Aqua Regia-Free Removal of Cr-Pt Hard Masks Using Thin Ag or Au Sacrificial Layers for High-Fidelity LiTaO3 Metasurfaces
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
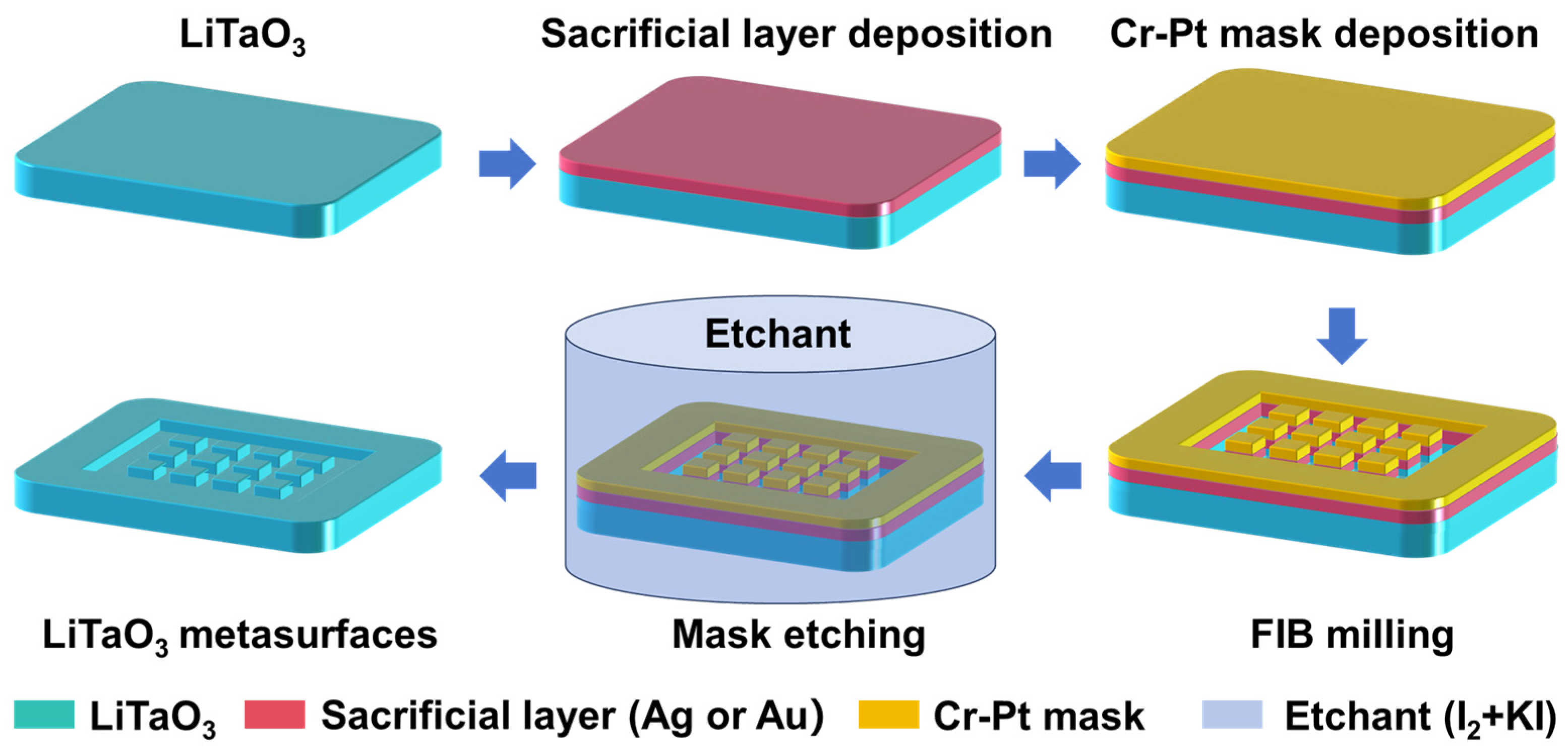
3.1. Concept Description
3.2. Mask Materials Survey
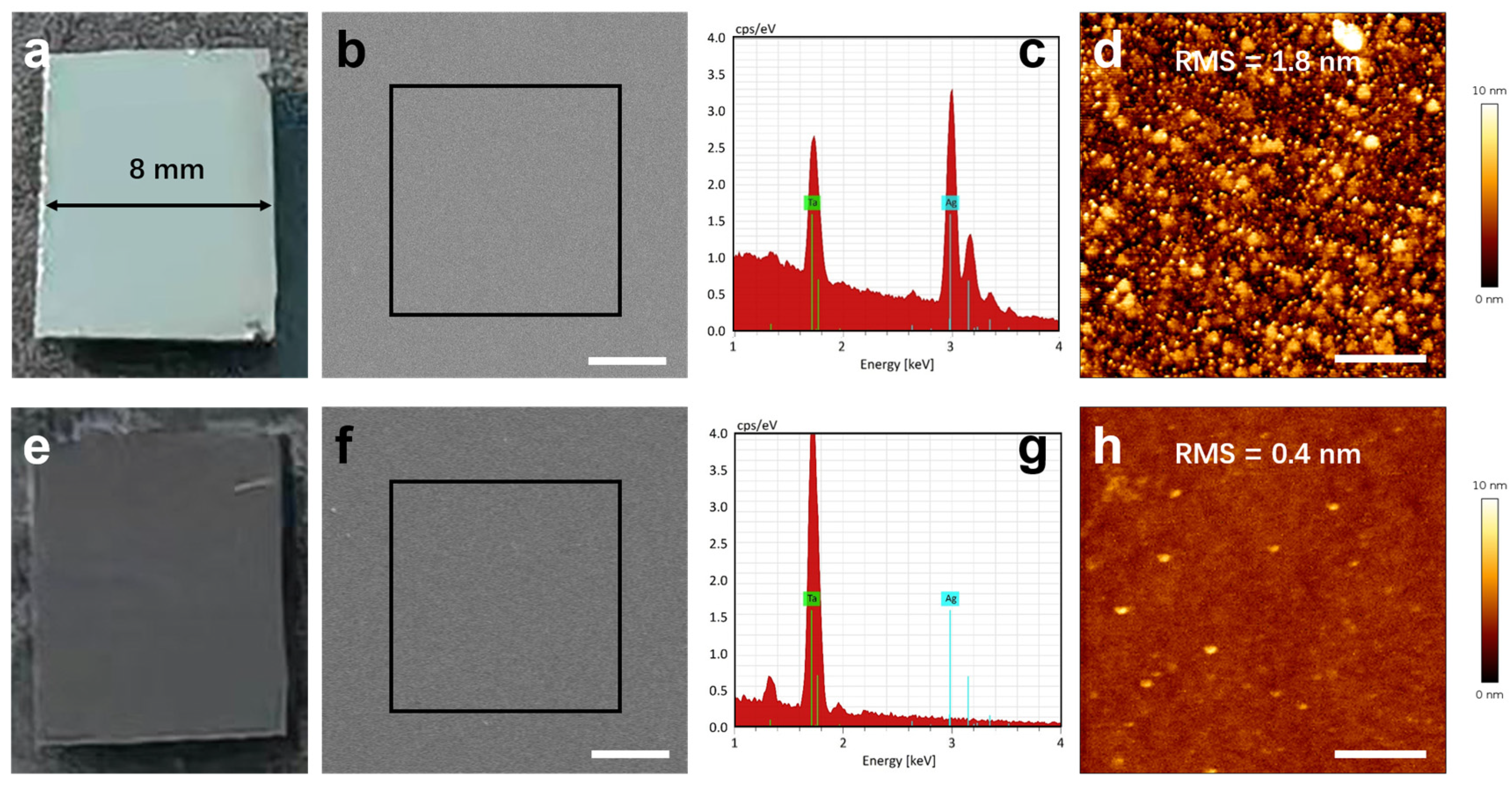
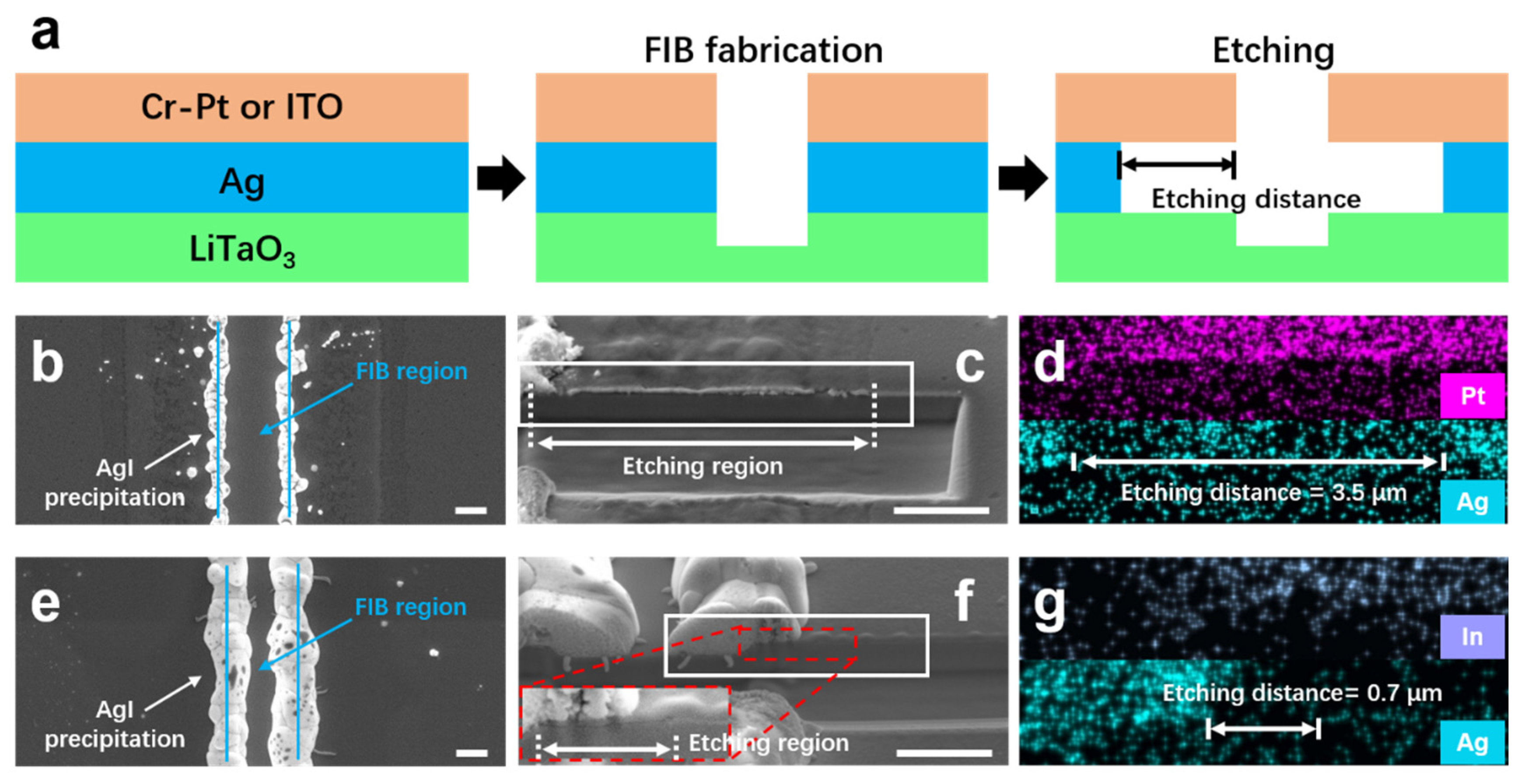
3.3. Experimental Validation
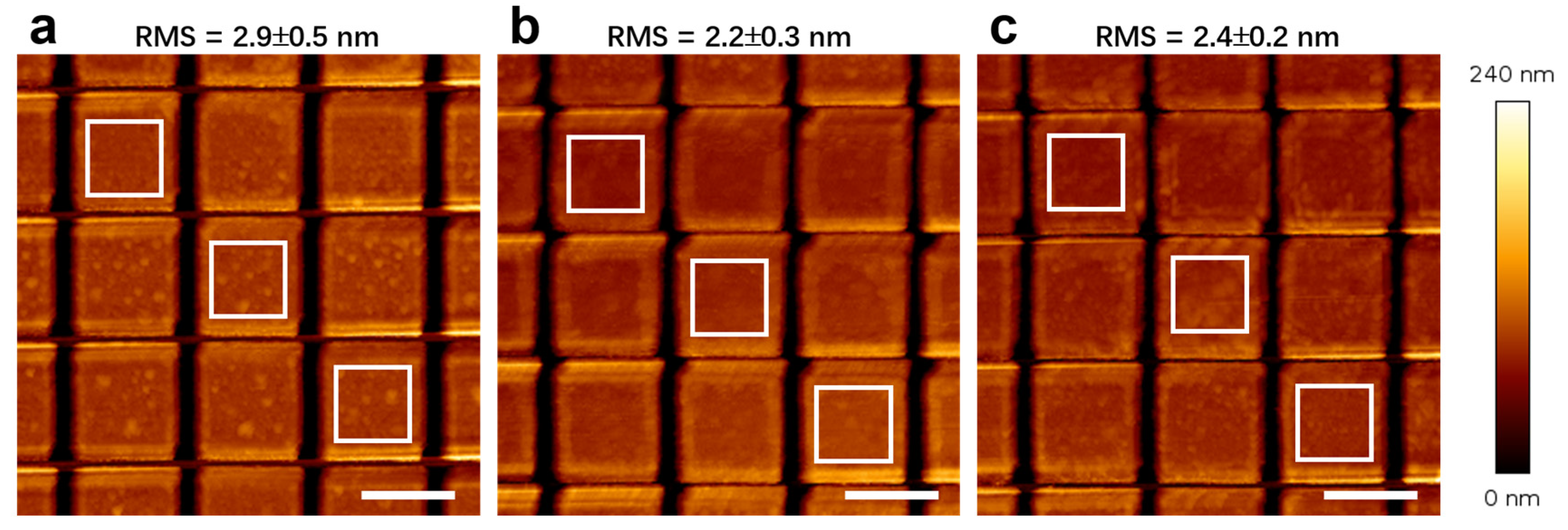
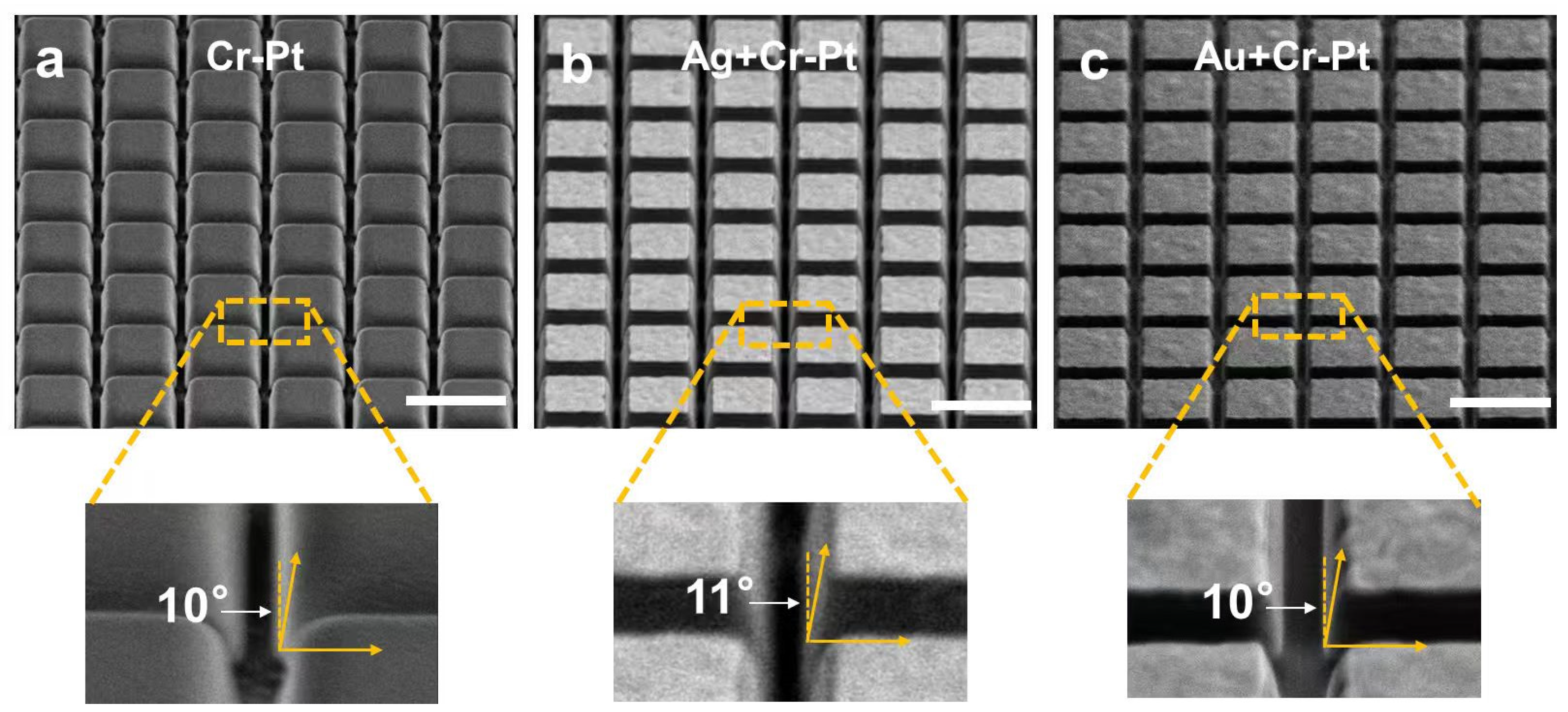
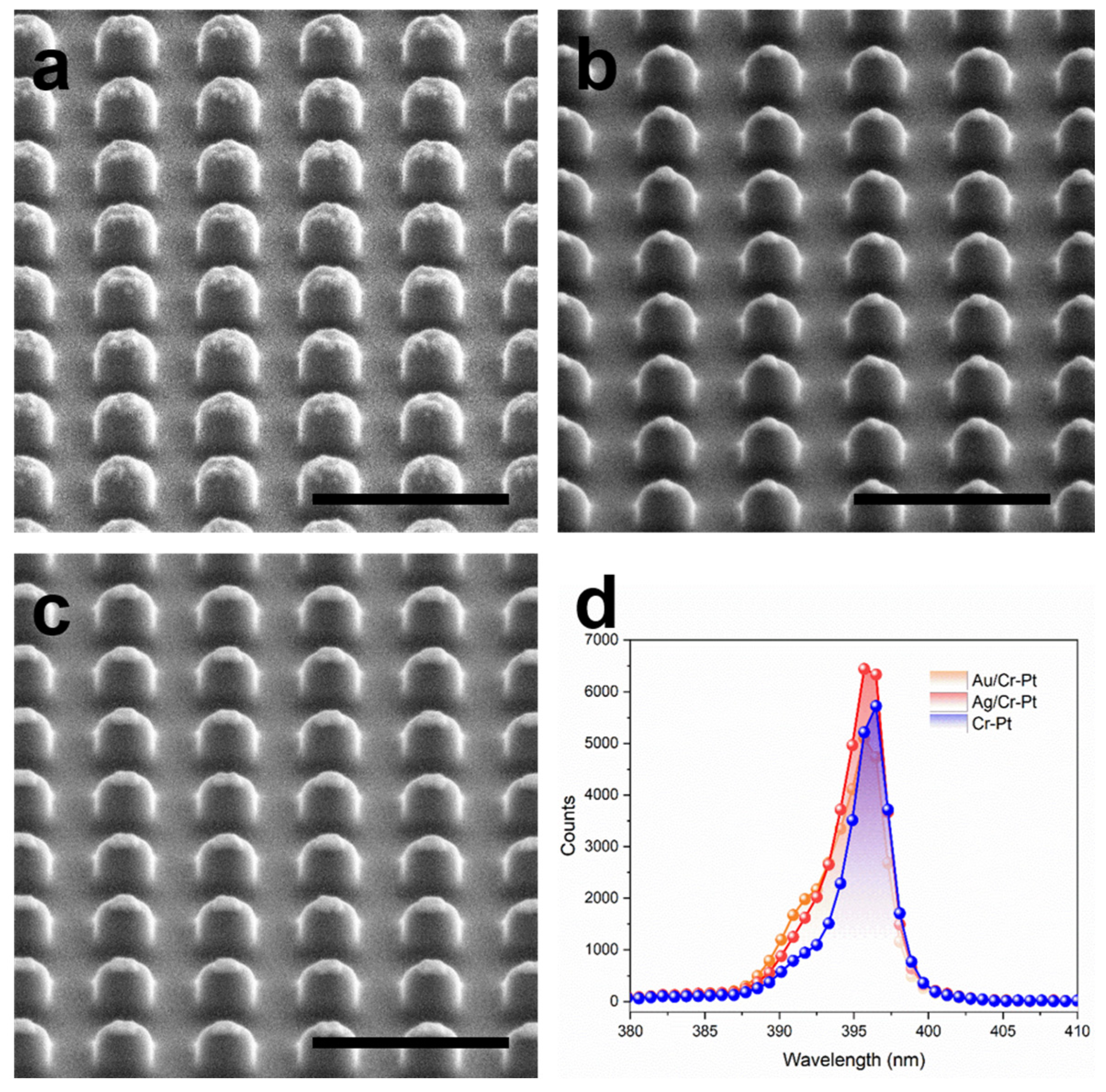
3.4. Fabrication Quality Verification
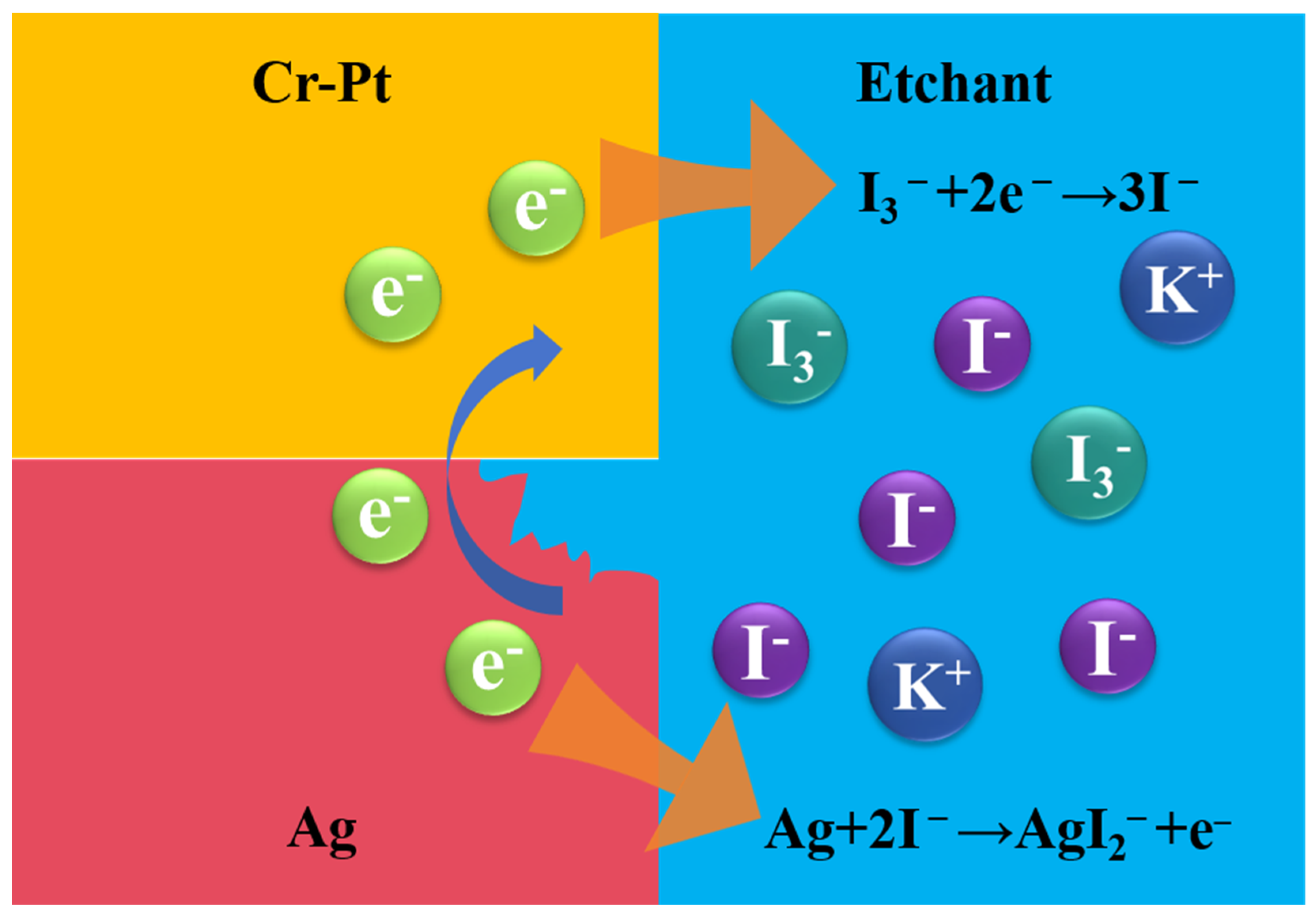
3.5. Discussion
4. Conclusions
- (1)
- Rationalized sacrificial layer selection: systematic evaluation identifies Au or Ag as optimal sacrificial layer materials, combining high sputtering yield for efficient FIB patterning with rapid dissolution kinetics in low-toxicity KI + I2.
- (2)
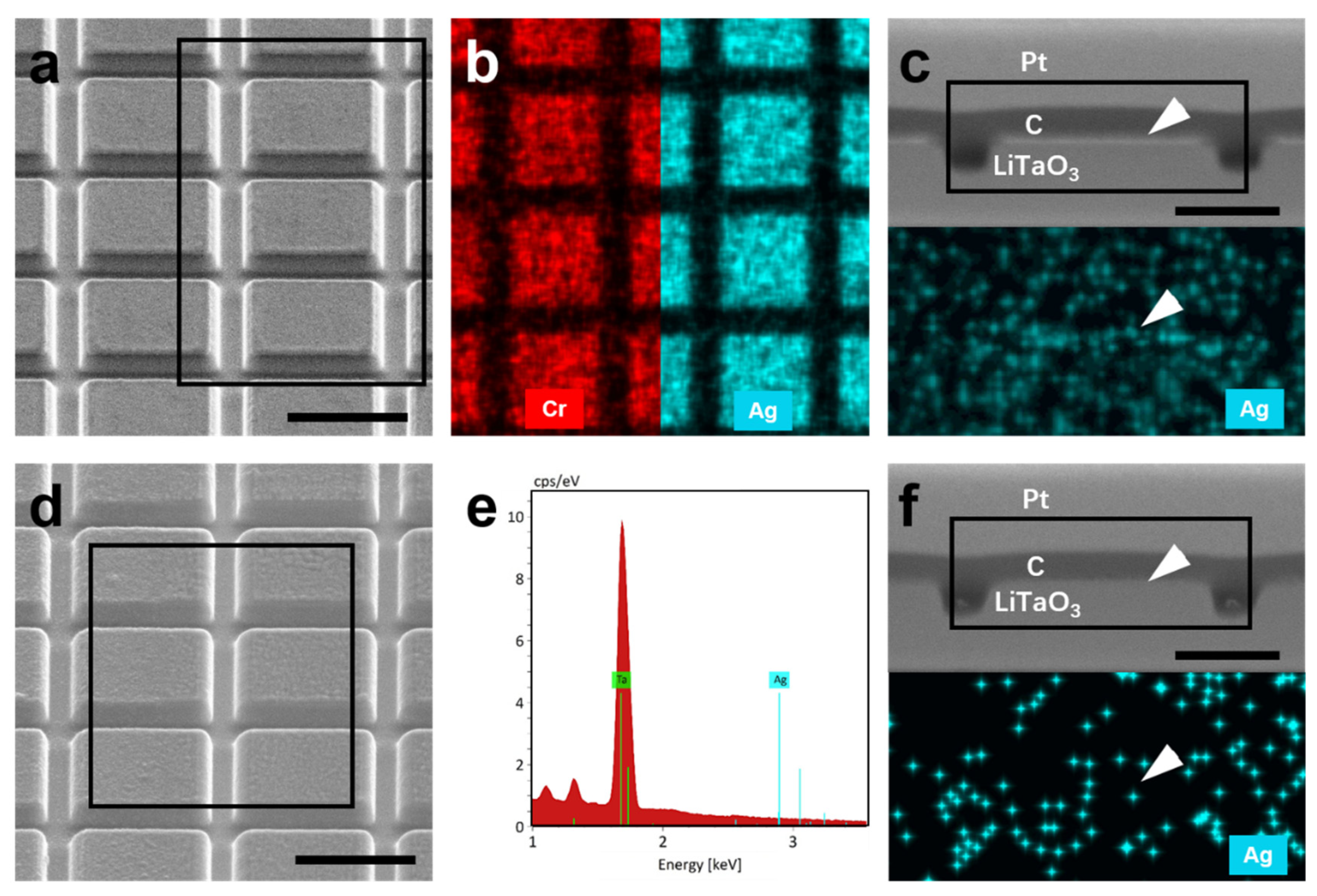
- Validated process efficacy: SEM observation reveals well-defined sidewall profiles (10–11°), demonstrating that sacrificial layer incorporation causes no degradation in milling quality. EDS analysis confirms the complete removal of the mask through KI + I2 solution. Additionally, AFM results confirm post-lift-off surface roughness comparable to conventional aqua regia processing. The SHG measurements show no notable difference in intensity with the introduction of Ag or Au sacrificial layers, suggesting comparable nonlinear optical performance.
- (3)
- Revealed electrochemical enhancement: the rapid dissolution of Ag—despite its lower intrinsic etch rate—is mechanistically explained by a galvanic coupling effect with the Cr-Pt mask. It has been verified by comparative experiments that lateral corrosion depth of the Ag layer in the Ag/Cr-Pt sample is substantially greater than that in the Ag/ITO sample. This electrochemical acceleration enables highly efficient lift-off of Cr-Pt mask, surpassing the limitations of purely chemical dissolution.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brongersma, M.L.; Pala, R.A.; Altug, H.; Capasso, F.; Chen, W.T.; Majumdar, A.; Atwater, H.A. The second optical metasurface revolution: Moving from science to technology. Nat. Rev. Electr. Eng. 2025, 2, 125–143. [Google Scholar] [CrossRef]
- Gu, T.; Kim, H.J.; Rivero-Baleine, C.; Hu, J. Reconfigurable metasurfaces towards commercial success. Nat. Photonics 2023, 17, 48–58. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Ansari, M.A.; Cheng, H.; Tian, J.; Chen, X.; Chen, S. Design Strategies and Applications of Dimensional Optical Field Manipulation Based on Metasurfaces. Adv. Mater. 2023, 35, 2208884. [Google Scholar] [CrossRef] [PubMed]
- Boes, A.; Chang, L.; Langrock, C.; Yu, M.; Zhang, M.; Lin, Q.; Lončar, M.; Fejer, M.; Bowers, J.; Mitchell, A. Lithium niobate photonics: Unlocking the electromagnetic spectrum. Science 2023, 379, eabj4396. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, M.; Chen, Z.; Ai, S.; Kentsch, U.; Zhou, S.; Jia, Y.; Chen, F.; Liu, H. A novel approach towards robust construction of physical colors on lithium niobate crystal. Opto-Electron. Adv. 2025, 8, 240193. [Google Scholar]
- Jia, Y.; Wang, L.; Chen, F. Ion-cut lithium niobate on insulator technology: Recent advances and perspectives. Appl. Phys. Rev. 2021, 8, 011307. [Google Scholar] [CrossRef]
- Kozlov, A.; Moskalev, D.; Salgaeva, U.; Bulatova, A.; Krishtop, V.; Volyntsev, A.; Syuy, A. Reactive Ion Etching of X-Cut LiNbO3 in an ICP/TCP System for the Fabrication of an Optical Ridge Waveguide. Appl. Sci. 2023, 13, 2097. [Google Scholar] [CrossRef]
- Shen, B.; Hu, D.; Dai, C.; Yu, X.; Tan, X.; Sun, J.; Jiang, J.; Jiang, A. Advanced Etching Techniques of LiNbO3 Nanodevices. Nanomaterials 2023, 13, 2789. [Google Scholar] [CrossRef]
- Höflich, K.; Hobler, G.; Allen, F.I.; Wirtz, T.; Rius, G.; McElwee-White, L.; Krasheninnikov, A.V.; Schmidt, M.; Utke, I.; Klingner, N.; et al. Roadmap for focused ion beam technologies. Appl. Phys. Rev. 2023, 10, 041311. [Google Scholar] [CrossRef]
- Si, G.; Danner, A.J.; Teo, S.L.; Teo, E.J.; Teng, J.; Bettiol, A.A. Photonic crystal structures with ultrahigh aspect ratio in lithium niobate fabricated by focused ion beam milling. J. Vac. Sci. Technol. B 2011, 29, 021205. [Google Scholar] [CrossRef]
- Lacour, F.; Courjal, N.; Bernal, M.P.; Sabac, A.; Bainier, C.; Spajer, M. Nanostructuring lithium niobate substrates by focused ion beam milling. Opt. Mater. 2005, 27, 1421–1425. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.A.; Ge, L.; Zhu, B.; Wu, J.; Chen, Y.; Chen, X. High optical damage threshold on-chip lithium tantalate microdisk resonator. Opt. Lett. 2020, 45, 4100–4103. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Z.; Lu, N.; Xu, L.; Han, Z.; Rahmani, M.; Jia, Y.; Chen, F. Lithium tantalate resonant metasurfaces for highly efficient ultraviolet harmonic generation. Appl. Phys. Rev. 2025, 12, 031404. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Lu, N.; Han, Z.; Xu, L.; Rahmani, M.; Jia, Y.; Chen, F. Highly Efficient Ultraviolet Harmonic Generation Based on Coupled Guided Mode Resonances in Lithium Niobate Metasurfaces. Laser Photonics Rev. 2025, 19, e01010. [Google Scholar] [CrossRef]
- Kim, K.H.; Akase, Z.; Suzuki, T.; Shindo, D. Charging Effects on SEM/SIM Contrast of Metal/Insulator System in Various Metallic Coating Conditions. Mater. Trans. 2010, 51, 1080–1083. [Google Scholar] [CrossRef]
- Lu, N.; Wang, Z.; Zang, Y.; Jia, Y. Mask Material-Driven Enhancement of Imaging Contrast for Lithium Tantalate Metasurface during Focused Ion Beam fabrication. Shandong University, Jinan, China. 2025; Submitted. [Google Scholar]
- Khamnualthong, N.; Siangchaew, K.; Limsuwan, P. Study of Chromium Hard Mask Formation and Wall Angle Control for Deep Etching Application. Procedia Eng. 2012, 32, 922–928. [Google Scholar] [CrossRef]
- Köllensperger, P.A.; Karl, W.J.; Ahmad, M.M.; Pike, W.T.; Green, M. Patterning of platinum (Pt) thin films by chemical wet etching in Aqua Regia. J. Micromech. Microeng. 2012, 22, 067001. [Google Scholar] [CrossRef]
- Kishimoto, S. Electron Moiré method. Theor. Appl. Mech. Lett. 2012, 2, 011001. [Google Scholar] [CrossRef]
- Aryal, A.; Stricklin, I.; Behzadirad, M.; Branch, D.W.; Siddiqui, A.; Busani, T. High-Quality Dry Etching of LiNbO3 Assisted by Proton Substitution through H2-Plasma Surface Treatment. Nanomaterials 2022, 12, 2836. [Google Scholar] [CrossRef]
- Mei, J.; Zhang, N.; Friend, J. Fabrication of Surface Acoustic Wave Devices on Lithium Niobate. J. Vis. Exp. 2020, 160, e61013. [Google Scholar] [CrossRef]
- Lu, N.; Wang, Z.; Zang, Y.; Jia, Y. Ion-Beam-induced Interfacial Blistering of Chromium Masks on Lithium Tantalate Substrate: Origins and Suppression Strategies. Shandong University, Jinan, China. 2025; Submitted. [Google Scholar]
- Choi, T.-S.; Hess, D.W. Chemical Etching and Patterning of Copper, Silver, and Gold Films at Low Temperatures. ECS J. Solid State Sci. Technol. 2015, 4, N3084. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Biersack, J.P.; Ziegler, M. SRIM—The stopping and range of ions in matter. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Yoshio Nishi, R.D. Handbook of Semiconductor Manufacturing Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Green, T.A. Gold etching for microfabrication. Gold Bull. 2014, 47, 205–216. [Google Scholar] [CrossRef]
- Verdingovas, V.; Müller, L.; Jellesen, M.S.; Grumsen, F.B.; Ambat, R. Effect of iodine on the corrosion of Au–Al wire bonds. Corros. Sci. 2015, 97, 161–171. [Google Scholar] [CrossRef]
- Bard, A.J. Standard Potentials in Aqueous Solution; Routledge: New York, NY, USA, 1985. [Google Scholar]
- Milazzo, G.; Caroli, S.; Braun, R.D. Tables of Standard Electrode Potentials. J. Electrochem. Soc. 1978, 125, 261C. [Google Scholar] [CrossRef]
| Mask Materials | Properties | ||
|---|---|---|---|
| Sputtering Yield | Compatible Etchants | Etchant Toxicity/Volatility | |
| Cr | 4.7 | HNO3 + (NH4)2Ce(NO3)6 | Severe |
| Ni | 7.9 | HNO3 | Severe |
| Al | 3.4 | H3PO4 or NaOH | Strong |
| Ti | 2.3 | HF + HNO3 | Severe |
| Au | 17.0 | KI + I2 | Weak |
| W | 7.4 | HF + HNO3 | Severe |
| Mo | 5.5 | HNO3 + H2SO4 | Severe |
| TiN | 3.0 | HF + HNO3 | Severe |
| Co | 7.6 | HNO3 | Severe |
| Ag | 13.2 | KI + I2 | Weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, Z.; Zang, Y.; Jia, Y.; Lu, N. Aqua Regia-Free Removal of Cr-Pt Hard Masks Using Thin Ag or Au Sacrificial Layers for High-Fidelity LiTaO3 Metasurfaces. Nanomaterials 2026, 16, 59. https://doi.org/10.3390/nano16010059
Wang Z, Zang Y, Jia Y, Lu N. Aqua Regia-Free Removal of Cr-Pt Hard Masks Using Thin Ag or Au Sacrificial Layers for High-Fidelity LiTaO3 Metasurfaces. Nanomaterials. 2026; 16(1):59. https://doi.org/10.3390/nano16010059
Chicago/Turabian StyleWang, Zhuoqun, Yufeng Zang, Yuechen Jia, and Ning Lu. 2026. "Aqua Regia-Free Removal of Cr-Pt Hard Masks Using Thin Ag or Au Sacrificial Layers for High-Fidelity LiTaO3 Metasurfaces" Nanomaterials 16, no. 1: 59. https://doi.org/10.3390/nano16010059
APA StyleWang, Z., Zang, Y., Jia, Y., & Lu, N. (2026). Aqua Regia-Free Removal of Cr-Pt Hard Masks Using Thin Ag or Au Sacrificial Layers for High-Fidelity LiTaO3 Metasurfaces. Nanomaterials, 16(1), 59. https://doi.org/10.3390/nano16010059







