Research Progress on the Synthesis of Nanostructured Photocatalysts and Their Environmental Applications
Abstract
1. Introduction
2. The Basic Principle of Nanostructure Photocatalysts
3. Synthesis of Typical Nanostructured Photocatalysts
3.1. Solvothermal/Hydrothermal Synthesis
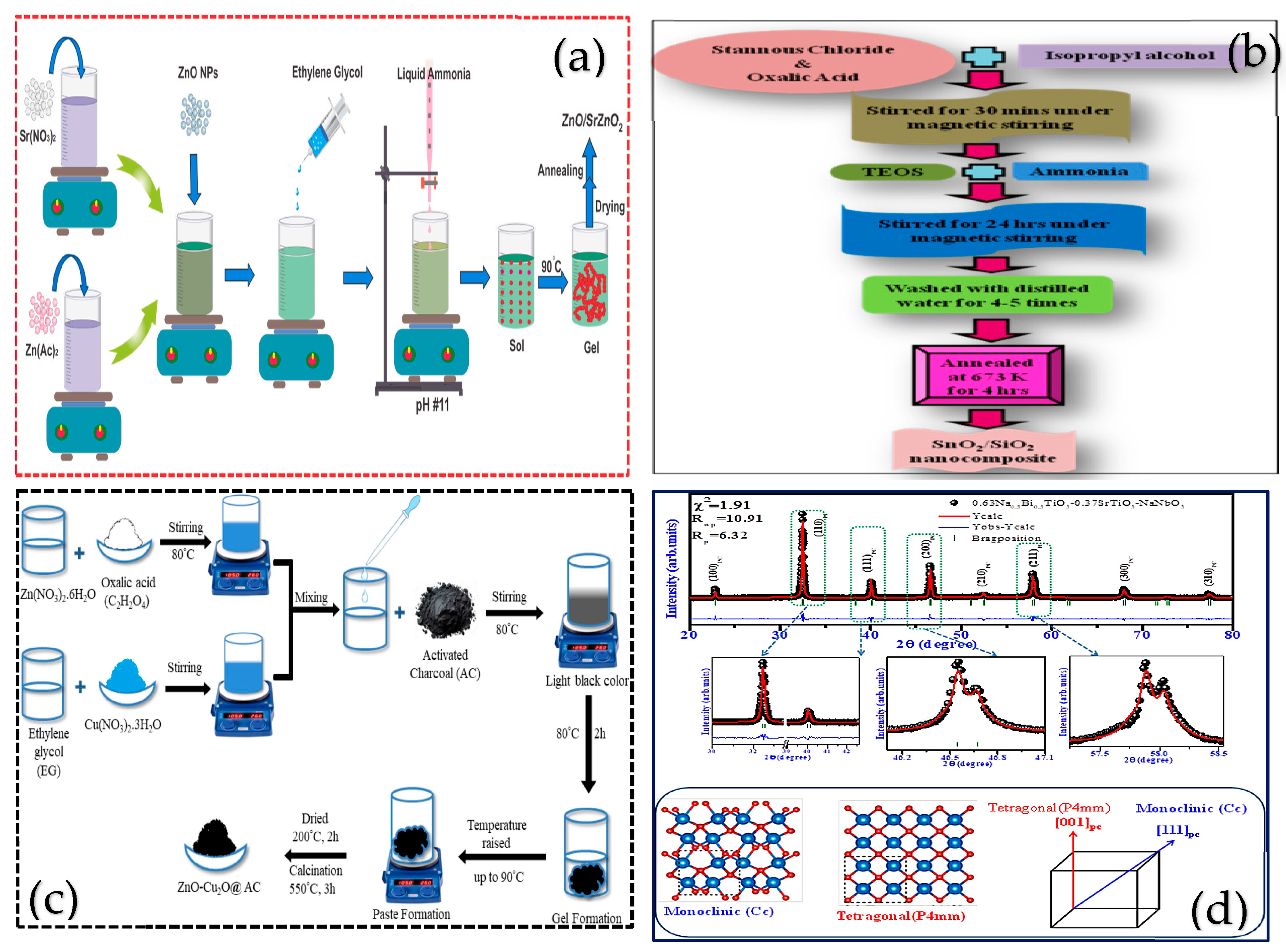
3.2. Sol-Gel Method
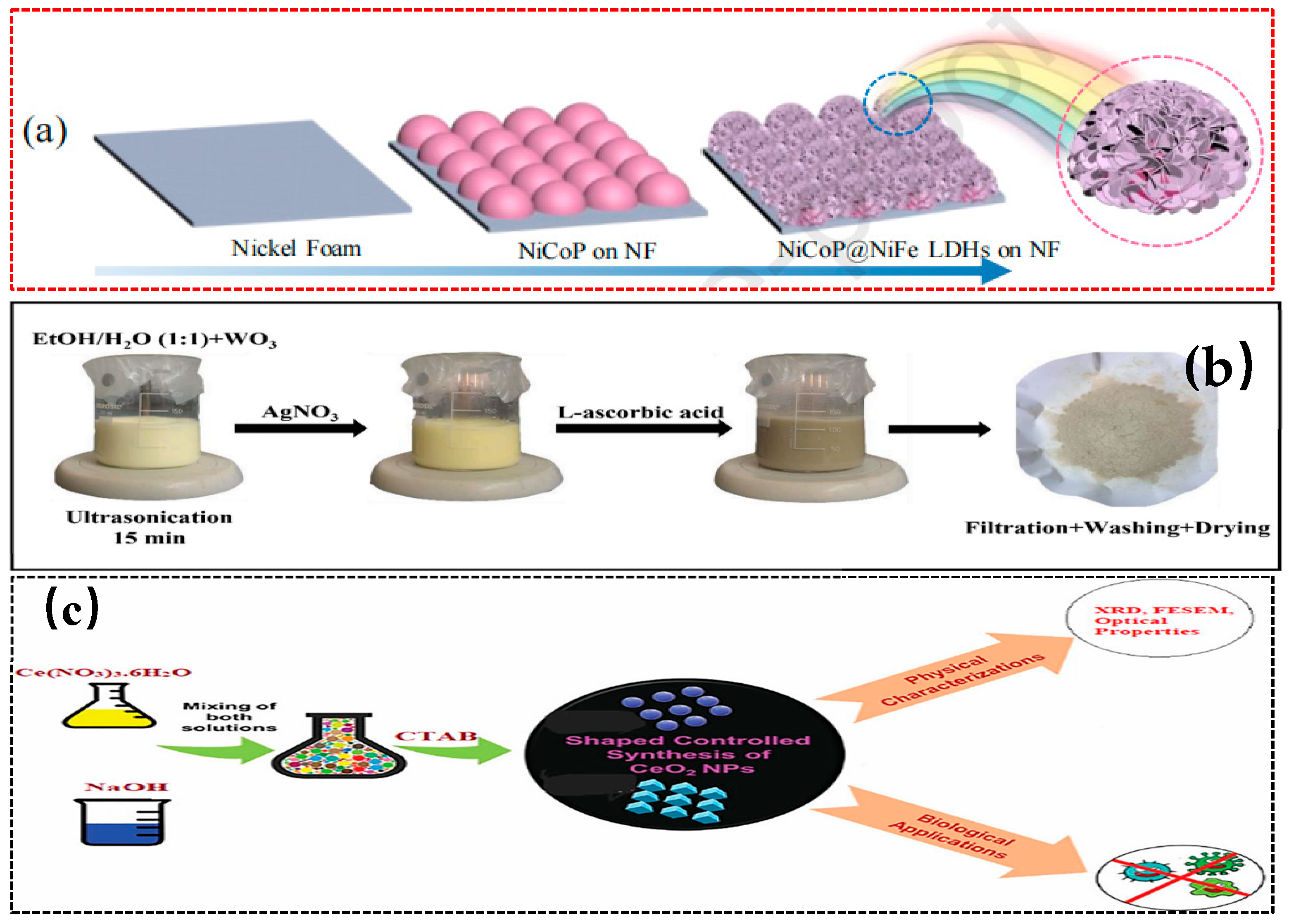
3.3. Chemical Vapor Deposition (CVD)
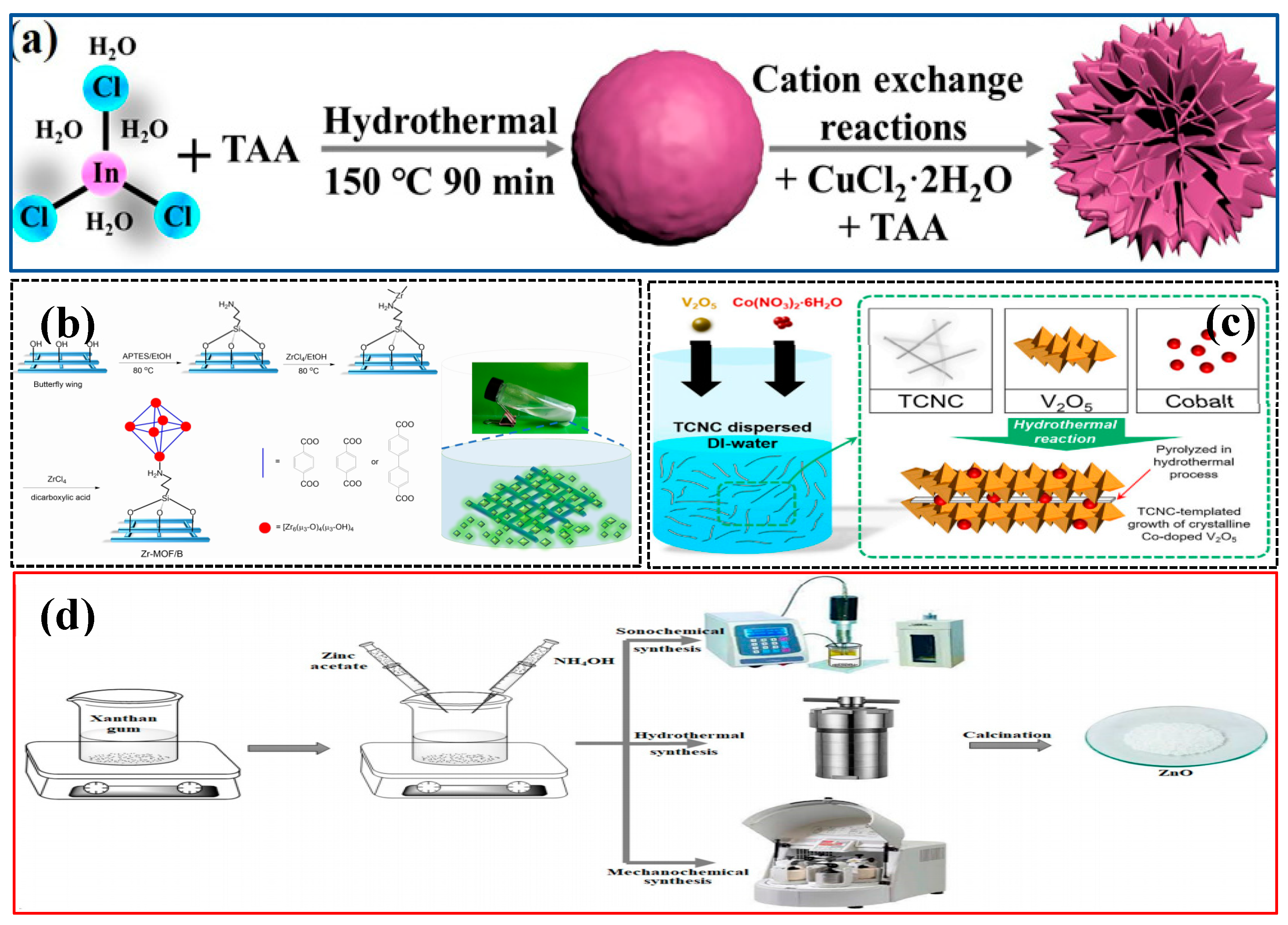
3.4. Template-Assisted Synthesis
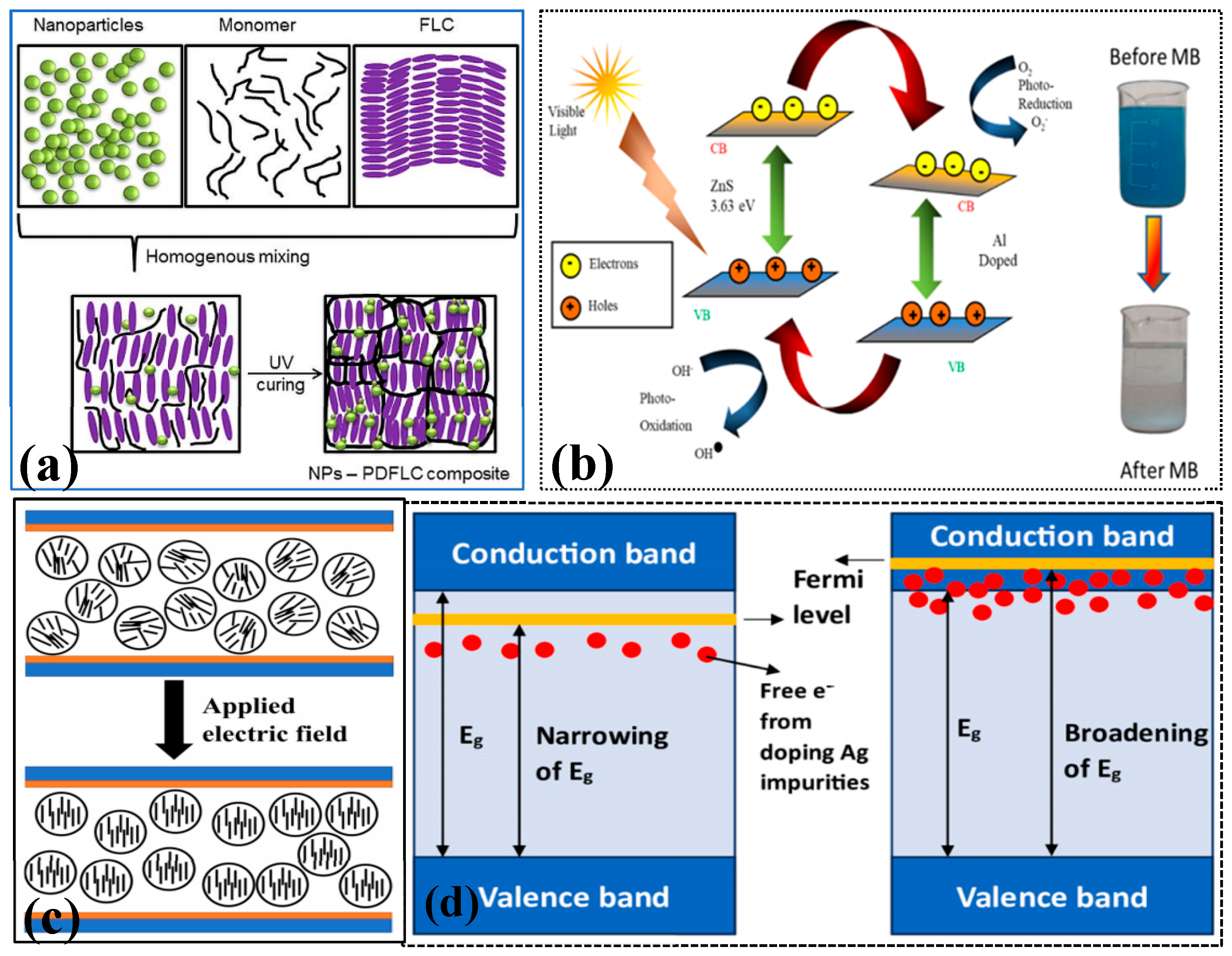
3.5. Other Methods
| Synthesis Method | Brief Principle | Precursor/Raw Material | Reaction Conditions | Name of Nanomaterials | Ref. |
|---|---|---|---|---|---|
| Solution Combustion Method, SCM | The high-temperature and high-pressure environment promotes the combination of zinc ions with oxygen | Metal salts, reducing agents, water | Atmospheric pressure combustion | ZnO nanoparticles | [92] |
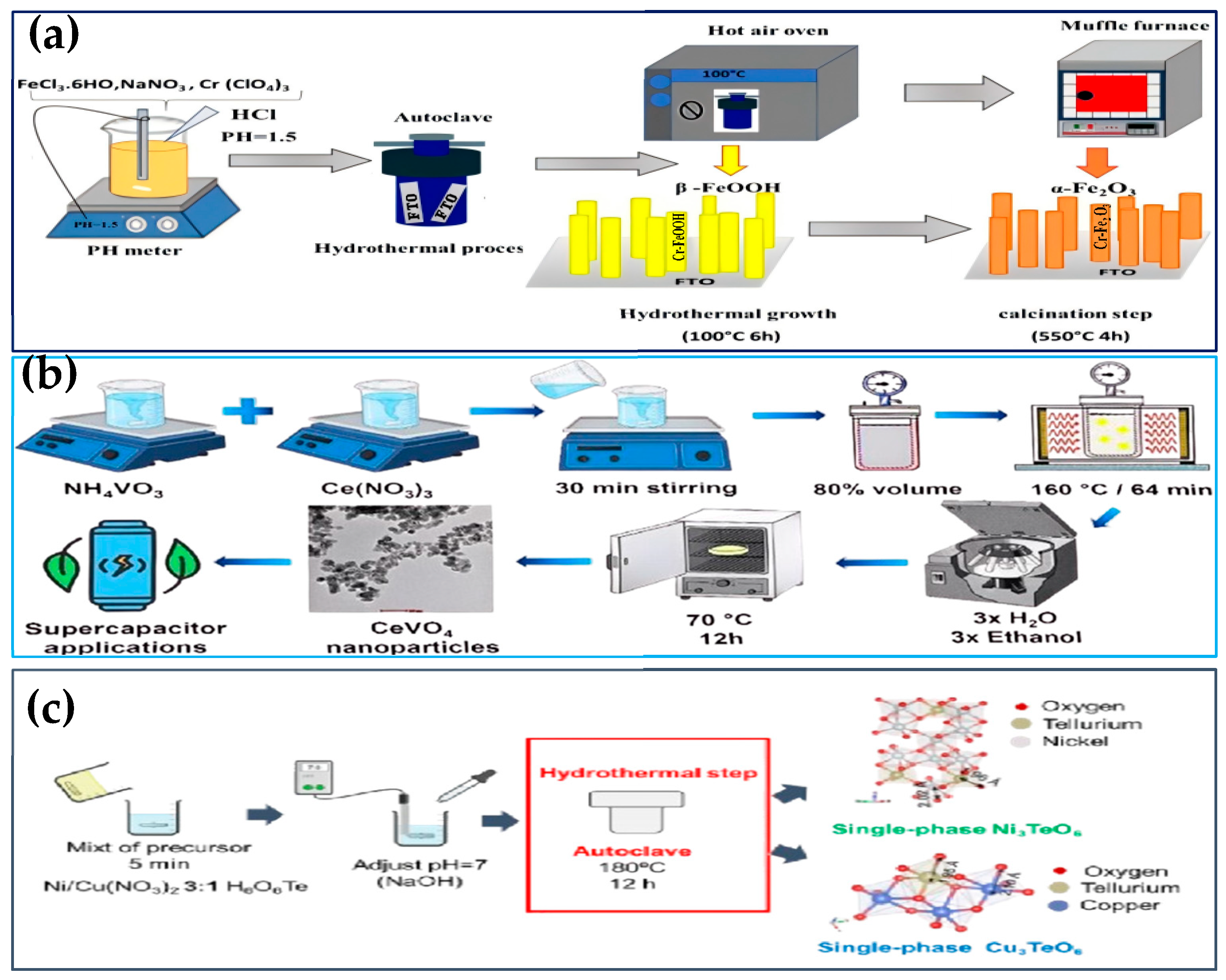
| Hydrothermal Method | Fe3+-β-FeOOH-α-Fe2O3-Cr3+ replace | Metal salts, deionized water, FTO conductive glass, etc. | Acidic conditions, high-temperature hydrothermal, and annealing | Cr-doped hematite, α-Fe2O3:Cr | [66] |
| Hydrothermal Method | The high temperature and high pressure in the hydrothermal environment control the growth of crystals | Metal salts, deionized water, NaOH, etc. | Alkaline, Constant temperature is maintained inside the autoclave | Strontium Titanate Nanocubes, STNCs | [93] |
| Binder-free hydrothermal method | Nanomaterials are formed in a high-temperature and high-pressure hydrothermal environment | Metal salts, CO (NH2)2 etc. | A sealed environment for high-temperature and high-pressure autoclaves | Binder-free ZnFe2O4 nanosheets on nickel foam | [63] |
| Sol-Gel Technique | The p-n heterojunction promotes the effective separation of e−-h+ pairs and enhances their activity | Metal salts, C2H6O2, deionized water, etc. | Alkaline, high-temperature calcination | Nanostructured ZnO/SrZnO2 Composite | [67] |
| Sol-Gel Technique | Hydrolysis generates metal complexes, which are then heated and calcined to prepare the materials | Metal salts, EG, deionized water, etc. | Alkaline high-temperature heating calcination | Ternary ZnO-Cu₂O@AC Nanocomposite | [71] |
| Sol-gel Method | Band gap regulation and Charge separation enhancement | Metal salts, deionized water, etc. | stir, gel drying, High-temperature calcination | Mn-Sr co-doped TiO2 | [69] |
| Sol-gel Method | Lattice doping and structure regulation | Metal salts, NH3·H2O, etc. | Alkaline, gel drying, high-temperature calcination | Sn:NiMn2O4 | [70] |
| Sol-gel Method | The hydrolysis and polycondensation reactions of the precursor form a sol, which is then dried and calcined | SnCl2, NH3·H2O, etc. | Mix and stir, filter, and calcine | SnO2/SiO2 | [15] |
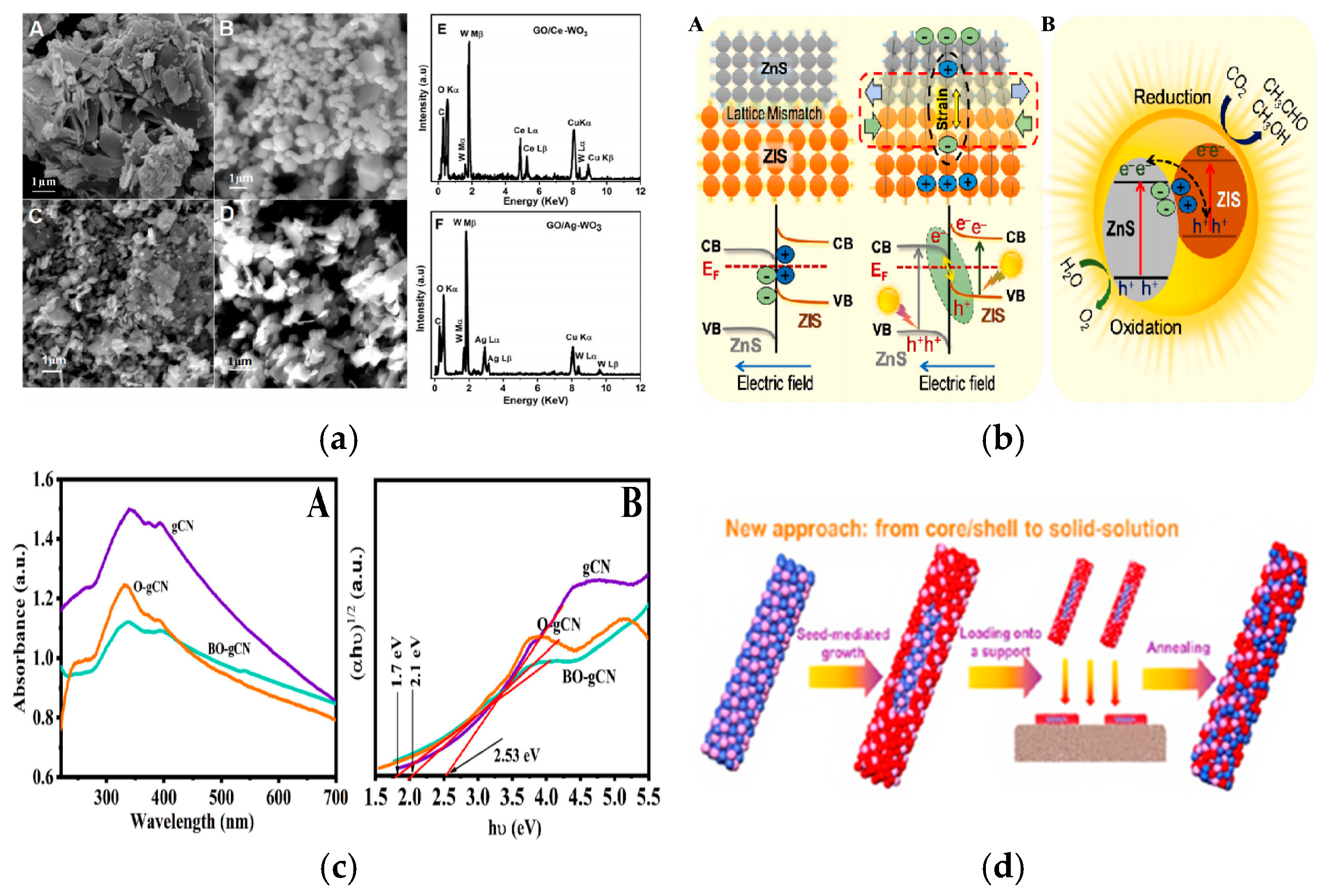
| CVD | Construction of heterogeneous structures | WO3, AgNO3, deionized water, etc. | Ultrasonic, stirring, drying, high-temperature calcination | Ag/WO₃ nanocomposite | [75] |
| CVD | The hydroxides of zinc and gadolinium were co-precipitated, then filtered, washed, dried, and calcined | Metal salts, NaOH, etc. | Stir, wash, dry, and calcine | ZnO:Gd nanoparticles | [73] |
| CVD | The precursor solution of cerium ions reacts with the precipitating agent to precipitate and form CeO2 nanoparticles | Metal salts, CTAB, NaOH, etc. | Mix and stir, evaporate and calcine | C-CeO2 | [77] |
| CVD | Template preparation, CVD growth | InAs nanowire array, C2H2, H2, etc. | High temperature and high pressure, hydrogen pretreatment, and electron bombardment of Fe catalyst | CNF/InAs hybrid nanostructures | [76] |
| CVD | Adjust the pH to make Zn2+ and Lu3+ react with HCO3− for calcination and decomposition. | Metal salts, NaHCO3, etc. | Magnetic stirring, PH adjustment, centrifugation, washing, drying, calcination | ZnO/Lu2O3 nanocomposites | [78] |
| Template-Assisted Synthesis | Template-guided growth, crystallization, and doping | Metal salts, V2O5, TEMPO, etc. | High-temperature water heat, filtration, and drying | CoVO-NBs | [83] |
| Template-Assisted Synthesis | Template-guided growth, no template for comparison | Metal salts, deionized water, ethanol, etc. | Ultrasonic, high-temperature reaction in hydrothermal reactor, ball milling | ZnO-TS, ZnO-TH, ZnO-TM | [82] |
| Template-Assisted Synthesis | Template-guided growth, cation exchange reaction | Metal salts, TAA, ethylene glycol, etc. | The reaction vessel is heated at high temperature | In2S3/CuInS2 | [81] |
| Ionothermal treatment | Al doping significantly enhances the hydrolysis activity of SrTiO₃ | SrTiO3, Al2O3 nanopowder and SrCl2 | Use air as the atmosphere and conduct for 10 h at 1423 K. | SrTiO3:Al | [85] |
| Photodeposition Method | Photoexcitation and charge separation, deposition of NiO nanoparticles | KNbO3 nanorods, CH4N2S, etc. | Irradiation with 300 W xenon lamp, photodeposition after nitrogen purging, centrifugal washing and drying | NiO/KNbO3 | [87] |
4. Modification Strategies for Nanostructured Photocatalysts
4.1. Doping Engineering
4.2. Construction of Heterojunctions
4.3. Interface Engineering and Surface Modification
5. The Application of Nanostructured Photocatalysts in the Environmental Field
5.1. Water Pollution Treatment
5.2. Air Pollution Control
5.3. Carbon Dioxide Reduction and Resource Utilization
5.4. Antibacterial and Biological Pollution Control
6. Conclusions and Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.; Cheng, M.; Lai, C.; Wei, Z.; Zhang, G.; Li, L.; Tang, C.; Du, L.; Wang, G.; Liu, H. The Collision between g-C3N4 and QDs in the Fields of Energy and Environment: Synergistic Effects for Efficient Photocatalysis. Small 2023, 19, 2205902. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Xu, Y.; Xie, M.; Li, Z.; Wu, C.; Zhao, H.; Wang, J.; Wang, H.; Yan, Y.; Zhong, N.; et al. Piezo-photocatalysts in the field of energy and environment: Designs, applications, and prospects. Nano Energy 2023, 112, 108508. [Google Scholar] [CrossRef]
- Li, M.; Han, N.; Zhang, X.; Wang, S.; Jiang, M.; Bokhari, A.; Zhang, W.; Race, M.; Shen, Z.; Chen, R.; et al. Perovskite oxide for emerging photo(electro)catalysis in energy and environment. Environ. Res. 2022, 205, 112544. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wei, J.; Fu, Z.; Zhang, M.; Zhao, S.; Xu, G.; Li, C.; Zhang, J.; Zhou, T.J.A.M. Bio-inspired synthetic hydrogen-bonded organic frameworks for efficient proton conduction. Adv. Mater. 2023, 35, 2208625. [Google Scholar] [CrossRef]
- Wang, C.; Wang, K.; Feng, Y.; Li, C.; Zhou, X.; Gan, L.; Feng, Y.; Zhou, H.; Zhang, B.; Qu, X.; et al. Co and Pt Dual-Single-Atoms with Oxygen-Coordinated Co–O–Pt Dimer Sites for Ultrahigh Photocatalytic Hydrogen Evolution Efficiency. Adv. Mater. 2021, 33, 2003327. [Google Scholar] [CrossRef]
- Merdoud, R.; Aoudjit, F.; Mouni, L.; Ranade, V.V. Degradation of methyl orange using hydrodynamic Cavitation, H2O2, and photo-catalysis with TiO2-Coated glass Fibers: Key operating parameters and synergistic effects. Ultrason. Sonochem. 2024, 103, 106772. [Google Scholar] [CrossRef]
- Safartoobi, A.; Mazloom, J.; Ghodsi, F.E. Novel electrospun bead-like Ag2MoO4 nanofibers coated on Ni foam for visible light-driven heterogeneous photocatalysis and high-performance supercapacitor electrodes. Phys. Chem. Chem. Phys. 2024, 26, 430–444. [Google Scholar] [CrossRef]
- Shi, H.; Shi, Q.; Gu, X.; Wang, B.; Lumbers, B.; Li, G. Integrating the 2D/2D heterostructure of the MXene monolayer and BiOBr nano-sheets for superior photo-catalysis. J. Colloid Interface Sci. 2024, 673, 527–536. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N.; Shah, N.K. Applications of nano-catalyst in new era. J. Saudi Chem. Soc. 2012, 16, 307–325. [Google Scholar] [CrossRef]
- Noor, S.; Sajjad, S.; Leghari, S.A.K.; Yousaf, Z.; El-Bahy, S.M. Comparative role of Ag and Ce doping on WO3 and GO modified nanostructures for bi-functional effective photocatalyst and electro catalyst. J. Phys. Chem. Solids 2024, 193, 112183. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Y.-M.; Cai, Y.; Yang, B.; Gu, C.; Zhang, S.X.-A. Advances in nanomaterials for electrochromic devices. Chem. Soc. Rev. 2020, 49, 8687–8720. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhi, J.; Wang, W.; Li, Q.; Jiang, D.; Chen, X.; Chen, Z. A mini-review on the research progress and application of nanomaterials in electrochemiluminescent sensors in the detection of water environmental pollutants. Microchim. Acta 2025, 192, 130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Rong, L.-H.; Cao, P.-F.; Advincula, R. Core–shell gold nanoparticle-star copolymer composites with gradient transfer and transport properties: Toward electro-optical sensors and catalysis. ACS Appl. Nano Mater. 2021, 4, 1394–1400. [Google Scholar] [CrossRef]
- Ding, Q.; Kang, Z.; Cao, L.; Lin, M.; Lin, H.; Yang, D.-P. Conversion of waste eggshell into difunctional Au/CaCO3 nanocomposite for 4-Nitrophenol electrochemical detection and catalytic reduction. Appl. Surf. Sci. 2020, 510, 145526. [Google Scholar] [CrossRef]
- Kumar, P.N.; Mary, J.S.S.; Chandrakala, V.; Jeyarani, W.J.; Shyla, J.M. Investigation of superior electro-optical properties of SnO2/SiO2 nanocomposite over its individual counterpart SnO2 nanoparticles. Mater. Chem. Phys. 2017, 193, 234–243. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of zero-dimensional nanomaterials in biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, T.; Li, H.; She, M.; Chen, J.; Wang, Z.; Zhang, S.; Li, J. Zero-dimensional carbon nanomaterials for fluorescent sensing and imaging. Chem. Rev. 2023, 123, 11047–11136. [Google Scholar] [CrossRef]
- Wu, X.; Guo, Y.; Zhang, Q.; Ye, Y.; Wang, Q.; Zhao, Y.; Zheng, Z.; Tao, L. CVD-Grown One-Dimensional and Two-Dimensional Sb2Se3 Semiconductor Nanomaterials for Ultrafast Fiber Laser Generation. ACS Appl. Nano Mater. 2024, 7, 28296–28305. [Google Scholar] [CrossRef]
- Yu, Y.; Bu, X.; Qi, J.; Zhang, Z.; Geng, J. Preparation and optical properties of one-dimensional magnetically oriented halloysite@ Fe3O4 nanomaterials. Appl. Clay Sci. 2024, 258, 107479. [Google Scholar] [CrossRef]
- Qian, W.; Xu, S.; Zhang, X.; Li, C.; Yang, W.; Bowen, C.R.; Yang, Y. Differences and similarities of photocatalysis and electrocatalysis in two-dimensional nanomaterials: Strategies, traps, applications and challenges. Nano-Micro Lett. 2021, 13, 156. [Google Scholar] [CrossRef]
- Shi, Z.; Ge, Y.; Yun, Q.; Zhang, H. Two-dimensional nanomaterial-templated composites. Acc. Chem. Res. 2022, 55, 3581–3593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bi, X.; Gou, M.; Sun, M.; Tao, L.; Chen, G.; Liu, X.; Meng, X.; Zhao, P. Oxidative degradation of acid red 73 in aqueous solution over a three-dimensional OMS-2 nanomaterial. Sep. Purif. Technol. 2021, 263, 118397. [Google Scholar] [CrossRef]
- Urs, K.M.; Katiyar, N.K.; Kumar, R.; Biswas, K.; Singh, A.K.; Tiwary, C.; Kamble, V. Multi-component (Ag–Au–Cu–Pd–Pt) alloy nanoparticle-decorated p-type 2D-molybdenum disulfide (MoS2) for enhanced hydrogen sensing. Nanoscale 2020, 12, 11830–11841. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, N.; Shahedi, M.; Habibi, Z.; Yousefi, M.; Ghasemi, S.; Mohammadi, M. A multi-component approach for co-immobilization of lipases on silica-coated magnetic nanoparticles: Improving biodiesel production from waste cooking oil. Bioprocess Biosyst. Eng. 2022, 45, 2043–2060. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Jia, Z.; Zhao, W.; Wu, G. Multicomponent nanoparticles synergistic one-dimensional nanofibers as heterostructure absorbers for tunable and efficient microwave absorption. Nano-Micro Lett. 2023, 15, 13. [Google Scholar] [CrossRef]
- Zheng, S.; Duley, W.W.; Peng, P.; Zhou, N. Laser modification of Au–CuO–Au structures for improved electrical and electro-optical properties. Nanotechnology 2022, 33, 245205. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Li, Y.; Wen, H.; Huang, H.; Huang, Z.; Situ, W.; Song, X. Preparation of nano-Ag-Bi2WO6–TiO2/starch bionanocomposite membranes and mechanism of enhancing visible light degradation of ethylene. Ceram. Int. 2023, 49, 30989–30998. [Google Scholar] [CrossRef]
- Ahmed, G.; Mohamed, W.; Hasaneen, M.; Saadallah, H.; Ali, H.; Ibrahim, E. Sn-doped ZnO nanostructure deposited with free-catalyst chemical vapor deposition route: Optical, electrical and photocatalytic properties. Phys. Scr. 2024, 100, a0154–a0159. [Google Scholar] [CrossRef]
- Sabbah, A.; Shown, I.; Qorbani, M.; Fu, F.-Y.; Lin, T.-Y.; Wu, H.-L.; Chung, P.-W.; Wu, C.-I.; Santiago, S.R.M.; Shen, J.-L. Boosting photocatalytic CO2 reduction in a ZnS/ZnIn2S4 heterostructure through strain-induced direct Z-scheme and a mechanistic study of molecular CO2 interaction thereon. Nano Energy 2022, 93, 106809. [Google Scholar] [CrossRef]
- Vasu, D.; Meenakshi, G.A.; Akila, B.; You, Y.-F.; Pichumani, M.; Chiu, T.-W. Heterogeneous single-atom doped 2D-layered graphitic carbon nitride electrocatalyst for oxygen evolution reaction and removal of toxic heavy metal ion Cr from Wastewater. Mater. Res. Bull. 2024, 170, 112597. [Google Scholar] [CrossRef]
- Cao, E.; Cao, Y.; Sun, M. Surface plasmonic core–shell nanostructures in surface enhanced raman scattering and photocatalysis. Anal. Chem. 2024, 96, 11623–11638. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhu, X. Plasmonics in nanostructures. Adv. Mater. 2013, 25, 3840–3856. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kong, L.; Chen, W.; Wang, H.; Zhang, J.; Gao, Z.; Xin, Y.; Xu, W.; Zuo, Y. Catalytic activation of peracetic acid for pelargonic acid vanillylamide degradation by Co3O4 nanoparticles in-situ anchored carbon-coated MXene nanosheets: Performance and mechanism insight. J. Colloid Interface Sci. 2024, 657, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Alprol, A.E.; Mansour, A.T.; Abdelwahab, A.M.; Ashour, M. Advances in green synthesis of metal oxide nanoparticles by marine algae for wastewater treatment by adsorption and photocatalysis techniques. Catalysts 2023, 13, 888. [Google Scholar] [CrossRef]
- Ullah, H.; Dilpazir, S.; Haider, A.; Shahzadi, A.; Ul-Hamid, A.; Ali, G.; Hussain, S.; Fouda, A.M.; Ikram, M. Chitosan and PVP doped cobalt/zinc catalysts for dye degradation and antimicrobial activity supported by molecular docking analysis. Mater. Today Commun. 2025, 42, 111150. [Google Scholar] [CrossRef]
- Fang, J.; Wang, D.; Xu, H.; Sun, F.; Fan, Y.; Chen, R.; Liu, Q. Unleashing solar energy’s full potential: Synergetic thermo-photo catalysis for enhanced hydrogen production with metal-free carbon nitrides. Energy Convers. Manag. 2024, 300, 117995. [Google Scholar] [CrossRef]
- Chausali, N.; Saxena, J.; Prasad, R. Nanotechnology as a sustainable approach for combating the environmental effects of climate change. J. Agric. Food Res. 2023, 12, 100541. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Qu, J.; Xie, H.; Lu, R.; Fan, F.; Li, C. Boosting photocatalytic water oxidation on photocatalysts with ferroelectric single domains. Adv. Mater. 2023, 35, 2210374. [Google Scholar] [CrossRef]
- Yang, J.; Sou, C.-K.; Lu, Y. Cell-free biocatalysis coupled with photo-catalysis and electro-catalysis: Efficient CO2-to-chemical conversion. Green Energy Environ. 2024, 9, 1366–1383. [Google Scholar] [CrossRef]
- Biswas, R.; Roy, T.; Chatterjee, S. Study of electro-optical performance and interfacial charge transfer dynamics of dye sensitized solar cells based on ZnO nanostructures and natural dyes. J. Nanoelectron. Optoelectron. 2019, 14, 99–108. [Google Scholar] [CrossRef]
- Yu, M.; Zhao, X.; Wang, X.; Chen, L.; Chen, X.; Huang, M.; Chen, W.-J.; Pan, X. Fabrication of the CdS-Cd2Nb2O7 Nanocomposite Heterojunction with Cadmium Vacancy for Efficient Piezocatalytic H2 Evolution. ACS Appl. Nano Mater. 2024, 7, 26155–26163. [Google Scholar] [CrossRef]
- Breczko, J.; Wysocka-Żołopa, M.; Grądzka, E.; Winkler, K. Zero-Dimensional carbon nanomaterials for electrochemical energy storage. ChemElectroChem 2024, 11, e202300752. [Google Scholar] [CrossRef]
- Sarfraz, J.; Rosqvist, E.; Ihalainen, P.; Peltonen, J. Electro-optical gas sensor consisting of nanostructured paper coating and an ultrathin sensing element. Chemosensors 2019, 7, 23. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, F.-W.; Liao, P.-H.; Lee, M.-L.; Sheu, J.-K. Stable photoelectrochemical water splitting using p–n GaN junction decorated with nickel oxides as photoanodes. J. Phys. Chem. C 2021, 125, 16776–16783. [Google Scholar] [CrossRef]
- Pawar, R.C.; Kang, S.; Park, J.H.; Kim, J.-h.; Ahn, S.; Lee, C.S. Room-temperature synthesis of nanoporous 1D microrods of graphitic carbon nitride (g-C3N4) with highly enhanced photocatalytic activity and stability. Sci. Rep. 2016, 6, 31147. [Google Scholar] [CrossRef]
- Liu, W.; Xie, J.; Fang, X.; Wang, D.; Wang, J. Electro-optical-magnetic-thermal coupling branched plasmonic heterojunction for efficient electrocatalytic hydrogen evolution and its dynamic process. Chem. Eng. J. 2024, 483, 149262. [Google Scholar] [CrossRef]
- Li, Q.; Gong, Z.; Li, Y.; Liu, H.; Feng, L.; Liu, S.; Yun, F. Electro-optical properties of low-temperature growth indium-tin-oxide nanowires using polystyrene spheres as catalyst. Nanoscale Res. Lett. 2016, 11, 131. [Google Scholar] [CrossRef]
- Qu, Y.; Medina, H.; Wang, S.W.; Wang, Y.C.; Chen, C.W.; Su, T.Y.; Manikandan, A.; Wang, K.; Shih, Y.C.; Chang, J.W. Wafer scale phase-engineered 1T-and 2H-MoSe2/Mo core–shell 3D-hierarchical nanostructures toward efficient electrocatalytic hydrogen evolution reaction. Adv. Mater. 2016, 28, 9831–9838. [Google Scholar] [CrossRef]
- Singh, P.; Singh, K.R.; Verma, R.; Singh, J.; Singh, R.P. Efficient electro-optical characteristics of bioinspired iron oxide nanoparticles synthesized by Terminalia chebula dried seed extract. Mater. Lett. 2022, 307, 131053. [Google Scholar] [CrossRef]
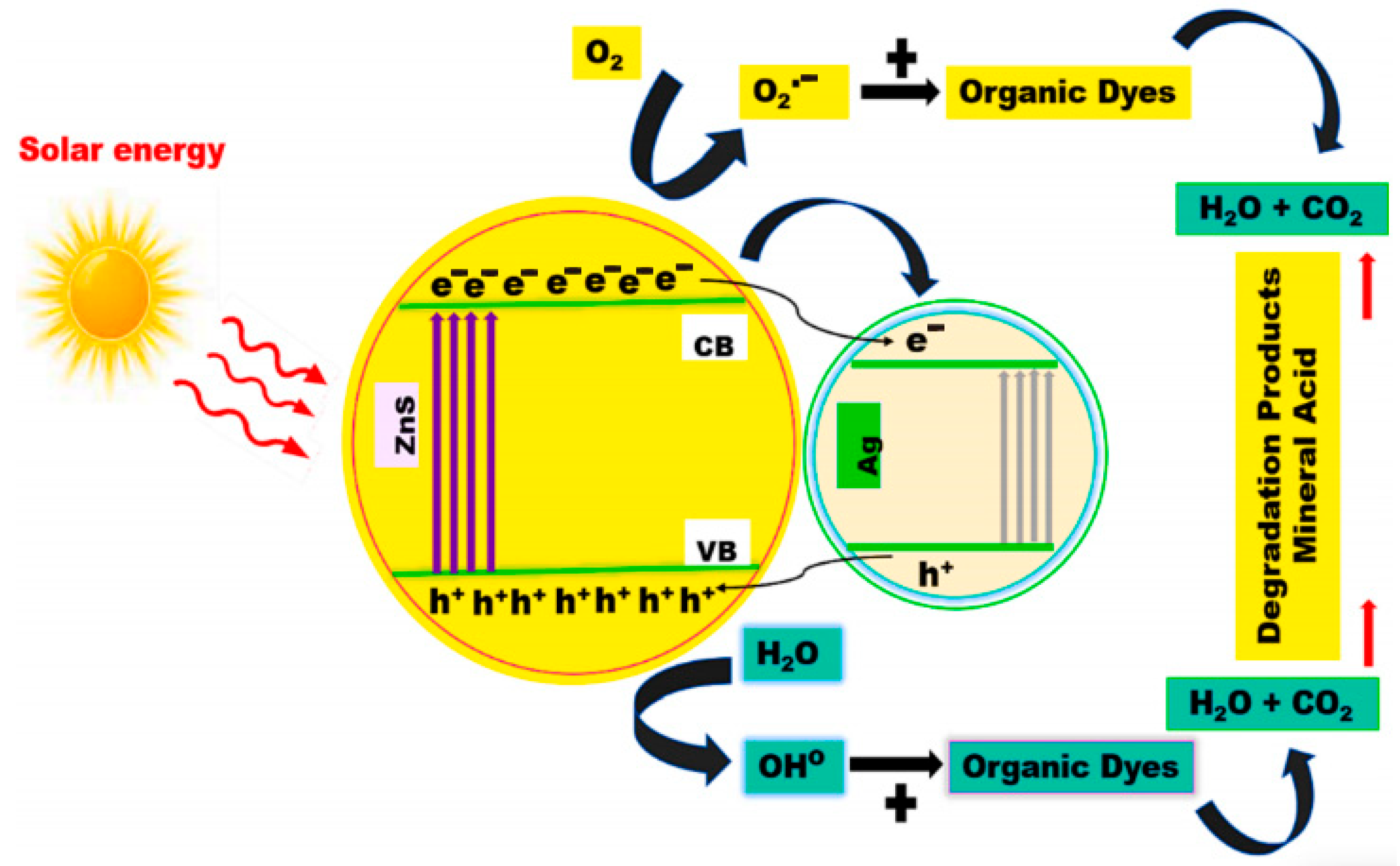
- Jothibas, M.; Suganya, S.; Muthuvel, A.; Paulson, E. The effects of Ag-ions on the physiochemical characteristics and visible-light catalytic activity of ZnS nanoparticles. Inorg. Chem. Commun. 2023, 150, 110511. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.; Lee, S.; Shim, H.; Suh, E.; Nahm, K. Characterization of ZnO needle-shaped nanostructures grown on NiO catalyst-coated Si substrates. Synth. Met. 2004, 144, 61–68. [Google Scholar] [CrossRef]
- Mhatre, M.M.; Katariya-Jain, A.; Saeed, M.H.; Deshmukh, R. Effects of multifunctional thiol monomers and BaTiO3 nanoparticles on electro-optical and dielectric properties of polymer dispersed liquid crystal films. J. Mol. Liq. 2024, 413, 125945. [Google Scholar] [CrossRef]
- Kader, M.A.; Azmi, N.S.; Kafi, A. Recent advances in gold nanoparticles modified electrodes in electrochemical nonenzymatic sensing of chemical and biological compounds. Inorg. Chem. Commun. 2023, 153, 110767. [Google Scholar] [CrossRef]
- Moumen, A.; Kaur, N.; Poli, N.; Zappa, D.; Comini, E. One dimensional ZnO nanostructures: Growth and chemical sensing performances. Nanomaterials 2020, 10, 1940. [Google Scholar] [CrossRef]
- Ravi, A.; Lims, S.C.; Aswathappa, S.; Sivakumar, M.; Dhas, S.S.J.; Almansour, A.I. Tunable band gap energy of l-Cysteine-assisted formation of Mn-doped ZnS interconnected nanoparticles for electro-optic applications. Opt. Mater. 2024, 150, 115293. [Google Scholar] [CrossRef]
- Yeh, C.C.; Zan, H.W.; Soppera, O. Solution-Based Micro-and Nanoscale Metal Oxide Structures Formed by Direct Patterning for Electro-Optical Applications. Adv. Mater. 2018, 30, 1800923. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Maeda, K.; Shiraishi, Y.; Xu, J.; Shiraki, H.; Toshima, N.; Kobayashi, S. Frequency modulation response of a tunable birefringent mode nematic liquid crystal electrooptic device fabricated by doping nanoparticles of Pd covered with liquid-crystal molecules. Jpn. J. Appl. Phys. 2002, 41, L1315. [Google Scholar] [CrossRef]
- Zhang, J.Z. Ultrafast studies of electron dynamics in semiconductor and metal colloidal nanoparticles: Effects of size and surface. Acc. Chem. Res. 1997, 30, 423–429. [Google Scholar] [CrossRef]
- Davel, C.; Bassiri-Gharb, N.; Correa-Baena, J.-P. Machine Learning in X-ray Scattering for Materials Discovery and Characterization. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Dubey, S.; Kumar, V.; Dubey, K.; Sahu, C.; Modi, A.; Gautam, U.; Sharma, R.; Haque, F.Z.; Pagare, G.; Gaur, N. Tailoring the structural and electro-optical properties of avisible-light emitting BaZrO3 photocatalyst: Integrating DFT and comprehensive experimental analysis. Nanoscale 2024, 16, 18086–18107. [Google Scholar] [CrossRef]
- Kohila Rani, K.; Xiao, Y.-H.; Devasenathipathy, R.; Gao, K.; Wang, J.; Kang, X.; Zhu, C.; Chen, H.; Jiang, L.; Liu, Q. Raman Monitoring of the Electro-Optical Synergy-Induced Enhancements in Carbon–Bromine Bond Cleavage, Reaction Rate, and Product Selectivity of p-Bromothiophenol. ACS Appl. Mater. Interfaces 2024, 16, 27831–27840. [Google Scholar] [CrossRef]
- Ahmadvand, N.; Mohammadi-Manesh, E. Effect of Nd and Cu Impurities on the Electro-Optical Properties of Recombinant of Graphene/CH3NH3PbCl3/Borophene for Photovoltaic and Biosensing Applications. Surf. Interfaces 2022, 29, 101798. [Google Scholar] [CrossRef]
- Tiwari, N.; Kadam, S.; Ingole, R.; Kulkarni, S. Facile hydrothermal synthesis of ZnFe2O4 nanostructures for high-performance supercapacitor application. Ceram. Int. 2022, 48, 29478–29483. [Google Scholar] [CrossRef]
- Barros, F.J.S.; Cardozo, K.L.P.; Cruvinel, G.H.; Longo, E.; Garcia, M.A.S.; Tanaka, A.A.; Pinatti, I.M. Microwave-assisted hydrothermal synthesis of CeVO4 nanostructures: Exploring their applicability in supercapacitor technologies. J. Mater. Sci. 2024, 59, 4236–4251. [Google Scholar] [CrossRef]
- Bouhjar, F.; Mollar, M.; Chourou, M.; Marí, B.; Bessais, B. Hydrothermal synthesis of nanostructured Cr-doped hematite with enhanced photoelectrochemical activity. Electrochim. Acta 2018, 260, 838–846. [Google Scholar] [CrossRef]
- Fernández-Catalá, J.; Singh, H.; Wang, S.; Huhtinen, H.; Paturi, P.; Bai, Y.; Cao, W. Hydrothermal synthesis of Ni3TeO6 and Cu3TeO6 nanostructures for magnetic and photoconductivity applications. ACS Appl. Nano Mater. 2023, 6, 4887–4897. [Google Scholar] [CrossRef]
- Ahmad, S.; Aadil, M.; Ejaz, S.R.; Akhtar, M.U.; Noor, H.; Haider, S.; Alsafari, I.A.; Yasmin, G. Sol-gel synthesis of nanostructured ZnO/SrZnO2 with boosted antibacterial and photocatalytic activity. Ceram. Int. 2022, 48, 2394–2405. [Google Scholar] [CrossRef]
- Giordano, C.; Antonietti, M. Synthesis of crystalline metal nitride and metal carbide nanostructures by sol–gel chemistry. Nano Today 2011, 6, 366–380. [Google Scholar] [CrossRef]
- Laghrib, S.; Bouchikhi, N.; Gherdaoui, C.E.; Hamroun, M.S.E.; Belgherbi, O.; Alaoui, C.; Djamaa, Z. Strontium and manganese co-doped TiO2 nanoparticles for the enhanced photocatalytic degradation of methylene blue dye. React. Kinet. Mech. Catal. 2024, 137, 2899–2916. [Google Scholar] [CrossRef]
- Martin Mark, J.A.; Nallusamy, S.; Pandiaraj, S.; Alodhayb, A.N.; Alzahrani, K.E. Integrating Sn4+ ions to spinel nickel manganite (NiMn2O4) by sol–gel approach to enhance its electrochemical and photocatalytic properties. J. Sol-Gel Sci. Technol. 2025, 1–20. [Google Scholar] [CrossRef]
- Chitalkar, K.; Hase, D.; Gurav, S.; Musmade, S.; Gaikar, R.; Sillanpää, M.; Murade, V.; Aher, H. Sol-gel Synthesis of Ternary ZnO-Cu2O@AC Nanocomposite: Characterization and Photocatalytic Application for Environmental Remediation. J. Inorg. Organomet. Polym. Mater. 2025, 1–15. [Google Scholar] [CrossRef]
- Satyanarayana, P.E.; Roja, K.; Babu, N.G.; Dhangar, B.K.; Akram, S.; Kiran, G.B.; Narendrudu, T.; Babu, N.C.R.; Kalpana, A. Improvment of electrocaloric energy storage properties in eco-friendly 0.63Na0.5Bi0.5TiO3-0.37SrTiO3-NaNbO3 ceramic synthesized by sol–gel route. J. Mater. Sci. Mater. Electron. 2025, 36, 410. [Google Scholar] [CrossRef]
- Mazhdi, M.; Tafreshi, M.J. The effects of gadolinium doping on the structural, morphological, optical, and photoluminescence properties of zinc oxide nanoparticles prepared by co-precipitation method. Appl. Phys. A 2018, 124, 863. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Yang, P.; Li, R.; Wang, D.; Ren, P.; Ji, S.; Lu, X.; Meng, F.; Zhang, J. Interfacial engineering induced highly efficient CoNiP@NiFe layered double hydroxides bifunctional electrocatalyst for water splitting. Mater. Today Energy 2022, 25, 100975. [Google Scholar] [CrossRef]
- Matalkeh, M.; Nasrallah, G.K.; Shurrab, F.M.; Al-Absi, E.S.; Mohammed, W.; Elzatahry, A.; Saoud, K.M. Visible light photocatalytic activity of Ag/WO3 nanoparticles and its antibacterial activity under ambient light and in the dark. Results Eng. 2022, 13, 100313. [Google Scholar] [CrossRef]
- Arshad, M.; Sorba, L.; Rudolf, P.; Cepek, C. Growth and Characterization of Carbon Nanofibers Grown on Vertically Aligned InAs Nanowires via Chemical Vapour Deposition. Nanomaterials 2023, 13, 3083. [Google Scholar] [CrossRef]
- Sebastiammal, S.; Bezy, N.A.; Somaprabha, A.; Henry, J.; Biju, C.; Fathima, A.L. Chemical and sweet basil leaf mediated synthesis of cerium oxide (CeO2) nanoparticles: Antibacterial action toward human pathogens. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 237–243. [Google Scholar] [CrossRef]
- Alsaedi, W.; Aljuhani, A.; Alahmadi, M.; Qassium, H.; Hussein, B.H.; Alawad, M.O.; Khushaim, M.; Abu-Dief, A.M. Fabrication of a novel ZnO/Lu2O3 nanomaterial for the photocatalytic disposal of methylene blue dye under solar cell illumination. J. Mater. Sci. Mater. Electron. 2025, 36, 327. [Google Scholar] [CrossRef]
- Sun, S.; Xiao, Y.; He, L.; Tong, Y.; Liu, D.; Zhang, J. Zr-Based Metal-Organic Framework Films Grown on Bio-Template for Photoelectrocatalysis. ChemistrySelect 2020, 5, 13855–13861. [Google Scholar] [CrossRef]
- Huo, X.; Luo, B.; Zhang, Y.; Li, H. Facile template-assisted synthesis of PtBiTe nanoplates for CO-free methanol oxidation in alkaline electrolytes. Green Chem. 2025, 27, 3980–3989. [Google Scholar] [CrossRef]
- Liao, A.; Liu, Z.; Wei, Y.; Xie, Q.; Kong, T.; Zeng, M.; Wang, W.; Yang, C.; Zhang, L.; Xu, Y. Synthesis of Sulfur Vacancy-Bearing In2S3/CuInS2 Microflower Heterojunctions via a Template-Assisted Strategy and Cation-Exchange Reaction for Photocatalytic CO2 Reduction. Molecules 2024, 29, 3334. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Mehta, V.S.; Kaur, G.; Sud, D. Biopolymer templated strategized greener protocols for fabrication of ZnO nanostructures and their application in photocatalytic technology for phasing out priority pollutants. Environ. Sci. Pollut. Res. 2023, 30, 25663–25681. [Google Scholar] [CrossRef]
- Youn, C.; Shin, S.; Shin, K.; Kim, C.; Park, C.-L.; Choi, J.; Kim, S.H.; Yeo, S.Y.; Shin, M.W.; Henkelman, G. Template-assisted synthesis of single-atom catalysts supported on highly crystalline vanadium pentoxide for stable oxygen evolution. Chem Catalysis 2022, 2, 1191–1210. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Antonietti, M. Metal poly (heptazine imides) as multifunctional photocatalysts for solar fuel production. Angew. Chem. Int. Ed. 2024, 63, e202406290. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Hisatomi, T.; Wang, Q.; Higashi, T.; Ishikiriyama, K.; Maeda, T.; Sakata, Y.; Okunaka, S.; Tokudome, H.; Katayama, M. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2018, 2, 509–520. [Google Scholar] [CrossRef]
- Manukumar, K.; Nagaraju, G.; Praveen Kumar, D.; Shankar, M. Facile ionothermal synthesis of TiO2 nanorods for photocatalytic H2 generation. J. Mater. Sci. Mater. Electron. 2019, 30, 1076–1083. [Google Scholar] [CrossRef]
- Xing, P.; Zhang, W.; Chen, L.; Dai, X.; Zhang, J.; Zhao, L.; He, Y. Preparation of a NiO/KNbO3 nanocomposite via a photodeposition method and its superior performance in photocatalytic N2 fixation. Sustain. Energy Fuels 2020, 4, 1112–1117. [Google Scholar] [CrossRef]
- Yadav, S.M.; Desai, M.A.; Sartale, S.D. Simplistic synthesis of ZnO/g-C3N4 heterojunction photocatalyst for improved photodegradation performance. J. Mater. Sci. Mater. Electron. 2024, 35, 1116. [Google Scholar] [CrossRef]
- Fauziah, N.; Syarifuddin, S.; Heryanto, H.; Tahir, D. Nanocrystal composite (CoFe2O4)/(Mg) for photocatalyst of methylene blue and Congo red: Stability structural properties from X-ray diffraction and chemical bonding from infra-red spectroscopy. J. Mater. Res. 2023, 38, 2059–2071. [Google Scholar] [CrossRef]
- Liu, W.-J.; Zeng, F.-X.; Jiang, H.; Zhang, X.-S.; Li, W.-W. Composite Fe2O3 and ZrO2/Al2O3 photocatalyst: Preparation, characterization, and studies on the photocatalytic activity and chemical stability. Chem. Eng. J. 2012, 180, 9–18. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.; Hou, J.; Yang, Y. Visible photocatalysis and photostability of Ag3PO4 photocatalyst. Appl. Surf. Sci. 2014, 319, 332–338. [Google Scholar] [CrossRef]
- Masoumbaigi, H.; Rezaee, A.; Hosseini, H.; Hashemi, S. Water disinfection by zinc oxide nanoparticle prepared with solution combustion method. Desalination Water Treat. 2015, 56, 2376–2381. [Google Scholar] [CrossRef]
- Kiran, K.; Shashanka, R.; Lokesh, S. Enhanced photocatalytic activity of hydrothermally synthesized perovskite Strontium titanate nanocubes. Top. Catal. 2022, 1–10. [Google Scholar] [CrossRef]
- Butt, T.M.; Janjua, N.K.; Mujtaba, A.; Zaman, S.A.; Ansir, R.; Rafique, A.; Sumreen, P.; Mukhtar, M.; Pervaiz, M.; Yaqub, A. B-site doping in lanthanum cerate nanomaterials for water electrocatalysis. J. Electrochem. Soc. 2020, 167, 026503. [Google Scholar] [CrossRef]
- Jayoti, D.; Malik, P.; Prasad, S.K. Effect of ZnO nanoparticles on the morphology, dielectric, electro-optic and photo luminescence properties of a confined ferroelectric liquid crystal material. J. Mol. Liq. 2018, 250, 381–387. [Google Scholar] [CrossRef]
- Selvaraj, V.; Mahboub, H.H.; Ganapathi, U.; Chandran, S.K.; Al-Onazi, W.; Al-Mohaimeed, A.M.; Chen, T.-W.; Faggio, C.; Paulraj, B. Enhanced photodegradation of methylene blue from aqueous solution using Al-doped ZnS nanoparticles. Environ. Sci. Pollut. Res. 2022, 29, 73528–73541. [Google Scholar] [CrossRef]
- Iqbal, T.; Munir, R.M.; Farooq, H.; Afsheen, S.; Younas, A.; Pham, P.V.; Syed, A.; AL-Shwaiman, H.A.; Wong, L.S. Novel Fe doped NiO-based electrode material for photoactivated catalyst and supercapacitor application. J. Energy Storage 2024, 103, 114284. [Google Scholar] [CrossRef]
- Veni, K.K.; Nehru, L.; Kavitha, R.; Sagadevan, S. Synergistic effect of sonophotocatalytic degradation of crystal violet and alizarin red S dyes using Ag-doped ZnSnO3 nanoparticles. Surf. Interfaces 2024, 51, 104590. [Google Scholar] [CrossRef]
- Rogolino, A.; Silva, I.F.; Tarakina, N.V.; da Silva, M.A.; Rocha, G.F.; Antonietti, M.; Teixeira, I.F. Modified poly (heptazine imides): Minimizing H2O2 decomposition to maximize oxygen reduction. ACS Appl. Mater. Interfaces 2022, 14, 49820–49829. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Yu, Y.; Zhao, Y.; Guo, Z.; Yao, R.; Gao, J.; Zhang, Y.; Wang, D. Electro-optical characteristics of polymer dispersed liquid crystal doped with MgO nanoparticles. Molecules 2022, 27, 7265. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, Q.; Yuan, S.; Zhang, Y.; Zhang, M. One-pot facile synthesis of branched Ag-ZnO heterojunction nanostructure as highly efficient photocatalytic catalyst. Appl. Surf. Sci. 2015, 353, 949–957. [Google Scholar] [CrossRef]
- Qin, Q.; Shi, Q.; Meng, J.; Wan, J.; Hu, Z. Visible-Light Response and High-Efficiency Photocatalytic Elimination of Polycyclic Organic Pollutants of Layer-By-Layer Assembled Ternary Nanotubular Catalysts. ChemistrySelect 2018, 3, 11414–11421. [Google Scholar] [CrossRef]
- Yi, P.; Song, Y.; Liu, Z.; Xie, P.; Liang, G.; Liu, R.; Chen, L.; Sun, J. Boosting alkaline urea oxidation with a nickel sulfide/cobalt oxide heterojunction catalyst via interface engineering. Adv. Compos. Hybrid Mater. 2023, 6, 228. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Hu, T.; Zhu, X.; Wang, H.; Zhang, W.; Chen, S.; Cheng, S.; Cao, Y.; Li, Y. Enhanced photocatalytic performance of multifunctional composite Ag-Ag2Se@ CdSe/3DOM TiO2 with dual Z-scheme heterostructures coupling Ag NPs: Degradation, hydrogen production and antibacterial activity. J. Alloys Compd. 2025, 1021, 179571. [Google Scholar] [CrossRef]
- Tong, H.; Odutola, J.; Song, J.; Peng, L.; Tkachenko, N.; Antonietti, M.; Pelicano, C.M. Boosting the quantum efficiency of ionic carbon nitrides in photocatalytic H2O2 evolution via controllable n→π* electronic transition activation. Adv. Mater. 2024, 36, 2412753. [Google Scholar] [CrossRef]
- Bizarro, M.; Tapia-Rodríguez, M.; Ojeda, M.; Alonso, J.; Ortiz, A. Photocatalytic activity enhancement of TiO2 films by micro and nano-structured surface modification. Appl. Surf. Sci. 2009, 255, 6274–6278. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, J.; Liu, J.; Wang, D.; Zhao, Z.; Cheng, K.; Li, J. Enhanced hydrothermal stability of Cu-ZSM-5 catalyst via surface modification in the selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2016, 375, 186–195. [Google Scholar] [CrossRef]
- Zhou, Z.; Orcutt, E.K.; Anderson, H.C.; Stowers, K.J. Hydrogen surface modification of a carbon nanotube catalyst for the improvement of ethane oxidative dehydrogenation. Carbon 2019, 152, 924–931. [Google Scholar] [CrossRef]
- Alkhalifa, R.A.; Albadri, A.E.; Ali, R.; Alluhayb, A.H.; Younis, A.M.; Saleh, S.M. Sustainable Synthesis of Zirconium Dioxide (ZrO2) Nanoparticles Utilizing Asphodelus fistulosus Extract for Congo Red Degradation. Catalysts 2025, 15, 123. [Google Scholar] [CrossRef]
- Chen, T.; Li, Y.; Ma, F.; Sun, M.; Fu, P.; Liu, X.; Zhou, Y.; Wang, J. Amino-tethered Ni3S2/MoS2 heterojunction for coupling electrochemical 5-hydroxymethylfurfural oxidation with 4-nitrophenol hydrogenation. J. Mater. Chem. A 2024, 12, 12237–12249. [Google Scholar] [CrossRef]
- Sun, K.; Yuan, H.; Yan, Y.; Qin, H.; Sun, L.; Tan, L.; Guo, F.; Du, X.; Shi, W. Visible-light-response 2D/2D Bi2Fe4O9/ZnIn2S4 van der Waals S-scheme heterojunction with efficient photocatalysis-self-Fenton degradation of antibiotics. J. Water Process Eng. 2024, 58, 104803. [Google Scholar] [CrossRef]
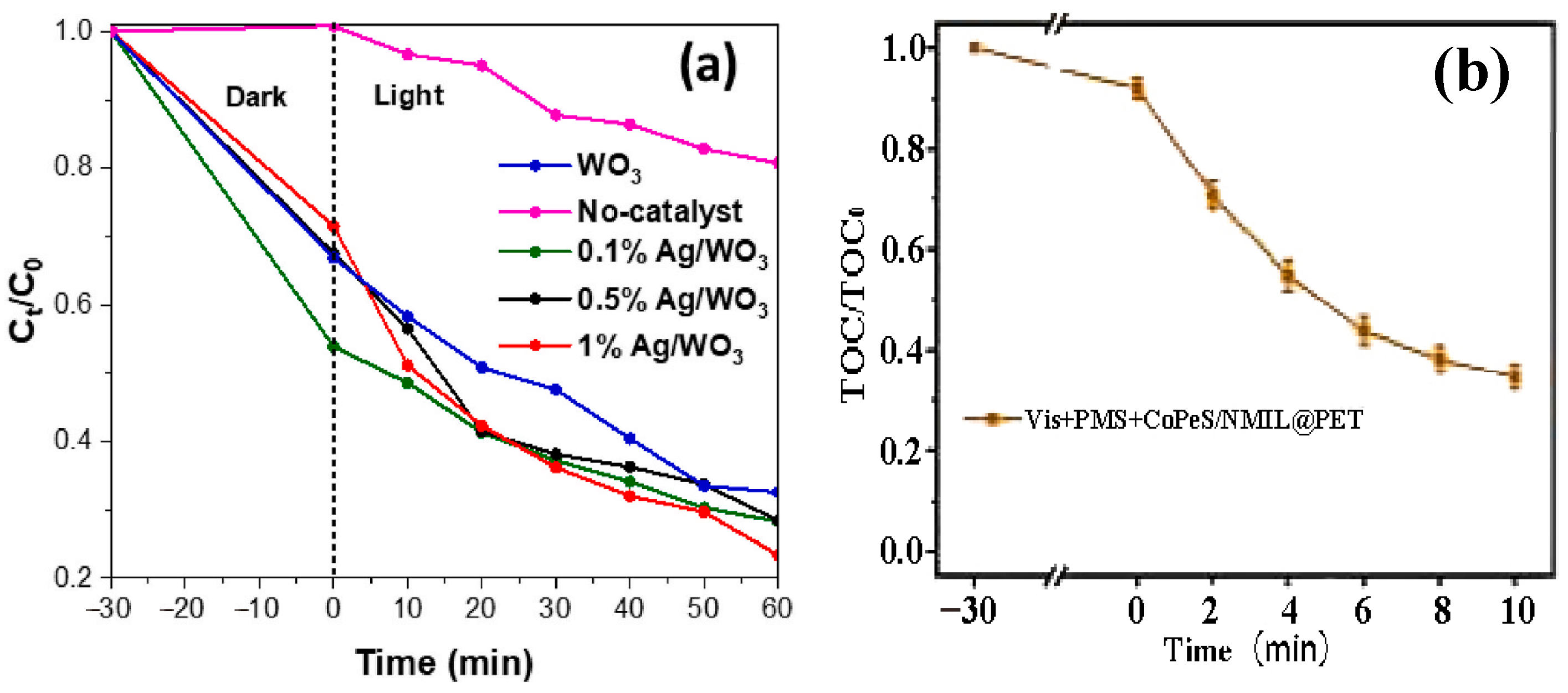
- Wu, Z.; Xue, J.; Chen, Z.; Lv, Y.; Ma, S.; Ma, J.; Wang, M. Sulfonated cobalt phthalocyanine/NH2-MIL-125 heterojunction modified electrospun nanofiber membrane for emulsion separation and PMS-photocatalysis synergistic degradation. Process Saf. Environ. Prot. 2024, 187, 707–720. [Google Scholar] [CrossRef]
- Xue, Q.; Song, B.; Feng, Q.; Yu, Z.; Hu, K.; Yang, Y.; Shen, X. Efficiently removal of tetracycline via synergistic photocatalysis with Fenton reaction with biochar/FeOOH. Appl. Surf. Sci. 2024, 645, 158869. [Google Scholar] [CrossRef]
- Mera, A.C.; Martínez-de la Cruz, A.; Pérez-Tijerina, E.; Meléndrez, M.; Valdés, H. Nanostructured BiOI for air pollution control: Microwave-assisted synthesis, characterization and photocatalytic activity toward NO transformation under visible light irradiation. Mater. Sci. Semicond. Process. 2018, 88, 20–27. [Google Scholar] [CrossRef]
- Lin, J.; Li, Q.; Lu, S.; Chen, X.; Liew, K.M. Cu-Mn-Ce ternary oxide catalyst coupled with KOH sorbent for air pollution control in confined space. J. Hazard. Mater. 2020, 389, 121946. [Google Scholar] [CrossRef]
- Le Pivert, M.; Kerivel, O.; Zerelli, B.; Leprince-Wang, Y. ZnO nanostructures based innovative photocatalytic road for air purification. J. Clean. Prod. 2021, 318, 128447. [Google Scholar] [CrossRef]
- Bhandari, S.; Khatun, R.; Poddar, M.K.; Kothari, A.C.; Bal, R.J.M.C. Direct oxidation of cyclohexane to adipic acid in air over Co3O4@ ZrO2 nanostructured catalyst. Mol. Catal. 2022, 528, 112473. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Meng, Y.; Zhang, C.; Li, X.; Ni, Z.; Zhang, X. Fabrication of site activated and synergistic double vacancy ZnIn2S4 for highly efficient bifunctional photocatalysis: Nitrogen reduction and oxidative degradation. J. Mater. Chem. A 2024, 12, 2294–2308. [Google Scholar] [CrossRef]
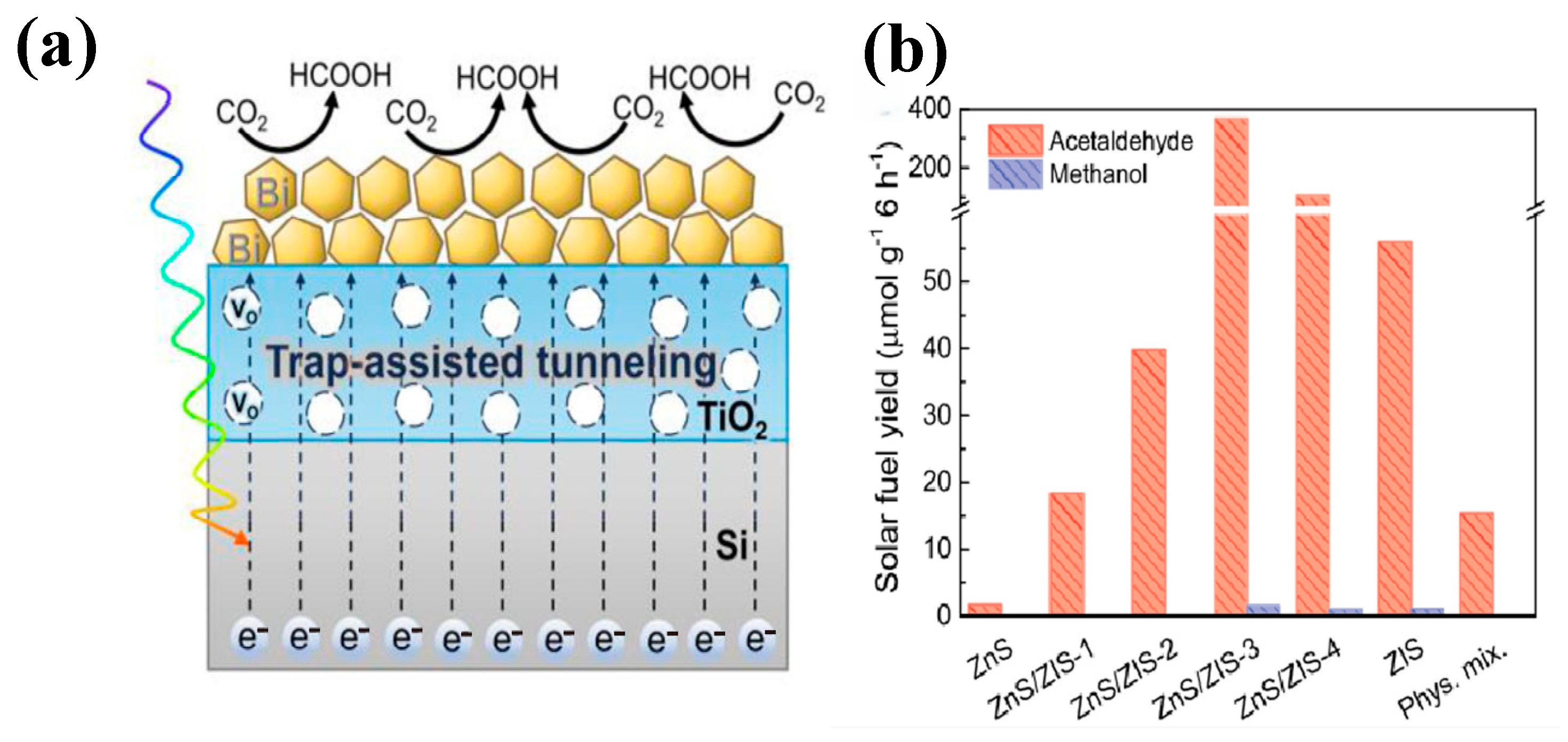
- Xing, J.; Shen, J.; Wei, Z.; Zheng, Z.; Cao, Y.; Chen, C.; Olu, P.Y.; Dong, W.; Peng, Y.; Shen, M. Dual Effect of Oxygen Vacancy-Enriched TiO2 Interlayer in Si Photocathode for Enhanced Photoelectrochemical CO2 Reduction to HCOOH. Small 2025, 21, 2502226. [Google Scholar] [CrossRef]
- He, X.; Wang, W.-N. MOF-based ternary nanocomposites for better CO2 photoreduction: Roles of heterojunctions and coordinatively unsaturated metal sites. J. Mater. Chem. A 2018, 6, 932–940. [Google Scholar] [CrossRef]
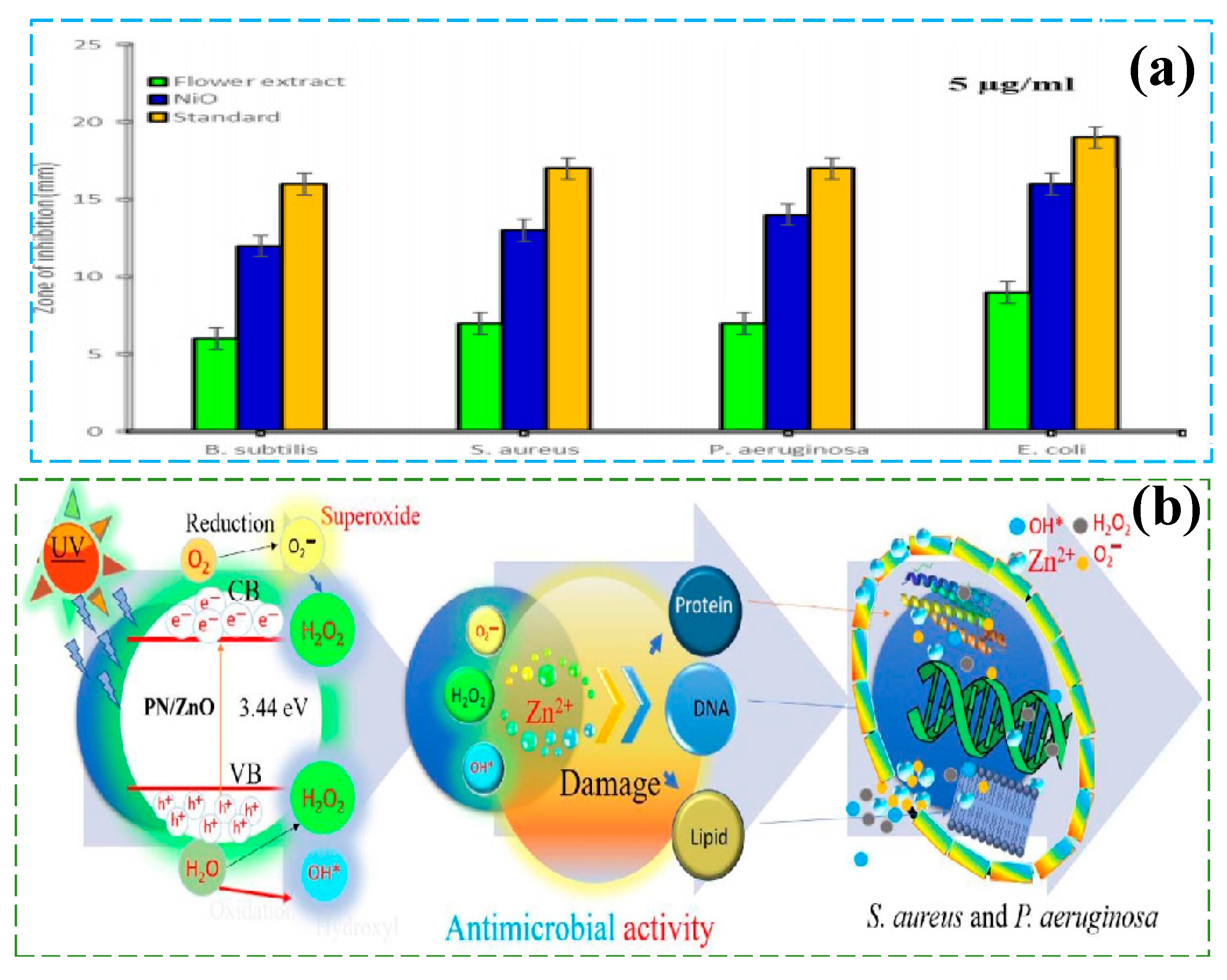
- Al-Zaqri, N.; Umamakeshvari, K.; Mohana, V.; Muthuvel, A.; Boshaala, A. Green synthesis of nickel oxide nanoparticles and its photocatalytic degradation and antibacterial activity. J. Mater. Sci. Mater. Electron. 2022, 33, 11864–11880. [Google Scholar] [CrossRef]
- Amiri, M.R.; Alavi, M.; Taran, M.; Kahrizi, D. Antibacterial, antifungal, antiviral, and photocatalytic activities of TiO2 nanoparticles, nanocomposites, and bio-nanocomposites: Recent advances and challenges. J. Public Heal. Res. 2022, 11, 22799036221104151. [Google Scholar] [CrossRef]
- Gaur, J.; Kumar, S.; Kaur, H.; Pal, M.; Bala, K.; Batoo, K.M.; Momoh, J.O.; Hussain, S. Eco-friendly innovation: Harnessing nature’s blueprint for enhanced photocatalysis and antimicrobial potential in multi-structured PN/ZnO nanoparticles. Funct. Compos. Struct. 2024, 6, 015005. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Deng, H.; Li, Z.; Xue, B.; Liu, A.; Shen, B.; Hao, D.; Zhu, H.; Wang, Q. Schottky heterojunction-based photocatalysis-in-situ-self-Fenton system: Removal of tetracycline hydrochloride and biotoxicity evaluation of intermediates. Appl. Catal. B Environ. Energy 2025, 360, 124533. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Ren, X.; Yuan, S.; Zhao, L.; Zhuang, L.; Teng, B.; Wu, Y.; He, Y. Heterophase structure of ZnSnO3 (rhombohedral and orthorhombic) for efficient dye degradation and N2-to-NH3 conversion via piezocatalysis and piezo-photocatalysis. Chem. Eng. J. 2024, 498, 155202. [Google Scholar] [CrossRef]
- Karthika, M.; Balu, A.; Vinitha, G.; Delci, Z.; Suganya, M.; Devi, S.C.; Devendran, K.; Sriramraj, M. Electrochemical, third order nonlinear optical and antibacterial properties of chitosan, a cationic polymer loaded CuS: Co nanoparticles. Ceram. Int. 2023, 49, 17806–17817. [Google Scholar] [CrossRef]
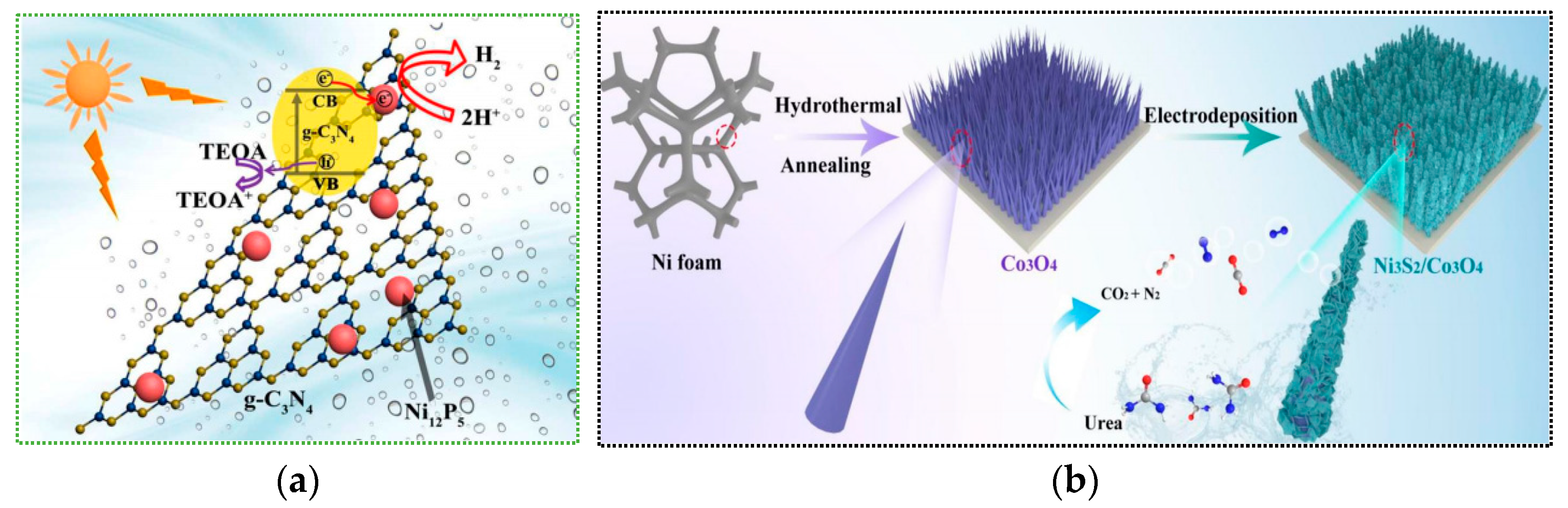


| Materials | Synthesis Method | Application Direction | Performance Index | Test Conditions (Light Source, Reaction Time, Catalyst Dosage, etc.) | Ref. |
|---|---|---|---|---|---|
| Bi2Fe4O9/ZnIn2S4 | Hydrothermal method (HTM) | Antibiotic (TC) degradation | 88.8% degradation rate of TC (120 min) | Visible light, 120 min, TC concentration: 50 mg/L | [111] |
| Ag/WO3 | Sol-Gel Technique | Degradation of dye (MB) | 80% degradation rate of MB (120 min) | Visible light and UV light, 120 min, 1% Ag/WO3 | [75] |
| BC/FeOOH | Carbonization-Hydrothermal Method | Antibiotic (TC) degradation | 92% degradation rate of TC (90 min) | Visible light, TC concentration: 20 mg/L, pH = 9 | [113] |
| CS/PVP-Co3O4/ZnO | Low-temperature Coprecipitation Method (LTCP) | Degradation of dye (RhB) | 83.3%degradation rate of RhB | Visible light, Neutral medium, 4%–CS/PVP-Co3O4/ZnO | [35] |
| Cu-Mn-Ce ternary catalyst | Wet/Solid-state Impregnation Method | Oxidation of CO | 50% conversion rate of CO | Heat from 5 °C/min to 150 °C, Nitrogen flow | [115] |
| ZnSnO3 heterogeneous junction | HTM and PM | conversion of N2 to NH3 | the yield of NH3 is 389 μmol/(L·g·h) | Ultrasonic vibration, atmosphere | [125] |
| BiOI nanostructure | Microwave—Assisted Solvothermal Method (MAM) | NO conversion | 61% conversion rate of NO | 24 WLED light source, 200 mg catalyst | [114] |
| ZnIn2S4VZn+S | Solvothermal Method (STM) | conversion of N2 to NH3 | the yield of NH3 is 198.71 μmol/(g·h) | Visible light, reaction for 1 h, isotope labeling | [118] |
| ZnS-ZIS | Hydrothermal Method (HTM) | CO2 reduction | the yields of acetaldehyde and methanol are 367.63 μmol/(g·h) and 1.37 μmol/(g·h). | Visible light, reaction for 3 h, | [29] |
| Si/dT/Bi and Si/T/Bi | Photoelectrod eposition Method (PED) | CO2 reduction | faradaic efficiencies of Si/dT/Bi and Si/T/Bi are 82.7% and 45.5%, respectively. | Simulate sunlight, 28 h and 2 h | [119] |
| TiO2/Cu2O/Cu3(BTC)2 | Aerosol Method (AM) | CO2 reduction | the CO yield of 310 μmol/(g·h) and the CH4 yield of μmol/(g·h) | 450 W Xe lamp, 7 h | [120] |
| PN/ZnO NPs | Hydrothermal method (HTM) | Antibacterial performance (S. aureus), (P. aeruginosa) | the diameters of the antibacterial zones are respectively 2.93 cm and 3.145 cm | 6 W ultraviolet lamp, 37 °C, 24 h, Catalyst dosage 10–40 µL | [123] |
| CuS:Co NPs | Precipitation Method (PM) | Antibacterial performance (S. aureus) | diameter of the antibacterial zone: 2.55 cm | Continuous-wave Nd: YAG laser, 6 mg | [126] |
| NiO nanoparticles | Green Synthesis Method (GSM) | Antibacterial performance (E. coli) | diameter of the antibacterial zone: 23 mm | Sunlight, 37 °C, 24–28 h, Catalyst dosage: 0.2 g/L | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Shi, Q.; Peng, T.; Cao, X.; Lv, Y. Research Progress on the Synthesis of Nanostructured Photocatalysts and Their Environmental Applications. Nanomaterials 2025, 15, 681. https://doi.org/10.3390/nano15090681
Niu Y, Shi Q, Peng T, Cao X, Lv Y. Research Progress on the Synthesis of Nanostructured Photocatalysts and Their Environmental Applications. Nanomaterials. 2025; 15(9):681. https://doi.org/10.3390/nano15090681
Chicago/Turabian StyleNiu, Yanan, Qi Shi, Tai Peng, Xi Cao, and Yuguang Lv. 2025. "Research Progress on the Synthesis of Nanostructured Photocatalysts and Their Environmental Applications" Nanomaterials 15, no. 9: 681. https://doi.org/10.3390/nano15090681
APA StyleNiu, Y., Shi, Q., Peng, T., Cao, X., & Lv, Y. (2025). Research Progress on the Synthesis of Nanostructured Photocatalysts and Their Environmental Applications. Nanomaterials, 15(9), 681. https://doi.org/10.3390/nano15090681







