Rapid and Ultrasensitive Short-Chain PFAS (GenX) Detection in Water via Surface-Enhanced Raman Spectroscopy with a Hierarchical Nanofibrous Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospun Nylon Nanofibers
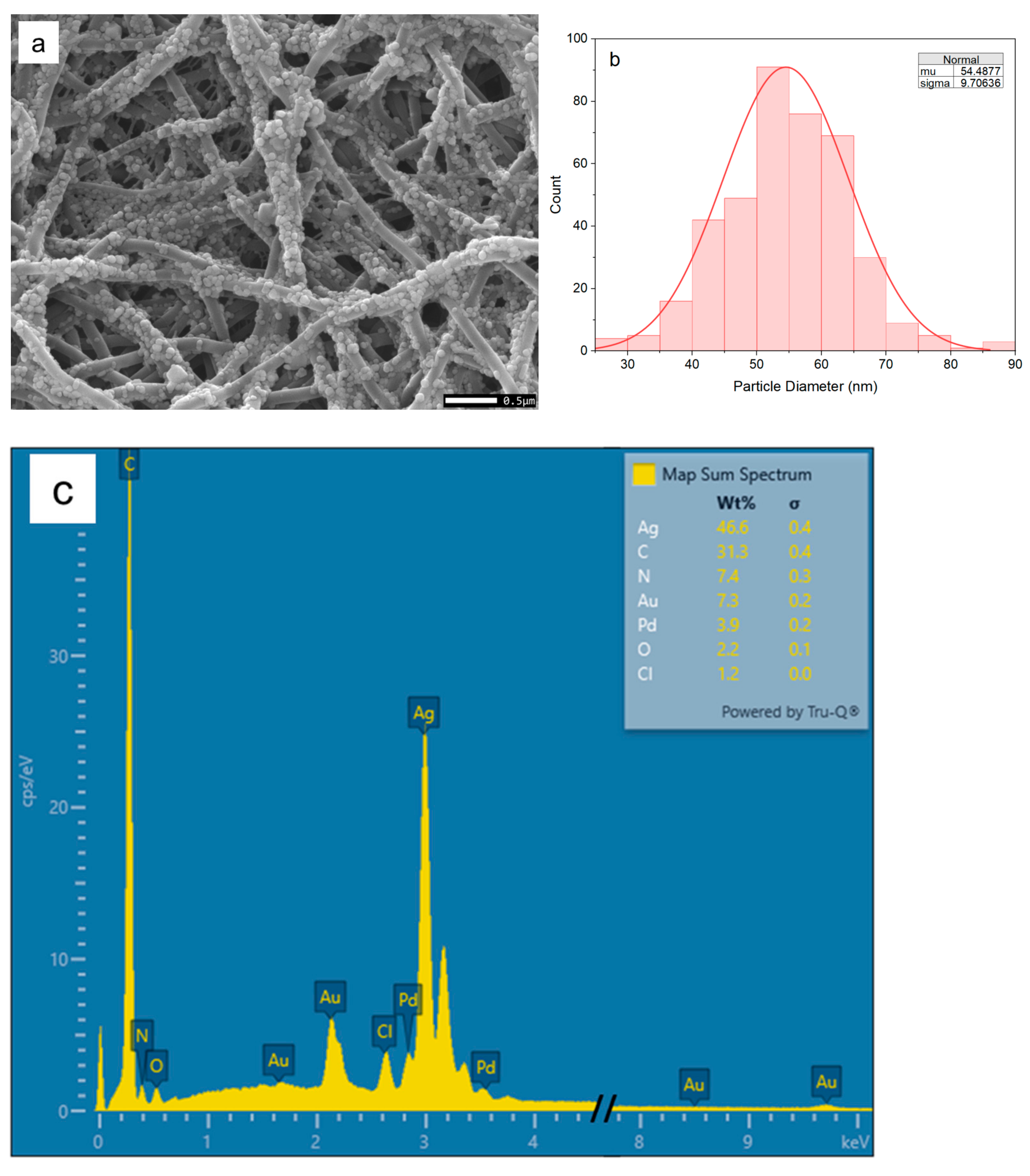
2.3. Assembly of Silver Nanoparticles on Nylon Nanofibers
2.4. Characterization
3. Results and Discussion
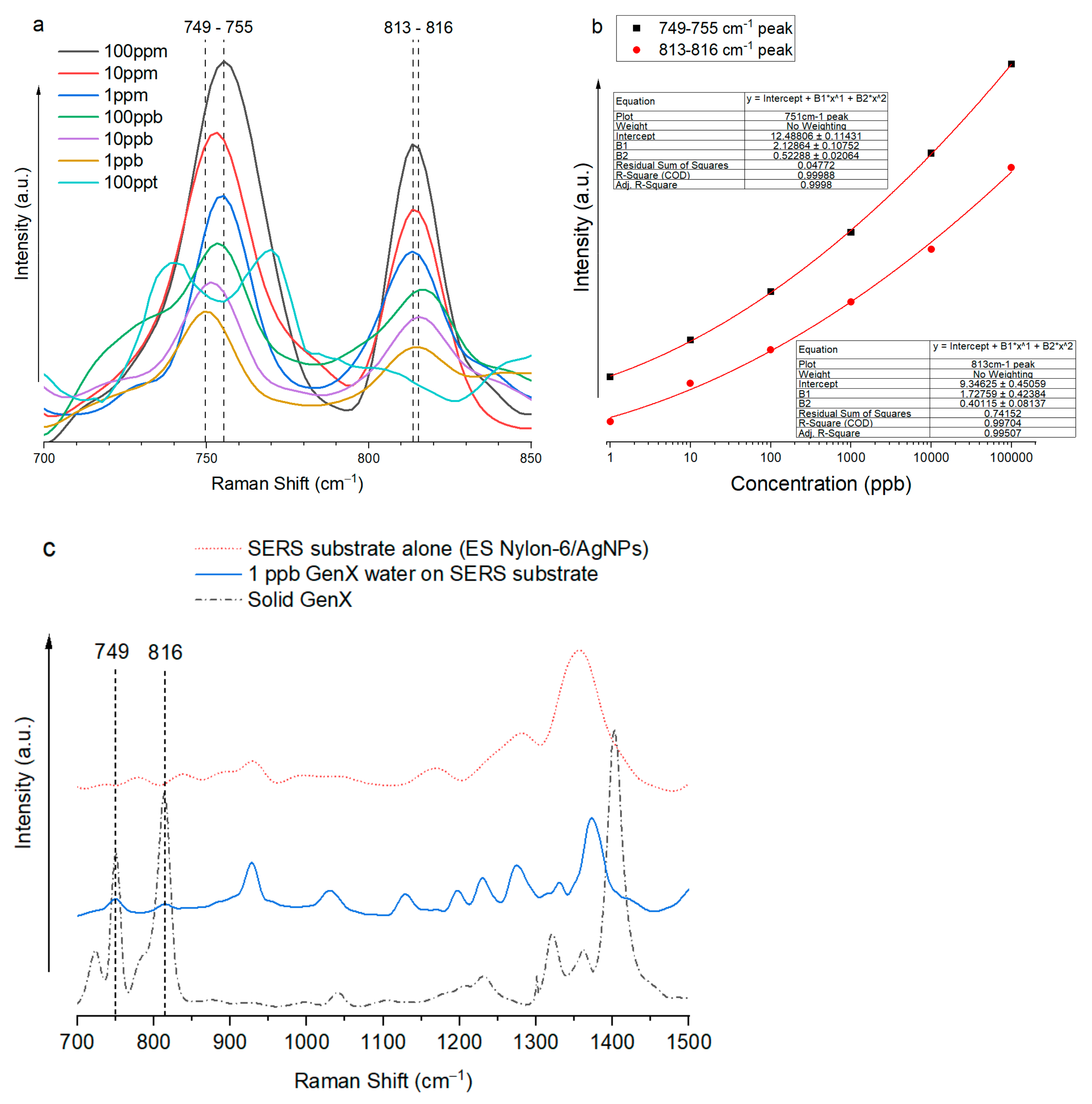
3.1. Determining the Unique Fingerprint Raman Signal of GenX Molecules
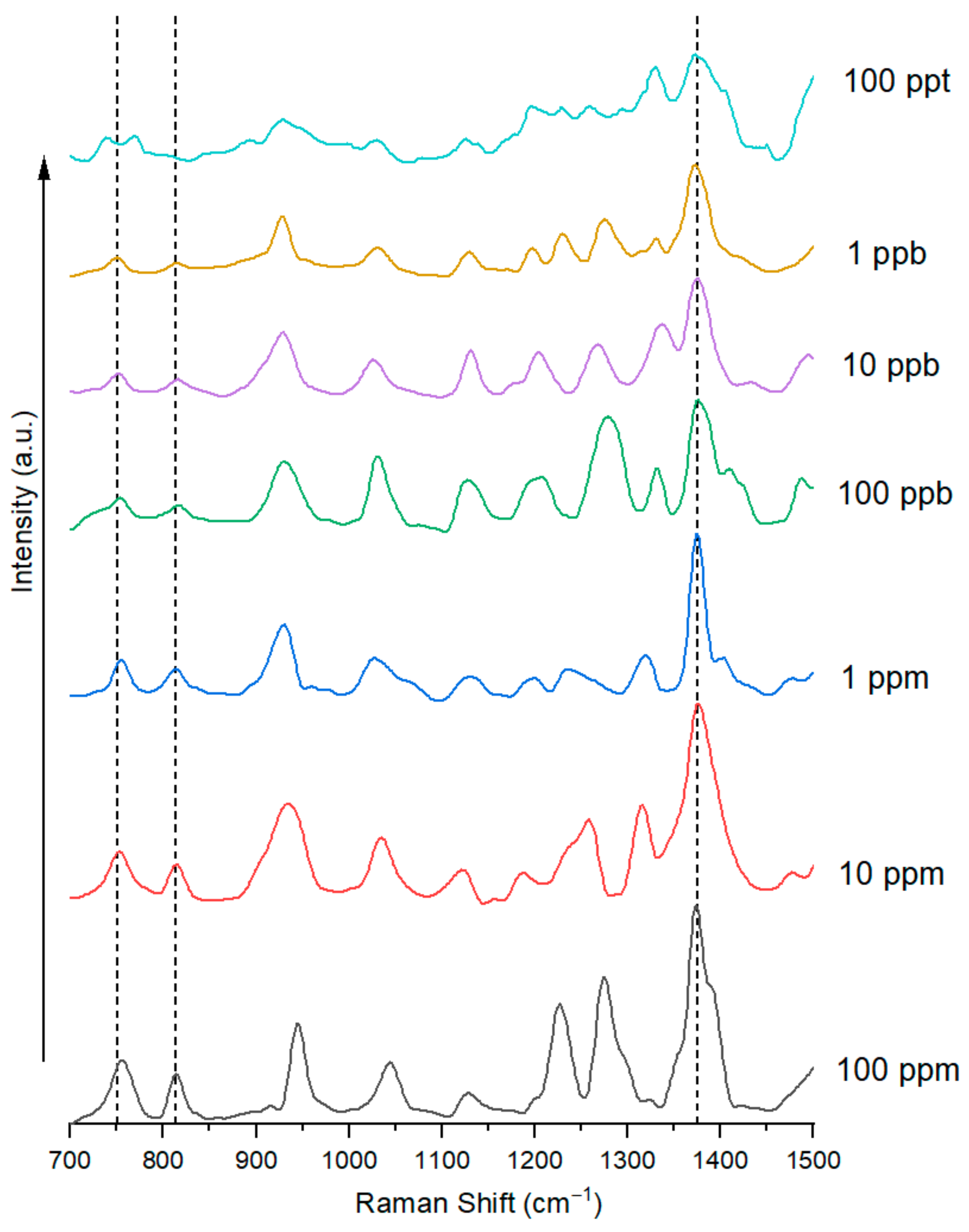
3.2. Highly Sensitive GenX Detection in Water Using SERS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manojkumar, Y.; Pilli, S.; Rao, P.V.; Tyagi, R.D. Sources, occurrence and toxic effects of emerging per- and polyfluoroalkyl substances (PFAS). Neurotoxicology Teratol. 2023, 97, 107174. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- Feng, Y.; Dai, J.; Wang, C.; Zhou, H.; Li, J.; Ni, G.; Zhang, M.; Huang, Y. Ag Nanoparticle/Au@Ag Nanorod Sandwich Structures for SERS-Based Detection of Perfluoroalkyl Substances. ACS Appl. Nano Mater. 2023, 6, 13974–13983. [Google Scholar] [CrossRef]
- Park, H.; Park, J.; Kim, W.; Kim, W.; Park, J. Ultra-sensitive SERS detection of perfluorooctanoic acid based on self-assembled p-phenylenediamine nanoparticle complex. J. Hazard. Mater. 2023, 453, 131384. [Google Scholar] [CrossRef]
- McDonnell, C.; Albarghouthi, F.M.; Selhorst, R.; Kelley-Loughnane, N.; Franklin, A.D.; Rao, R. Aerosol Jet Printed Surface-Enhanced Raman Substrates: Application for High-Sensitivity Detection of Perfluoroalkyl Substances. ACS Omega 2022, 8, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.C.; Cui, J.; Yang, Y.; Chen, X.; Zhao, Y. Ultra-sensitive detection of PFASs using surface enhanced Raman scattering and machine learning: A promising approach for environmental analysis. Sens. Diagn. 2024, 3, 1272–1284. [Google Scholar] [CrossRef]
- Almaviva, S.; Artuso, F.; Giardina, I.; Lai, A.; Pasquo, A. Fast Detection of Different Water Contaminants by Raman Spectroscopy and Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 8338. [Google Scholar] [CrossRef]
- Bhavya, M.; Rhakho, N.; Jena, S.R.; Yadav, S.; Altaee, A.; Saxena, M.; Samal, A.K. Detection of PFAS via Surface Enhanced Raman Scattering: Challenges and future Perspectives. Sustain. Chem. Environ. 2023. [Google Scholar] [CrossRef]
- Fang, C.; Megharaj, M.; Naidu, R. Surface-enhanced Raman scattering (SERS) detection of fluorosurfactants in firefighting foams. RSC Adv. 2016, 6, 11140–11145. [Google Scholar] [CrossRef]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Weifeng, J.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Mantripragada, S.; Obare, S.O.; Zhang, L. Addressing Short-Chain PFAS Contamination in Water with Nanofibrous Adsorbent/Filter Material from Electrospinning. Accounts Chem. Res. 2023, 56, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Kancharla, S.; Choudhary, A.; Davis, R.T.; Dong, D.; Bedrov, D.; Tsianou, M.; Alexandridis, P. GenX in water: Interactions and self-assembly. J. Hazard. Mater. 2022, 428, 128137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.-J.; Meng, J.-H.; Chen, S.; Panneerselvam, R.; Li, C.-Y.; Jamali, S.B.; Li, X.; Yang, Z.-L.; Li, J.-F.; et al. A facile method for the synthesis of large-size Ag nanoparticles as efficient SERS substrates. J. Raman Spectrosc. 2016, 47, 662–667. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, X.; Bao, Y.; Zhao, Y.; Xi, M.; Jiang, C.; Fong, H. Electrospun Nanofibrous Membranes Surface-Decorated with Silver Nanoparticles as Flexible and Active/Sensitive Substrates for Surface-Enhanced Raman Scattering. Langmuir 2012, 28, 14433–14440. [Google Scholar] [CrossRef]
- Pankin, D.; Kolesnikov, I.; Vasileva, A.; Pilip, A.; Zigel, V.; Manshina, A. Raman fingerprints for unambiguous identification of organotin compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 158–163. [Google Scholar] [CrossRef]
- Li, C.; Fang, X.; Li, H.; Zhang, X. Direct and Rapid Sensing of Per- and Polyfluoroalkyl Substances Using SERS-Active Optical Fibers. ACS Appl. Opt. Mater. 2024, 2, 610–616. [Google Scholar] [CrossRef]
- Marchetti, M.; Offroy, M.; Abdat, F.; Branchu, P.; Bourson, P.; Jobard, C.; Durmont, J.-F.; Casteran, G. Chemometrics-Assisted Monitoring in Raman Spectroscopy for the Biodegradation Process of an Aqueous Polyfluoroalkyl Ether from a Fire-Fighting foam in an Environmental Matrix. Environments 2020, 7, 4. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2001; Volume 199. [Google Scholar]
- de Resende, L.F.T.; Basilio, F.C.; Filho, P.A.; Therézio, E.M.; Silva, R.A.; Oliveira, O.N.; Marletta, A.; Campana, P.T. Revisiting the conformational transition model for the pH dependence of BSA structure using photoluminescence, circular dichroism, and ellipsometric Raman spectroscopy. Int. J. Biol. Macromol. 2024, 259, 129142. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Araújo, A.; Caro, C.; Mendes, M.J.; Nunes, D.; Fortunato, E.; Franco, R.; Águas, H.; Martins, R. Highly efficient nanoplasmonic SERS on cardboard packaging substrates. Nanotechnology 2014, 25, 415202. [Google Scholar] [CrossRef]
- Tong, Q.; Wang, W.; Fan, Y.; Dong, L. Recent progressive preparations and applications of silver-based SERS substrates. TrAC Trends Anal. Chem. 2018, 106, 246–258. [Google Scholar] [CrossRef]
- Dong, H.; Wang, D.; Sun, G.; Hinestroza, J.P. Assembly of Metal Nanoparticles on Electrospun Nylon 6 Nanofibers by Control of Interfacial Hydrogen-Bonding Interactions. Chem. Mater. 2008, 20, 6627–6632. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of Colloidal Silver Nanoparticles: Capping Action of Citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
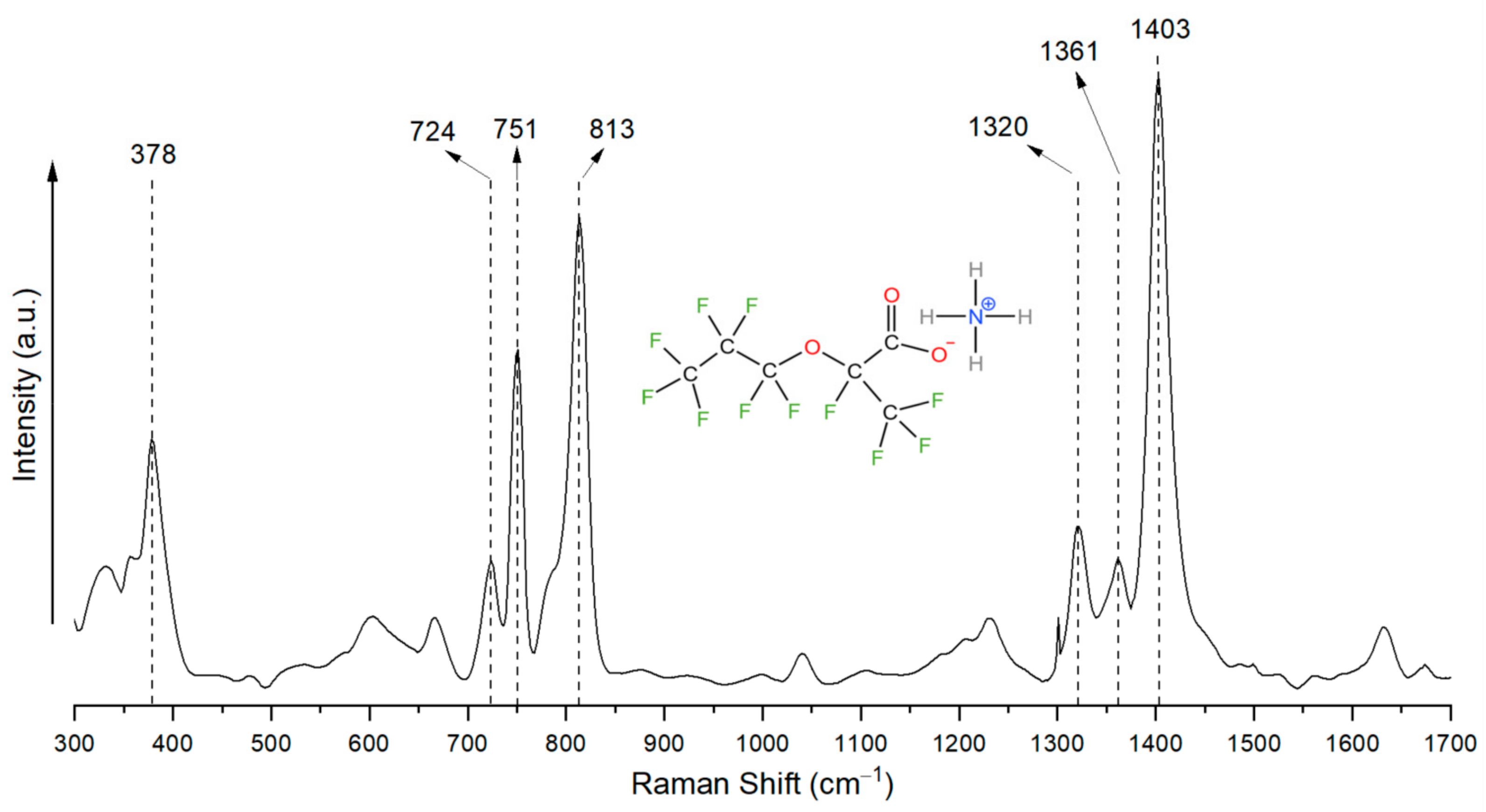
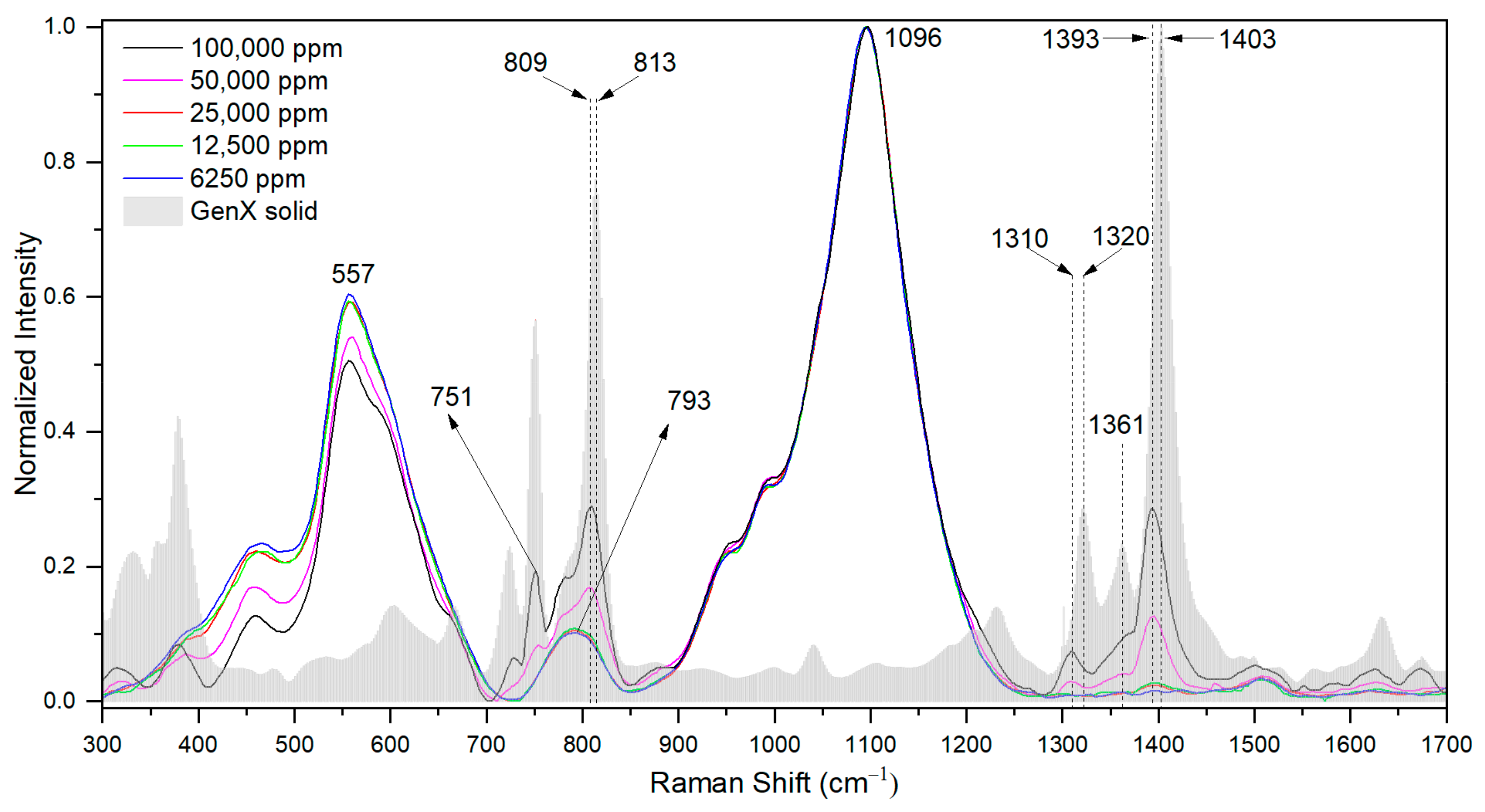


| Raman Shift for Solid State (cm−1) | Raman Shift When Dissolved in Water (cm−1) | Chemical Bond Assignment | Reference |
|---|---|---|---|
| 378 | 378 | –CF2– | [15] |
| 724 | 728 | C–C, –CF2–, –CF3 | [5,6,15] |
| 751 | 751 | –CF3, –CF2– | [6,16,17] |
| 813 | 809 | –CF2–,–CF3, –C–O–C– | [5,6,16,17,18] |
| 1320 | 1310 | –CF2–, –CF3 | [5,18] |
| 1361 | n.i. | –CF<, –COO− | [6,18] |
| 1403 | 1393 | –CF3, NH4+ | [6,18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, A.K.; Mantripragada, S.; Zhao, R.; Obare, S.O.; Zhang, L. Rapid and Ultrasensitive Short-Chain PFAS (GenX) Detection in Water via Surface-Enhanced Raman Spectroscopy with a Hierarchical Nanofibrous Substrate. Nanomaterials 2025, 15, 655. https://doi.org/10.3390/nano15090655
Ismail AK, Mantripragada S, Zhao R, Obare SO, Zhang L. Rapid and Ultrasensitive Short-Chain PFAS (GenX) Detection in Water via Surface-Enhanced Raman Spectroscopy with a Hierarchical Nanofibrous Substrate. Nanomaterials. 2025; 15(9):655. https://doi.org/10.3390/nano15090655
Chicago/Turabian StyleIsmail, Ali K., Shobha Mantripragada, Renzun Zhao, Sherine O. Obare, and Lifeng Zhang. 2025. "Rapid and Ultrasensitive Short-Chain PFAS (GenX) Detection in Water via Surface-Enhanced Raman Spectroscopy with a Hierarchical Nanofibrous Substrate" Nanomaterials 15, no. 9: 655. https://doi.org/10.3390/nano15090655
APA StyleIsmail, A. K., Mantripragada, S., Zhao, R., Obare, S. O., & Zhang, L. (2025). Rapid and Ultrasensitive Short-Chain PFAS (GenX) Detection in Water via Surface-Enhanced Raman Spectroscopy with a Hierarchical Nanofibrous Substrate. Nanomaterials, 15(9), 655. https://doi.org/10.3390/nano15090655







