Activated Carbon from Spartina alterniflora and Its N-Doped Material for Li-Ion Battery Anode
Abstract
:1. Introduction
2. Experimental
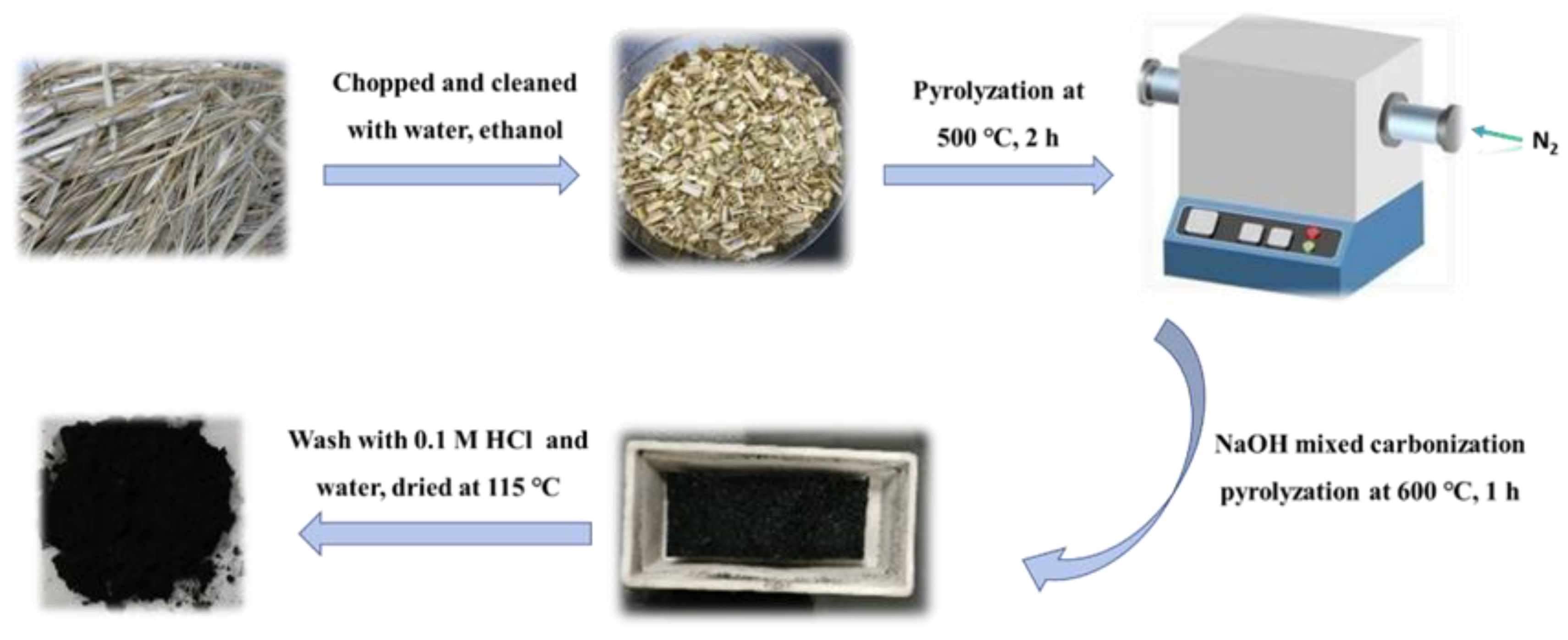
2.1. Material Synthesis
2.2. Characterization
2.3. Electrochemical Testing
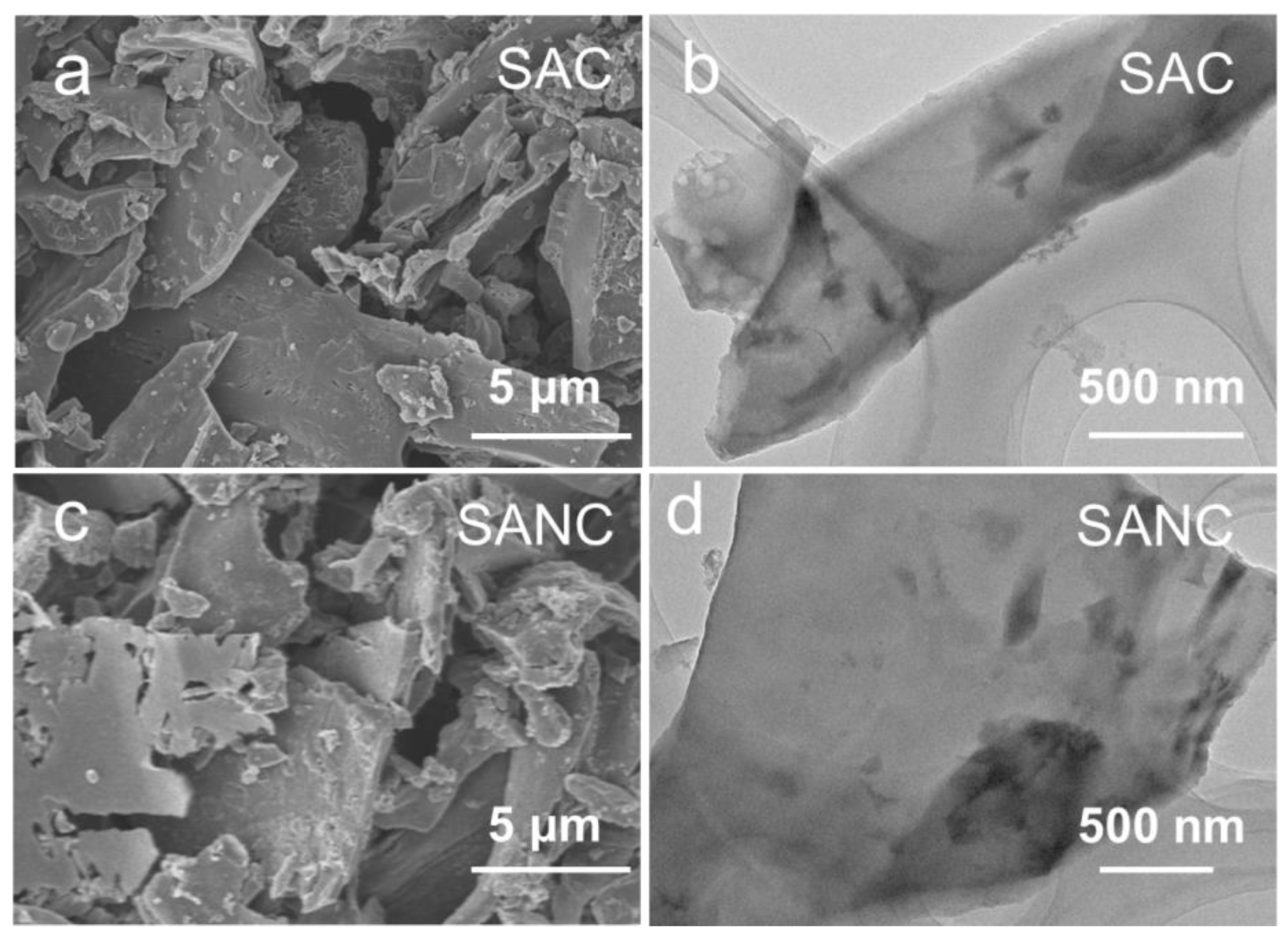
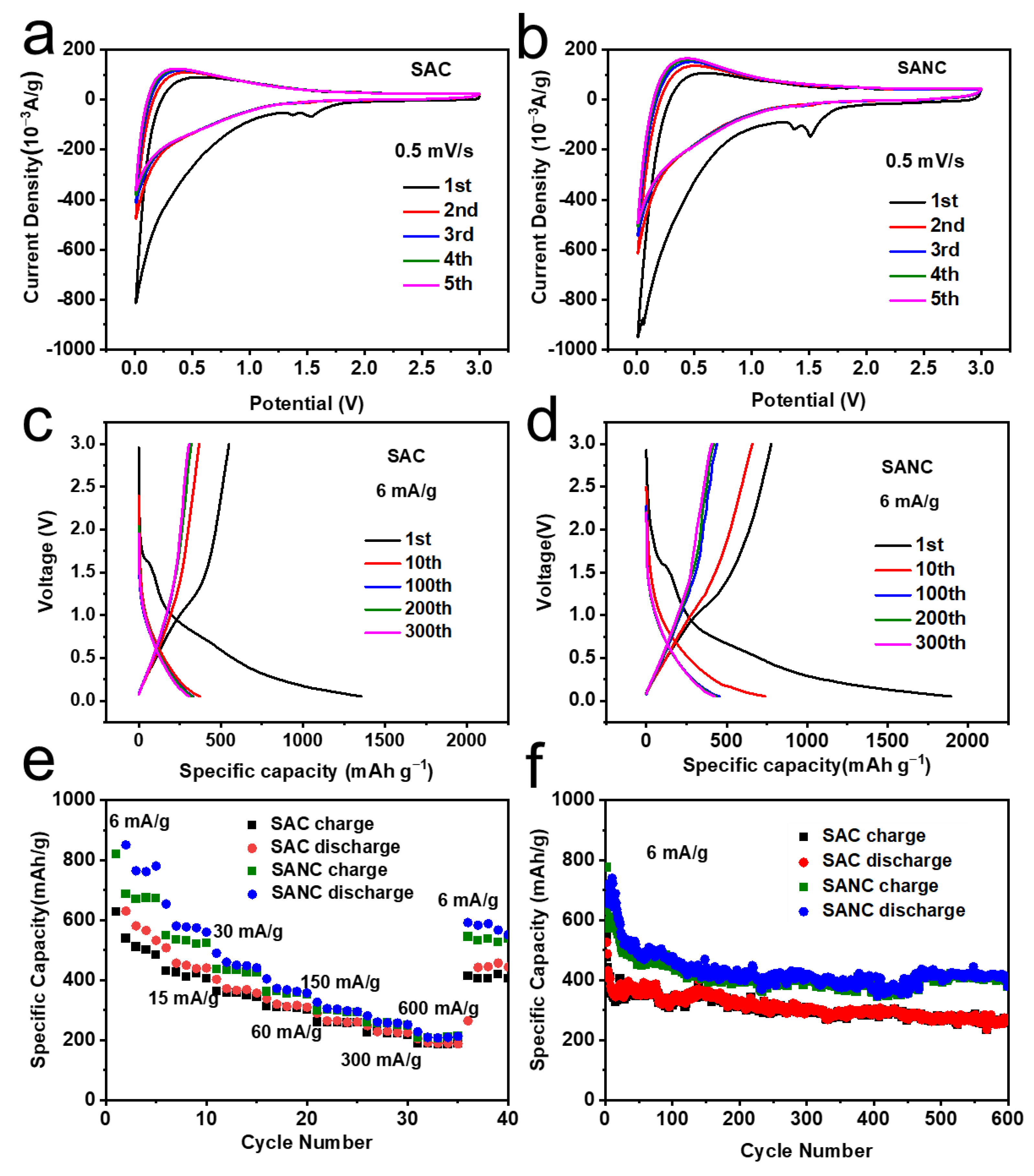
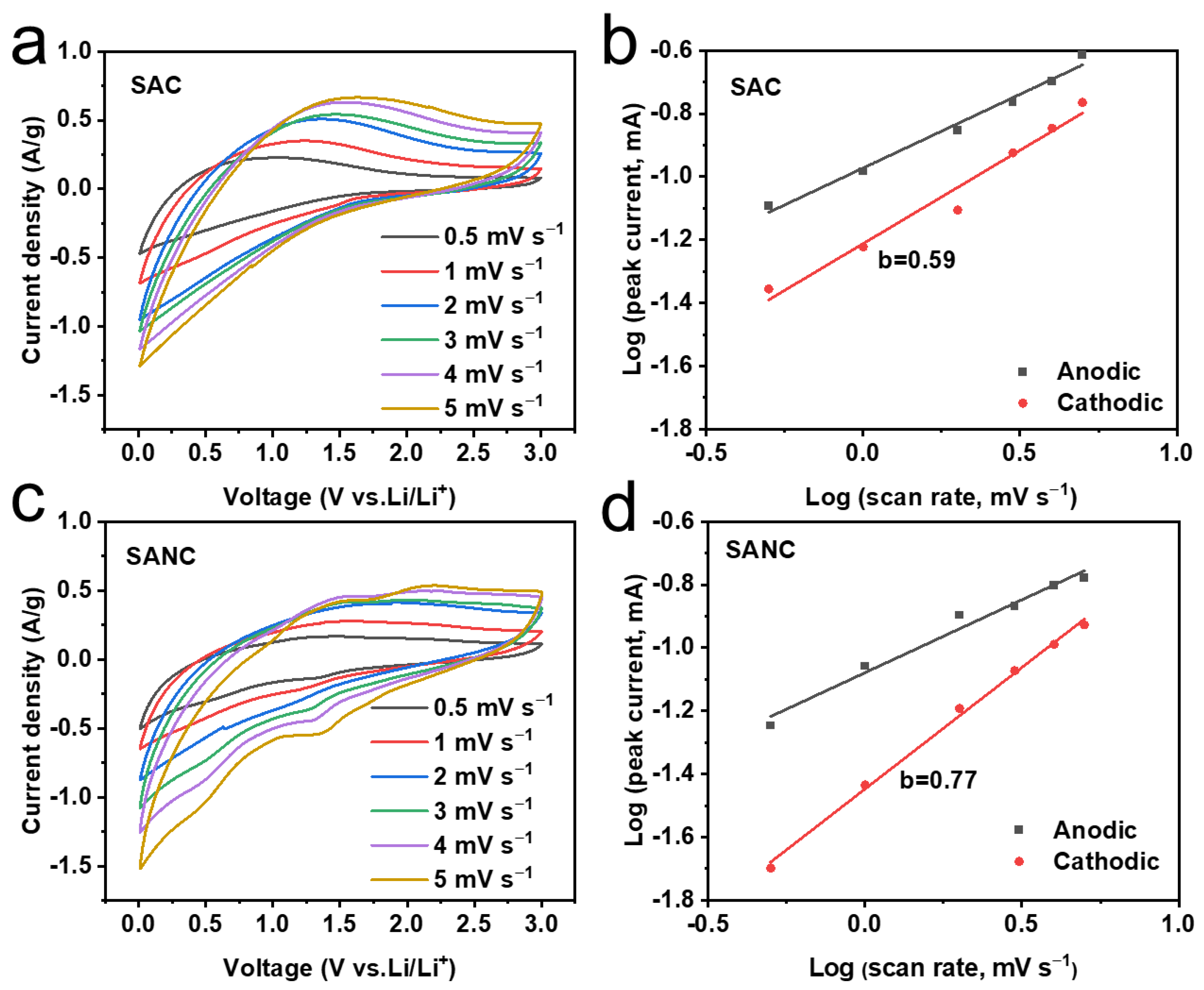
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, S.; Wang, G. Advanced lithium-ion batteries for practical applications: Technology, development, and future perspectives. Adv. Mater. Technol. 2018, 3, 1870034. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Li, X.; Deng, C.; Liu, M.; Xiong, J.; Zhang, X.; Yan, Q.; Lin, J.; Chen, C.; Wu, F.; Zhao, Y.; et al. Reutilization and upcycling of spent graphite for sustainable lithium-ion batteries: Progress and perspectives. eScience 2025, 100394. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Yu, H. Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ. Sci. 2019, 12, 1751–1779. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. A review of the next-generation biochar production from waste biomass for material applications. Sci. Total Environ. 2023, 15, 167171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Men, Y.; Zhang, W.; Yang, W.; Wang, F.; Zhang, Y.; Zhang, Y.; Zeng, X.; Xiao, J.; Tang, C.; et al. Versatile carbon-based materials from biomass for advanced electrochemical energy storage systems. eScience 2024, 4, 100249. [Google Scholar] [CrossRef]
- Jin, C.; Nai, J.; Sheng, O.; Yuan, H.; Zhang, W.; Tao, X.; Lou, X.W. Biomass-based materials for green lithium secondary batteries. Energy Environ. Sci. 2021, 14, 1326. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Vivekanandhan, S.; Das, O.; Millán, L.M.R.; Klinghoffer, N.B.; Nzihou, A.; Misra, M. Biocarbon materials. Nat. Rev. Methods Primers 2024, 4, 19. [Google Scholar] [CrossRef]
- Liedel, C. Sustainable battery materials from biomass. ChemSusChem 2020, 13, 2110–2141. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Park, J. Biomass-derived N-doped porous carbon nanosheets for energy technologies. Chem. Eng. J. 2021, 425, 129017. [Google Scholar] [CrossRef]
- Ariharan, A.; Ramesh, K.; Vinayagamoorthi, R.; Rani, M.S.; Viswanathan, B.; Ramaprabhu, S.; Nandhakumar, V. Biomass derived phosphorous containing porous carbon material for hydrogen storage and high-performance supercapacitor applications. J. Energy Storage 2021, 35, 102185. [Google Scholar] [CrossRef]
- Ma, L.; Hu, X.; Liu, W.; Li, H.; Lam, P.K.S.; Zeng, R.J.; Yu, H. Constructing N, P-dually doped biochar materials from biomass wastes for high-performance bifunctional oxygen electrocatalysts. Chemosphere 2021, 278, 130508. [Google Scholar] [CrossRef]
- Aristote, N.T.; Song, Z.; Deng, W.; Hou, H.; Zou, G.; Ji, X. Effect of double and triple-doping of sulfur, nitrogen and phosphorus on the initial coulombic efficiency and rate performance of the biomass derived hard carbon as anode for sodium-ion batteries. J. Power Sources 2023, 558, 232517. [Google Scholar] [CrossRef]
- Li, J.; Deng, W.; Li, H.; Chen, L.; Zhang, Y.; Li, J.; Song, Y.; Duan, H. Biomass-derived N-P double-doped porous carbon spheres and their lithium storage mechanism. Int. J. Hydrogen Energy 2024, 56, 828–836. [Google Scholar] [CrossRef]
- Wan, H.; Hu, X. Nitrogen doped biomass-derived porous carbon as anode materials of lithium ion batteries. Solid State Ionics 2019, 341, 115030. [Google Scholar] [CrossRef]
- Xiong, J.; Pan, Q.; Zheng, F.; Xiong, X.; Yang, C.; Hu, D.; Huang, C. N/S Co-doped carbon derived from cotton as high performance anode materials for lithium ion batteries. Front. Chem. 2018, 6, 78. [Google Scholar] [CrossRef]
- Butcha, S.; Rajrujithong, C.; Sattayarut, V.; Youngjan, S.; Nakajima, H.; Supruangnet, R.; Wittayakun, J.; Prayoonpokarach, S.; Faungnawakij, K.; Chanthad, C.; et al. Sustainable production of multifunctional hierarchical carbon from weed water hyacinth: Assessment for lithium-ion battery and supercapacitor. J. Energy Storage 2023, 72, 108578. [Google Scholar] [CrossRef]
- Yu, K.; Wang, J.; Wang, X.; Liang, J.; Liang, C. Sustainable application of biomass by-products: Corn straw-derived porous carbon nanospheres using as anode materials for lithium ion batteries. Mater. Chem. Phys. 2020, 243, 122644. [Google Scholar] [CrossRef]
- Zheng, S.; Luo, Y.; Zhang, K.; Liu, H.; Hu, G.; Qin, A. Nitrogen and phosphorus Co-doped mesoporous carbon nanosheets derived from bagasse for lithium-ion batteries. Mater. Lett. 2021, 290, 129459. [Google Scholar] [CrossRef]
- Cheng, H.; Tang, Z.; Luo, X.; Zheng, Z. Spartina alterniflora-derived porous carbon using as anode material for sodium-ion battery. Sci. Total Environ. 2021, 777, 146120. [Google Scholar] [CrossRef]
- Xia, L.; Yang, W.; Geng, Q.; Jeelani, N.; An, S. Research on spartina alterniflora using molecular biological techniques: An overview. Mar. Freshwater Res. 2020, 71, 1564–1571. [Google Scholar] [CrossRef]
- Xu, X.; Wu, F.; Yang, W.; Dai, X.; Wang, T.; Zhou, J.; Wang, J.; Guo, D. Silicon@natural nitrogen-doped biomass carbon composites derived from “silicon Tofu” as green and efficient anode materials for lithium-ion batteries. ACS Sustain. Chem. Eng. 2021, 9, 13215–13224. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Xu, R.; Gao, X.; Zhou, D.; Yuan, T.; Chen, Y.; Cui, L. In-situ embedding of ultrasmall MnO nanoparticles into pollen biomass-derived hollow porous N heteroatoms enriched carbon microspheres as high-performance anode for lithium-ion batteries. Chem. Eng. J. 2023, 473, 145117. [Google Scholar] [CrossRef]
- Bartoli, M.; Piovano, A.; Elia, G.A.; Meligrana, G.; Pedraza, R.; Pianta, N.; Tealdi, C.; Pagot, G.; Negro, E.; Triolo, C.; et al. Pristine and engineered biochar as Na-ion batteries anode material: A comprehensive overview. Renew. Sustain. Energy Rev. 2024, 194, 114304. [Google Scholar] [CrossRef]
- Yang, L.; Lei, Y.; Liang, X.; Qu, L.; Xu, K.; Hua, Y.; Feng, J. SnO2 nanoparticles composited with biomass N-doped carbon microspheres as low cost, environmentally friendly and high-performance anode material for sodium-ion and lithium-ion batteries. J. Power Sources 2022, 541, 232032. [Google Scholar] [CrossRef]
- Zeng, D.; Xiong, H.; Qi, K.; Guo, X.; Qiu, Y. Constructing N-doping biomass-derived carbon with hierarchically porous architecture to boost fast reaction kinetics for high-performance lithium storage. J. Colloid Interface Sci. 2021, 605, 741–751. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Lin, H.; Feng, P.; Xie, Y.; Liang, Y.; Zheng, M.; Liu, X.; Liu, Y.; Xiao, Y. Exfoliating waste biomass into porous carbon with multi-structural levels for dual energy storage. ACS Appl. Energy Mater. 2022, 5, 12090–12098. [Google Scholar] [CrossRef]
- Duan, Y.; Li, Z.; Zhang, S.; Su, T.; Ma, Y.; Jiao, A.; Fu, Z. Metal-organic frameworks (MOFs)-derived Mn2SnO4@C anode based on dual lithium storage mechanism for high-performance lithium-ion capacitors. Chem. Eng. J. 2023, 477, 146914. [Google Scholar] [CrossRef]
- Sobaszek, M.; Brzhezinskaya, M.; Olejnik, A.; Mortet, V.; Alam, M.; Sawczak, M.; Ficek, M.; Gazda, M.; Weiss, Z.; Bogdanowicz, R. Highly occupied surface states at deuterium-grown boron-doped diamond interfaces for efficient photoelectrochemistry. Small 2023, 19, 2208265. [Google Scholar] [CrossRef]
- Plavniece, A.; Kaare, K.; Simkunaite, D.; Balciunaite, A.; Jasulaitiene, V.; Niaura, G.; Volperts, A.; Dobele, G.; Colmenares-Rausseo, L.; Kruusenberg, I.; et al. Manganese- and nitrogen-doped biomass-based carbons as catalysts for the oxygen reduction reaction. Catalysts 2024, 14, 92. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Mishakov, I.V.; Bauman, Y.I.; Shubin, Y.V.; Maksimova, T.A.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A. One-pot functionalization of catalytically derived carbon nanostructures with heteroatoms for toxic-free environment. Appl. Surf. Sci. 2022, 590, 153055. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Liao, Y.; Zhang, Z.; Luo, S.; Li, L.; Wu, Y.; Qing, Y. Tuning carbonized wood fiber via sacrificial template-assisted hydrothermal synthesis for high-performance lithium/sodium-ion batteries. J. Power Sources 2022, 546, 231993. [Google Scholar] [CrossRef]
- Sun, C.; Pan, J.; Zhao, X.; Jiao, C.; Yao, W.; Wang, C.; Fu, X.; Ma, D.; Xue, H.; Liu, J.; et al. Effective coating of Si@NiO nanoflowers with nitrogen-doped wheat protein-derived biochar for efficient lithium-ion and lithium-sulfur batteries anode materials. J. Alloys Compd. 2023, 968, 171922. [Google Scholar] [CrossRef]
- Li, H.; Gong, Y.; Fu, C.; Zhou, H.; Yang, W.; Guo, M.; Li, M.; Kuang, Y. A novel method to prepare a nanotubes@mesoporous carbon composite material based on waste biomass and its electrochemical performance. J. Mater. Chem. A 2017, 5, 3875–3887. [Google Scholar] [CrossRef]
- Schneidermann, C.; Kensy, C.; Otto, P.; Oswald, S.; Giebeler, L.; Leistenschneider, D.; Grätz, S.; Dörfler, S.; Kaskel, S.; Borchardt, L. Nitrogen-doped biomass-derived carbon formed by mechanochemical synthesis for lithium-sulfur batteries. ChemSusChem 2018, 12, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, X.; Li, Z.; Zhang, X.; Zhai, X.; Zhang, Q.; Li, L.; Gao, N.; He, G.; Li, H. Nanowires framework supported porous lotus-carbon anode boosts lithium-ion and sodium-ion batteries. Small Methods 2023, 8, 2300746. [Google Scholar] [CrossRef] [PubMed]
- Sheng, O.; Jin, C.; Yang, T.; Ju, Z.; Luo, J.; Tao, X. Designing biomass-integrated solid polymer electrolytes for safe and energy-dense lithium metal batteries. Energy Environ. Sci. 2023, 16, 2804–2824. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Yan, X. Hierarchically porous and nitrogen, sulfur-codoped graphene-like microspheres as a high capacity anode for lithium ion batteries. Chem. Commun. 2015, 51, 2134–2137. [Google Scholar] [CrossRef]
- Tao, L.; Liu, L.; Chang, R.F.; He, H.B.; Zhao, P.; Liu, J. Structural and interface design of hierarchical porous carbon derived from soybeans as anode materials for potassium-ion batteries. J. Power Sources 2020, 463, 228172. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, R.; Pang, M.; Wang, H.; Zeng, S.; Yue, X.; Ni, L.; Qiu, S.; Zhang, Z. Hierarchical composites of ultrathin carbon self-coated TiO2 nanosheets on reduced graphene oxide with enhanced lithium storage capability. Chem. Eng. J. 2015, 280, 614–622. [Google Scholar] [CrossRef]
- Zhu, H.; Li, J.; Wu, D.; Zhang, G.; Sun, Y.; Wang, A.; Sun, K. Construction of the hierarchical porous biochar with an ultrahigh specific surface area for application in high-performance lithium-ion capacitor cathode. Biochar 2023, 5, 46. [Google Scholar] [CrossRef]
- Bhatia, D.; Saroha, A.K. Rice straw-derived biochar as anode material for lithium-ion battery: A step toward sustainable development. Energy Technol. 2024, 12, 6. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Jin, Y.H.; Han, T.; Yang, H.M.; Gond, R.; Subasi, Y.; Asfaw, H.D.; Younesi, R.; Jönsson, P.G.; Yang, W.H. Bio-based anode material production for lithium-ion batteries through catalytic graphitization of biochar: The deployment of hybrid catalysts. Sci. Rep. 2024, 14, 3966. [Google Scholar] [CrossRef] [PubMed]
- Belmesov, A.A.; Glukhov, A.A.; Kayumov, R.R.; Podlesniy, D.N.; Latkovskaya, E.M.; Repina, M.A.; Ivanov, N.P.; Tsvetkov, M.V.; Shichalin, O.O. Using aquatic plant-derived biochars as carbon materials for the negative electrodes of Li-ion batteries. Coatings 2023, 13, 2075. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, H.Y.; Ma, T.; Deng, H.; Zha, Z.H. Effect of cotton stalk particle size on the structure of biochar and the performance of anode for lithium-ion battery. J. Phys. Chem. Solids 2022, 169, 110845. [Google Scholar] [CrossRef]
- Jiang, R.T.; Yang, G. Biomass-derived N-doping porous carbon spheres by N2 plasma for lithium-ion batteries. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]

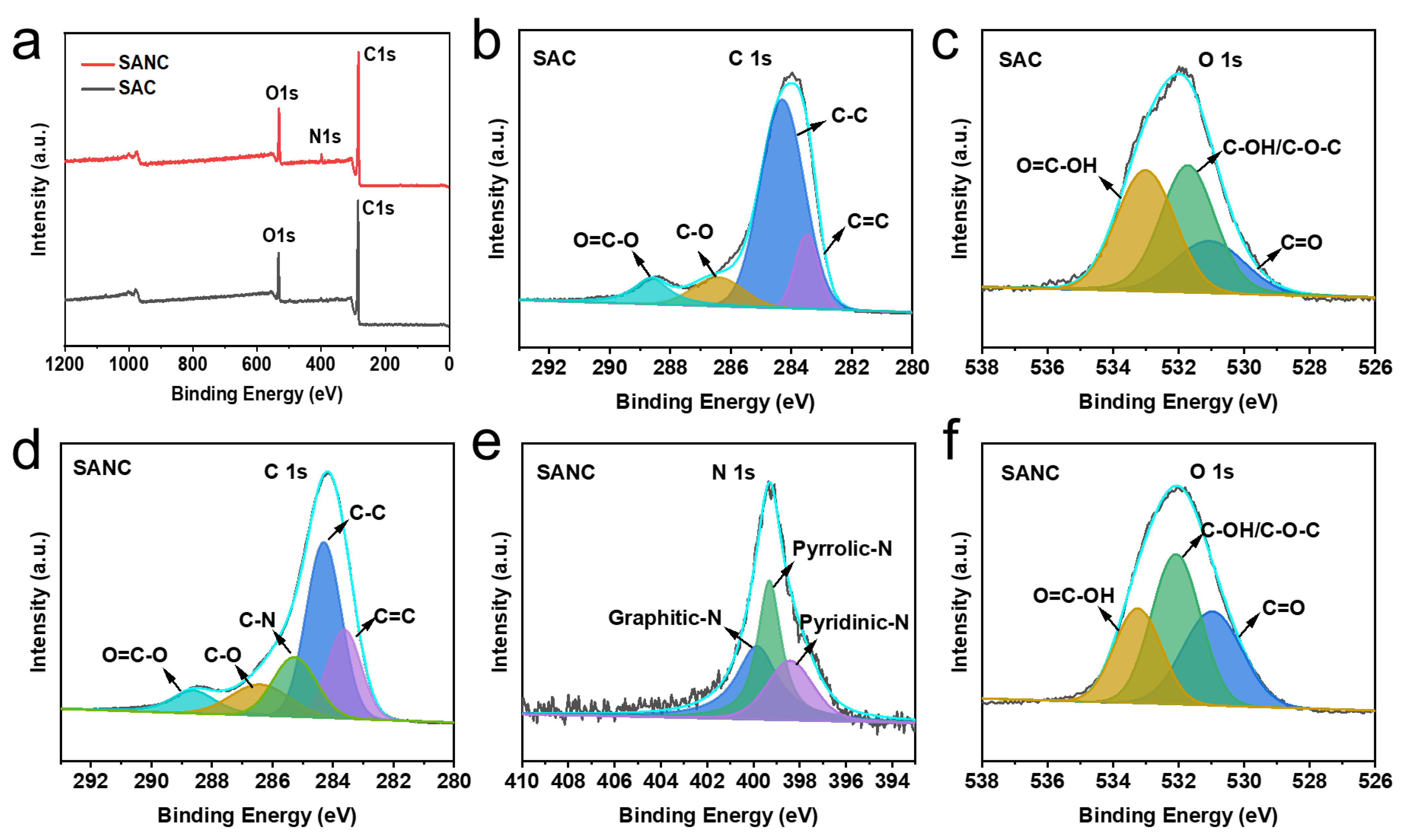
| Sample | Peaks and Their Positions (eV) | ||||
| C=C | C−C | C−N | C−O | O=C−OH | |
| 283.62 | 284.29 | 285.27 | 286.41 | 288.62 | |
| SAC | 18.1% | 45.0% | 14.9% | 12.8% | 9.2% |
| SANC | 17.6% | 57.9% | 13.14% | 11.34% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, H.; Hao, X.; Zhou, Y.; Peng, J.; Guo, L.; Li, H.; Sun, B. Activated Carbon from Spartina alterniflora and Its N-Doped Material for Li-Ion Battery Anode. Nanomaterials 2025, 15, 658. https://doi.org/10.3390/nano15090658
Shang H, Hao X, Zhou Y, Peng J, Guo L, Li H, Sun B. Activated Carbon from Spartina alterniflora and Its N-Doped Material for Li-Ion Battery Anode. Nanomaterials. 2025; 15(9):658. https://doi.org/10.3390/nano15090658
Chicago/Turabian StyleShang, Hong, Xinmeng Hao, Yougui Zhou, Jia Peng, Lihua Guo, Huipeng Li, and Bing Sun. 2025. "Activated Carbon from Spartina alterniflora and Its N-Doped Material for Li-Ion Battery Anode" Nanomaterials 15, no. 9: 658. https://doi.org/10.3390/nano15090658
APA StyleShang, H., Hao, X., Zhou, Y., Peng, J., Guo, L., Li, H., & Sun, B. (2025). Activated Carbon from Spartina alterniflora and Its N-Doped Material for Li-Ion Battery Anode. Nanomaterials, 15(9), 658. https://doi.org/10.3390/nano15090658







