leong, K.W. Quantum-dots-FRET nanosensors for detecting unamplified nucleic acids by single molecule detection. Nanomedicine 2006, 1, 119–122. [Google Scholar] [CrossRef]
- Ho, Y.P.; Chen, H.H.; Leong, K.W.; Wang, T.H. Evaluating the intracellular stability and unpacking of DNA nanocomplexes by quantum dots-FRET. J. Control. Release 2006, 116, 83–89. [Google Scholar]
- Chen, H.H.; Ho, Y.P.; Jiang, X.; Mao, H.Q.; Wang, T.H.; Leong, K.W. Quantitative comparison of intracellular unpacking kinetics of polyplexes by a model constructed from quantum Dot-FRET. Mol. Ther. 2008, 16, 324–332. [Google Scholar] [CrossRef]
- Rieger, S.; Kulkarni, R.P.; Darcy, D.; Fraser, S.E.; Koster, R.W. Quantum dots are powerful multipurpose vital labeling agents in zebrafish embryos. Dev. Dynam. 2005, 234, 670–681. [Google Scholar]
- Slotkin, J.R.; Chakrabarti, L.; Dai, H.N.; Carney, R.S.E.; Hirata, T.; Bregman, B.S.; Gallicano, G.I.; Corbin, J.G.; Haydar, T.F. In vivo quantum dot labeling of mammalian stem and progenitor cells. Dev. Dynam. 2007, 236, 3393–3401. [Google Scholar] [CrossRef]
- Gaponik, N.; Rogach, A.L. Thiol-capped CdTe nanocrystals: Progress and perspectives of the related research fields. PCCP Phys. Chem. Chem. Phys. 2010, 12, 8685–8693. [Google Scholar] [CrossRef]
- Zintchenko, A.; Susha, A.S.; Concia, M.; Feldmann, J.; Wagner, E.; Rogach, A.L.; Ogris, M. Drug nanocarriers labeled with near-infrared-emitting quantum dots (quantoplexes): Imaging fast dynamics of distribution in living animals. Mol. Ther. 2009, 17, 1849–1856. [Google Scholar] [CrossRef]
- Kim, S.; Lim, Y.T.; Soltesz, E.G.; De Grand, A.M.; Lee, J.; Nakayama, A.; Parker, J.A.; Mihaljevic, T.; Laurence, R.G.; Dor, D.M.; Cohn, L.H.; Bawendi, M.G.; Frangioni, J.V. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004, 22, 93–97. [Google Scholar]
- Tsay, J.M.; Pflughoefft, M.; Bentolila, L.A.; Weiss, S. Hybrid approach to the synthesis of highly luminescent CdTe/ZnS and CdHgTe/ZnS nanocrystals. J. Am. Chem. Soc. 2004, 126, 1926–1927. [Google Scholar]
- Rogach, A.L.; Harrison, M.T.; Kershaw, S.V.; Kornowski, A.; Burt, M.G.; Eychmuller, A.; Weller, H. Colloidally prepared CdHgTe and HgTe quantum dots with strong near-infrared luminescence. Phys. Status Solid B Basic Re. 2001, 224, 153–158. [Google Scholar]
- Zimmer, J.P.; Kim, S.W.; Ohnishi, S.; Tanaka, E.; Frangioni, J.V.; Bawendi, M.G. Size series of small indium arsenide-zinc selenide core-shell nanocrystals and their application to in vivo imaging. J. Am. Chem. Soc. 2006, 128, 2526–2527. [Google Scholar]
- Kim, S.W.; Zimmer, J.P.; Ohnishi, S.; Tracy, J.B.; Frangioni, J.V.; Bawendi, M.G. Engineering InAsxP1-x/InP/ZnSe III-V alloyed core/shell quantum dots for the near-infrared. J. Am. Chem. Soc. 2005, 127, 10526–10532. [Google Scholar]
- Jasieniak, J.; Califano, M.; Watkins, S.E. Size-dependent valence and conduction band-edge energies of semiconductor nanocrystals. ACS Nano 2011, 5, 5888–5902. [Google Scholar] [CrossRef]
- Dai, Q.Q.; Wang, Y.N.; Li, X.B.; Zhang, Y.; Pellegrino, D.J.; Zhao, M.X.; Zou, B.; Seo, J.; Wang, Y.D.; Yu, W.W. Size-dependent composition and molar extinction coefficient of PbSe semiconductor nanocrystals. ACS Nano 2009, 3, 1518–1524. [Google Scholar]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Guo, G.N.; Liu, W.; Liang, J.G.; He, Z.K.; Xu, H.B.; Yang, X.L. Probing the cytotoxicity of CdSe quantum dots with surface modification. Mater. Lett. 2007, 61, 1641–1644. [Google Scholar] [CrossRef]
- Cho, S.J.; Maysinger, D.; Jain, M.; Roder, B.; Hackbarth, S.; Winnik, F.M. Long-term exposure to CdTe quantum dots causes functional impairments in live cells. Langmuir 2007, 23, 1974–1980. [Google Scholar]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 65–172. [Google Scholar]
- Kirchner, C.; Liedl, T.; Kudera, S.; Pellegrino, T.; Javier, A.M.; Gaub, H.E.; Stolzle, S.; Fertig, N.; Parak, W.J. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005, 5, 331–338. [Google Scholar]
- Xie, R.G.; Peng, X.G. Synthesis of Cu-doped InP nanocrystals (d-dots) with ZnSe diffusion barrier as efficient and color-tunable NIR emitters. J. Am. Chem. Soc. 2009, 131, 10645–10651. [Google Scholar]
- Allen, P.M.; Bawendi, M.G. Ternary I-III-VI quantum dots luminescent in the red to near-infrared. J. Am, Chem. Soc. 2008, 130, 9240–9241. [Google Scholar]
- Park, J.; Dvoracek, C.; Lee, K.H.; Galloway, J.F.; Bhang, H.E.C.; Pomper, M.G.; Searson, P.C. CuInSe/ZnS Core/Shell NIR quantum dots for biomedical imaging. Small 2011, 7, 3148–3152. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhong, X.H. Facile synthesis of ZnS-CuInS(2)-alloyed nanocrystals for a color-tunable fluorchrome and photocatalyst. Inorg. Chem. 2011, 50, 4065–4072. [Google Scholar]
- Pons, T.; Pic, E.; Lequeux, N.; Cassette, E.; Bezdetnaya, L.; Guillemin, F.; Marchal, F.; Dubertret, B. Cadmium-free CuInS(2)/ZnS quantum dots for sentinel lymph node imaging with reduced toxicity. ACS Nano 2010, 4, 2531–2538. [Google Scholar]
- Smith, A.M.; Dave, S.; Nie, S.M.; True, L.; Gao, X.H. Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev. Mol. Diagn. 2006, 6, 231–244. [Google Scholar] [CrossRef]
- Alivisatos, A.P.; Gu, W.W.; Larabell, C. Quantum dots as cellular probes. Ann. Rev. Biomed. Eng. 2005, 7, 55–76. [Google Scholar] [CrossRef]
- Uyeda, H.T.; Medintz, I.L.; Jaiswal, J.K.; Simon, S.M.; Mattoussi, H. Synthesis of compact multidentate ligands to prepare stable hydrophilic quantum dot fluorophores. J. Am. Chem. Soc. 2005, 127, 3870–3878. [Google Scholar]
- Carion, O.; Mahler, B.; Pons, T.; Dubertret, B. Synthesis, encapsulation, purification and coupling of single quantum dots in phospholipid micelles for their use in cellular and in vivo imagin. Nat. Protoc. 2007, 2, 2383–2390. [Google Scholar] [CrossRef]
- Pic, E.; Pons, T.; Bezdetnaya, L.; Leroux, A.; Guillemin, F.; Dubertret, B.; Marchall, F. Fluorescence imaging and whole-body biodistribution of near-infrared-emitting quantum dots after subcutaneous injection for regional lymph node mapping in mice. Mol. Imaging Biol. 2010, 12, 394–405. [Google Scholar] [CrossRef]
- Vogel, E.M. Technology and metrology of new electronic materials and devices. Nat. Nanotechnol. 2007, 2, 25–32. [Google Scholar] [CrossRef]
- Melinon, P.; Masenelli, B.; Tournus, F.; Perez, A. Playing with carbon and silicon at the nanoscale. Nat. Mater. 2007, 6, 479–490. [Google Scholar]
- Fujioka, K.; Hiruoka, M.; Sato, K.; Manabe, N.; Miyasaka, R.; Hanada, S.; Hoshino, A.; Tilley, R.D.; Manome, Y.; Hirakuri, K.; Yamamoto, K. Luminescent passive-oxidized silicon quantum dots as biological staining labels and their cytotoxicity effects at high concentration. Nanotechnology 2008, 19, 415102. [Google Scholar]
- Park, J.H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar]
- Erogbogbo, F.; Yong, K.T.; Roy, I.; Xu, G.X.; Prasad, P.N.; Swihart, M.T. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano 2008, 2, 873–878. [Google Scholar] [CrossRef]
- Erogbogbo, F.; Yong, K.T.; Hu, R.; Law, W.C.; Ding, H.; Chang, C.W.; Prasad, P.N.; Swihart, M.T. Biocompatible magnetofluorescent probes: Luminescent silicon quantum dots coupled with superparamagnetic iron(III) oxide. ACS Nano 2010, 4, 5131–5138. [Google Scholar]
- Li, X.G.; He, Y.Q.; Talukdar, S.S.; Swihart, M.T. Process for preparing macroscopic quantities of brightly photoluminescent silicon nanoparticles with emission spanning the visible spectrum. Langmuir 2003, 19, 8490–8496. [Google Scholar] [CrossRef]
- Hua, F.J.; Erogbogbo, F.; Swihart, M.T.; Ruckenstein, E. Organically capped silicon nanoparticles with blue photoluminescence prepared by hydrosilylation followed by oxidation. Langmuir 2006, 22, 4363–4370. [Google Scholar]
- Li, Z.F.; Ruckenstein, E. Water-soluble poly(acrylic acid) grafted luminescent silicon nanoparticles and their use as fluorescent biological staining labels. Nano Lett. 2004, 4, 1463–1467. [Google Scholar] [CrossRef]
- Sun, Y.; Balasubramanian, K.; Rao, T.U.B.; Pradeep, T. First principles studies of two luminescent molecular quantum clusters of silver, Ag(7)(H(2)MSA)(7) and Ag(8)(H(2)MSA)(8), based on experimental fluorescence spectra. J. Phys. Chem. C 2011, 115, 20380–20387. [Google Scholar]
- Yuan, X.; Luo, Z.T.; Zhang, Q.B.; Zhang, X.H.; Zheng, Y.G.; Lee, J.Y.; Xie, J.P. Synthesis of highly fluorescent metal (Ag, Au, Pt, and Cu) nanoclusters by electrostatically induced reversible phase transfer. ACS Nano 2011, 5, 8800–8808. [Google Scholar] [CrossRef]
- Selvam, T.S.; Chi, K.M. Synthesis of hydrophobic gold nanoclusters: Growth mechanism study, luminescence property and catalytic application. J. Nanopart. Res. 2011, 13, 1769–1780. [Google Scholar] [CrossRef]
- Devadas, M.S.; Kim, J.; Sinn, E.; Lee, D.; Goodson, T.; Ramakrishna, G. Unique ultrafast visible luminescence in monolayer-protected Au(25) clusters. J. Phys. Chem. C 2010, 114, 22417–22423. [Google Scholar]
- Wu, Z.K.; Jin, R.C. On the ligand’s role in the fluorescence of gold nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef]
- Guo, S.J.; Wang, E.K. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 2011, 6, 240–264. [Google Scholar] [CrossRef]
- Zheng, J.; Nicovich, P.R.; Dickson, R.M. Highly fluorescent noble-metal quantum dots, in Annual Review of Physical Chemistry. Annu. Rev. Phys. Chem. 2007, 58, 409–431. [Google Scholar]
- Shang, L.; Dong, S.J. Sensitive detection of cysteine based on fluorescent silver clusters. Biosens. Bioelectron. 2009, 24, 1569–1573. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, C.T.; Shiang, Y.C.; Lin, Z.H.; Chang, H.T. Synthesis of fluorescent carbohydrate-protected Au nanodots for detection of concanavalin A and escherichia coli. Anal. Chem. 2009, 81, 875–882. [Google Scholar]
- Wei, H.; Wang, Z.D.; Yang, L.M.; Tian, S.L.; Hou, C.J.; Lu, Y. Lysozyme-stabilized gold fluorescent cluster: Synthesis and application as Hg(2+) sensor. Analyst 2010, 135, 1406–1410. [Google Scholar] [CrossRef]
- Yu, J.; Patel, S.A.; Dickson, R.M. In vitro and intracellular production of peptide-encapsulated fluorescent silver nanoclusters. Angew. Chem. Int. Ed. 2007, 46, 2028–2030. [Google Scholar]
- Richards, C.I.; Choi, S.; Hsiang, J.C.; Antoku, Y.; Vosch, T.; Bongiorno, A.; Tzeng, Y.L.; Dickson, R.M. Oligonucleotide-stabilized Ag nanocluster fluorophores. J. Am. Chem. Soc. 2008, 130, 5038–5039. [Google Scholar]
- Yu, J.H.; Choi, S.; Dickson, R.M. Shuttle-based fluorogenic silver-cluster biolabels. Angew. Chem. Int. Ed. 2009, 48, 318–320. [Google Scholar]
- Negishi, Y.; Takasugi, Y.; Sato, S.; Yao, H.; Kimura, K.; Tsukuda, T. Magic-numbered Au-n clusters protected by glutathione monolayers (n = 18, 21, 25, 28, 32, 39): Isolation and spectroscopic characterization. J. Am. Chem. Soc. 2004, 126, 6518–6519. [Google Scholar]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-protected gold clusters revisited: Bridging the gap between gold(I)-thiolate complexes and thiolate-protected gold nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar]
- Negishi, Y.; Chaki, N.K.; Shichibu, Y.; Whetten, R.L.; Tsukuda, T. Origin of magic stability of thiolated gold clusters: A case study on Au-25(SC6H13)(18). J. Am. Chem. Soc. 2007, 129, 11322–11323. [Google Scholar]
- Wang, G.L.; Huang, T.; Murray, R.W.; Menard, L.; Nuzzo, R.G. Near-IR luminescence of monolayer-protected metal clusters. J. Am. Chem. Soc. 2005, 127, 812–813. [Google Scholar]
- Shichibu, Y.; Negishi, Y.; Tsunoyama, H.; Kanehara, M.; Teranishi, T.; Tsukuda, T. Extremely high stability of glutathionate-protected Au-25 clusters against core etching. Small 2007, 3, 835–839. [Google Scholar] [CrossRef]
- Duan, H.W.; Nie, S.M. Etching colloidal gold nanocrystals with hyperbranched and multivalent polymers: A new route to fluorescent and water-soluble atomic clusters. J. Am. Chem. Soc. 2007, 129, 2412–2413. [Google Scholar] [CrossRef]
- Jana, N.R.; Peng, X.G. Single-phase and gram-scale routes toward nearly monodisperse Au and other noble metal nanocrystals. J. Am. Chem. Soc. 2003, 125, 14280–14281. [Google Scholar]
- Lin, C.A.J.; Yang, T.Y.; Lee, C.H.; Huang, S.H.; Sperling, R.A.; Zanella, M.; Li, J.K.; Shen, J.L.; Wang, H.H.; Yeh, H.I.; Parak, W.J.; Chang, W.H. Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling application. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef]
- Bao, Y.; Yeh, H.C.; Zhong, C.; Ivanov, S.A.; Sharma, J.K.; Neidig, M.L.; Vu, D.M.; Shreve, A.P.; Dyer, R.B.; Werner, J.H.; Martinez, J.S. Formation and stabilization of fluorescent gold nanoclusters using small molecules. J. Phys. Chem. C 2010, 114, 15879–15882. [Google Scholar]
- Xie, J.P.; Zheng, Y.G.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar]
- Wu, X.; He, X.; Wang, K.; Xie, C.; Zhou, B.; Qing, Z. Ultrasmall near-infrared gold nanoclusters for tumor fluorescence imaging in vivo. Nanoscale 2010, 2, 2244–2249. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Feng, L.Z.; Liu, Z.A. Graphene in biomedicine: opportunities and challenges. Nanomedicine 2011, 6, 317–324. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H.J. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar] [CrossRef]
- Welsher, K.; Liu, Z.; Sherlock, S.P.; Robinson, J.T.; Chen, Z.; Daranciang, D.; Dai, H.J. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 2009, 4, 773–780. [Google Scholar] [CrossRef]
- Dai, H.J. Carbon nanotubes: Synthesis, integration, and propertie. Account. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef]
- Welsher, K.; Sherlock, S.P.; Dai, H.J. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Nat. Acad. Sci. USA 2011, 108, 8943–8948. [Google Scholar] [CrossRef]
© 2012 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Quek, C.-H.; Leong, K.W. Near-Infrared Fluorescent Nanoprobes for in Vivo Optical Imaging. Nanomaterials 2012, 2, 92-112. https://doi.org/10.3390/nano2020092
Quek C-H, Leong KW. Near-Infrared Fluorescent Nanoprobes for in Vivo Optical Imaging. Nanomaterials. 2012; 2(2):92-112. https://doi.org/10.3390/nano2020092
Chicago/Turabian StyleQuek, Chai-Hoon, and Kam W. Leong. 2012. "Near-Infrared Fluorescent Nanoprobes for in Vivo Optical Imaging" Nanomaterials 2, no. 2: 92-112. https://doi.org/10.3390/nano2020092
APA StyleQuek, C.-H., & Leong, K. W. (2012). Near-Infrared Fluorescent Nanoprobes for in Vivo Optical Imaging. Nanomaterials, 2(2), 92-112. https://doi.org/10.3390/nano2020092




