Investigation on the Interfaces in Organic Devices by Photoemission Spectroscopy
Abstract
1. Introduction
2. Substrate-Controlled Growth of Organic Molecular Thin Films
2.1. Growth of Organic Molecules
2.1.1. Organic Molecular Growth Modes
2.1.2. Temperature and Rate Control of Organic Molecular Growth
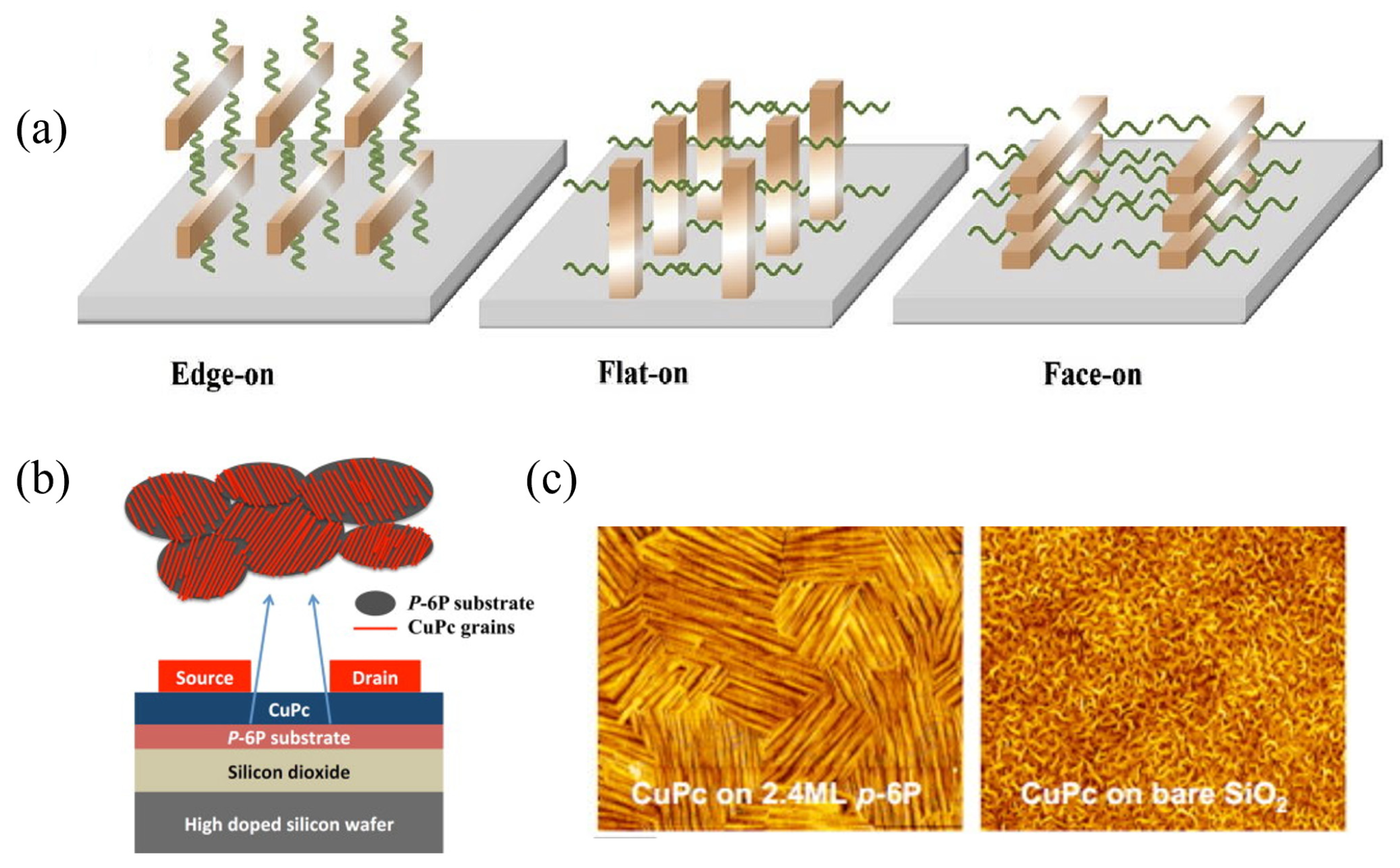
2.2. Organic Molecular Orientation
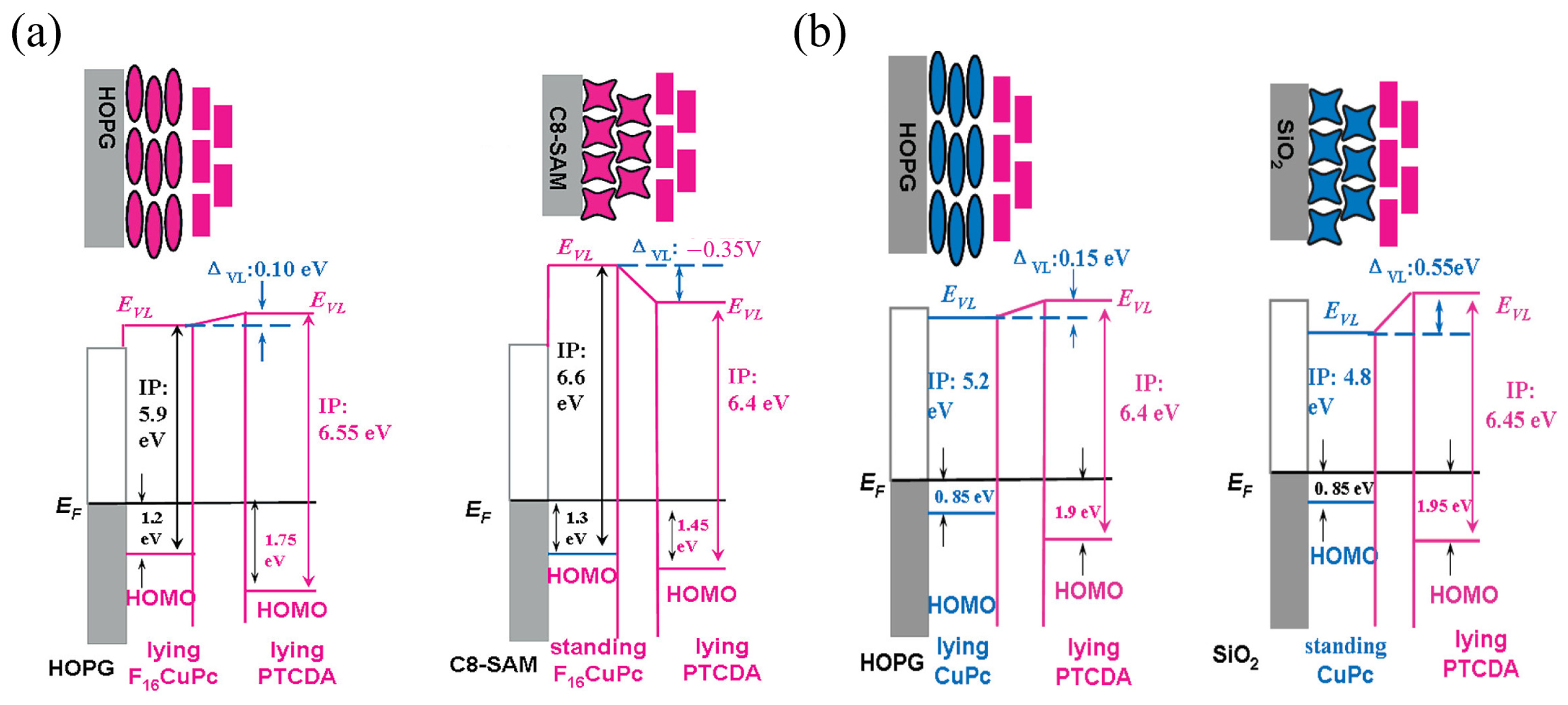
2.2.1. Influence of Molecular Orientation on Charge Transport and Interface Energy Levels
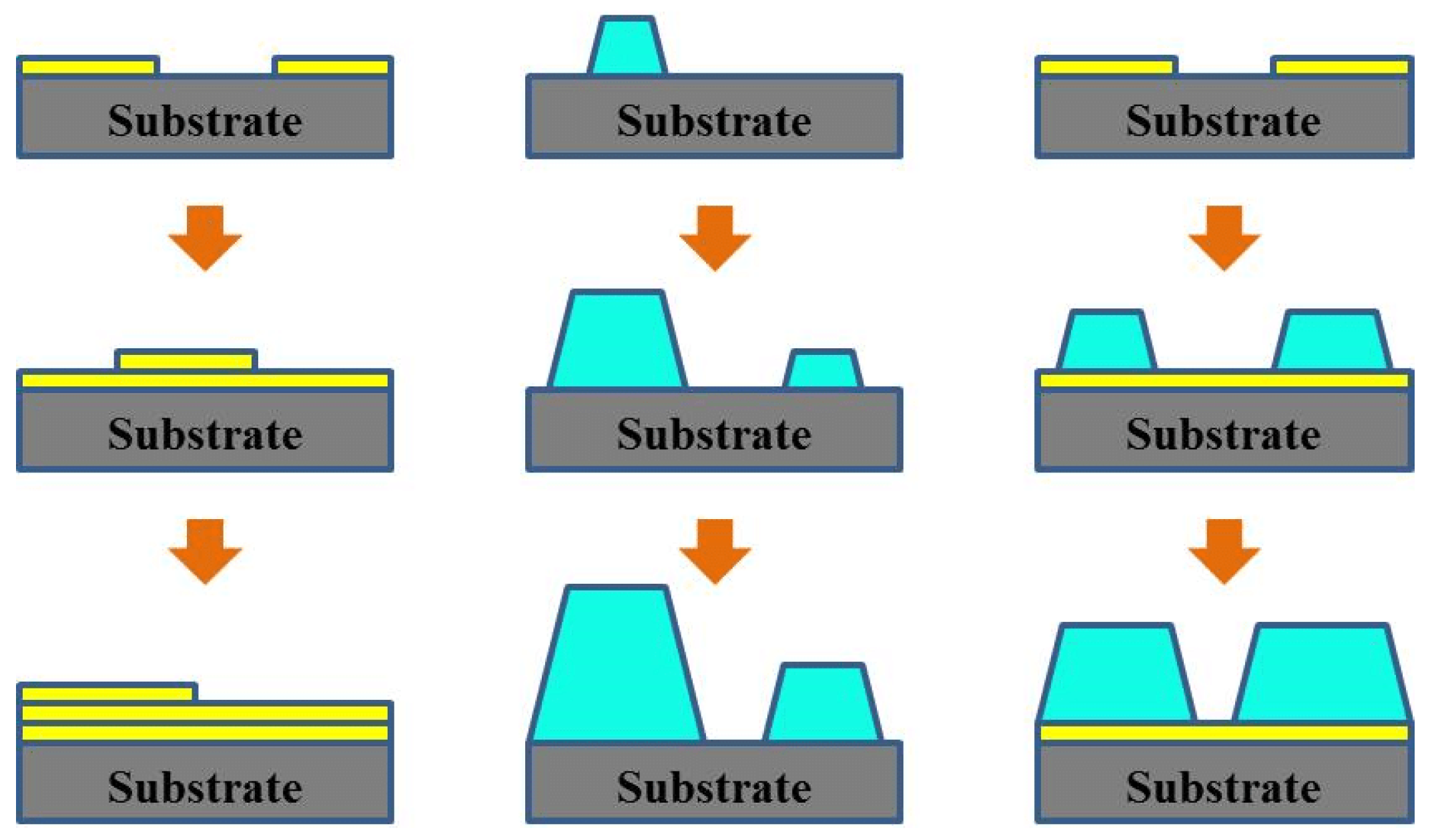
2.2.2. Control of Molecular Orientation
3. Interfacial Electronic Structure and Modulation
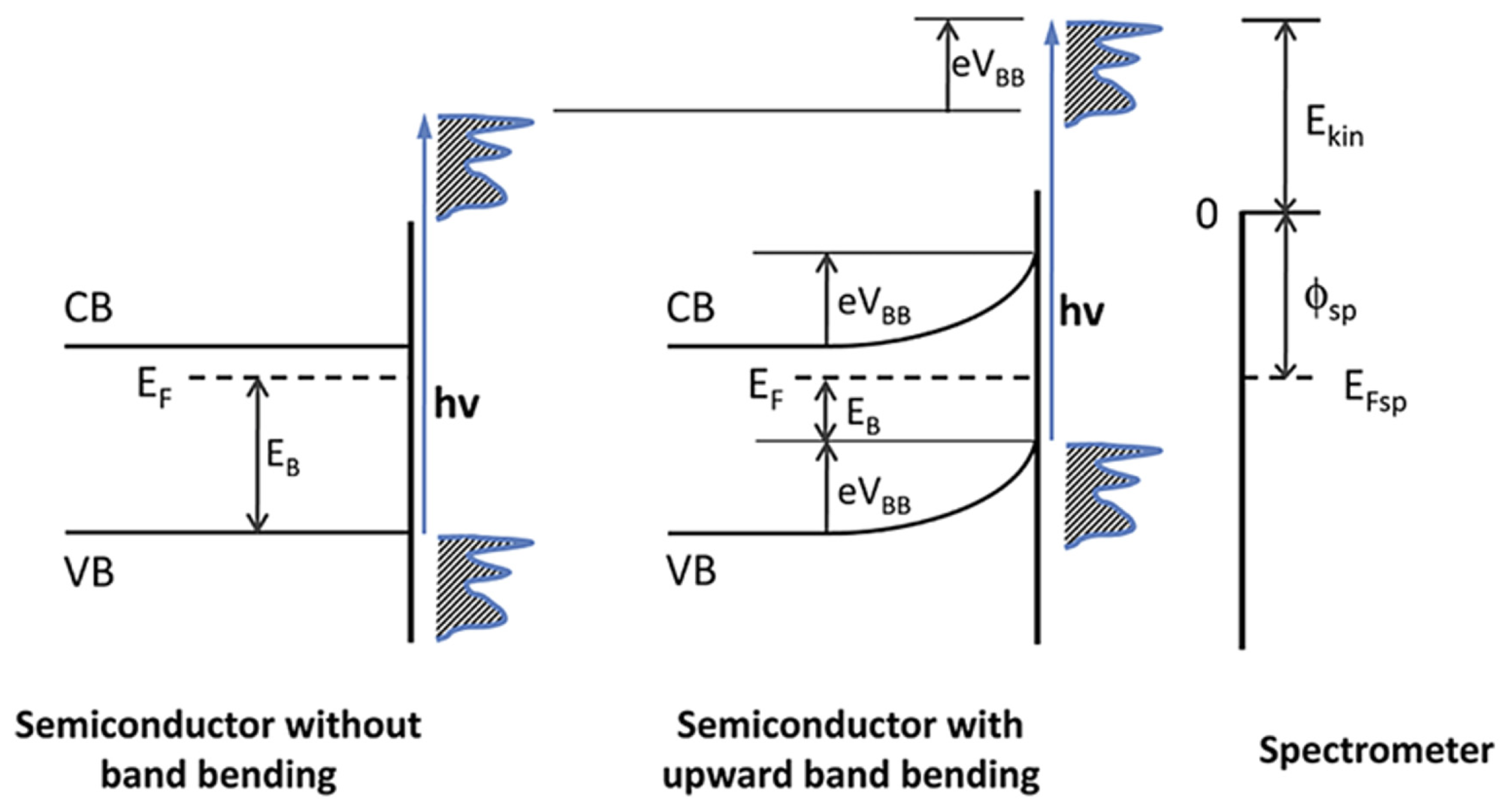
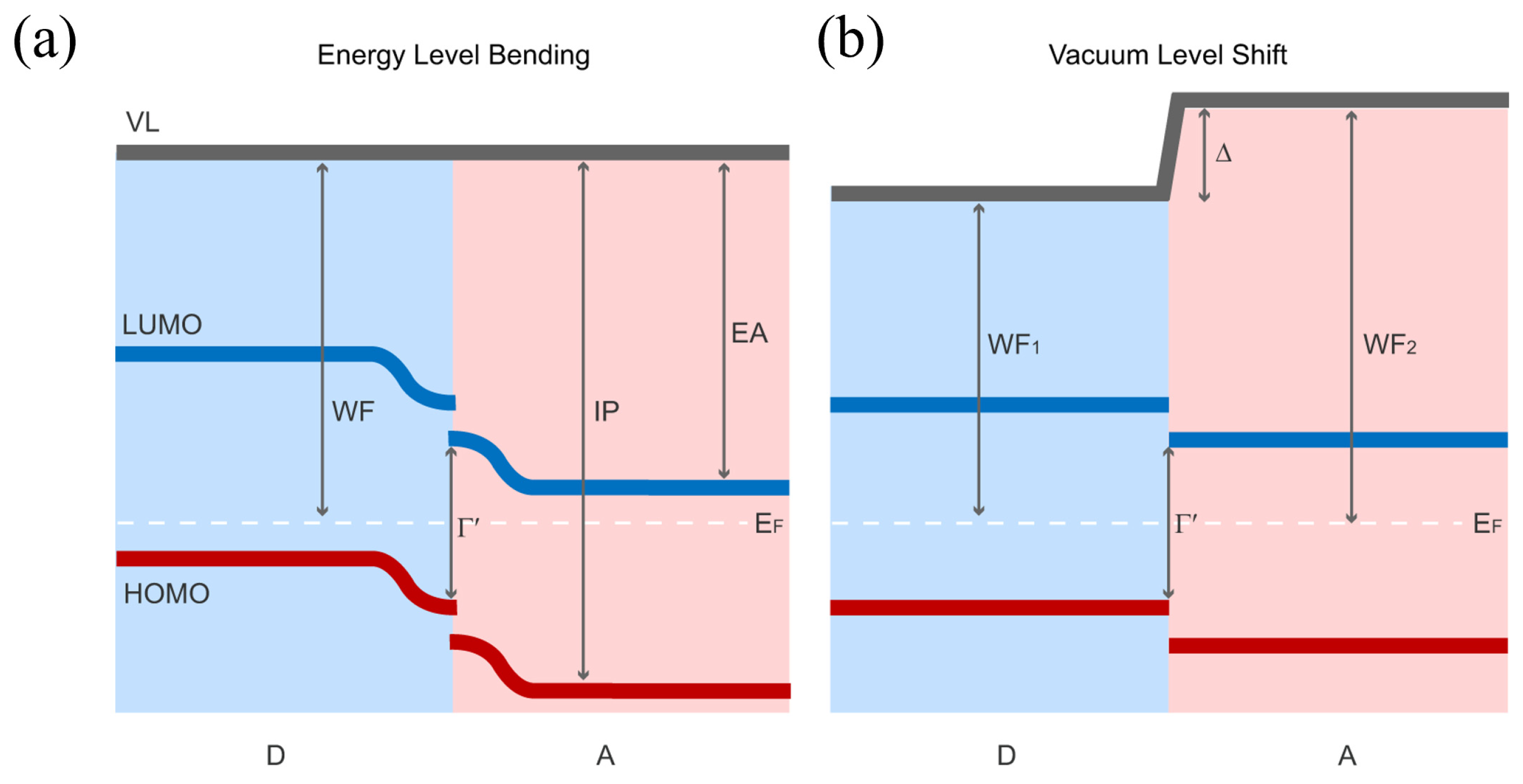
3.1. Mechanisms of Interface Band Bending
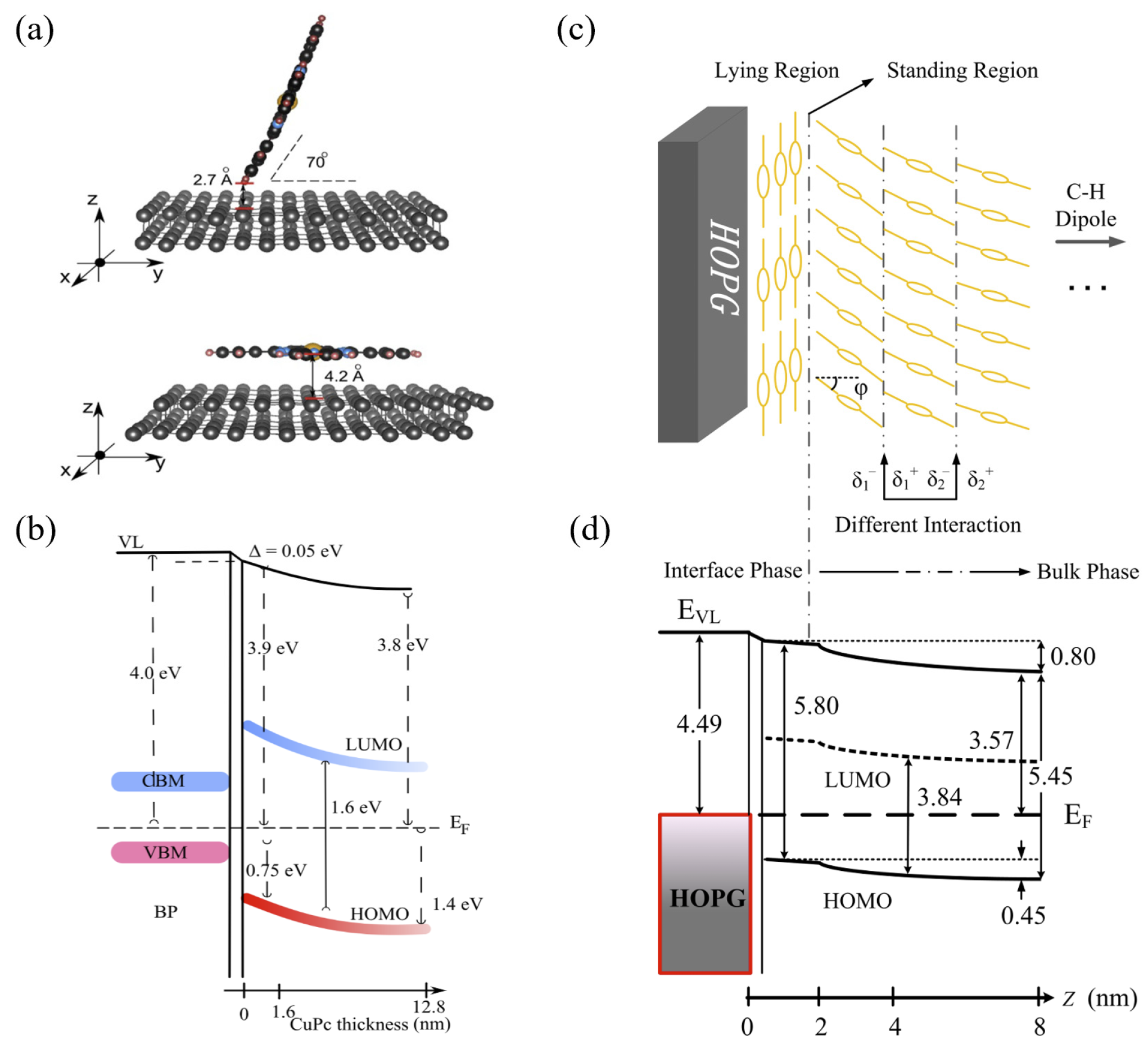
3.1.1. Interface Band Bending Induced by Molecular Orientation
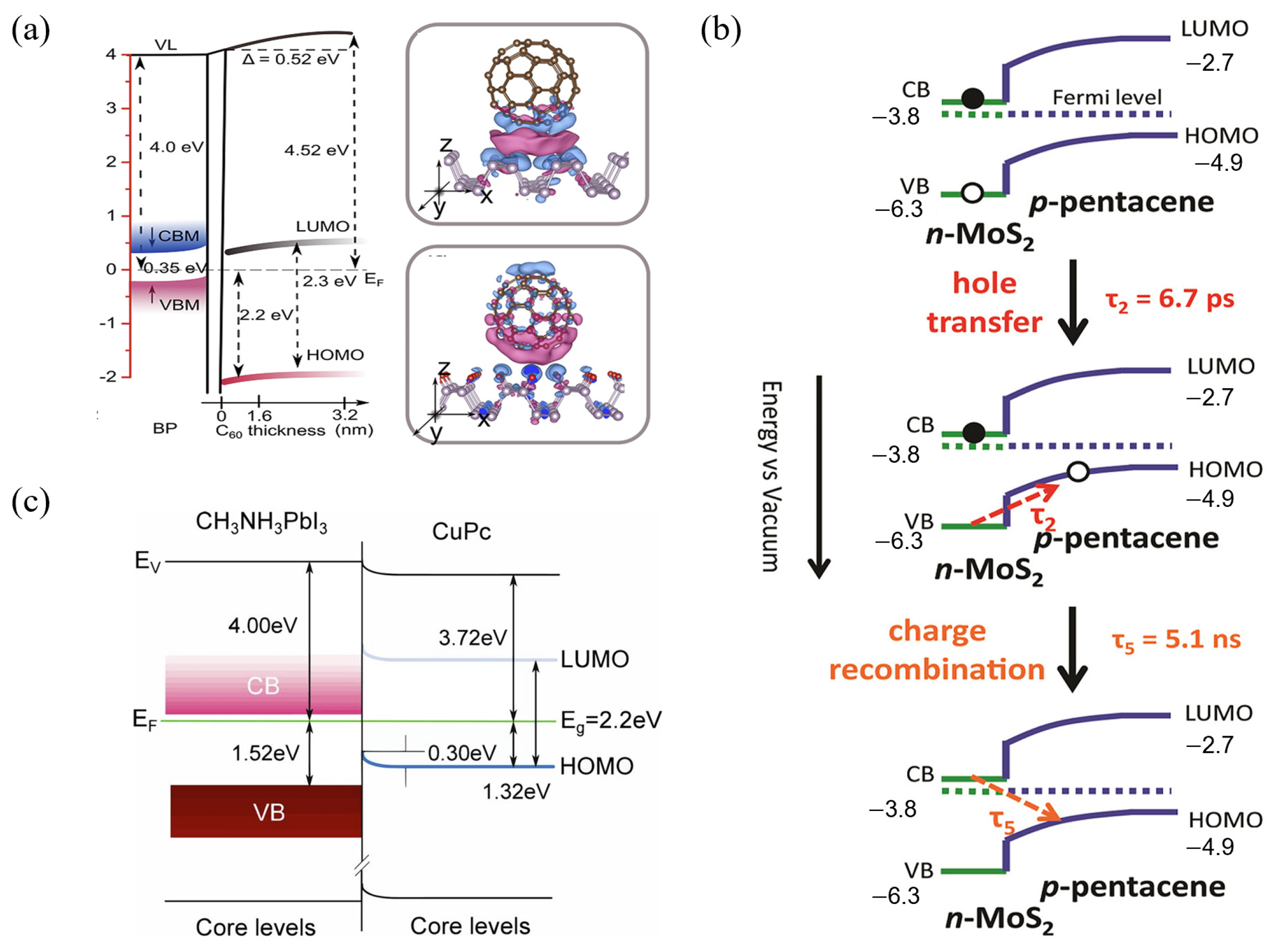
3.1.2. Interface Band Bending Arising from Interfacial Charge Transfer
3.1.3. Interface Band Bending Caused by Interfacial Chemical Reactions
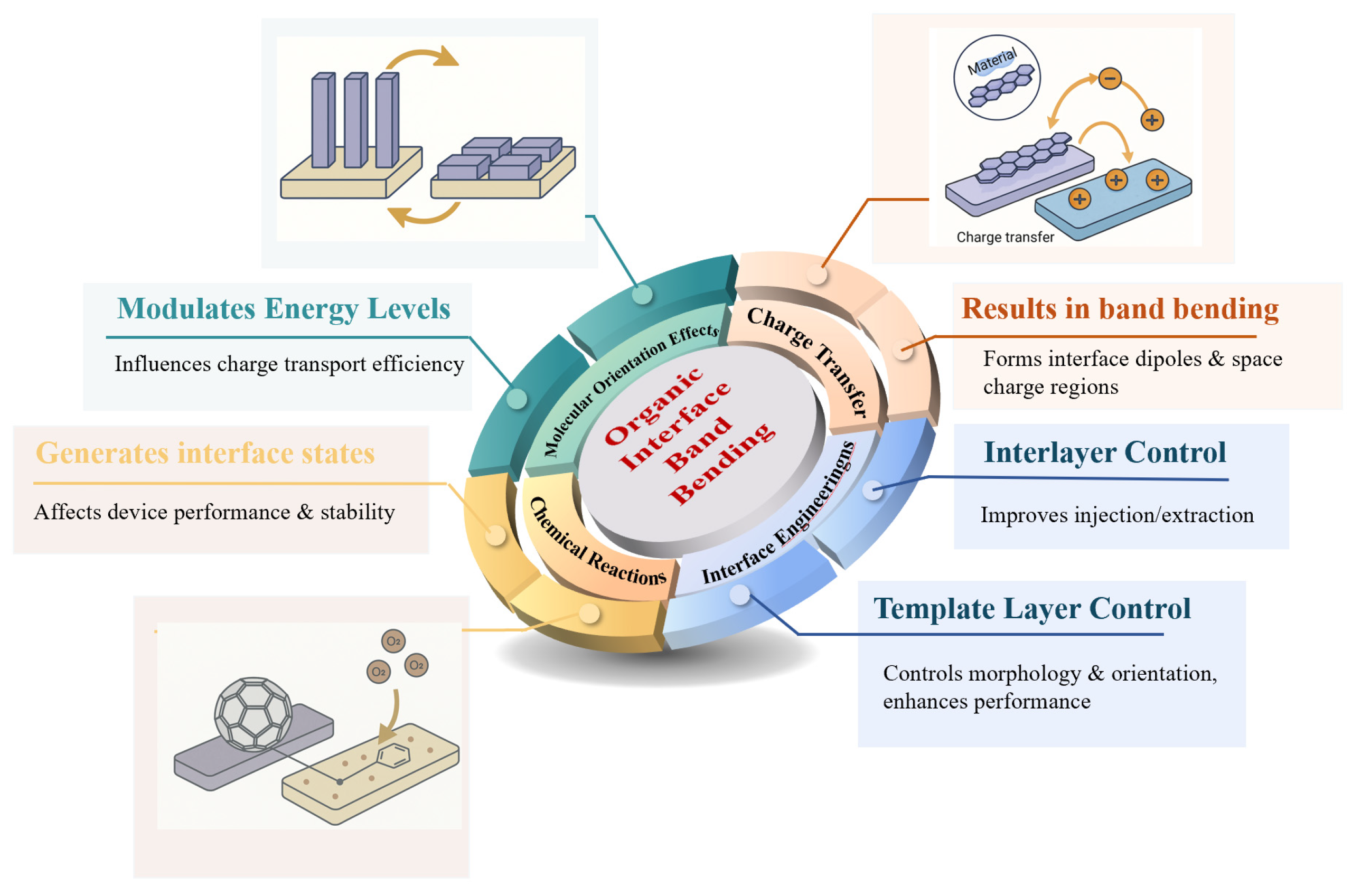
3.2. Interface Engineering
3.2.1. Interlayer Control
3.2.2. Template Layer Control
4. Conclusions
Funding
Conflicts of Interest
References
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burn, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Gelinck, G.H.; Huitema, H.E.A.; Van Veenendaal, E.; Cantatore, E.; Schrijnemakers, L.; van der Putten, J.B.P.H.; Geuns, T.C.T.; Beenhakkers, M.; Giesbers, J.B.; Huisman, B.H.; et al. Flexible active-matrix displays and shift registers based on solution-processed organic transistors. Nat. Mater. 2004, 3, 106–110. [Google Scholar] [CrossRef]
- Rogers, J.A.; Bao, Z. Printed plastic electronics and paperlike displays. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3327–3334. [Google Scholar] [CrossRef]
- Kang, J.; Mun, J.; Zheng, Y.; Koizumi, M.; Matsuhisa, N.; Wu, H.C.; Chen, S.; Tok, B.-H.; Lee, G.H.; Jin, L.; et al. Tough-interface-enabled stretchable electronics using non-stretchable polymer semiconductors and conductors. Nat. Nanotechnol. 2022, 17, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, W.; Jiang, Y.; Wang, Y.-X.; Wu, Y.; Lai, J.-C.; Niu, S.; Xu, C.; Shih, C.-C.; Wang, C.; et al. High-brightness all-polymer stretchable LED with charge-trapping dilution. Nature 2022, 603, 624–630. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Niu, S.; O’Neill, S.J.K.; Kang, J.; Ochiai, Y.; Katsumata, T.; Wu, H.C.; Ashizawa, M.; Wang, G.J.N.; Zhong, D.; et al. High-frequency and intrinsically stretchable polymer diodes. Nature 2021, 600, 246–252. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M.R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 2018, 3, 18020. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, M.; Yan, Y.; Xin, J.; Wang, T.; Ma, W.; Tang, C.; Zheng, Q. Ladder-Type Dithienonaphthalene-Based Small-Molecule Acceptors for Efficient Nonfullerene Organic Solar Cells. Chem. Mater. 2017, 29, 7942–7952. [Google Scholar] [CrossRef]
- Muccini, M. A bright future for organic field-effect transistors. Nat. Mater. 2006, 5, 605–613. [Google Scholar] [CrossRef]
- Wang, J.; Xue, P.; Jiang, Y.; Huo, Y.; Zhan, X. The principles, design and applications of fused-ring electron acceptors. Nat. Rev. Chem. 2022, 6, 614–634. [Google Scholar] [CrossRef] [PubMed]
- Dreiser, J.; Pacchioni, G.E.; Donati, F.; Gragnaniello, L.; Cavallin, A.; Pedersen, K.S.; Bendix, J.; Delley, B.; Pivetta, M.; Rusponi, S.; et al. Out-of-Plane Alignment of Er(trensal) Easy Magnetization Axes Using Graphene. ACS Nano 2016, 10, 2887–2892. [Google Scholar] [CrossRef] [PubMed]
- Di, C.-a.; Xu, W.; Zhu, D. Organic thermoelectrics for green energy. Natl. Sci. Rev. 2016, 3, 269–271. [Google Scholar] [CrossRef]
- Ivanco, J.; Toader, T.; Firsov, A.; Brzhezinskaya, M.; Sperling, M.; Braun, W.; Zahn, D.R.T. Indium on a copper phthalocyanine thin film: Not a reactive system. Phys. Rev. B 2010, 81, 115325. [Google Scholar] [CrossRef]
- Lebedev, A.M.; Sukhanov, L.P.; Brzhezinskaya, M.; Menshikov, K.A.; Svechnikov, N.Y.; Chumakov, R.G.; Stankevich, V.G. On the interaction of self-assembled C60F18 polar molecules with the Ni(100) surface. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2017, 11, 814–822. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Yan, X.; Shi, J.; Tian, H.; Geng, Y.; Yan, D. Ambipolar organic field-effect transistors with air stability, high mobility, and balanced transport. Appl. Phys. Lett. 2006, 88, 133508. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, D. Organic semiconductor heterojunctions as charge generation layers and their application in tandem organic light-emitting diodes for high power efficiency. J. Mater. Chem. 2012, 22, 18718–18734. [Google Scholar] [CrossRef]
- Oehzelt, M.; Koch, N.; Heimel, G. Organic semiconductor density of states controls the energy level alignment at electrode interfaces. Nat. Commun. 2014, 5, 4174. [Google Scholar] [CrossRef]
- Wang, C.; Niu, D.; Liu, B.; Wang, S.; Wei, X.; Liu, Y.; Xie, H.; Gao, Y. Charge Transfer at the PTCDA/Black Phosphorus Interface. J. Phys. Chem. C 2017, 121, 18084–18094. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, Y.; Xie, H.; Wei, J.; Zhao, Y.; Wang, S.; Zhang, Y.; Niu, D.; Gao, Y. Interfacial electronic structure at rubrene/NiFe heterostructure. Results Phys. 2021, 29, 104692. [Google Scholar] [CrossRef]
- Lee, H. Energy Level Alignment of Indium Tin Oxide/Pentacene/C60. Appl. Sci. Converg. Technol. 2024, 33, 72–75. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, Z.; Zhao, S.; Zhao, D.; Yuan, G.; Cheng, Z. Improved performance of organic light emitting diodes by pentacene as hole transporting layer. Applied Surface Science 2008, 255, 1942–1945. [Google Scholar] [CrossRef]
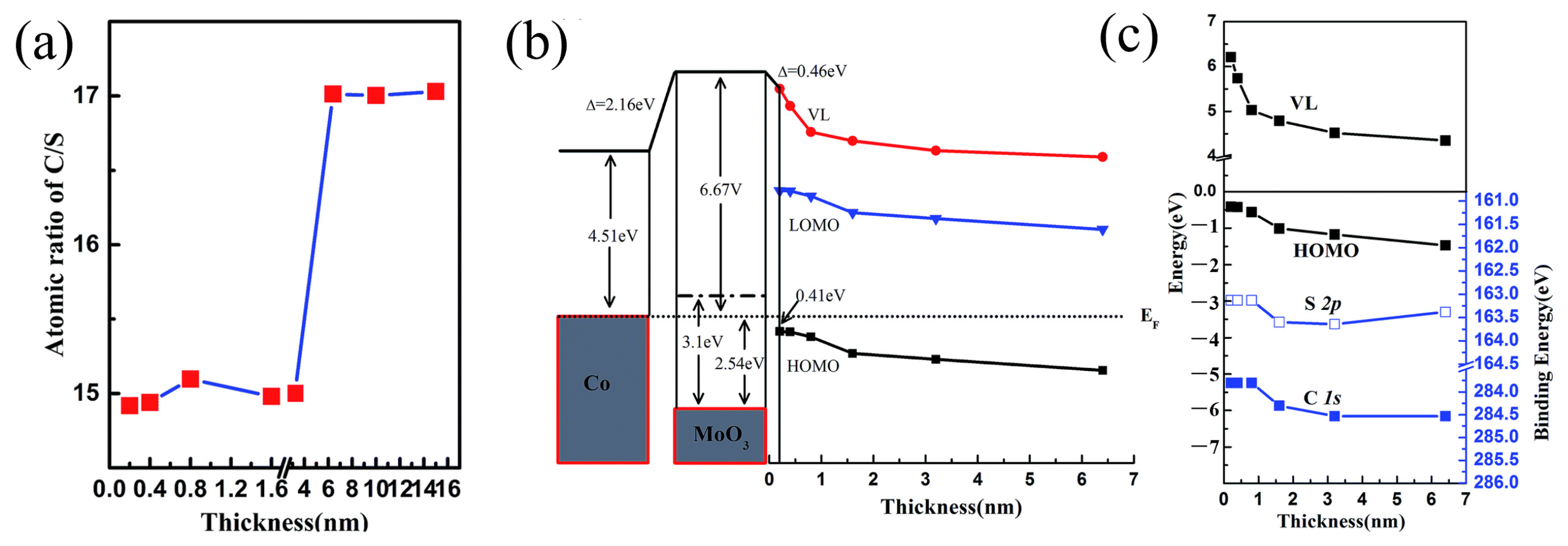
- Zhu, M.; Lyu, L.; Niu, D.; Zhang, H.; Wang, S.; Gao, Y. Effect of a MoO3 buffer layer between C8-BTBT and Co(100) single-crystal film. RSC Adv. 2016, 6, 112403–112408. [Google Scholar] [CrossRef]
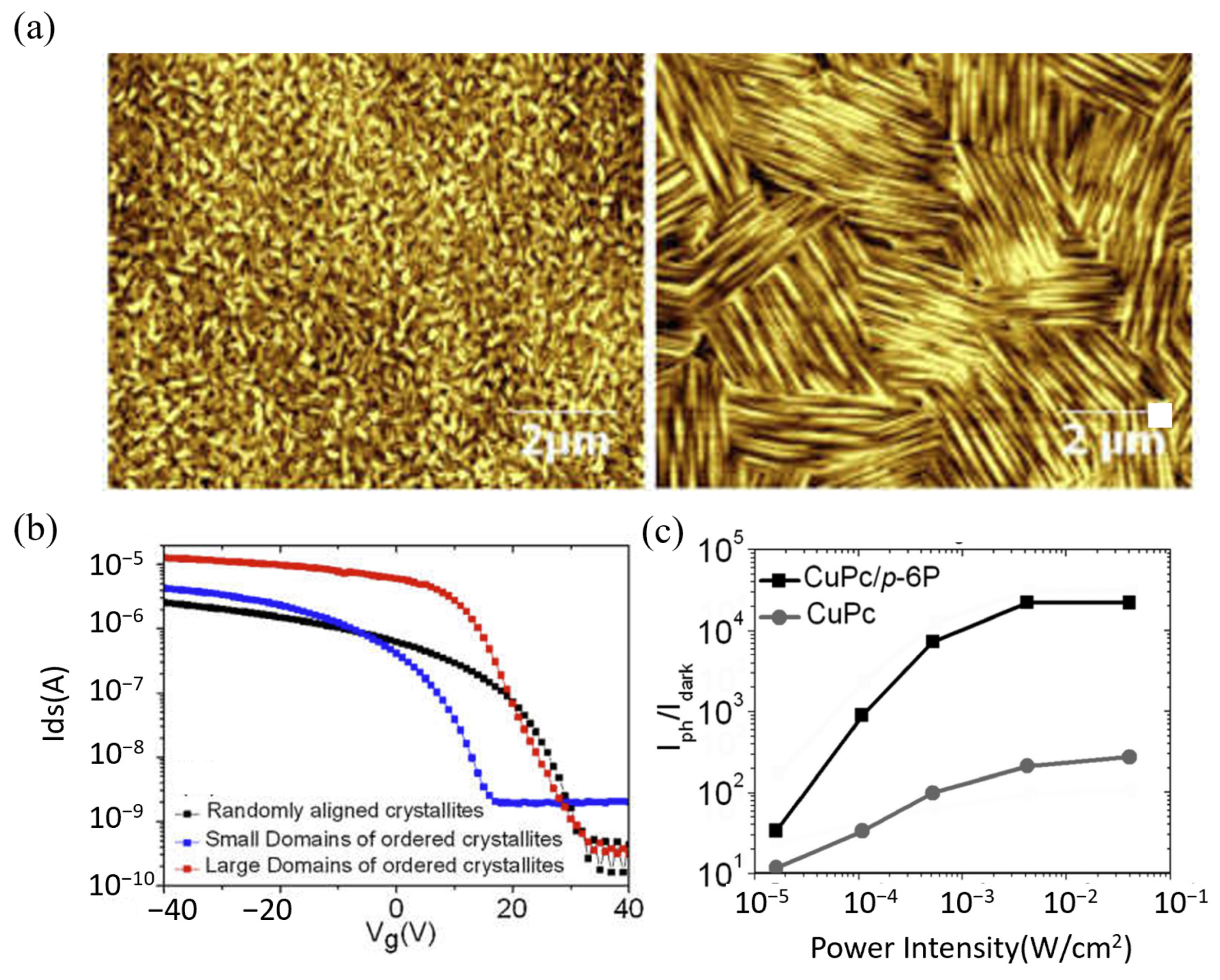
- Qian, C.; Sun, J.; Kong, L.-A.; Gou, G.; Zhu, M.; Yuan, Y.; Huang, H.; Gao, Y.; Yang, J. High-Performance Organic Heterojunction Phototransistors Based on Highly Ordered Copper Phthalocyanine/para-Sexiphenyl Thin Films. Adv. Funct. Mater. 2017, 27, 1604933. [Google Scholar] [CrossRef]
- Ewenike, R.B.; King, B.; Battaglia, A.M.; Quezada Borja, J.D.; Lin, Z.S.; Manion, J.G.; Brusso, J.L.; Kelly, T.L.; Seferos, D.S.; Lessard, B.H. Toward Weak Epitaxial Growth of Silicon Phthalocyanines: How the Choice of the Optimal Templating Layer Differs from Traditional Phthalocyanines. ACS Appl. Electron. Mater. 2023, 5, 7023–7033. [Google Scholar] [CrossRef]
- Ewenike, R.B.; Lin, Z.S.; Cranston, R.R.; Lamontagne, H.R.; Shuhendler, A.J.; Kim, C.-H.; Brusso, J.L.; Lessard, B.H. Engineering the Template Layer for Silicon Phthalocyanine-Based Organic Thin Film Transistors. Adv. Funct. Mater. 2024, 34, 2408779. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, X.K.; Xiao, J.; Chow, P.C.Y.; Ren, M.; Kupgan, G.; Jiao, X.; Chan, C.C.S.; Du, X.; Xia, R.; et al. Delocalization of exciton and electron wavefunction in non-fullerene acceptor molecules enables efficient organic solar cells. Nat. Commun. 2020, 11, 3943. [Google Scholar] [CrossRef]
- Meyer zu Heringdorf, F.J.; Reuter, M.C.; Tromp, R.M. Growth dynamics of pentacene thin films. Nature 2001, 412, 517–520. [Google Scholar] [CrossRef]
- Verlaak, S.; Steudel, S.; Heremans, P.; Janssen, D.; Deleuze, M.S. Nucleation of organic semiconductors on inert substrates. Phys. Rev. B 2003, 68, 195409. [Google Scholar] [CrossRef]
- Tsuchie, K.; Nagao, T.; Hasegawa, S. Structure of C60 layers on the Si(111) − √3 × √3 − Ag surface. Phys. Rev. B 1999, 60, 11131–11136. [Google Scholar] [CrossRef]
- Haddon, R.C.; Perel, A.S.; Morris, R.C.; Palstra, T.T.M.; Hebard, A.F.; Fleming, R.M. C60 thin film transistors. Appl. Phys. Lett. 1995, 67, 121–123. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kim, T.-G.; Jung, E.; Park, J.W. Hydrogen-bonding-facilitated layer-by-layer growth of ultrathin organic semiconducting films. ACS Appl. Mater. Interfaces 2013, 5, 6837–6842. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Pot, I.; Chang, M.; Xu, H.; Aneliunas, V.; Kwok, T.; Newitt, R.; Aebersold, R.; Boone, C.; Brown, G.W.; et al. Identification of Protein Complexes Required for Efficient Sister Chromatid Cohesion. Mol. Biol. Cell 2004, 15, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Virkar, A.A.; Mannsfeld, S.; Oh, J.H.; Toney, M.; Locklin, J.; Bao, Z. Crystalline ultrasmooth self-assembled monolayers of alkylsilanes for organic field-effect transistors. J. Am. Chem. Soc. 2009, 131, 9396–9404. [Google Scholar] [CrossRef]
- Steudel, S.; Janssen, D.; Verlaak, S.; Genoe, J.; Heremans, P. Patterned growth of pentacene. Appl. Phys. Lett. 2004, 85, 5550–5552. [Google Scholar] [CrossRef]
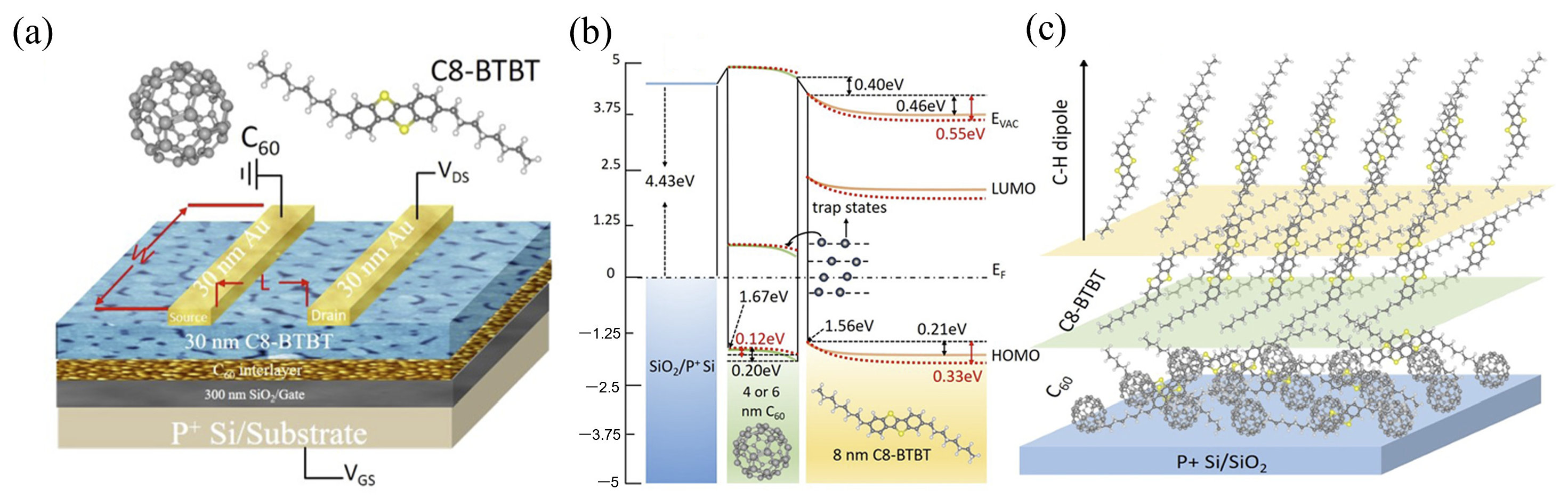
- Wang, S.; Niu, D.; Lyu, L.; Huang, Y.; Wei, X.; Wang, C.; Xie, H.; Gao, Y. Interface electronic structure and morphology of 2,7-dioctyl[1]benzothieno[3,2-b]benzothiophene (C8-BTBT) on Au film. Appl. Surf. Sci. 2017, 416, 696–703. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Wang, B.; Zheng, Y. Ultrathin Ferroelectric Films: Growth, Characterization, Physics and Applications. Materials 2014, 7, 6377–6485. [Google Scholar] [CrossRef]
- Kowarik, S.; Gerlach, A.; Schreiber, F. Organic molecular beam deposition: Fundamentals, growth dynamics, and in situ studies. J. Phys. Condens. Matter 2008, 20, 184005. [Google Scholar] [CrossRef]
- Pancaldi, A.; Raimondo, L.; Minotto, A.; Sassella, A. Post-Growth Dynamics and Growth Modeling of Organic Semiconductor Thin Films. Langmuir 2023, 39, 3266–3272. [Google Scholar] [CrossRef]
- Kim, C.; Bang, K.; An, I.; Kang, C.J.; Kim, Y.-S.; Jeon, D. Morphology and ellipsometry study of pentacene films grown on native SiO2 and glass substrates. Curr. Appl. Phys. 2006, 6, 925–930. [Google Scholar] [CrossRef]
- Salvan, G.; Tenne, D.A.; Das, A.; Kampen, T.U.; Zahn, D.R.T. Influence of deposition temperature on the structure of 3,4,9,10-perylene tetracarboxylic dianhydride thin films on H-passivated silicon probed by Raman spectroscopy. Org. Electron. 2000, 1, 49–56. [Google Scholar] [CrossRef]
- Reisz, B.; Empting, E.; Zwadlo, M.; Hodas, M.; Duva, G.; Belova, V.; Zeiser, C.; Hagenlocher, J.; Maiti, S.; Hinderhofer, A.; et al. Thin film growth of phase-separating phthalocyanine-fullerene blends: A combined experimental and computational study. Phys. Rev. Mater. 2021, 5, 045601. [Google Scholar] [CrossRef]
- Yang, H.; Ling, M.-M.; Yang, L. Temperature-Dependent Pentacene Nanostructures on Hydrophobic Gate-Dielectrics Correlated with Charge Carrier Mobilities. J. Phys. Chem. C 2007, 111, 12508–12511. [Google Scholar] [CrossRef]
- Moser, A.; Novák, J.; Flesch, H.-G.; Djuric, T.; Werzer, O.; Haase, A.; Resel, R. Temperature stability of the pentacene thin-film phase. Appl. Phys. Lett. 2011, 99, 221911. [Google Scholar] [CrossRef]
- Yagi, I.; Tsukagoshi, K.; Aoyagi, Y. Growth control of pentacene films on SiO2/Si substrates towards formation of flat conduction layers. Thin Solid Film. 2004, 467, 168–171. [Google Scholar] [CrossRef]
- Kato, M.; Yoshizawa, H.; Nakaya, M.; Kitagawa, Y.; Okamoto, K.; Yamada, T.; Yoshino, M.; Tanaka, K.; Onoe, J. Unraveling the reasons behind lead phthalocyanine acting as a good absorber for near-infrared sensitive devices. Sci. Rep. 2022, 12, 8810. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Lv, W.; Luo, X.; Sun, L.; Zhong, J.; Zhao, F.; Huang, F.; Peng, Y. Substrate temperature dependent performance of near infrared photoresponsive organic field effect transistors based on lead phthalocyanine. Synth. Met. 2015, 205, 190–194. [Google Scholar] [CrossRef]
- Pratontep, S.; Brinkmann, M.; Nüesch, F.; Zuppiroli, L. Nucleation and growth of ultrathin pentacene films on silicon dioxide: Effect of deposition rate and substrate temperature. Synth. Met. 2004, 146, 387–391. [Google Scholar] [CrossRef]
- Isoda, S.; Kawakubo, H.; Nishikawa, S.; Wada, O. Preparation of C60 single crystalline thin film by ionized cluster beam deposition and ion implantation into single crystalline C60 thin film. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1996, 112, 94–98. [Google Scholar] [CrossRef]
- Babajanyan, A.; Enkhtur, L.; Khishigbadrakh, B.-E.; Melikyan, H.; Yoon, Y.; Kim, S.; Lee, H.; Kim, T.; Lee, K.; Friedman, B. Anisotropic electric properties of Copper-(II)-Phthalocyanine thin films characterized by a near-field microwave microscope. Curr. Appl. Phys. 2011, 11, 166–170. [Google Scholar] [CrossRef]
- Yogev, S.; Matsubara, R.; Nakamura, M.; Rosenwaks, Y. Local charge accumulation and trapping in grain boundaries of pentacene thin film transistors. Org. Electron. 2010, 11, 1729–1735. [Google Scholar] [CrossRef]
- Ochiai, S.; Palanisamy, K.; Kannappan, S.; Shin, P.-K. Pentacene Active Channel Layers Prepared by Spin-Coating and Vacuum Evaporation Using Soluble Precursors for OFET Applications. Int. Sch. Res. Not. 2012, 2012, 313285. [Google Scholar] [CrossRef]
- Yunus, Y.; Mahadzir, N.A.; Mohamed Ansari, M.N.; Tg Abd Aziz, T.H.; Mohd Afdzaluddin, A.; Anwar, H.; Wang, M.; Ismail, A.G. Review of the Common Deposition Methods of Thin-Film Pentacene, Its Derivatives, and Their Performance. Polymers 2022, 14, 1112. [Google Scholar] [CrossRef]
- Podzorov, V.; Sysoev, S.E.; Loginova, E.; Pudalov, V.M.; Gershenson, M.E. Single-crystal organic field effect transistors with the hole mobility 8 cm2/Vs. Appl. Phys. Lett. 2003, 83, 3504–3506. [Google Scholar] [CrossRef]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-Level Alignment at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Xin, Y.; Zeng, G.; OuYang, J.; Zhao, X.; Yang, X. Enhancing thermal stability of nonfullerene organic solar cells via fluoro-side-chain engineering. J. Mater. Chem. C 2019, 7, 9513–9522. [Google Scholar] [CrossRef]
- Anthony, J.E. Small-Molecule, Nonfullerene Acceptors for Polymer Bulk Heterojunction Organic Photovoltaics. Chem. Mater. 2011, 23, 583–590. [Google Scholar] [CrossRef]
- Zhang, S.; Talnack, F.; Jousselin-Oba, T.; Bhat, V.; Wu, Y.; Lei, Y.; Tomo, Y.; Gong, H.; Michalek, L.; Zhong, D.; et al. Shear-aligned large-area organic semiconductor crystals through extended π–π interaction. J. Mater. Chem. C 2023, 11, 8992–9001. [Google Scholar] [CrossRef]
- Strohriegl, P.; Grazulevicius, J.V. Charge-Transporting Molecular Glasses. Adv. Mater. 2002, 14, 1439–1452. [Google Scholar] [CrossRef]
- Yokoyama, D. Molecular orientation in small-molecule organic light-emitting diodes. J. Mater. Chem. 2011, 21, 19187–19202. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Zhang, Z.; Cai, J.; Zhang, H.; Gurney, R.S.; Liu, D.; Yu, J.; Tang, W.; Wang, T. Retarding the Crystallization of a Nonfullerene Electron Acceptor for High-Performance Polymer Solar Cells. Adv. Funct. Mater. 2019, 29, 1807662. [Google Scholar] [CrossRef]
- He, X.; Chan, C.C.S.; Zou, X.; Zhang, S.; Fong, P.W.K.; Kim, J.; Li, G.; Hu, X.; Ma, W.; Wong, K.S.; et al. Modulating the Mixing Gibbs Free Energy to Enhance Solid–Liquid Phase Separation for High-Performance Organic Solar Cells. Adv. Energy Mater. 2023, 13, 2203697. [Google Scholar] [CrossRef]
- Yamada, K.; Okamoto, M.; Sakurai, M.; Suenobu, T.; Nakayama, K.-i. Solution-processable reduced graphene oxide template layer for molecular orientation control of organic semiconductors. RSC Adv. 2019, 9, 32940–32945. [Google Scholar] [CrossRef]
- Schünemann, C.; Wynands, D.; Eichhorn, K.-J.; Stamm, M.; Leo, K.; Riede, M. Evaluation and Control of the Orientation of Small Molecules for Strongly Absorbing Organic Thin Films. J. Phys. Chem. C 2013, 117, 11600–11609. [Google Scholar] [CrossRef]
- Gu, W.; Hu, Y.; Zhu, Z.; Liu, N.; Zhang, J.; Wang, J. Preparing highly ordered copper phthalocyanine thin-film by controlling the thickness of the modified layer and its application in organic transistors. Solid-State Electron. 2013, 89, 101–104. [Google Scholar] [CrossRef]
- Liang, Q.; Duan, M.; Geng, Z.; Zhang, M.; Xu, W.; Geng, H.; He, Z.; Liu, J. Regulation of molecular orientation in organic solar cells. Chem. Eng. J. 2024, 488, 150783. [Google Scholar] [CrossRef]
- Garnier, F.; Yassar, A.; Hajlaoui, R.; Horowitz, G.; Deloffre, F.; Servet, B.; Ries, S.; Alnot, P. Molecular engineering of organic semiconductors: Design of self-assembly properties in conjugated thiophene oligomers. J. Am. Chem. Soc. 1993, 115, 8716–8721. [Google Scholar] [CrossRef]
- Duhm, S.; Heimel, G.; Salzmann, I.; Glowatzki, H.; Johnson, R.L.; Vollmer, A.; Rabe, J.P.; Koch, N. Orientation-dependent ionization energies and interface dipoles in ordered molecular assemblies. Nat. Mater. 2008, 7, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Javaid, S.; Lee, G. The impact of molecular orientation on carrier transfer characteristics at a phthalocyanine and halide perovskite interface. RSC Adv. 2021, 11, 31776–31782. [Google Scholar] [CrossRef]
- Ihm, K.; Kim, B.; Kang, T.H.; Kim, K.J.; Joo, M.H.; Kim, T.H.; Yoon, S.S.; Chung, S. Molecular orientation dependence of hole-injection barrier in pentacene thin film on the Au surface in organic thin film transistor. Appl. Phys. Lett. 2006, 89, 100. [Google Scholar] [CrossRef]
- Kera, S.; Fukagawa, H.; Kataoka, T.; Hosoumi, S.; Yamane, H.; Ueno, N. Spectroscopic evidence of strong π-π interorbital interaction in a lead-phthalocyanine bilayer film attributed to the dimer nanostructure. Phys. Rev. B 2007, 75, 121305. [Google Scholar] [CrossRef]
- Friedlein, R.; Crispin, X.; Pickholz, M.; Keil, M.; Stafström, S.; Salaneck, W.R. High intercalation levels in lithium perylene stoichiometric compounds. Chem. Phys. Lett. 2002, 354, 389–394. [Google Scholar] [CrossRef]
- Ivanco, J.; Winter, B.; Netzer, F.P.; Ramsey, M.G. Substrate-Mediated Electronic Structure and Properties of Sexiphenyl Films. Adv. Mater. 2003, 15, 1812–1815. [Google Scholar] [CrossRef]
- Koller, G.; Berkebile, S.P.; Ivančo, J.; Netzer, F.P.; Ramsey, M.G. Device relevant organic films and interfaces: A surface science approach. Surf. Sci. 2007, 601, 5683–5689. [Google Scholar] [CrossRef]
- Tepavcevic, S.; Wroble, A.T.; Bissen, M.; Wallace, D.J.; Choi, Y.; Hanley, L. Photoemission Studies of Polythiophene and Polyphenyl Films Produced via Surface Polymerization by Ion-Assisted Deposition. J. Phys. Chem. B 2005, 109, 7134–7140. [Google Scholar] [CrossRef]
- Peumans, P.; Uchida, S.; Forrest, S.R. Efficient bulk heterojunction photovoltaic cells using small-molecular-weight organic thin films. Nature 2003, 425, 158–162. [Google Scholar] [CrossRef]
- Zhong, S.; Zhong, J.Q.; Wee, A.T.S.; Chen, W. Molecular orientation and electronic structure at organic heterojunction interfaces. J. Electron Spectrosc. Relat. Phenom. 2015, 204, 12–22. [Google Scholar] [CrossRef]
- Xue, J.; Rand, B.P.; Uchida, S.; Forrest, S.R. A Hybrid Planar-Mixed Molecular Heterojunction Photovoltaic Cell. Adv. Mater. 2005, 17, 66–71. [Google Scholar] [CrossRef]
- Chen, W.; Qi, D.C.; Huang, Y.L.; Huang, H.; Wang, Y.Z.; Chen, S.; Gao, X.Y.; Wee, A.T.S. Molecular Orientation Dependent Energy Level Alignment at Organic−Organic Heterojunction Interfaces. J. Phys. Chem. C 2009, 113, 12832–12839. [Google Scholar] [CrossRef]
- Wang, C.; Niu, D.; Xie, H.; Liu, B.; Wang, S.; Zhu, M.; Gao, Y. Electronic structures at the interface between CuPc and black phosphorus. J. Chem. Phys. 2017, 147, 064702. [Google Scholar] [CrossRef]
- Lyu, L.; Niu, D.; Xie, H.; Cao, N.; Zhang, H.; Zhang, Y.; Liu, P.; Gao, Y. Orientation-dependent energy level alignment and film growth of 2,7-diocty[1]benzothieno[3,2-b]benzothiophene (C8-BTBT) on HOPG. J. Chem. Phys. 2016, 144, 034701. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, S.; Qian, D.; Gautam, B.; Yang, G.; Zhao, J.; Bergqvist, J.; Zhang, F.; Ma, W.; Ade, H.; et al. Fast charge separation in a non-fullerene organic solar cell with a small driving force. Nat. Energy 2016, 1, 16089. [Google Scholar] [CrossRef]
- Li, X.e.; Zhang, Q.; Yu, J.; Xu, Y.; Zhang, R.; Wang, C.; Zhang, H.; Fabiano, S.; Liu, X.; Hou, J.; et al. Mapping the energy level alignment at donor/acceptor interfaces in non-fullerene organic solar cells. Nat. Commun. 2022, 13, 2046. [Google Scholar] [CrossRef] [PubMed]
- Duhm, S.; Gerlach, A.; Salzmann, I.; Bröker, B.; Johnson, R.L.; Schreiber, F.; Koch, N. PTCDA on Au(111), Ag(111) and Cu(111): Correlation of interface charge transfer to bonding distance. Org. Electron. 2008, 9, 111–118. [Google Scholar] [CrossRef]
- Aihara, T.; Abd-Rahman, S.A.; Yoshida, H. Metal screening effect on energy levels at metal/organic interface: Precise determination of screening energy using photoelectron and inverse-photoelectron spectroscopies. Phys. Rev. B 2021, 104, 085305. [Google Scholar] [CrossRef]
- Rana, O.; Srivastava, R.; Chauhan, G.; Zulfequar, M.; Husain, M.; Srivastava, P.C.; Kamalasanan, M.N. Modification of metal–organic interface using F4-TCNQ for enhanced hole injection properties in optoelectronic devices. Phys. Status Solidi 2012, 209, 2539–2545. [Google Scholar] [CrossRef]
- Okuyama, H.; Kuwayama, S.; Nakazawa, Y.; Hatta, S.; Aruga, T. Structure and electronic states of strongly interacting metal-organic interfaces: CuPc on Cu(100) and Cu(110). Surf. Sci. 2022, 723, 122126. [Google Scholar] [CrossRef]
- Carlotto, S.; Verdini, A.; Zamborlini, G.; Cojocariu, I.; Feyer, V.; Floreano, L.; Casarin, M. A local point of view of the Cu(100) → NiTPP charge transfer at the NiTPP/Cu(100) interface. Phys. Chem. Chem. Phys. 2023, 25, 26779–26786. [Google Scholar] [CrossRef]
- Wang, C.; Niu, D.; Wang, S.; Zhao, Y.; Tan, W.; Li, L.; Huang, H.; Xie, H.; Deng, Y.; Gao, Y. Energy Level Evolution and Oxygen Exposure of Fullerene/Black Phosphorus Interface. J. Phys. Chem. Lett. 2018, 9, 5254–5261. [Google Scholar] [CrossRef]
- Bettis Homan, S.; Sangwan, V.K.; Balla, I.; Bergeron, H.; Weiss, E.A.; Hersam, M.C. Ultrafast Exciton Dissociation and Long-Lived Charge Separation in a Photovoltaic Pentacene–MoS2 van der Waals Heterojunction. Nano Lett. 2017, 17, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Goh, T.W.; Sabba, D.; Chua, J.; Mathews, N.; Huan, C.H.A.; Sum, T.C. Energy level alignment at the methylammonium lead iodide/copper phthalocyanine interface. APL Mater. 2014, 2, 081512. [Google Scholar] [CrossRef]
- Schulz, P.; Edri, E.; Kirmayer, S.; Hodes, G.; Cahen, D.; Kahn, A. Interface energetics in organo-metal halide perovskite-based photovoltaic cells. Energy Environ. Sci. 2014, 7, 1377–1381. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, W.; Ju, H.; Zhang, L.; Ye, Y.; Zhang, W.; Zhu, J. Electronic structures and chemical reactions at the interface between Li and regioregular poly (3-hexylthiophene). Org. Electron. 2012, 13, 1060–1067. [Google Scholar] [CrossRef]
- Wei, H.; Scudiero, L.; Eilers, H. Infrared and photoelectron spectroscopy study of vapor phase deposited poly (3-hexylthiophene). Appl. Surf. Sci. 2009, 255, 8593–8597. [Google Scholar] [CrossRef]
- Zhang, X.; Su, Z.; Zhao, B.; Yang, Y.; Xiong, Y.; Gao, X.; Qi, D.C.; Cao, L. Chemical interaction dictated energy level alignment at the N,N′-dipentyl-3,4,9,10-perylenedicarboximide/CH3NH3PbI3 interface. Appl. Phys. Lett. 2018, 113, 113901. [Google Scholar] [CrossRef]
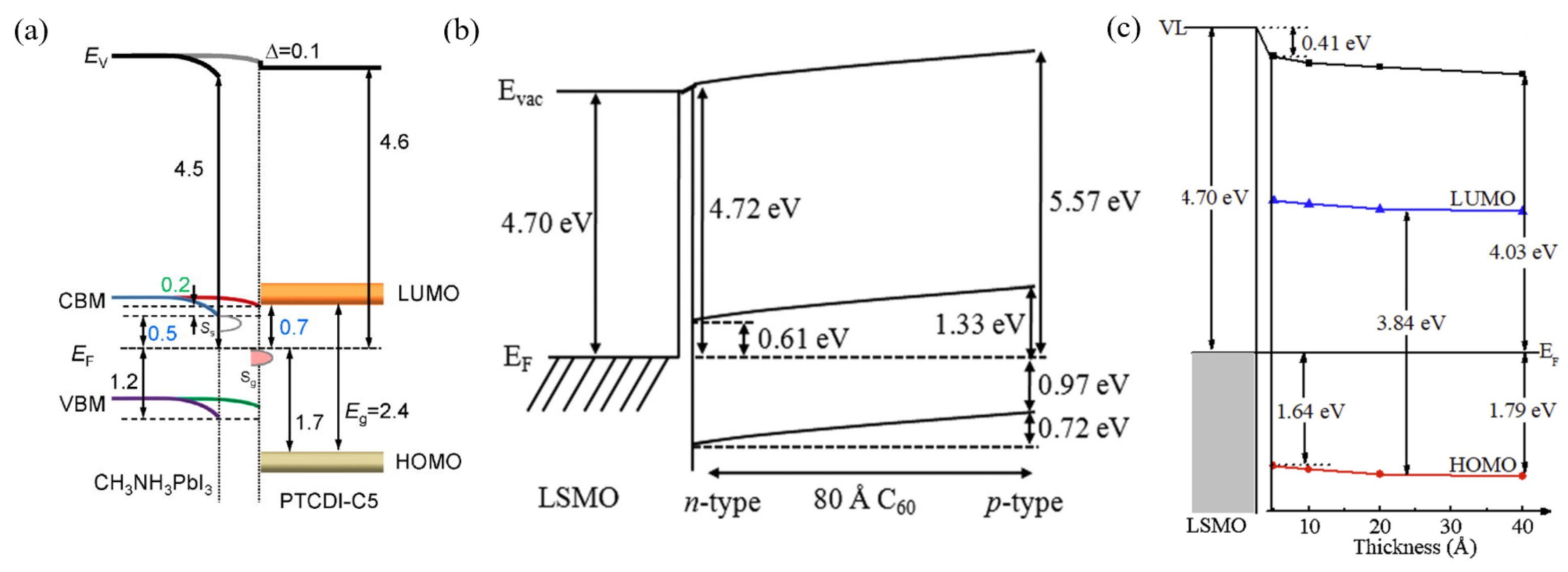
- Xie, H.; Niu, D.; Lyu, L.; Zhang, H.; Zhang, Y.; Liu, P.; Wang, P.; Wu, D.; Gao, Y. Evolution of the electronic structure of C60/La0.67Sr0.33MnO3 interface. Appl. Phys. Lett. 2016, 108, 011603. [Google Scholar] [CrossRef]
- Xie, H.; Niu, D.; Zhao, Y.; Wang, S.; Liu, B.; Liu, Y.; Huang, H.; Wang, P.; Wu, D.; Gao, Y. Photoemission studies of C8-BTBT/La0.67Sr0.33MnO3 interface. Synth. Met. 2020, 260, 116261. [Google Scholar] [CrossRef]
- Shi, M.; Huang, S.; Dong, N.; Liu, Z.; Gan, F.; Wang, J.; Chen, Y. Donor–acceptor type blends composed of black phosphorus and C60 for solid-state optical limiters. Chem. Commun. 2017, 54, 366. [Google Scholar] [CrossRef]
- Liu, X.; Yi, S.; Wang, C.; Wang, C.; Gao, Y. Electronic structure evolution and energy level alignment at C60/4,4′-cyclohexylidenebis[N,N-bis(4-methylphenyl) benzenamine]/MoOx/indium tin oxide interfaces. J. Appl. Phys. 2014, 115, 183. [Google Scholar] [CrossRef]
- Kim, D.Y.; Subbiah, J.; Sarasqueta, G.; So, F.; Ding, H.; Irfan; Gao, Y. The effect of molybdenum oxide interlayer on organic photovoltaic cells. Appl. Phys. Lett. 2009, 95, 093304. [Google Scholar] [CrossRef]
- Liu, P.; Liu, X.; Lyu, L.; Xie, H.; Zhang, H.; Niu, D.; Huang, H.; Bi, C.; Xiao, Z.; Huang, J.; et al. Interfacial electronic structure at the CH3NH3PbI3/MoOx interface. Appl. Phys. Lett. 2015, 106, 193903. [Google Scholar] [CrossRef]
- Chu, C.-W.; Li, S.-H.; Chen, C.-W.; Shrotriya, V.; Yang, Y. High-performance organic thin-film transistors with metal oxide/metal bilayer electrode. Appl. Phys. Lett. 2005, 87, 193508. [Google Scholar] [CrossRef]
- An, Z.; Wu, Q.; Xiao, J.; Chen, R.; Yin, J.; Shi, H.; Tao, Y.; Wang, Y.; Wang, Z.; Li, H.; et al. Modulation of singlet and triplet excited states through σ spacers in ternary 1,3,5-triazines. RSC Adv. 2013, 3, 13782–13788. [Google Scholar] [CrossRef]
- Kumaki, D.; Umeda, T.; Tokito, S. Reducing the contact resistance of bottom-contact pentacene thin-film transistors by employing a MoOx carrier injection layer. Appl. Phys. Lett. 2008, 92, 013301. [Google Scholar] [CrossRef]
- Irfan; Ding, H.; Gao, Y.; Kim, D.Y.; Subbiah, J.; So, F. Energy level evolution of molybdenum trioxide interlayer between indium tin oxide and organic semiconductor. Appl. Phys. Lett. 2010, 96, 073304. [Google Scholar] [CrossRef]
- Kröger, M.; Hamwi, S.; Meyer, J.; Riedl, T.; Kowalsky, W.; Kahn, A. Role of the deep-lying electronic states of MoO3 in the enhancement of hole-injection in organic thin films. Appl. Phys. Lett. 2009, 95, 123301. [Google Scholar] [CrossRef]
- Kanai, K.; Koizumi, K.; Ouchi, S.; Tsukamoto, Y.; Sakanoue, K.; Ouchi, Y.; Seki, K. Electronic structure of anode interface with molybdenum oxide buffer layer. Org. Electron. 2010, 11, 188–194. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhang, M.; Ding, H.; Tang, C.; Gao, Y. Strong interface p-doping and band bending in C60 on MoOx. Org. Electron. 2011, 12, 1588–1593. [Google Scholar] [CrossRef]
- Wang, C.; Irfan, I.; Liu, X.; Gao, Y. Role of molybdenum oxide for organic electronics: Surface analytical studies. J. Vac. Sci. Technol. B 2014, 32, 040801. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Lo, M.F.; Ng, T.W.; Lee, C.-S.; Yan, D.; Lee, S.-T. Organic-inorganic heterojunction field-effect transistors. J. Appl. Phys. 2010, 107, 024510. [Google Scholar] [CrossRef]
- Lee, J.Y. Efficient hole injection in organic light-emitting diodes using C60 as a buffer layer for Al reflective anodes. Appl. Phys. Lett. 2006, 88, 073512. [Google Scholar] [CrossRef]
- Yao, B.; Lv, W.; Chen, D.; Fan, G.; Zhou, M.; Peng, Y. Photoresponsivity enhancement of pentacene organic phototransistors by introducing C60 buffer layer under source/drain electrodes. Appl. Phys. Lett. 2012, 101, 163301. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Feng, G.; Lyu, L.; Li, L.; Wang, S.; Jiang, J.; Li, Y.; Niu, D.; Xie, H.; et al. Modification of C60 nano-interlayers on organic field-effect transistors based on 2,7-diocty[1]benzothieno-[3,2-b]benzothiophene (C8-BTBT)/SiO2. Results Phys. 2020, 19, 103590. [Google Scholar] [CrossRef]
- Ehrler, B.; Musselman, K.P.; Böhm, M.L.; Friend, R.H.; Greenham, N.C. Hybrid pentacene/a-silicon solar cells utilizing multiple carrier generation via singlet exciton fission. Appl. Phys. Lett. 2012, 101, 153507. [Google Scholar] [CrossRef]
- Mooser, S.; Cooper, J.F.K.; Banger, K.K.; Wunderlich, J.; Sirringhaus, H.; Drouhin, H.J.; Wegrowe, J.E.; Razeghi, M. Evidence for spin injection and transport in solution-processed TIPS-pentacene at room temperature. In Proceedings of the Spintronics V, San Diego, CA, USA, 12–16 August 2012; pp. 1109–1124. [Google Scholar]
- Chen, X.; Taguchi, D.; Manaka, T.; Iwamoto, M. Study of blocking effect of Cu-phthalocyanine layer in zinc oxide/pentacene/CuPc/C60/Al organic solar cells by electric field-induced optical second harmonic generation measurement. Org. Electron. 2013, 14, 320–325. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, D.Y.; Lee, S.G.; Cho, K. High-Mobility Organic Single-Crystal Microtubes of Soluble Pentacene Semiconductors with Hollow Tetragonal Structures. Chem. Mater. 2012, 24, 2752–2756. [Google Scholar] [CrossRef]
- Benavides, C.M.; Biele, M.; Schmidt, O.; Brabec, C.J.; Tedde, S.F. TIPS Pentacene as a Beneficial Interlayer for Organic Photodetectors in Imaging Applications. IEEE Trans. Electron Devices 2018, 65, 1516–1522. [Google Scholar] [CrossRef]
- Pan, F.; Tian, H.; Qian, X.; Huang, L.; Geng, Y.; Yan, D. High performance vanadyl phthalocyanine thin-film transistors based on fluorobenzene end-capped quaterthiophene as the inducing layer. Org. Electron. 2011, 12, 1358–1363. [Google Scholar] [CrossRef]
- Chua, L.-L.; Zaumseil, J.; Chang, J.-F.; Ou, E.C.W.; Ho, P.K.H.; Sirringhaus, H.; Friend, R.H. General observation of n-type field-effect behaviour in organic semiconductors. Nature 2005, 434, 194–199. [Google Scholar] [CrossRef]
- Puigdollers, J.; Voz, C.; Fonrodona, M.; Cheylan, S.; Stella, M.; Andreu, J.; Vetter, M.; Alcubilla, R. Copper phthalocyanine thin-film transistors with polymeric gate dielectric. J. Non-Cryst. Solids 2006, 352, 1778–1782. [Google Scholar] [CrossRef]
- Shi, J.; Wang, H.; Song, D.; Tian, H.; Geng, Y.; Yan, D. Ambipolar organic heterojunction transistors with various p-type semiconductors. Thin Solid Film. 2008, 516, 3270–3273. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, Y.; Guo, Y.; Yu, G.; Wan, L.; Zhu, D. Influence of self-assembly monolayers on the characteristics of copper phthalacyanine thin film transistor. Appl. Phys. A 2005, 80, 1541–1545. [Google Scholar] [CrossRef]
- Wang, H.; Song, D.; Yang, J.; Yu, B.; Geng, Y.; Yan, D. High mobility vanadyl-phthalocyanine polycrystalline films for organic field-effect transistors. Appl. Phys. Lett. 2007, 90, 253510. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, F.; Liu, C.; Treske, U.; Grobosch, M.; Tian, H.; Zhang, J.; Geng, Y.; Knupfer, M.; Yan, D. Crystalline Organic Heterostructures Engineering Based on Vanadyl Phthalocyanine and Rod-Like Conjugated Organic Semiconductors with Selected Central Groups. Adv. Funct. Mater. 2012, 22, 4598–4607. [Google Scholar] [CrossRef]
- Zhu, F.; Grobosch, M.; Treske, U.; Knupfer, M.; Huang, L.; Ji, S.; Yan, D. Interfacial energy level bending in a crystalline p/p-type organic heterostructure. Appl. Phys. Lett. 2011, 98, 203303. [Google Scholar] [CrossRef]
- Qian, C.; Sun, J.; Zhang, L.; Xie, H.; Huang, H.; Yang, J.; Gao, Y. Air-stable and high-performance organic field-effect transistors based on ordered, large-domain phthalocyanine copper thin film. Synth. Met. 2015, 210, 336–341. [Google Scholar] [CrossRef]
- Chauhan, K.V.; Sullivan, P.; Yang, J.L.; Jones, T.S. Efficient Organic Photovoltaic Cells through Structural Modification of Chloroaluminum Phthalocyanine/Fullerene Heterojunctions. J. Phys. Chem. C 2010, 114, 3304–3308. [Google Scholar] [CrossRef]
- Sullivan, P.; Jones, T.S.; Ferguson, A.J.; Heutz, S. Structural templating as a route to improved photovoltaic performance in copper phthalocyanine/fullerene (C60) heterojunctions. Appl. Phys. Lett. 2007, 91, 233114. [Google Scholar] [CrossRef]
- Rochford, L.A.; Jones, T.S.; Nielsen, C.B. Epitaxial Templating of C60 with a Molecular Monolayer. J. Phys. Chem. Lett. 2016, 7, 3487–3490. [Google Scholar] [CrossRef]
- Hagara, J.; Kim, H.; Hagenlocher, J.; Zaluzhnyy, I.; Gerlach, A.; Hinderhofer, A.; Roth, S.V.; Brütting, W.; Schreiber, F. Improved order and transport in C60 thin films grown on SiO2 via use of transient templates. Appl. Phys. Lett. 2022, 121, 182101. [Google Scholar] [CrossRef]
- Liu, X.; Luo, X.; Nan, H.; Guo, H.; Wang, P.; Zhang, L.; Zhou, M.; Yang, Z.; Shi, Y.; Hu, W.; et al. Epitaxial Ultrathin Organic Crystals on Graphene for High-Efficiency Phototransistors. Adv. Mater. 2016, 28, 5200–5205. [Google Scholar] [CrossRef] [PubMed]
- Moh, A.; Khoo, P.L.; Sasaki, K.; Watase, S.; Shinagawa, T.; Izaki, M. Growth and Characteristics of C8-BTBT Layer on C-Sapphire Substrate by Thermal Evaporation. Phys. Status Solidi (a) 2018, 215, 1700862. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Cheng, X.; Huang, H. Investigation on the Interfaces in Organic Devices by Photoemission Spectroscopy. Nanomaterials 2025, 15, 680. https://doi.org/10.3390/nano15090680
Xie H, Cheng X, Huang H. Investigation on the Interfaces in Organic Devices by Photoemission Spectroscopy. Nanomaterials. 2025; 15(9):680. https://doi.org/10.3390/nano15090680
Chicago/Turabian StyleXie, Haipeng, Xianjun Cheng, and Han Huang. 2025. "Investigation on the Interfaces in Organic Devices by Photoemission Spectroscopy" Nanomaterials 15, no. 9: 680. https://doi.org/10.3390/nano15090680
APA StyleXie, H., Cheng, X., & Huang, H. (2025). Investigation on the Interfaces in Organic Devices by Photoemission Spectroscopy. Nanomaterials, 15(9), 680. https://doi.org/10.3390/nano15090680






