Evaluation of Factors Influencing Fluoride Release from Dental Nanocomposite Materials: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Protocol
2.3. Eligibility Criteria
- Nanocomposite dental materials;
- Fluoride release evaluation;
- In vitro studies;
- Studies in English;
- Full-text articles.
- Other than nanocomposite dental materials;
- Evaluation of other physical, chemical, or mechanical properties without evaluation of fluoride release;
- Non-English papers;
- Systematic review articles;
- Review articles;
- No full-text accessible;
- Duplicated publications.
2.4. Information Sources, Search Strategy, and Study Selection
2.5. Data Collection Process and Data Items
2.6. Risk of Bias and Quality Assessment
2.7. Quality Assessment
3. Results
3.1. Study Selection
3.2. General Characteristics of the Included Studies
3.3. Main Study Outcomes
3.3.1. Sample Size/Volume and Material Composition
3.3.2. Storage Conditions
3.3.3. Measurement Methods
3.3.4. Fluoride Release Results
3.3.5. Estimated Daily Fluoride Release (Converted to μg/cm2/day)
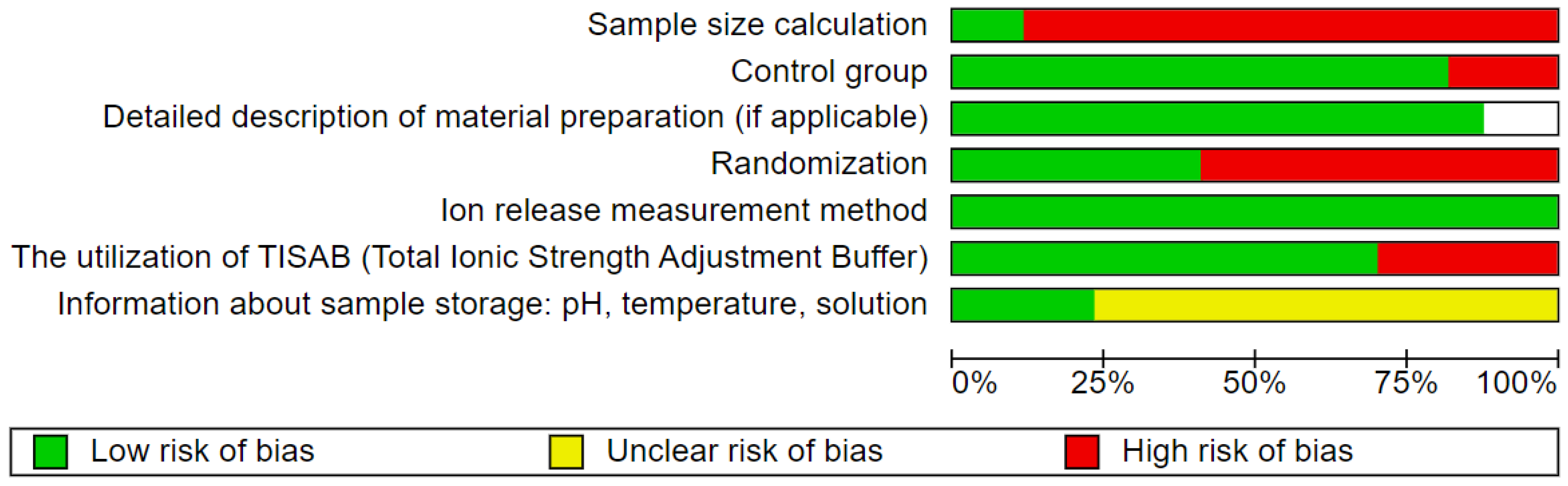
3.4. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jandt, K.D.; Watts, D.C. Nanotechnology in Dentistry: Present and Future Perspectives on Dental Nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Păstrav, M.; Păstrav, O.; Chisnoiu, A.M.; Chisnoiu, R.M.; Cuc, S.; Petean, I.; Saroși, C.; Feștilă, D. Properties of Nanohybrid Dental Composites—A Comparative In Vitro Study. Biomedicines 2024, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of Filler Characteristics on the Performance of Dental Composites: A Comprehensive Review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Sun, H.; Liu, Y.; Liu, W.; Su, B.; Li, S. The Development of Filler Morphology in Dental Resin Composites: A Review. Materials 2021, 14, 5612. [Google Scholar] [CrossRef]
- Chen, M.-H. Update on Dental Nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef]
- Beun, S.; Glorieux, T.; Devaux, J.; Vreven, J.; Leloup, G. Characterization of Nanofilled Compared to Universal and Microfilled Composites. Dent. Mater. 2007, 23, 51–59. [Google Scholar] [CrossRef]
- Mitra, S.B.; Wu, D.; Holmes, B.N. An Application of Nanotechnology in Advanced Dental Materials. J. Am. Dent. Assoc. 2003, 134, 1382–1390. [Google Scholar] [CrossRef]
- Gronwald, B.; Kozłowska, L.; Kijak, K.; Lietz-Kijak, D.; Skomro, P.; Gronwald, K.; Gronwald, H. Nanoparticles in Dentistry—Current Literature Review. Coatings 2023, 13, 102. [Google Scholar] [CrossRef]
- Nikolaidis, A.K.; Koulaouzidou, E.A.; Gogos, C.; Achilias, D.S. Synthesis of Novel Dental Nanocomposite Resins by Incorporating Polymerizable, Quaternary Ammonium Silane-Modified Silica Nanoparticles. Polymers 2021, 13, 1682. [Google Scholar] [CrossRef]
- Ausiello, P.; Ciaramella, S.; De Benedictis, A.; Lanzotti, A.; Tribst, J.P.M.; Watts, D.C. The Use of Different Adhesive Filling Material and Mass Combinations to Restore Class II Cavities under Loading and Shrinkage Effects: A 3D-FEA. Comput. Biomech. Biomed. Eng. 2021, 24, 485–495. [Google Scholar] [CrossRef]
- Alzraikat, H.; Burrow, M.F.; Maghaireh, G.A.; Taha, N.A. Nanofilled Resin Composite Properties and Clinical Performance: A Review. Oper. Dent. 2018, 43, E173–E190. [Google Scholar] [CrossRef] [PubMed]
- Saridou, M.; Nikolaidis, A.K.; Koulaouzidou, E.A.; Achilias, D.S. Synthesis and Characterization of Dental Nanocomposite Resins Reinforced with Dual Organomodified Silica/Clay Nanofiller Systems. J. Funct. Biomater. 2023, 14, 405. [Google Scholar] [CrossRef] [PubMed]
- Łukomska-Szymańska, M.; Zarzycka, B.; Grzegorczyk, J.; Sokołowski, K.; Półtorak, K.; Sokołowski, J.; Łapińska, B. Antibacterial Properties of Calcium Fluoride-Based Composite Materials: In Vitro Study. BioMed Res. Int. 2016, 2016, 1048320. [Google Scholar] [CrossRef]
- Boaro, L.C.C.; Campos, L.M.; Varca, G.H.C.; Dos Santos, T.M.R.; Marques, P.A.; Sugii, M.M.; Saldanha, N.R.; Cogo-Müller, K.; Brandt, W.C.; Braga, R.R.; et al. Antibacterial Resin-Based Composite Containing Chlorhexidine for Dental Applications. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, 909–918. [Google Scholar] [CrossRef]
- Mitwalli, H.; Balhaddad, A.A.; AlSahafi, R.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel CaF2 Nanocomposites with Antibacterial Function and Fluoride and Calcium Ion Release to Inhibit Oral Biofilm and Protect Teeth. J. Funct. Biomater. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Moreau, J.L.; Sun, L.; Chow, L.C. Novel CaF2 Nanocomposite with High Strength and Fluoride Ion Release. J. Dent. Res. 2010, 89, 739–745. [Google Scholar] [CrossRef]
- Tian, K.V.; Yang, B.; Yue, Y.; Bowron, D.T.; Mayers, J.; Donnan, R.S.; Dobó-Nagy, C.; Nicholson, J.W.; Fang, D.-C.; Greer, A.L.; et al. Atomic and Vibrational Origins of Mechanical Toughness in Bioactive Cement during Setting. Nat. Commun. 2015, 6, 8631. [Google Scholar] [CrossRef]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Sayyedan, F.S.; Fathi, M.; Edris, H.; Doostmohammadi, A.; Mortazavi, V.; Shirani, F. Fluoride Release and Bioactivity Evaluation of Glass Ionomer: Forsterite Nanocomposite. Dent. Res. J. 2013, 10, 452–459. [Google Scholar]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Fluoride Exchange by Glass-Ionomer Dental Cements and Its Clinical Effects: A Review. Biomater. Investig. Dent. 2023, 10, 2244982. [Google Scholar] [CrossRef]
- Mitra, S.B.; Oxman, J.D.; Falsafi, A.; Ton, T.T. Fluoride Release and Recharge Behavior of a Nano-Filled Resin-Modified Glass Ionomer Compared with That of Other Fluoride Releasing Materials. Am. J. Dent. 2011, 24, 372–378. [Google Scholar] [PubMed]
- Neti, B.; Sayana, G.; Muddala, L.; Raju, S.; Yarram, A.; GVD, H. Fluoride Releasing Restorative Materials: A Review. Int. J. Dent. Mater. 2020, 2, 19–23. [Google Scholar] [CrossRef]
- Asmussen, E.; Peutzfeldt, A. Long-Term Fluoride Release from a Glass Ionomer Cement, a Compomer, and from Experimental Resin Composites. Acta Odontol. Scand. 2002, 60, 93–97. [Google Scholar] [CrossRef]
- Nigam, A.G.; Murthy, R.; Pandey, R. Estimation of Fluoride Release from Various Dental Materials in Different Media—An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2009, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Moreau, J.; Sun, L.; Chow, L.C. Strength and Fluoride Release Characteristics of a Calcium Fluoride Based Dental Nanocomposite. Biomaterials 2008, 29, 4261–4267. [Google Scholar] [CrossRef]
- Olczak-Kowalczyk, D.; Mielczarek, A.; Jackowska, T.; Mielnik-Błaszczak, M.; Turska-Szybka, A.; Opydo-Szymaczek, J.; Jurczak, A.; Kaczmarek, U. Fluoride agents in the prevention and treatment of dental caries and erosion in children, adolescents and adults–recommendations of Polish Experts. Update of recommendations: Individual fluoride prevention in children and adolescents–recommendations of Polish Experts. Nowa Stomatol. 2022, 27, 35–59. [Google Scholar] [CrossRef]
- Sicca, C.; Bobbio, E.; Quartuccio, N.; Nicolò, G.; Cistaro, A. Prevention of Dental Caries: A Review of Effective Treatments. J. Clin. Exp. Dent. 2016, 8, e604–e610. [Google Scholar] [CrossRef]
- Veiga, N.; Figueiredo, R.; Correia, P.; Lopes, P.; Couto, P.; Fernandes, G.V.O. Methods of Primary Clinical Prevention of Dental Caries in the Adult Patient: An Integrative Review. Healthcare 2023, 11, 1635. [Google Scholar] [CrossRef]
- Meyer, J.M.; Cattani-Lorente, M.A.; Dupuis, V. Compomers: Between Glass-Ionomer Cements and Composites. Biomaterials 1998, 19, 529–539. [Google Scholar] [CrossRef]
- Lee, Y. Diagnosis and Prevention Strategies for Dental Caries. J. Lifestyle Med. 2013, 3, 107–109. [Google Scholar]
- Mankar, N.; Kumbhare, S.; Nikhade, P.; Mahapatra, J.; Agrawal, P. Role of Fluoride in Dentistry: A Narrative Review. Cureus 2023, 15, e50884. [Google Scholar] [CrossRef] [PubMed]
- Nassar, Y.; Brizuela, M. The Role of Fluoride on Caries Prevention. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Di Lauro, A.; Di Duca, F.; Montuori, P.; Dal Piva, A.M.d.O.; Tribst, J.P.M.; Borges, A.L.S.; Ausiello, P. Fluoride and Calcium Release from Alkasite and Glass Ionomer Restorative Dental Materials: In Vitro Study. J. Funct. Biomater. 2023, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, A.; Di Duca, F.; Triassi, M.; Montuori, P.; Scippa, S.; Piscopo, M.; Ausiello, P. The Effect of Different pH and Temperature Values on Ca2+, F−, PO43−, OH−, Si, and Sr2+ Release from Different Bioactive Restorative Dental Materials: An In Vitro Study. Polymers 2025, 17, 640. [Google Scholar] [CrossRef]
- Vasisth, D.; Mehra, P.; Yadav, L.; Kumari, V.; Bhatia, U.; Garg, R. Fluoride and Its Implications on Oral Health: A Review. J. Pharm. Bioallied Sci. 2024, 16, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, C.H.; Manh, D.T.; Le, H. An Experimental and Clinically Controlled Study of the Prevention of Dental Caries Using 1.23% Fluoride Gel in Elderly Patients. J. Int. Soc. Prev. Community Dent. 2021, 11, 661–670. [Google Scholar] [CrossRef]
- Körner, P.; Georgis, L.; Wiedemeier, D.B.; Attin, T.; Wegehaupt, F.J. Potential of Different Fluoride Gels to Prevent Erosive Tooth Wear Caused by Gastroesophageal Reflux. BMC Oral Health 2021, 21, 183. [Google Scholar] [CrossRef]
- Medjedovic, E.; Medjedovic, S.; Deljo, D.; Sukalo, A. IMPACT OF FLUORIDE ON DENTAL HEALTH QUALITY. Mater. Socio-Medica 2015, 27, 395–398. [Google Scholar] [CrossRef]
- Nayak, A.K.; Mazumder, S.; Ara, T.J.; Ansari, M.T.; Hasnain, M.S. 2-Calcium Fluoride-Based Dental Nanocomposites. In Applications of Nanocomposite Materials in Dentistry; Asiri, A.M., Inamuddin, Mohammad, A., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 27–45. ISBN 978-0-12-813742-0. [Google Scholar]
- Moreau, J.L.; Xu, H.H.K. Fluoride Releasing Restorative Materials: Effects of pH on Mechanical Properties and Ion Release. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2010, 26, e227–e235. [Google Scholar] [CrossRef]
- Bahadure, R.N.; Pandey, R.K.; Kumar, R.; Gopal, K.; Singh, R.K. An Estimation of Fluoride Release from Various Dental Restorative Materials at Different pH: In Vitro Study. J. Indian Soc. Pedod. Prev. Dent. 2012, 30, 122–126. [Google Scholar] [CrossRef]
- Vuletic, L.; Peros, K.; Spalj, S.; Rogic, D.; Alajbeg, I. Time-Related Changes in pH, Buffering Capacity and Phosphate and Urea Concentration of Stimulated Saliva. Oral Health Prev. Dent. 2014, 12, 45–53. [Google Scholar] [CrossRef]
- Edgar, W.M. The Role of Saliva in the Control of pH Changes in Human Dental Plaque. Caries Res. 1976, 10, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Loke, C.; Lee, J.; Sander, S.; Mei, L.; Farella, M. Factors Affecting Intra-oral pH—A Review. J. Oral Rehabil. 2016, 43, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.A.; Kriven, W.M.; Casanova, H. Development of Mechanical Properties in Dental Resin Composite: Effect of Filler Size and Filler Aggregation State. Mater. Sci. Eng. C 2019, 101, 274–282. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tokarczuk, D.; Tokarczuk, O.; Kiryk, J.; Kensy, J.; Szablińska, M.; Dyl, T.; Dobrzyński, W.; Matys, J.; Dobrzyński, M. Fluoride Release by Restorative Materials after the Application of Surface Coating Agents: A Systematic Review. Appl. Sci. 2024, 14, 4956. [Google Scholar] [CrossRef]
- Oleniacz-Trawińska, M.; Kotela, A.; Kensy, J.; Kiryk, S.; Dobrzyński, W.; Kiryk, J.; Gerber, H.; Fast, M.; Matys, J.; Dobrzyński, M. Evaluation of Factors Affecting Fluoride Release from Compomer Restorative Materials: A Systematic Review. Materials 2025, 18, 1627. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, S.; Kiryk, J.; Kensy, J.; Michalak, M.; Matys, J.; Dobrzyński, M. Fluoride Release from Two Commercially Available Dental Fluoride Gels—In Vitro Study. Gels 2025, 11, 135. [Google Scholar] [CrossRef]
- Kiryk, J.; Kiryk, S.; Kensy, J.; Świenc, W.; Palka, B.; Zimoląg-Dydak, M.; Dobrzyński, W.; Matys, J.; Dobrzyński, M. Effectiveness of Laser-Assisted Teeth Bleaching: A Systematic Review. Appl. Sci. 2024, 14, 9219. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Taheri, M.M.; Abdul Kadir, M.R.; Shokuhfar, T.; Hamlekhan, A.; Shirdar, M.R.; Naghizadeh, F. Fluoridated Hydroxyapatite Nanorods as Novel Fillers for Improving Mechanical Properties of Dental Composite: Synthesis and Application. Mater. Des. 2015, 82, 119–125. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Lee, B.-S.; Chang, K.-C.; Chiu, H.-C.; Lin, F.-H.; Lin, C.-P. Characterization, Fluoride Release and Recharge Properties of Polymer–Kaolinite Nanocomposite Resins. Compos. Sci. Technol. 2007, 67, 3409–3416. [Google Scholar] [CrossRef]
- Liu, J.; Dai, Q.; Weir, M.D.; Schneider, A.; Zhang, C.; Hack, G.D.; Oates, T.W.; Zhang, K.; Li, A.; Xu, H.H.K. Biocompatible Nanocomposite Enhanced Osteogenic and Cementogenic Differentiation of Periodontal Ligament Stem Cells In Vitro for Periodontal Regeneration. Mater. 2020, 13, 4951. [Google Scholar] [CrossRef]
- Dai, Q.; Weir, M.D.; Ruan, J.; Liu, J.; Gao, J.; Lynch, C.D.; Oates, T.W.; Li, Y.; Chang, X.; Xu, H.H.K. Effect of Co-Precipitation plus Spray-Drying of Nano-CaF2 on Mechanical and Fluoride Properties of Nanocomposite. Dent. Mater. Off. Publ. Acad. Dent. Materials 2021, 37, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Mitwalli, H.; AlSahafi, R.; Albeshir, E.G.; Dai, Q.; Sun, J.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel Nano Calcium Fluoride Remineralizing and Antibacterial Dental Composites. J. Dent. 2021, 113, 103789. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Aamer, S.; Chaudhry, A.A.; Wong, F.S.L.; Ur Rehman, I. Synthesis and Characterizations of a Fluoride-Releasing Dental Restorative Material. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3458–3464. [Google Scholar] [CrossRef]
- Li, K.-Y.; Tsai, C.-C.; Fang, C.-H.; Wang, Y.-L.; Lin, F.-H.; Lin, C.-P. Fluorinated Montmorillonite Composite Resin as a Dental Pit and Fissure Sealant. Polymers 2019, 11, 1535. [Google Scholar] [CrossRef]
- Mitwalli, H.; AlSahafi, R.; Alhussein, A.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel Rechargeable Calcium Fluoride Dental Nanocomposites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2022, 38, 397–408. [Google Scholar] [CrossRef]
- Meng, L.; Shu, M.; Mei, P.; Liang, Y.; Xia, L. Size-Controllable Synthesis of Hydroxyapatite Nanorods via Fluorine Modulation: Applications in Dental Adhesives for Enhanced Enamel Remineralization. BMC Oral Health 2025, 25, 204. [Google Scholar] [CrossRef]
- Fei, X.; Li, Y.; Weir, M.D.; Baras, B.H.; Wang, H.; Wang, S.; Sun, J.; Melo, M.A.S.; Ruan, J.; Xu, H.H.K. Novel Pit and Fissure Sealant Containing Nano-CaF2 and Dimethylaminohexadecyl Methacrylate with Double Benefits of Fluoride Release and Antibacterial Function. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 1241–1253. [Google Scholar] [CrossRef]
- Leite, K.L.d.F.; Vieira, T.I.; Alexandria, A.K.; Silva, R.F.d.; Silva, A.S.d.S.; Lopes, R.T.; Fonseca-Gonçalves, A.; Neves, A.d.A.; Cabral, L.M.; Pithon, M.M.; et al. In Vitro Effect of Experimental Nanocomposites Solutions on the Prevention of Dental Caries around Orthodontic Brackets. Braz. Dent. J. 2021, 32, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Komalsingsakul, A.; Srisatjaluk, R.L.; Senawongse, P. Effect of Brushing on Surface Roughness, Fluoride Release, and Biofilm Formation with Different Tooth-Colored Materials. J. Dent. Sci. 2022, 17, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Morais, W.A.; Passos, V.F.; Lima, J.P.M.; Rodrigues, L.K.A. Fluoride Releasing and Enamel Demineralization around Orthodontic Brackets by Fluoride-Releasing Composite Containing Nanoparticles. Clin. Oral Investig. 2014, 18, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Kooshki, F.; Fatemi, S.M.; Darvishghaderi, S.; Vahedi, P. Comparison of the effects of fluoride varnish containing silver nanoparticles and conventional fluoride varnish on the surface microhardness of tooth enamel. Dent Med Probl. 2024, 61, 241–247. [Google Scholar] [CrossRef]
- Shalaby, H.A.; Soliman, N.K.; HardnessAl-Saudi, K.W. Antibacterial and preventive effects of newly developed modified nano-chitosan/glass-ionomer restoration on simulated initial enamel caries lesions: An in vitro study. Dent Med Probl. 2024, 61, 353–362. [Google Scholar] [CrossRef]
- O’Donnell, J.N.R.; Schumacher, G.E.; Antonucci, J.M.; Skrtic, D. Structure-Composition-Property Relationships in Polymeric Amorphous Calcium Phosphate-Based Dental Composites. Materials 2009, 2, 1929–1954. [Google Scholar] [CrossRef]
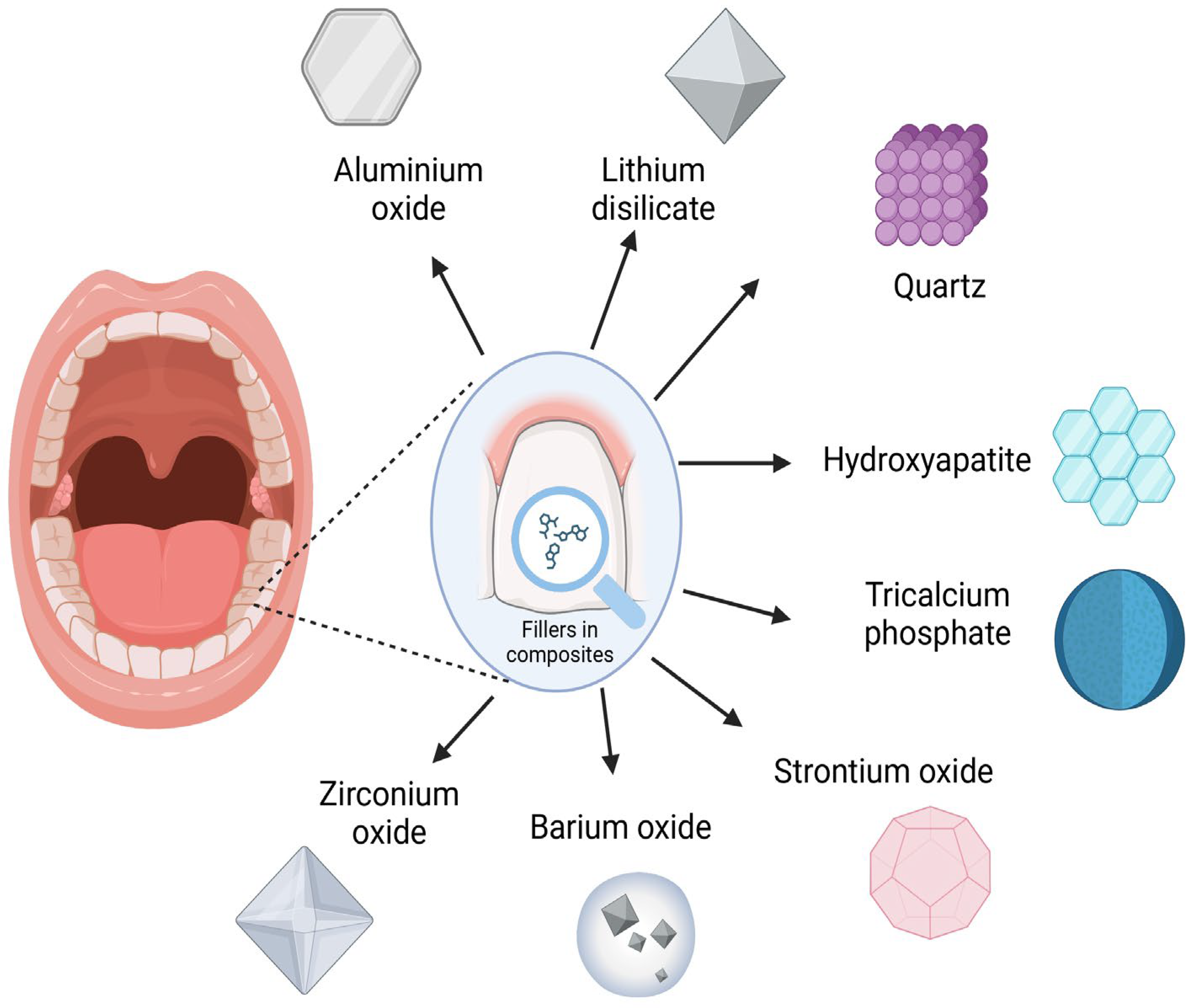

| Authors | Sample Size/Volume | Material Composition | Measurement Method | Storage Conditions | Fluoride Release Results | Fluoride Release Rate (μg/cm2/day) |
|---|---|---|---|---|---|---|
| Taheri [53] | 1 mm × 0.2 mm | Bis-GMA/TEGDMA + HA + NaF + I-819 | Ion chromatography |
| 0.97 ppm (μg/mL) at 21 days for 0.2 wt% FHA | 138.6 |
| Melo [65] | N/A | Orthocem (Dentscare Ltd.a., Joinville, SC, Brazil) | Fluoride-sensitive electrode |
| 1.48 (1.24) μg/g wet Biofilm Incubation time 5 days | 0.0362 (estimated; based on 1.48 μg/g wet biofilm, assuming 0.012 g biofilm mass and 0.09806 cm2 bracket contact area over 5 days) |
| Wang [54] | 6 mm diameter × 2 mm thickness | Kaolinite + diamine/acrylamide/acetate + Bis-GMA/TEGDMA (NaF only in diamine) | Ion-selective electrode |
| No numeric data | No numeric data |
| Mitwalli [15] | 2 mm × 2 mm × 12 mm | BT + CaF2 (+DMAHDM/+MPC/+DMAHDM + MPC) + glass | Ion-selective electrode |
| nCaF2 + MPC = 0.40 ± 0.02 mmol/L nCaF2 + DMAHDM + MPC = 0.25 ± 0.03 mmol/L nCaF2 + DMAHDM = 0.20 ± 0.03 mmol/L nCaF2 = 0.04 ± 0.01 mmol/L | nCaF2 + MPC: 2261.90 nCaF2 + DMAHDM + MPC: 1413.69 nCaF2 + DMAHDM: 1130.95 nCaF2: 226.19 |
| Dai [56] | 2 mm × 2 mm × 12 mm | Heliomolar (Ivoclar); Exp.: Bis-GMA/TEGDMA + CaF2 + glass + silane | Ion-selective electrode |
| 20% CaF2cp composite = 183.7 ± 5.8 g/cm2 20% nCaF2cpsd composite = 103.6 ± 9.4 g/cm2 Heliomolar = 1.6 ± 0.1 g/cm2 | 20% CaF2cp composite: 2.19 20% nCaF2cpsd composite: 1.23 Heliomolar: 0.019 |
| Xu [16] | 2 mm × 2 mm × 12 mm | Heliomolar (Ivoclar); Exp.: CaF2 + glass + Bis-GMA/TEGDMA + silane + additives | Ion-selective electrode |
| Nanocomposite30CaF2 = 327 ± 8 μg/cm2 Nanocomposite20CaF2 = 252 ± 8 μg/cm2 Nanocomposite10CaF2 = 47 ± 2 μg/cm2 Heliomolar = 4.7 ± 0.1 μg/cm2 | Nanocomposite 30% CaF2: 3.89 Nanocomposite 20% CaF2: 3.00 Nanocomposite 10% CaF2: 0.56 Heliomolar: 0.056 |
| Mitwalli [57] | 2 mm × 2 mm × 12 mm | Heliomolar (Ivoclar); BT/BTM + CaF2 ± DMAHDM + glass | Ion-selective electrode |
| BT + nCaF2 = 1.15 ± 0.01 mmol/L BT + nCaF2 + DMAHDM = 0.89 ± 0.01 mmol/L BTM + nCaF2 = 0.44 ± 0.01 mmol/L BTM + nCaF2 + DMAHDM = 0.22 ± 0.03 mmol/L Heliomolar = 0.02 ± 0.0008 mmol/L | BT + nCaF2: 6503 BT + nCaF2 + DMAHDM: 5033 BTM + nCaF2: 2488 BTM + nCaF2 + DMAHDM: 1244 Heliomolar: 113.10 |
| Komalsingsakul [64] | 5 mm diameter × 2 mm thickness | Filtek Z350XT (3M ESPE, St. Paul, MN, USA) | Ion-selective electrode |
| Without brushing = 0.0032(0.0015) ppm With brushing = 0.0040(0.0026) ppm | Z350 XT (without brushing): 66.67 Z350 XT (with brushing): 83.33 |
| Xu [25] | 2 mm × 2 mm × 12 mm | 1. Experimental composite: Bis-GMA, TEGDMA 2. Commercial control (TPH, Caulk/Dentsply, Milford, DE, USA) | Ion-selective electrode |
| The cumulative F release (0.15 ± 0.03) mmol/L at 1 day = (0.15 ± 0.03) 1 week = (0.68 ± 0.11), 10 weeks = (2.34 ± 0.26) | Average 21.84 |
| Liu [55] | 2 mm × 2 mm × 12 mm | Heliomolar (Ivoclar); Exp.: BT + glass ± 10–20% nCaF2 | Ion-selective electrode |
| No numeric data | No numeric data |
| Khan [58] | 15 mm × 15 mm × 1 mm | PU composites + 10–20% nFA (nano-fluorapatite) | Orion Ionplus fluoride electrode |
| 1 day = 0.0003 mg/L 7 days = 0.00094 mg/L The total release at 6 months = 0.0026 mg/L | Average 0.30 |
| Li [59] | 6 mm diameter × 2 mm thickness | ClinproTM (3M, Maplewood, MN, USA) Fluorinated montmorillonite (FMMT) | Ion chromatography |
| No numeric data | No numeric data |
| Mitwalli [60] | 2 mm × 2 mm × 12 mm | Vitremer (RMGI, 3M) Ketac Nano (RMGI, 3M) Heliomolar (fluoride composite, Ivoclar) Bis-GMA/TEGDMA Bis-GMA/TEGDMA + Bis-MEP PMGDM/EBPADMA | Selective electrode |
| Initial (mmol/L): Vitremer 2.89, PE-nCaF2 1.28, BT-nCaF2 1.15, BTM-nCaF2 0.44, Heliomolar 0 After 6 cycles: Vitremer 1.29, PE-nCaF2 0.63, BT-nCaF2 0.28, BTM-nCaF2 0.26, Heliomolar 0.02 No recharge: Vitremer 1.96, PE-nCaF2 1.19, BT-nCaF2 0.79, BTM-nCaF2 0.50 At 98 days: Helioseal F 0.0098, 0% DMAHDM 0.3377, 5% DMAHDM 0.2483 | Vitremer: 16,342 PE-nCaF2: 7238 BT-nCaF2: 6503 BTM-nCaF2: 2488 Heliomolar: 0 |
| Meng [58] | 10 mm diameter × 1 mm hight | Adhesive (MPA-epoxy) ± FHA nanorods (2–10% F) | IC (ICS-5000+, Thermo Fisher Scientific, USA) measurer |
| No numeric data | No numeric data |
| Fei [62] | 2 mm × 2 mm × 12 mm | Helioseal F (Ivoclar); Exp.: 20% nCaF2 ± 5% DMAHDM | Combination of a fluoride ion-selective electrode and a reference electrode |
| Measured after 98. Day (cumulative amount): Helioseal F 0.0098 ± 0.0019 mmol/L 0% DMAHDM + 20%nCaF2 0.3377 ± 0.0325 mmol/L 5%DMAHDM + 20%nCaF 0.2483 ± 0.0054 mmol/L | Helioseal F: 39.58 0% DMAHDM + 20% nCaF2: 1364.01 5% DMAHDM + 20% nCaF2: 1002.91 |
| Sayyeda [19] | 4 mm diameter × 6 mm thickness | GIC (Fuji II) + forsterite (Mg2SiO4) | Fluoride ion-selective electrode |
| Specific values:
| Fuji II GIC: 416,667 Nanocomposite: 372,024 |
| Leite [63] | N/A | MSCaTiF4: MS + TiF4 + Ca MSTiF4: MS + TiF4 MSCaNaF: MS + NaF + Ca MSNaF: MS + NaF | Fluoride ion-selective electrode |
| Specific TSF values (μg F⁻/mL): MSCaTiF4: 3.52 ± 1.68, MSTiF4: 1.39 ± 0.60, MSCaNaF: 3.25 ± 0.48, MSNaF: 1.16 ± 0.31, TiF4: 19.76 ± 9.88, NaF: 10.18 ± 4.34. After 24 h | TiF4: 411.67 MSCaTiF4: 73.33 MSCaNaF: 67.71 MSTiF4: 28.96 MSNaF: 24.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawska-Wilk, A.; Kensy, J.; Kiryk, S.; Kotela, A.; Kiryk, J.; Michalak, M.; Grychowska, N.; Fast, M.; Matys, J.; Dobrzyński, M. Evaluation of Factors Influencing Fluoride Release from Dental Nanocomposite Materials: A Systematic Review. Nanomaterials 2025, 15, 651. https://doi.org/10.3390/nano15090651
Morawska-Wilk A, Kensy J, Kiryk S, Kotela A, Kiryk J, Michalak M, Grychowska N, Fast M, Matys J, Dobrzyński M. Evaluation of Factors Influencing Fluoride Release from Dental Nanocomposite Materials: A Systematic Review. Nanomaterials. 2025; 15(9):651. https://doi.org/10.3390/nano15090651
Chicago/Turabian StyleMorawska-Wilk, Alicja, Julia Kensy, Sylwia Kiryk, Agnieszka Kotela, Jan Kiryk, Mateusz Michalak, Natalia Grychowska, Magdalena Fast, Jacek Matys, and Maciej Dobrzyński. 2025. "Evaluation of Factors Influencing Fluoride Release from Dental Nanocomposite Materials: A Systematic Review" Nanomaterials 15, no. 9: 651. https://doi.org/10.3390/nano15090651
APA StyleMorawska-Wilk, A., Kensy, J., Kiryk, S., Kotela, A., Kiryk, J., Michalak, M., Grychowska, N., Fast, M., Matys, J., & Dobrzyński, M. (2025). Evaluation of Factors Influencing Fluoride Release from Dental Nanocomposite Materials: A Systematic Review. Nanomaterials, 15(9), 651. https://doi.org/10.3390/nano15090651










