Scalable Production and Multifunctional Coating of Gold Nanostars for Catalytic Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Precursor Concentration on AuNSTs Growth
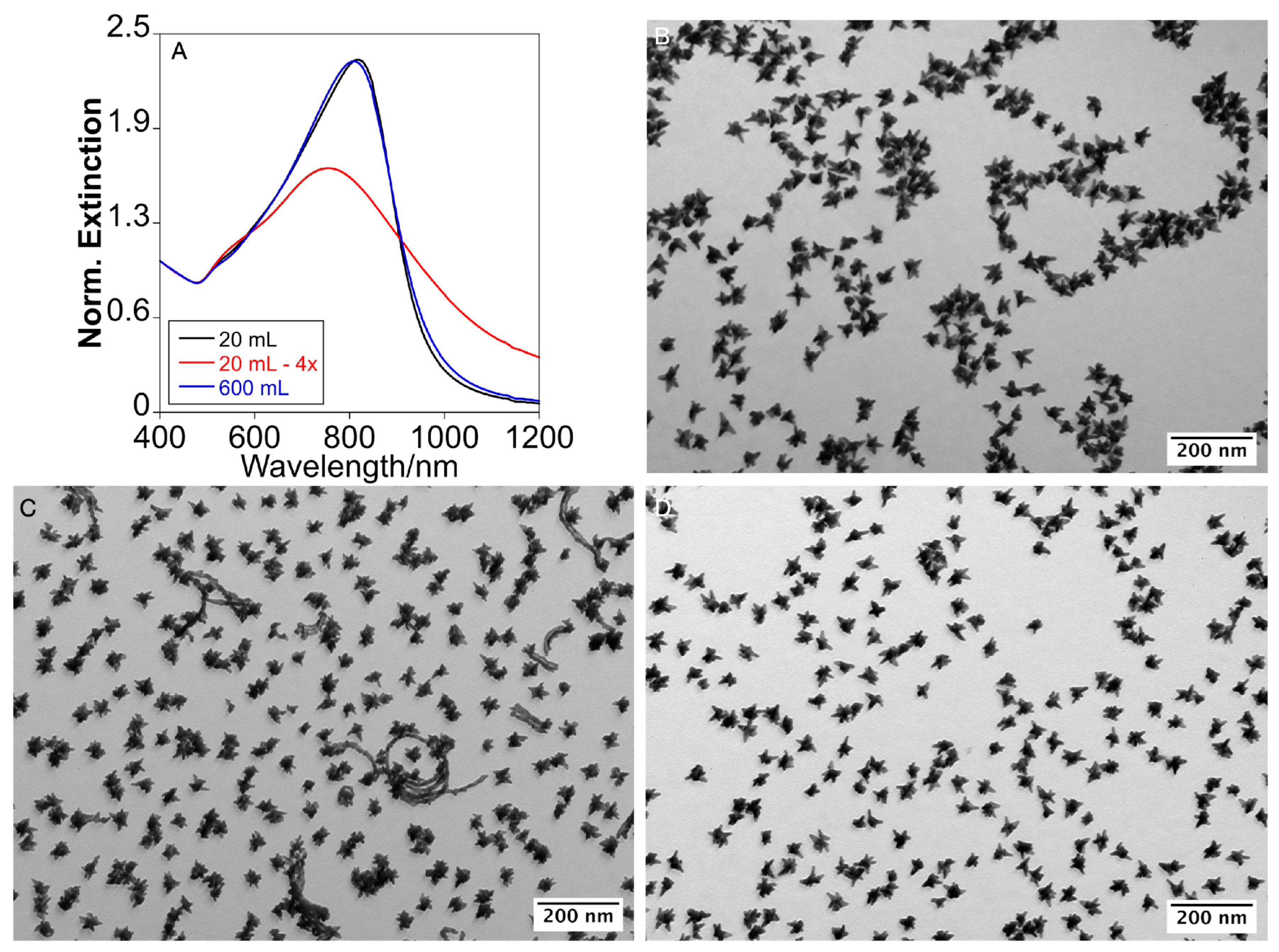
2.2. Scale-Up by Volume Expansion and Temperature Acceleration
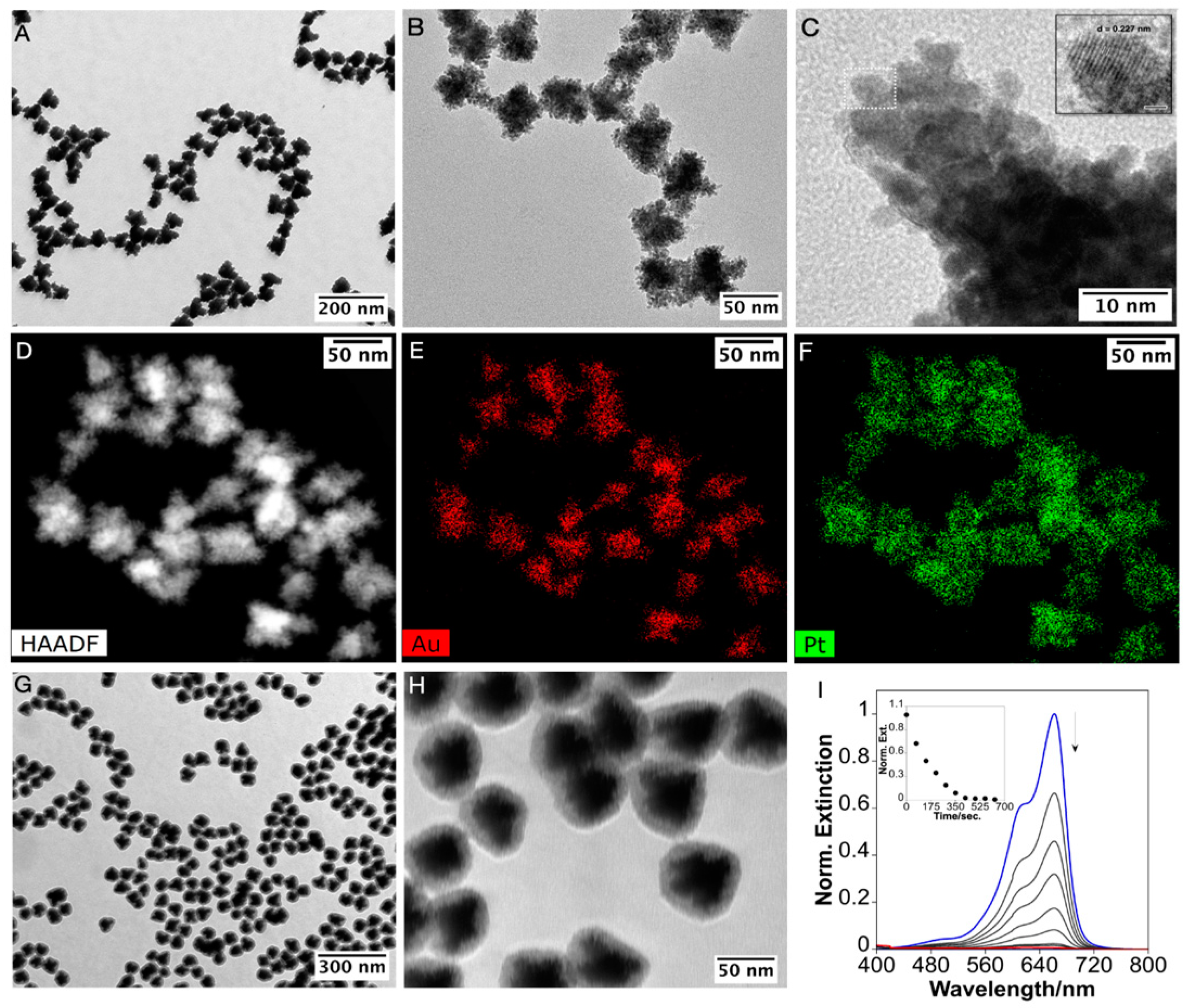
2.3. The AuNSTs Mesoporous Silica Coating
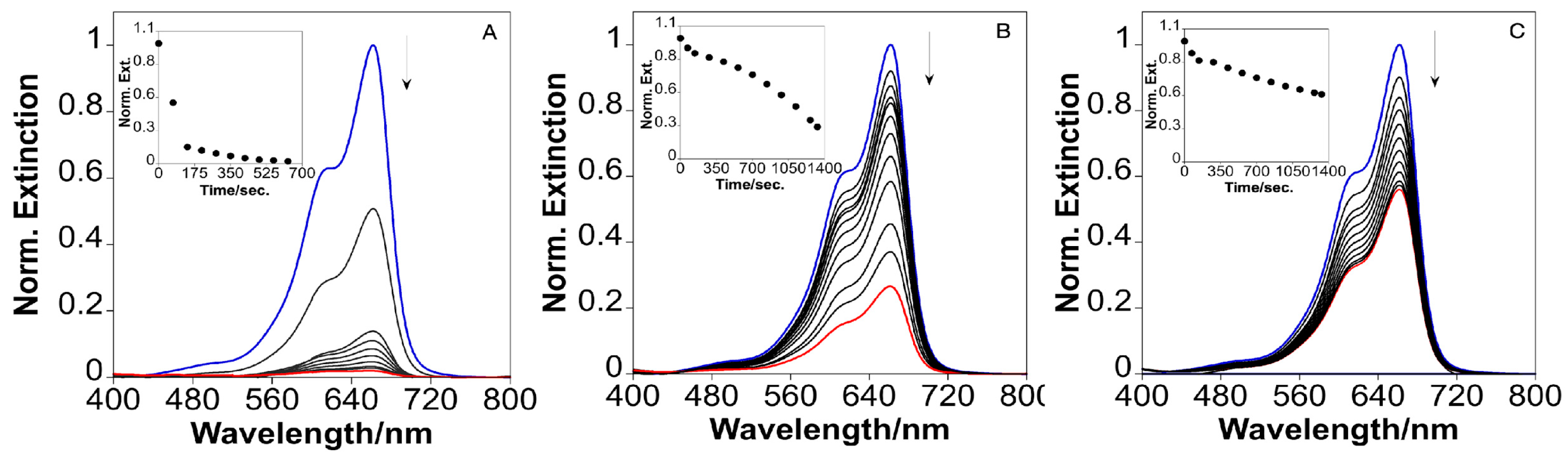
2.4. Catalytic Reduction of Methylene Blue with the Silica-Coated AuNSTs
2.5. Platinum and Mesoporous Silica Functionalization of the AuNSTs and Catalytic Applications
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Z.; Yu, G.; Zhang, Z.; Han, Y.; Guan, G.; Yang, W.; Han, M.Y. Intrinsic Optical Properties and Emerging Applications of Gold Nanostructures. Adv. Mater. 2023, 35, e2206700. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chemie Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Castillo, J.E.; Gallo-Villanueva, R.C.; Madou, M.J.; Perez-Gonzalez, V.H. Anisotropic Gold Nanoparticles: A Survey of Recent Synthetic Methodologies. Coord. Chem. Rev. 2020, 425, 213489. [Google Scholar] [CrossRef]
- Fernández-Lodeiro, C.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Nuti, S.; Lodeiro, C.; LaGrow, A.; Pérez-Juste, I.; Pérez-Juste, J.; Pastoriza-Santos, I. Synthesis of Tuneable Gold Nanostars: The Role of Adenosine Monophosphate. J. Mater. Chem. C 2023, 11, 12626–12636. [Google Scholar] [CrossRef]
- Nuti, S.; Fernández-Lodeiro, C.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Pérez-Juste, J.; Pastoriza-Santos, I.; LaGrow, A.P.; Schraidt, O.; Luis Capelo-Martínez, J.; Lodeiro, C. Polyallylamine Assisted Synthesis of 3D Branched AuNPs with Plasmon Tunability in the Vis-NIR Region as Refractive Index Sensitivity Probes. J. Colloid Interface Sci. 2022, 611, 695–705. [Google Scholar] [CrossRef]
- Fabris, L. Gold Nanostars in Biology and Medicine: Understanding Physicochemical Properties to Broaden Applicability. J. Phys. Chem. C 2020, 124, 26540–26553. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Barbosa, S.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Nanostars Shine Bright for You. Colloidal Synthesis, Properties and Applications of Branched Metallic Nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 118–127. [Google Scholar] [CrossRef]
- Donoso-González, O.; Hengsbach, R.; Ohlerth, T.; Noyong, M.; Sierpe, R.; Rozas-Castro, N.; Lodeiro, L.; Bolaños, K.; Melo, F.; Yutronic, N.; et al. Impact of Ligand Exchange on Gold Nanostars’ Reshaping, Stabilization, Photothermal Efficiency, and Cell Viability. ACS Appl. Nano Mater. 2024, 7, 1437–1449. [Google Scholar] [CrossRef]
- Siegel, A.L.; Baker, G.A. Bespoke Nanostars: Synthetic Strategies, Tactics, and Uses of Tailored Branched Gold Nanoparticles. Nanoscale Adv. 2021, 3, 3980–4004. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, X.; Zhang, H.; Bai, X.; Ai, R.; Shao, L.; Wang, J. Gold Nanorods: The Most Versatile Plasmonic Nanoparticles. Chem. Rev. 2021, 121, 13342–13453. [Google Scholar] [CrossRef]
- Ndokoye, P.; Zhao, Q.; Li, X.; Li, T.; Tade, M.O.; Wang, S. Branch Number Matters: Promoting Catalytic Reduction of 4-Nitrophenol over Gold Nanostars by Raising the Number of Branches and Coating with Mesoporous SiO2. J. Colloid Interface Sci. 2016, 477, 1–7. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, X.; Peng, G.; Shang, L.; Zhang, T. Silica Encapsulation Strategy for Protection and Controllable Synthesis of Nanocatalysts. Accounts Mater. Res. 2024, 5, 194–205. [Google Scholar] [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef] [PubMed]
- Nuti, S.; Fernández-Lodeiro, J.; Palomo, J.M.; Capelo-Martinez, J.-L.; Lodeiro, C.; Fernández-Lodeiro, A. Synthesis, Structural Analysis, and Peroxidase-Mimicking Activity of AuPt Branched Nanoparticles. Nanomaterials 2024, 14, 1166. [Google Scholar] [CrossRef] [PubMed]
- Hernández Montoto, A.; Llopis-Lorente, A.; Gorbe, M.; Terrés, J.M.; Cao-Milán, R.; Díaz de Greñu, B.; Alfonso, M.; Ibañez, J.; Marcos, M.D.; Orzáez, M.; et al. Janus Gold Nanostars–Mesoporous Silica Nanoparticles for NIR-Light-Triggered Drug Delivery. Chem. Eur. J. 2019, 25, 8471–8478. [Google Scholar] [CrossRef]
- Blanco-Formoso, M.; Sousa-Castillo, A.; Xiao, X.; Mariño-Lopez, A.; Turino, M.; Pazos-Perez, N.; Giannini, V.; Correa-Duarte, M.A.; Alvarez-Puebla, R.A. Boosting the Analytical Properties of Gold Nanostars by Single Particle Confinement into Yolk Porous Silica Shells. Nanoscale 2019, 11, 21872–21879. [Google Scholar] [CrossRef]
- Hernández Montoto, A.; Montes, R.; Samadi, A.; Gorbe, M.; Terrés, J.M.; Cao-Milán, R.; Aznar, E.; Ibañez, J.; Masot, R.; Marcos, M.D.; et al. Gold Nanostars Coated with Mesoporous Silica Are Effective and Nontoxic Photothermal Agents Capable of Gate Keeping and Laser-Induced Drug Release. ACS Appl. Mater. Interfaces 2018, 10, 27644–27656. [Google Scholar] [CrossRef]
- Scarabelli, L.; Sánchez-Iglesias, A.; Pérez-Juste, J.; Liz-Marzán, L.M. A “Tips and Tricks” Practical Guide to the Synthesis of Gold Nanorods. J. Phys. Chem. Lett. 2015, 6, 4270–4279. [Google Scholar] [CrossRef]
- Nuti, S.; Fernández-Lodeiro, A.; Galhano, J.; Oliveira, E.; Duarte, M.P.; Capelo-Martínez, J.L.; Lodeiro, C.; Fernández-Lodeiro, J. Tailoring Mesoporous Silica-Coated Silver Nanoparticles and Polyurethane-Doped Films for Enhanced Antimicrobial Applications. Nanomaterials 2024, 14, 462. [Google Scholar] [CrossRef]
- Fernández-Lodeiro, A.; Djafari, J.; Fernández-Lodeiro, J.; Duarte, M.P.; Muchagato Mauricio, E.; Capelo-Martínez, J.L.; Lodeiro, C. Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications. Nanomaterials 2021, 11, 1338. [Google Scholar] [CrossRef]
- Fernández-Lodeiro, C.; Tambosi, R.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Nuti, S.; Ouchane, S.; Kébaïli, N.; Pérez-Juste, J.; Pastoriza-Santos, I.; Lodeiro, C. Adenosine-Monophosphate-Assisted Homogeneous Silica Coating of Silver Nanoparticles in High Yield. Nanomaterials 2023, 13, 2788. [Google Scholar] [CrossRef]
- Wu, W.-C.; Tracy, J.B. Large-Scale Silica Overcoating of Gold Nanorods with Tunable Shell Thicknesses. Chem. Mater. 2015, 27, 2888–2894. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Gorelikov, I.; Matsuura, N. Single-Step Coating of Mesoporous Silica on Cetyltrimethyl Ammonium Bromide-Capped Nanoparticles. Nano Lett. 2008, 8, 369–373. [Google Scholar] [CrossRef]
- Ma, T.; Yang, W.; Liu, S.; Zhang, H.; Liang, F. A Comparison Reduction of 4-Nitrophenol by Gold Nanospheres and Gold Nanostars. Catalysts 2017, 7, 38. [Google Scholar] [CrossRef]
- Beagan, A.M. Investigating Methylene Blue Removal from Aqueous Solution by Cysteine-Functionalized Mesoporous Silica. J. Chem. 2021, 2021, 8839864. [Google Scholar] [CrossRef]
- Stephanie Stolle, H.L.K.; Kluitmann, J.J.; Csáki, A.; Köhler, J.M.; Fritzsche, W. Shape-Dependent Catalytic Activity of Gold and Bimetallic Nanoparticles in the Reduction of Methylene Blue by Sodium Borohydride. Catalysts 2021, 11, 1442. [Google Scholar] [CrossRef]
- Mouarrawis, V.; Plessius, R.; van der Vlugt, J.I.; Reek, J.N.H. Confinement Effects in Catalysis Using Well-Defined Materials and Cages. Front. Chem. 2018, 6, 623. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xiao, F.; Hu, Y.; Yuan, S.; Wang, S.; Qian, L.; Liu, Y. Hierarchical Nanoporous Gold-Platinum with Heterogeneous Interfaces for Methanol Electrooxidation. Sci. Rep. 2014, 4, 4370. [Google Scholar] [CrossRef]
- Moon, Y.; Mai, H.D.; Yoo, H. Platinum Overgrowth on Gold Multipod Nanoparticles: Investigation of Synergistic Catalytic Effects in a Bimetallic Nanosystem. Chemnanomat 2017, 3, 196–203. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Tsung, C.-K.; Yamada, Y.; Yang, P.; Somorjai, G.A. Thermally Stable Pt/Mesoporous Silica Core–Shell Nanocatalysts for High-Temperature Reactions. Nat. Mater. 2009, 8, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, Q.-H. Stable and Functionable Mesoporous Silica-Coated Gold Nanorods as Sensitive Localized Surface Plasmon Resonance (LSPR) Nanosensors. Langmuir 2009, 25, 9441–9446. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Ji, Y.; Leong, G.J.; Kovach, N.C.; Trewyn, B.G.; Richards, R.M. Hybrid Mesoporous Silica/Noble-Metal Nanoparticle Materials-Synthesis and Catalytic Applications. ACS Appl. Nano Mater. 2018, 1, 4386–4400. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Özuğur Uysal, B.; Pekcan, Ö.; Erim, F.B. Efficient Photocatalytic Degradation of Methylene Blue Dye from Aqueous Solution with Cerium Oxide Nanoparticles and Graphene Oxide-Doped Polyacrylamide. ACS Omega 2023, 8, 13004–13015. [Google Scholar] [CrossRef] [PubMed]
- Piella, J.; Merkoçi, F.; Genç, A.; Arbiol, J.; Bastús, N.G.; Puntes, V. Probing the surface reactivity of nanocrystals by the catalytic degradation of organic dyes: The effect of size, surface chemistry, and composition. J. Mater. Chem. A 2017, 5, 11917–11929. [Google Scholar] [CrossRef]
- Menges, F. Spectragryph-Optical Spectroscopy Software. 2022. Version 1. Available online: http://www.effemm2.de/spectragryph/ (accessed on 27 April 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuti, S.; Fernández-Lodeiro, A.; Ortiz-Gómez, I.; Lodeiro, C.; Fernández-Lodeiro, J. Scalable Production and Multifunctional Coating of Gold Nanostars for Catalytic Applications. Nanomaterials 2025, 15, 692. https://doi.org/10.3390/nano15090692
Nuti S, Fernández-Lodeiro A, Ortiz-Gómez I, Lodeiro C, Fernández-Lodeiro J. Scalable Production and Multifunctional Coating of Gold Nanostars for Catalytic Applications. Nanomaterials. 2025; 15(9):692. https://doi.org/10.3390/nano15090692
Chicago/Turabian StyleNuti, Silvia, Adrián Fernández-Lodeiro, Inmaculada Ortiz-Gómez, Carlos Lodeiro, and Javier Fernández-Lodeiro. 2025. "Scalable Production and Multifunctional Coating of Gold Nanostars for Catalytic Applications" Nanomaterials 15, no. 9: 692. https://doi.org/10.3390/nano15090692
APA StyleNuti, S., Fernández-Lodeiro, A., Ortiz-Gómez, I., Lodeiro, C., & Fernández-Lodeiro, J. (2025). Scalable Production and Multifunctional Coating of Gold Nanostars for Catalytic Applications. Nanomaterials, 15(9), 692. https://doi.org/10.3390/nano15090692






