Citrate-Stabilized Amorphous Calcium Phosphate Nanoparticles as an Effective Adsorbent for Defluorination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbent
2.3. Batch Adsorption Experiments
2.4. Characterization
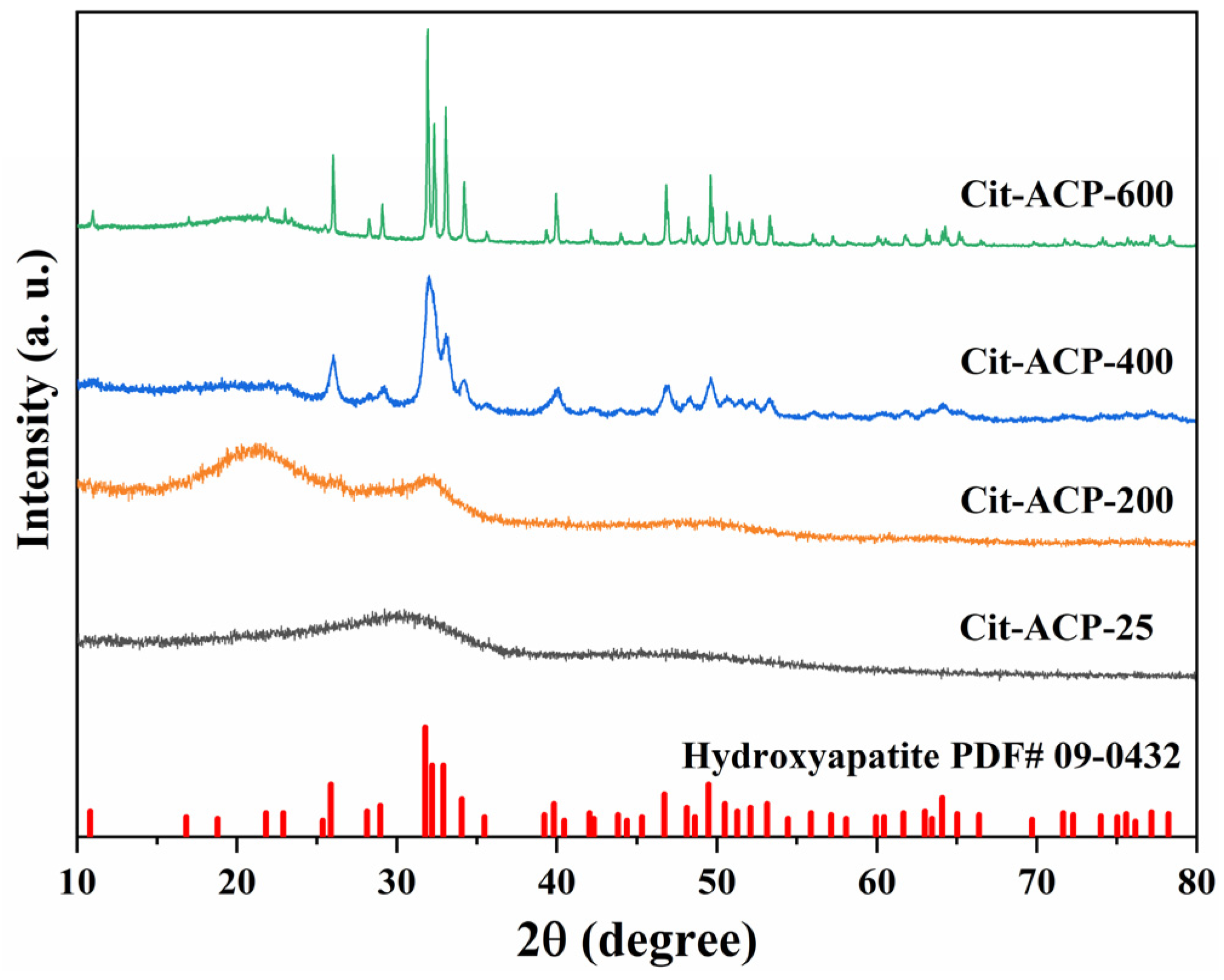
2.4.1. Wide-Angle X-Ray Diffraction (XRD)
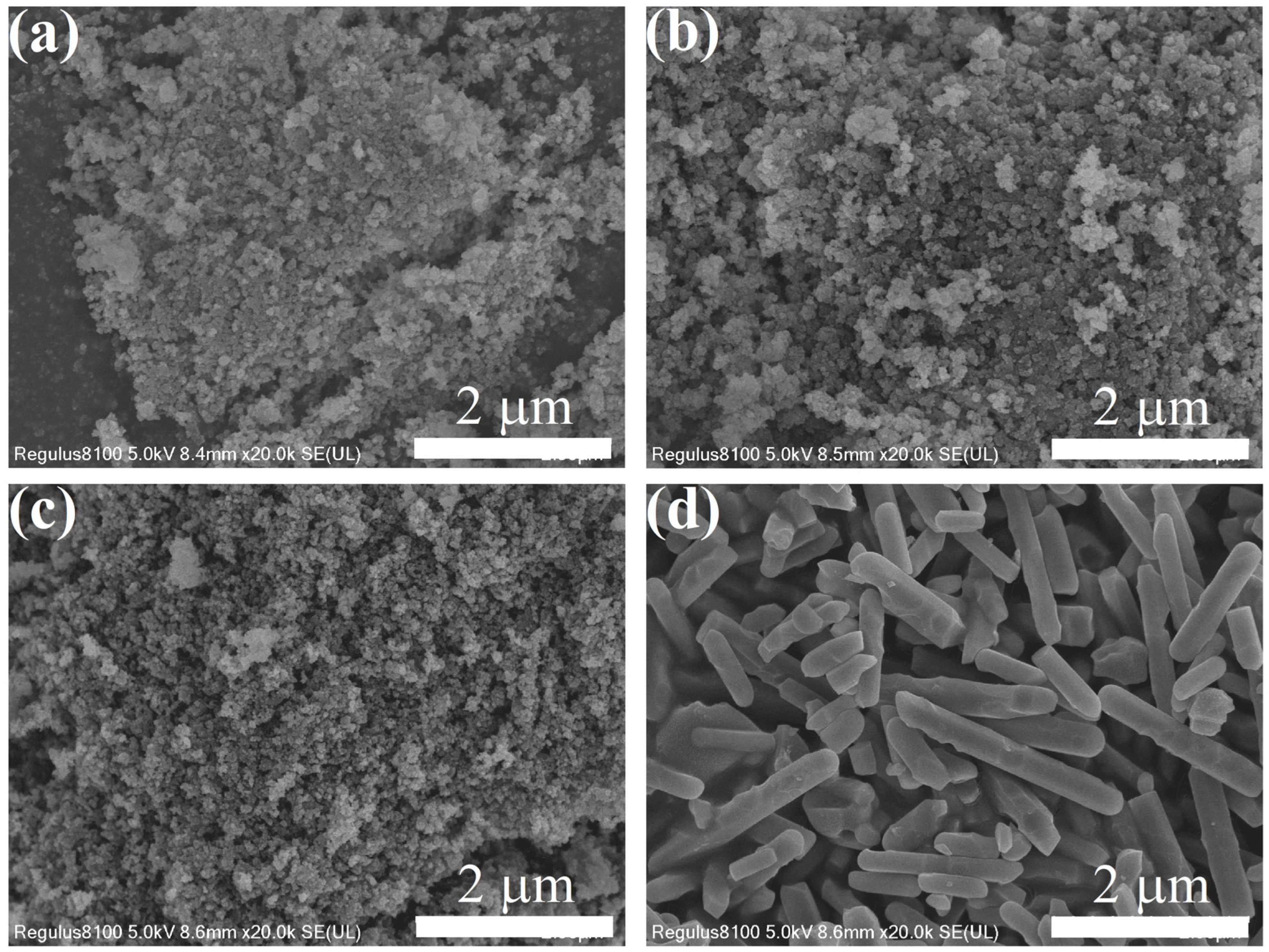
2.4.2. Field-Emission Scanning Electronic Microscopy (SEM)
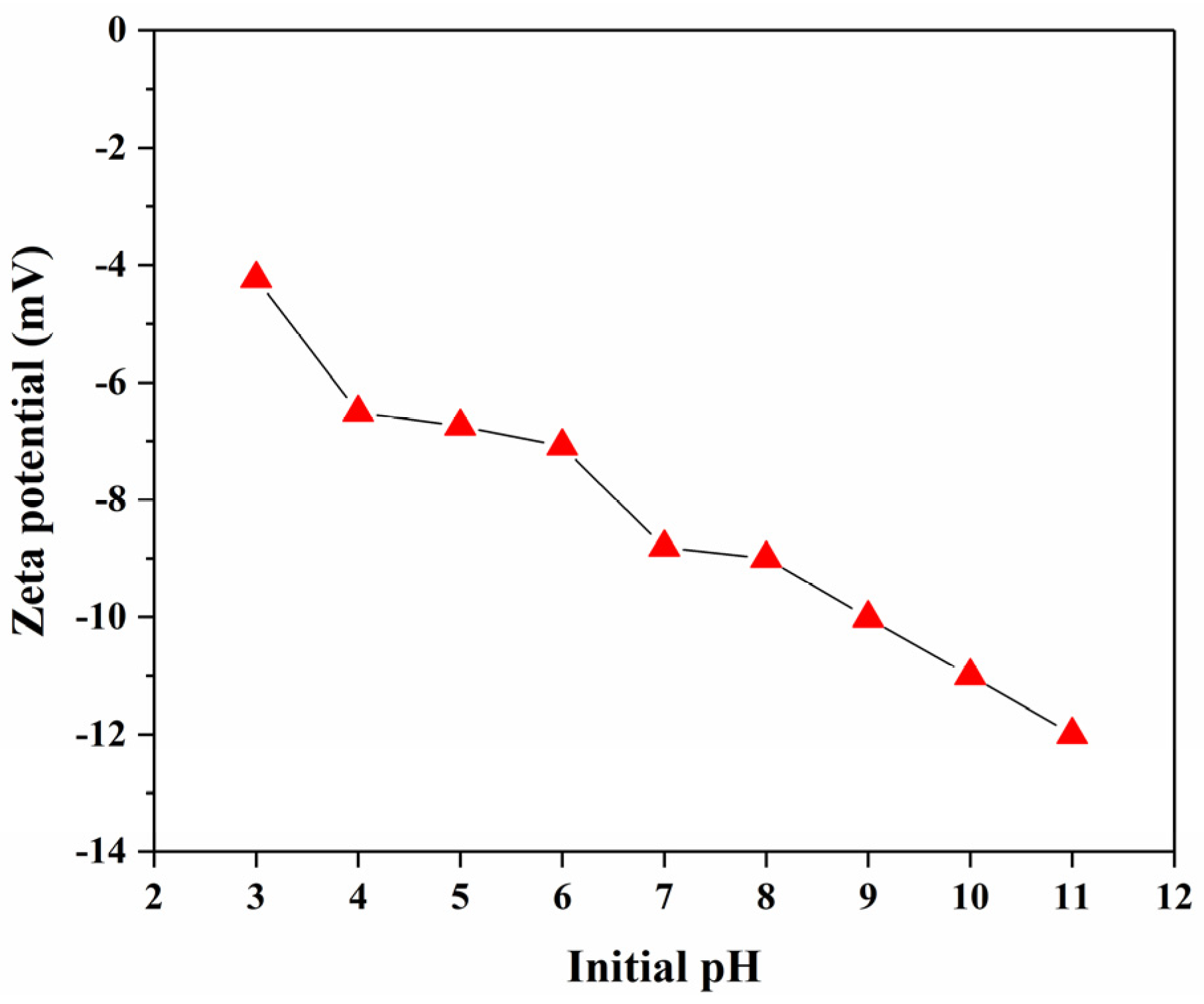
2.4.3. Zeta Potential Measurement
2.4.4. X-Ray Photoelectron Spectroscopy (XPS)
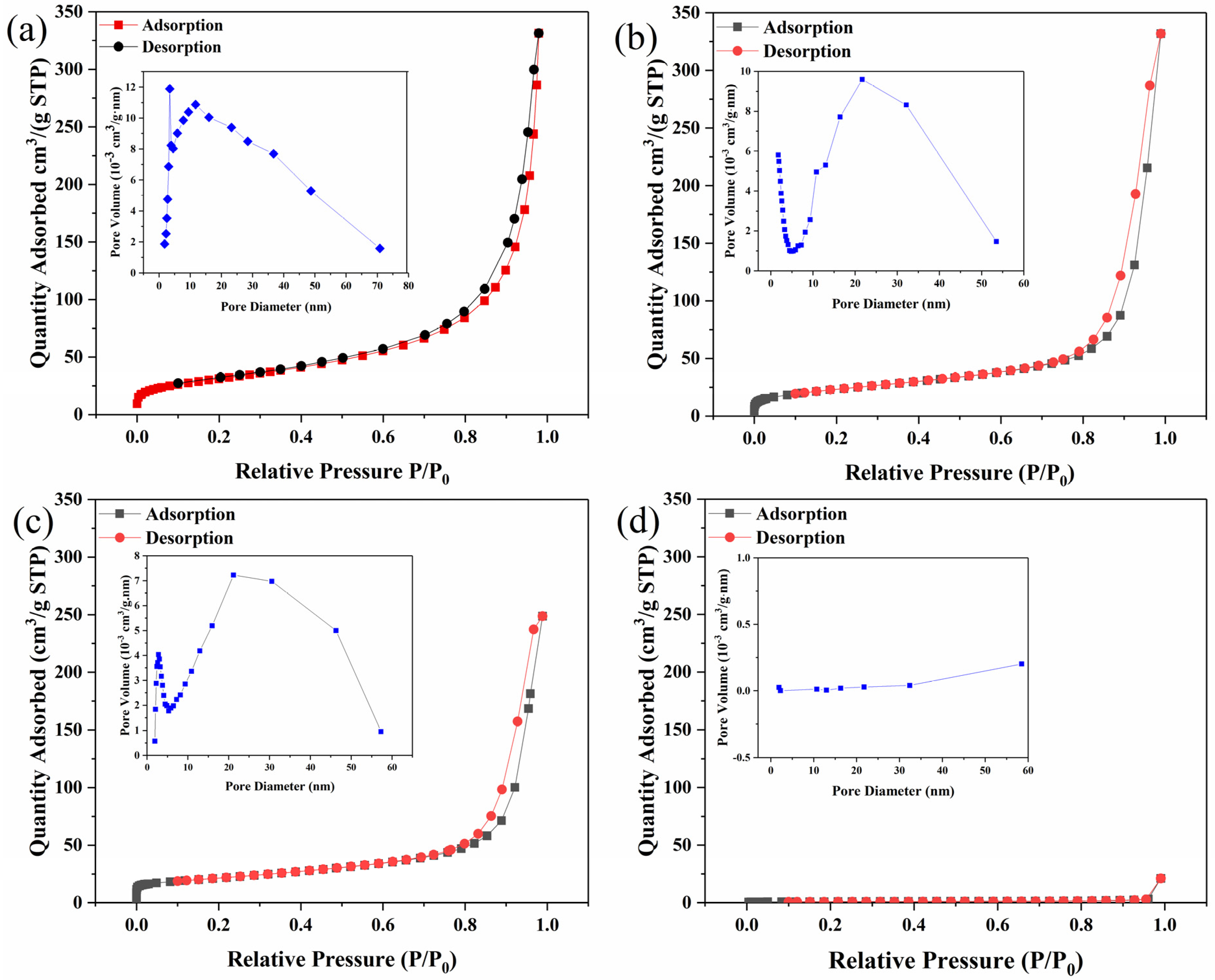
2.4.5. Brunauer–Emmett–Teller Measurement (BET)
3. Results and Discussion
3.1. Characterization of Adsorbents
3.2. Fluoride Ion Adsorption Behavior of the Samples
3.2.1. Adsorption of Time Dependence
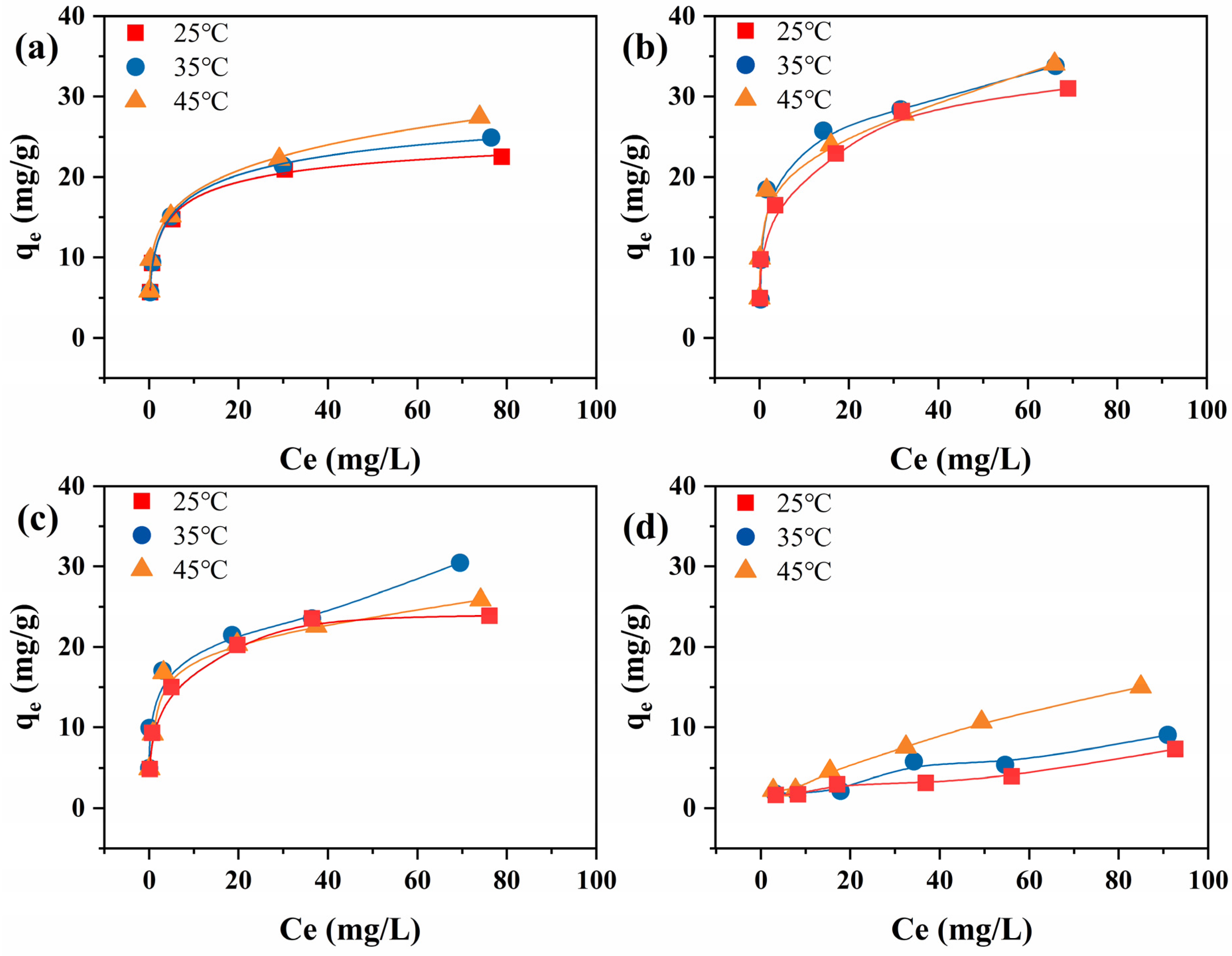
3.2.2. Adsorption Isotherms
3.2.3. Adsorption Thermodynamic Parameters Analysis
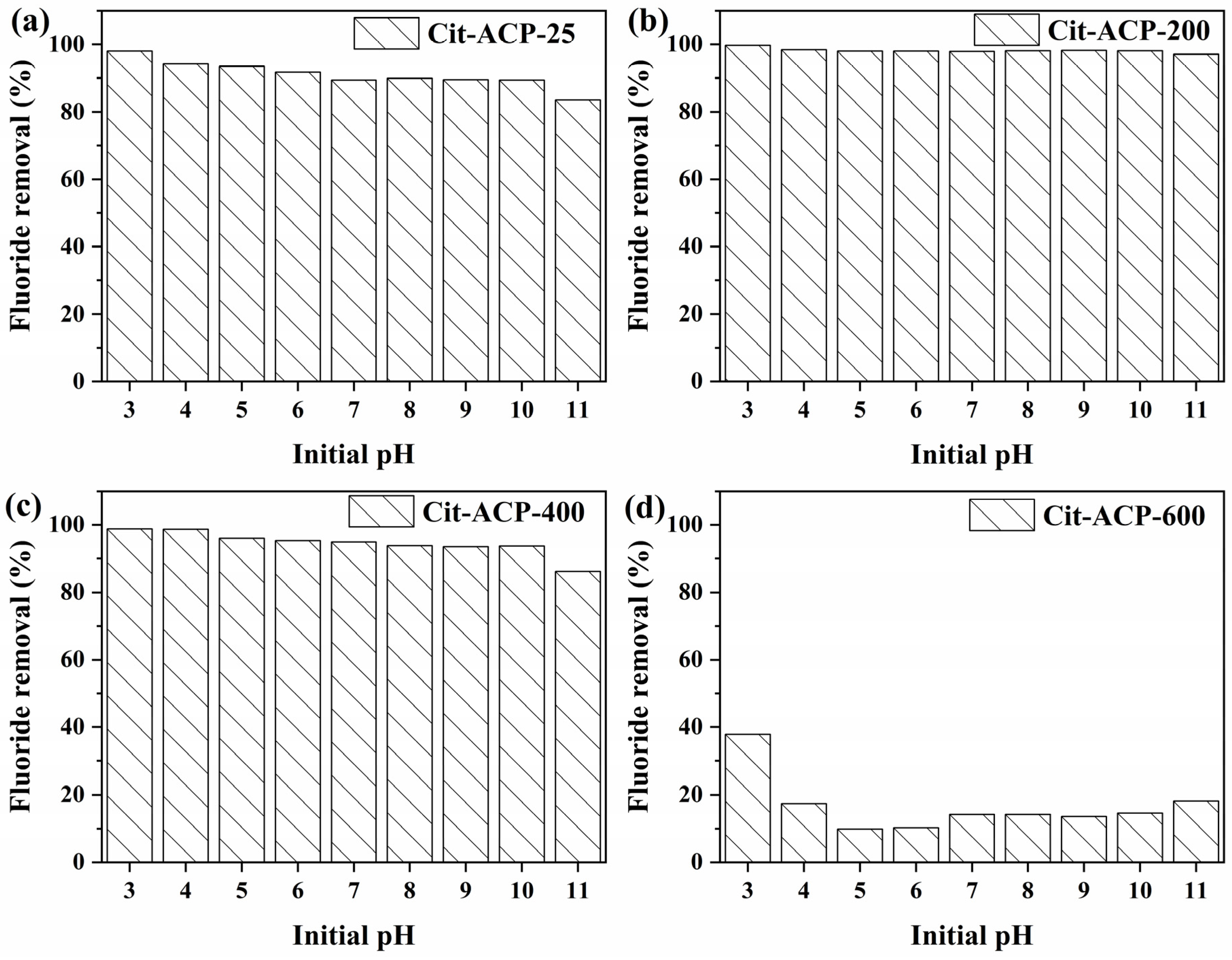
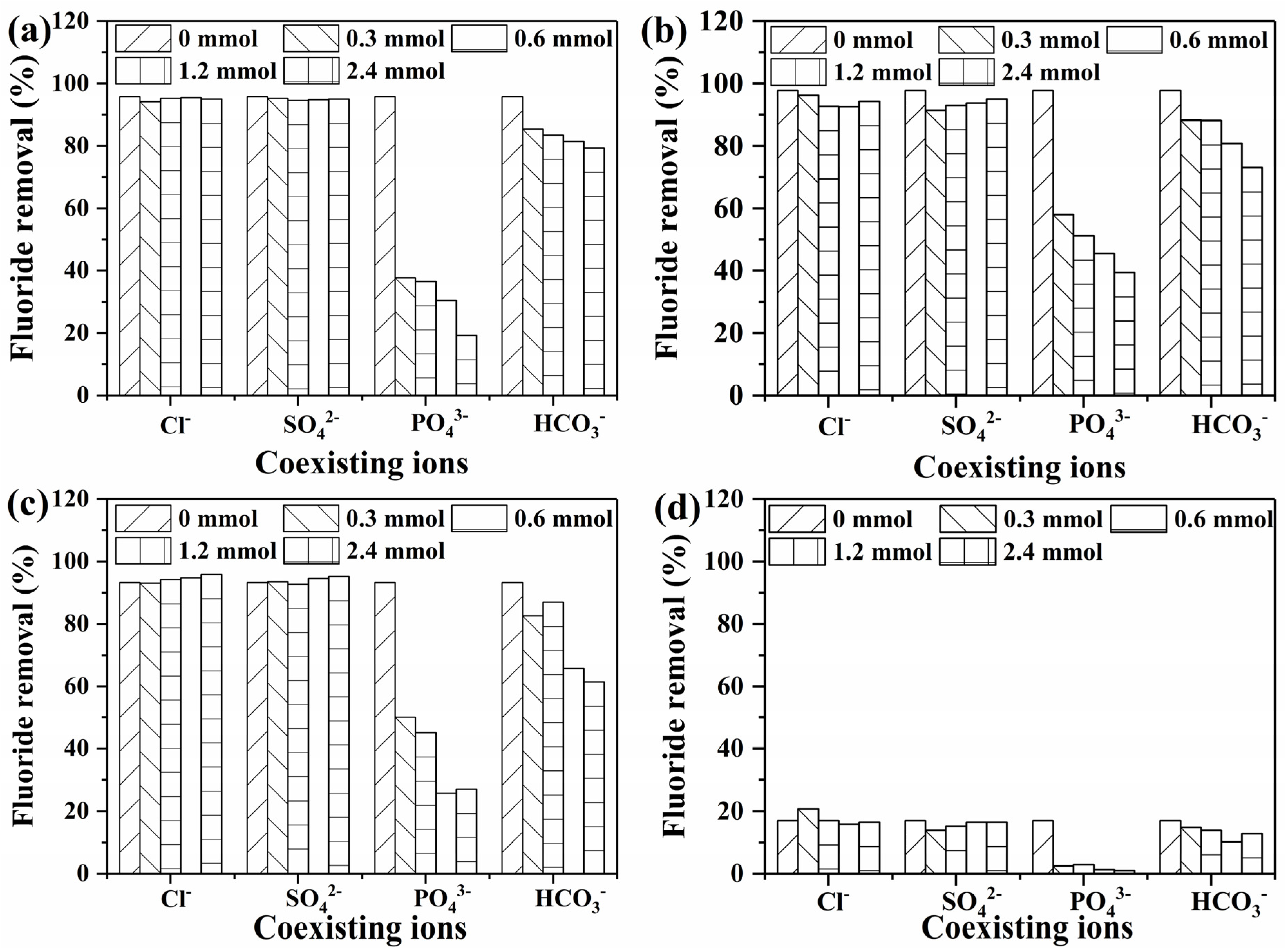
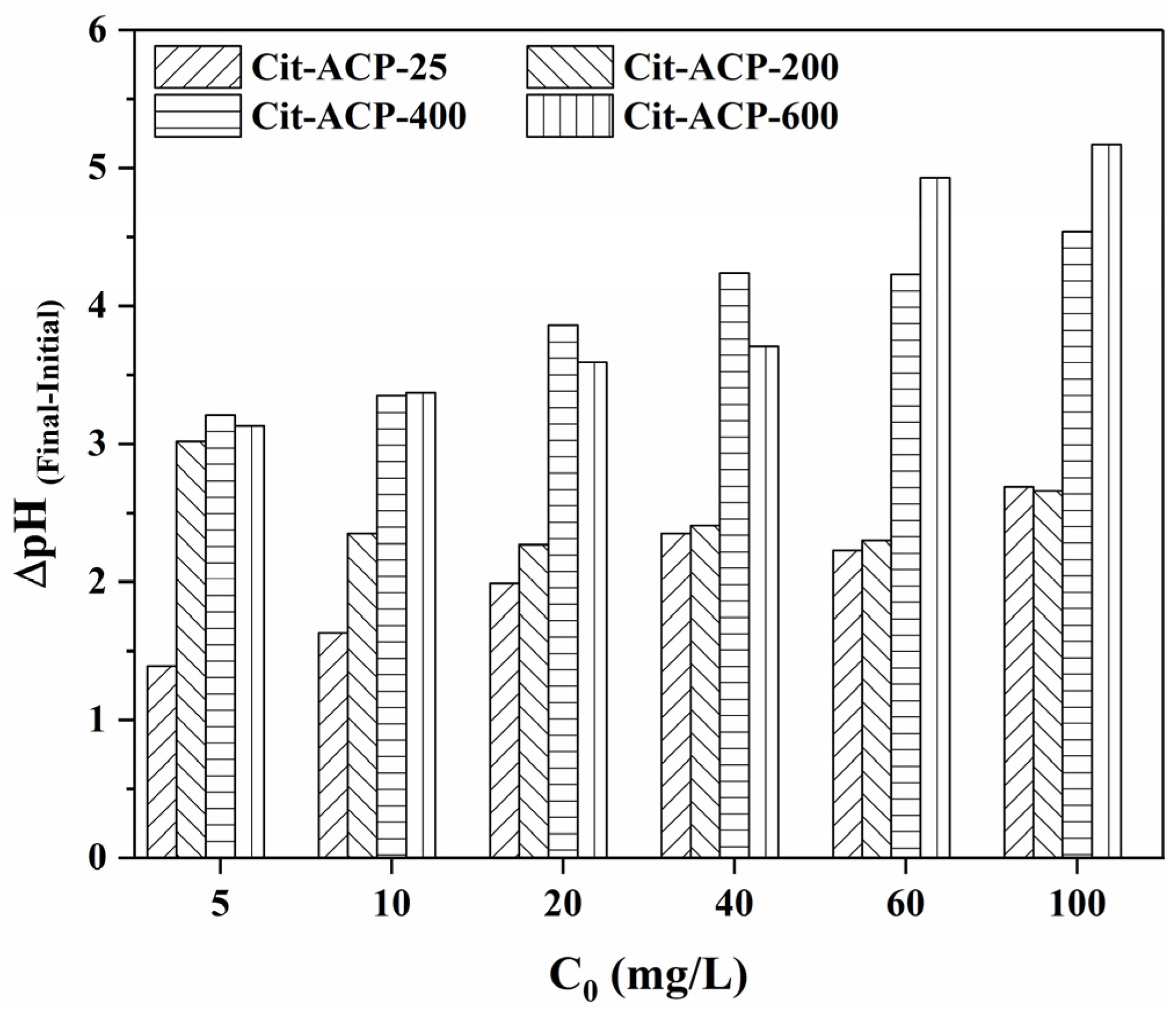
3.2.4. The Effect of pH and Co-Existing Ions
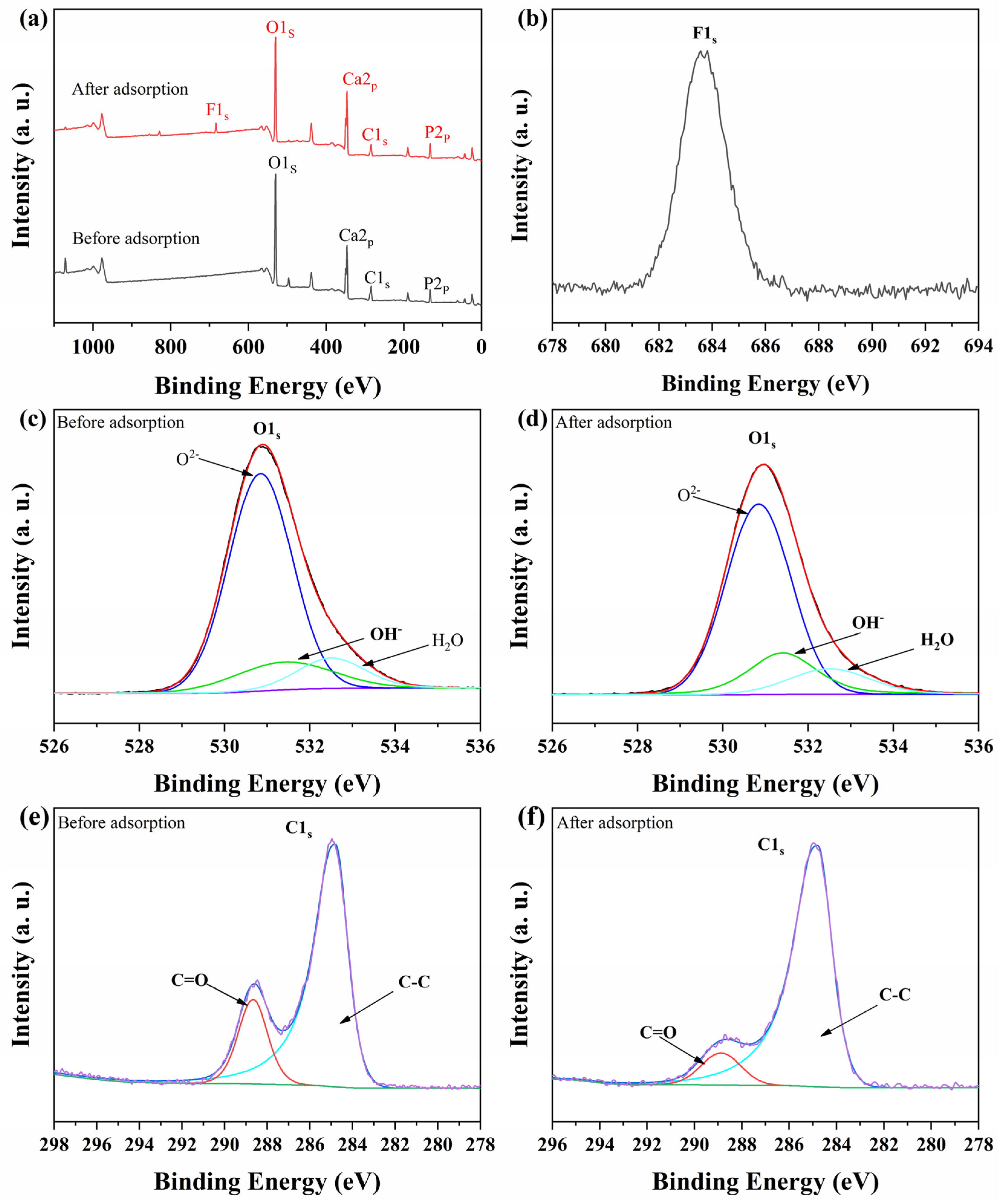
3.2.5. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohammadi, A.A.; Yousefi, M.; Yaseri, M.; Jalilzadeh, M.; Mahvi, A.H. Skeletal fluorosis in relation to drinking water in rural areas of West Azerbaijan, Iran. Sci. Rep. 2017, 7, 17300. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride contamination, consequences and removal techniques in water: A review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Kabay, N.; Arar, Ö.; Samatya, S.; Yüksel, Ü.; Yüksel, M. Separation of fluoride from aqueous solution by electrodialysis: Effect of process parameters and other ionic species. J. Hazard. Mater. 2008, 153, 107–113. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Ulu, F. Evaluation of operating parameters with respect to charge loading on the removal efficiency of arsenic from potable water by electrocoagulation. J. Environ. Chem. Eng. 2016, 4, 1484–1494. [Google Scholar] [CrossRef]
- Lacson, C.F.Z.; Lu, M.-C.; Huang, Y.-H. Fluoride-rich wastewater treatment by ballast-assisted precipitation with the selection of precipitants and discarded or recovered materials as ballast. J. Environ. Chem. Eng. 2021, 9, 105713. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Ali, A.; Zhang, R.; Yang, W.; Xu, L.; Shi, J.; Gao, Z. Synergistic removal of fluoride from groundwater by seed crystals and bacteria based on microbially induced calcium precipitation. Sci. Total Environ. 2022, 806, 150341. [Google Scholar] [CrossRef]
- He, Y.; Huang, L.; Song, B.; Wu, B.; Yan, L.; Deng, H.; Yang, Z.; Yang, W.; Wang, H.; Liang, Z.; et al. Defluorination by ion exchange of SO42− on alumina surface: Adsorption mechanism and kinetics. Chemosphere 2021, 273, 129678. [Google Scholar] [CrossRef]
- Zeidabadi, F.A.; Esfahani, E.B.; McBeath, S.T.; Mohseni, M. Managing PFAS exhausted Ion-exchange resins through effective regeneration/electrochemical process. Water Res. 2024, 255, 121529. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Lima, R.; Choudhary, G.; Sharma, P.R.; Ferdov, S.; Botelho, G.; Sharma, R.K.; Lanceros-Méndez, S. Highly efficient removal of fluoride from aqueous media through polymer composite membranes. Sep. Purif. Technol. 2018, 205, 1–10. [Google Scholar] [CrossRef]
- Han, M.J.; Baroña, G.N.B.; Jung, B. Effect of surface charge on hydrophilically modified poly(vinylidene fluoride) membrane for microfiltration. Desalination 2011, 270, 76–83. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Zhang, Z.; Wu, D. Removal of fluoride from water using aluminum hydroxide-loaded zeolite synthesized from coal fly ash. J. Hazard. Mater. 2022, 421, 126817. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Hu, X.; Ali, A.; Wu, Z. Isolation of biosynthetic crystals by microbially induced calcium carbonate precipitation and their utilization for fluoride removal from groundwater. J. Hazard. Mater. 2021, 406, 124748. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of fluoride removal from water environment by adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Nie, Y.; Hu, C.; Kong, C. Enhanced fluoride adsorption using Al (III) modified calcium hydroxyapatite. J. Hazard. Mater. 2012, 233–234, 194–199. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.-S.; He, J.-Y.; Xu, W.-H.; Huang, X.-J.; Liu, J.-H. Enhanced fluoride removal from water by sulfate-doped hydroxyapatite hierarchical hollow microspheres. Chem. Eng. J. 2016, 285, 616–624. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Zhao, N.; Yang, W.; You, X. Sulfate-doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water. Water Res. 2013, 47, 4040–4049. [Google Scholar] [CrossRef]
- Kumar, E.; Bhatnagar, A.; Kumar, U.; Sillanpää, M. Defluoridation from aqueous solutions by nano-alumina: Characterization and sorption studies. J. Hazard. Mater. 2011, 186, 1042–1049. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Farhang, M.; Alimohammadi, M.; Afsharnia, M.; McKay, G. Adsorptive removal of fluoride from water by activated carbon derived from CaCl2-modified Crocus sativus leaves: Equilibrium adsorption isotherms, optimization, and influence of anions. Chem. Eng. Commun. 2018, 205, 955–965. [Google Scholar] [CrossRef]
- Araga, R.; Soni, S.; Sharma, C.S. Fluoride adsorption from aqueous solution using activated carbon obtained from KOH-treated jamun (Syzygium cumini) seed. J. Environ. Chem. Eng. 2017, 5, 5608–5616. [Google Scholar] [CrossRef]
- Viswanathan, N.; Prabhu, S.M.; Meenakshi, S. Development of amine functionalized co-polymeric resins for selective fluoride sorption. J. Fluor. Chem. 2013, 153, 143–150. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Çoşkunırmak, M.H.; Kahya, N.; Erim, F.B. Aluminum Alginate–Montmorillonite Composite Beads for Defluoridation of Water. Water Air Soil Pollut. 2014, 226, 22571–22579. [Google Scholar] [CrossRef]
- Jeyaseelan, A.; Viswanathan, N.; Kumar, I.A.; Ansar, S. Effective defluoridation using lanthanum-organic frameworks encapsulated hydrotalcite based bio-hybrid beads. J. Solid State Chem. 2023, 328, 124301. [Google Scholar] [CrossRef]
- Uddin, M.K.; Ahmed, S.S.; Naushad, M. A mini update on fluoride adsorption from aqueous medium using clay materials. Desalination Water Treat. 2019, 145, 232–248. [Google Scholar] [CrossRef]
- Nagaraj, A.; Pillay, K.; Kumar, S.K.; Rajan, M. Dicarboxylic acid cross-linked metal ion decorated bentonite clay and chitosan for fluoride removal studies. RSC Adv. 2020, 10, 16791–16803. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Helmreich, B. Batch defluoridation appraisal of aluminium oxide infused diatomaceous earth. Chem. Eng. J. 2014, 258, 51–61. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Chen, S.; Drewes, J.E.; Helmreich, B. Characterization of granular matrix supported nano magnesium oxide as an adsorbent for defluoridation of groundwater. Chem. Eng. J. 2015, 281, 632–643. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, J.; Zhang, X.; Wang, C.; Wang, T.; Jiang, M.; Zhu, Q.; Xie, T.; Chen, D. Size controlled fabrication of enzyme encapsulated amorphous calcium phosphate nanoparticle and its intracellular biosensing application. Colloids Surf. B Biointerfaces 2021, 201, 111638. [Google Scholar] [CrossRef]
- Ding, G.-J.; Zhu, Y.-J.; Cheng, G.-F.; Ruan, Y.-J.; Qi, C.; Lu, B.-Q.; Chen, F.; Wu, J. Porous Microspheres of Casein/Amorphous Calcium Phosphate Nanocomposite: Room Temperature Synthesis and Application in Drug Delivery. Curr. Nanosci. 2015, 12, 70–78. [Google Scholar] [CrossRef]
- Safronova, T.V.; Mukhin, E.A.; Putlyaev, V.I.; Knotko, A.V.; Evdokimov, P.V.; Shatalova, T.B.; Filippov, Y.Y.; Sidorov, A.V.; Karpushkin, E.A. Amorphous calcium phosphate powder synthesized from calcium acetate and polyphosphoric acid for bioceramics application. Ceram. Int. 2017, 43, 1310–1317. [Google Scholar] [CrossRef]
- Ding, G.-J.; Zhu, Y.-J.; Qi, C.; Lu, B.-Q.; Chen, F.; Wu, J. Porous hollow microspheres of amorphous calcium phosphate: Soybean lecithin templated microwave-assisted hydrothermal synthesis and application in drug delivery. J. Mater. Chem. B 2015, 3, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Shahrezaee, M.; Raz, M.; Shishehbor, S.; Moztarzadeh, F.; Baghbani, F.; Sadeghi, A.; Bajelani, K.; Tondnevis, F. Synthesis of Magnesium Doped Amorphous Calcium Phosphate as a Bioceramic for Biomedical Application: In Vitro Study. Silicon 2017, 10, 1171–1179. [Google Scholar] [CrossRef]
- Pan, H.; Liu, X.Y.; Tang, R.; Xu, H.Y. Mystery of the transformation from amorphous calcium phosphate to hydroxyapatite. Chem. Commun. 2010, 46, 7415. [Google Scholar] [CrossRef]
- Du, L.-W.; Bian, S.; Gou, B.-D.; Jiang, Y.; Huang, J.; Gao, Y.-X.; Zhao, Y.-D.; Wen, W.; Zhang, T.-L.; Wang, K. Structure of Clusters and Formation of Amorphous Calcium Phosphate and Hydroxyapatite: From the Perspective of Coordination Chemistry. Cryst. Growth Des. 2013, 13, 3103–3109. [Google Scholar] [CrossRef]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef]
- Luo, J.; Qiu, S.; Zhou, X.; Lai, R.; Dong, P.; Xie, X. In situ grafting polyethylene glycol chains onto amorphous calcium phosphate nanoparticles to improve the storage stability and organic solvent redispersibility. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 81–88. [Google Scholar] [CrossRef]
- Garcia, A.C.; Vavrusova, M.; Skibsted, L.H. Supersaturation of calcium citrate as a mechanism behind enhanced availability of calcium phosphates by presence of citrate. Food Res. Int. 2018, 107, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Chatzipanagis, K.; Iafisco, M.; Roncal-Herrero, T.; Bilton, M.; Tampieri, A.; Kröger, R.; Delgado-López, J.M. Crystallization of citrate-stabilized amorphous calcium phosphate to nanocrystalline apatite: A surface-mediated transformation. Cryst. Eng. Comm. 2016, 18, 3170–3173. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Adamiano, A.; Tampieri, A.; Ramirez-Rodriguez, G.B.; Siliqi, D.; Giannini, C.; Ivanchenko, P.; Martra, G.; Lin, F.-H.; Delgado-López, J.M.; et al. Combined Effect of Citrate and Fluoride Ions on Hydroxyapatite Nanoparticles. Cryst. Growth Des. 2020, 20, 3163–3172. [Google Scholar] [CrossRef]
- Lin, K.A.; Liu, Y.T.; Chen, S.Y. Adsorption of fluoride to UiO-66-NH2 in water: Stability, kinetic, isotherm and thermodynamic studies. J. Colloid Interface Sci. 2016, 461, 79–87. [Google Scholar] [CrossRef]
- Jimenez-Reyes, M.; Solache-Rios, M. Sorption behavior of fluoride ions from aqueous solutions by hydroxyapatite. J. Hazard. Mater. 2010, 180, 297–302. [Google Scholar] [CrossRef]
- Maity, J.P.; Hsu, C.-M.; Lin, T.-J.; Lee, W.-C.; Bhattacharya, P.; Bundschuh, J.; Chen, C.-Y. Removal of fluoride from water through bacterial-surfactin mediated novel hydroxyapatite nanoparticle and its efficiency assessment: Adsorption isotherm, adsorption kinetic and adsorption Thermodynamics. Environ. Nanotechnol. Monit. Manag. 2018, 9, 18–28. [Google Scholar] [CrossRef]
- Joshi, K.J.; Shah, N.M. Structural, morphological & optical studies of Hydroxyapatite microplates synthesized using hydrothermal technique. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Y.; Gong, J.; Jin, X.; Cheng, C.; Zhang, R.; Chen, M. Non-aqueous liquid crystals of hydroxyapatite nanorods. Acta Biomater. 2020, 116, 383–390. [Google Scholar] [CrossRef]
- He, J.; Zhang, K.; Wu, S.; Cai, X.; Chen, K.; Li, Y.; Sun, B.; Jia, Y.; Meng, F.; Jin, Z.; et al. Performance of novel hydroxyapatite nanowires in treatment of fluoride contaminated water. J. Hazard. Mater. 2016, 303, 119–130. [Google Scholar] [CrossRef]
- Mehta, D.; Mondal, P.; Saharan, V.K.; George, S. In-vitro synthesis of marble apatite as a novel adsorbent for removal of fluoride ions from ground water: An ultrasonic approach. Ultrason. Sonochemistry 2018, 40, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Muro, N.M.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; Tapia-Picazo, J.C. Fluoride adsorption properties of cerium-containing bone char. J. Fluor. Chem. 2017, 197, 63–73. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Silvestre-Albero, J.; Aguayo-Villarreal, I.A.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A. A new synthesis route for bone chars using CO2 atmosphere and their application as fluoride adsorbents. Microporous Mesoporous Mater. 2015, 209, 38–44. [Google Scholar] [CrossRef]
- Singh, S.; Khare, A.; Chaudhari, S. Enhanced fluoride removal from drinking water using non-calcined synthetic hydroxyapatite. J. Environ. Chem. Eng. 2020, 8, 103704. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Liu, L.; Yang, J.; Liu, S.; Zhang, T. Preparation, characterization serpentine-loaded hydroxyapatite and its simultaneous removal performance for fluoride, iron and manganese. RSC Adv. 2021, 11, 16201–16215. [Google Scholar] [CrossRef]
- Ayinde, W.B.; Gitari, W.M.; Munkombwe, M.; Samie, A.; Smith, J.A. Green synthesis of AgMgOnHaP nanoparticles supported on chitosan matrix: Defluoridation and antibacterial effects in groundwater. J. Environ. Chem. Eng. 2020, 8, 104026. [Google Scholar] [CrossRef]
- Fernando, M.S.; Wimalasiri, A.K.D.V.K.; Ratnayake, S.P.; Jayasinghe, J.M.A.R.B.; William, G.R.; Dissanayake, D.P.; de Silva, K.M.N.; de Silva, R.M. Improved nanocomposite of montmorillonite and hydroxyapatite for defluoridation of water. RSC Adv. 2019, 9, 35588–35598. [Google Scholar] [CrossRef]
- Tang, Q.; Duan, T.; Li, P.; Zhang, P.; Wu, D. Enhanced Defluoridation Capacity From Aqueous Media via Hydroxyapatite Decorated With Carbon Nanotube. Front. Chem. 2018, 6, 104. [Google Scholar] [CrossRef]
- Raghav, S.; Sapna; Kumar, D. Cubical-Shaped Rods of Pectin–Hydroxyapatite Composite for Adsorption Studies of Fluoride by Statistical Method and Adsorption Experiments. ACS Omega 2018, 3, 9675–9688. [Google Scholar] [CrossRef]
- Horsfall Jnr, M.; Spiff, A.I. Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium bicolor (Wild Cocoyam) biomass. Electron. J. Biotechnol. 2005, 8, 162–169. [Google Scholar] [CrossRef]
- Jin, X.; Zhuang, J.; Zhang, Z.; Guo, H.; Tan, J. Hydrothermal synthesis of hydroxyapatite nanorods in the presence of sodium citrate and its aqueous colloidal stability evaluation in neutral pH. J. Colloid Interface Sci. 2015, 443, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Jin, X. Monodisperse, colloidal and luminescent calcium fluoride nanoparticles via a citrate-assisted hydrothermal route. J. Colloid Interface Sci. 2018, 531, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, L.; Markovic, S.; Ignjatovic, N.; Panseri, S.; Montesi, M.; Adamiano, A.; Fosca, M.; Rau, J.V.; Uskoković, V.; Iafisco, M. Thermal crystallization of amorphous calcium phosphate combined with citrate and fluoride doping: A novel route to produce hydroxyapatite bioceramics. J. Mater. Chem. B 2021, 9, 4832–4845. [Google Scholar] [CrossRef]
- He, J.; Chen, K.; Cai, X.; Li, Y.; Wang, C.; Zhang, K.; Jin, Z.; Meng, F.; Wang, X.; Kong, L.; et al. A biocompatible and novelly-defined Al-HAP adsorption membrane for highly effective removal of fluoride from drinking water. J. Colloid Interface Sci. 2017, 490, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, W.; Pan, H.; Jiang, S.; Tang, R. Stabilizing amorphous calcium phosphate phase by citrate adsorption. Cryst. Eng. Comm. 2014, 16, 1864–1867. [Google Scholar] [CrossRef]
- Kanno, C.M.; Sanders, R.L.; Flynn, S.M.; Lessard, G.; Myneni, S.C. Novel apatite-based sorbent for defluoridation: Synthesis and sorption characteristics of nano-micro-crystalline hydroxyapatite-coated-limestone. Environ. Sci. Technol. 2014, 48, 5798–5807. [Google Scholar] [CrossRef]
- Wimalasiri, A.K.D.V.K.; Fernando, M.S.; Williams, G.R.; Dissanayake, D.P.; de Silva, K.M.N.; de Silva, R.M. Microwave assisted accelerated fluoride adsorption by porous nanohydroxyapatite. Mater. Chem. Phys. 2021, 257, 123712. [Google Scholar] [CrossRef]
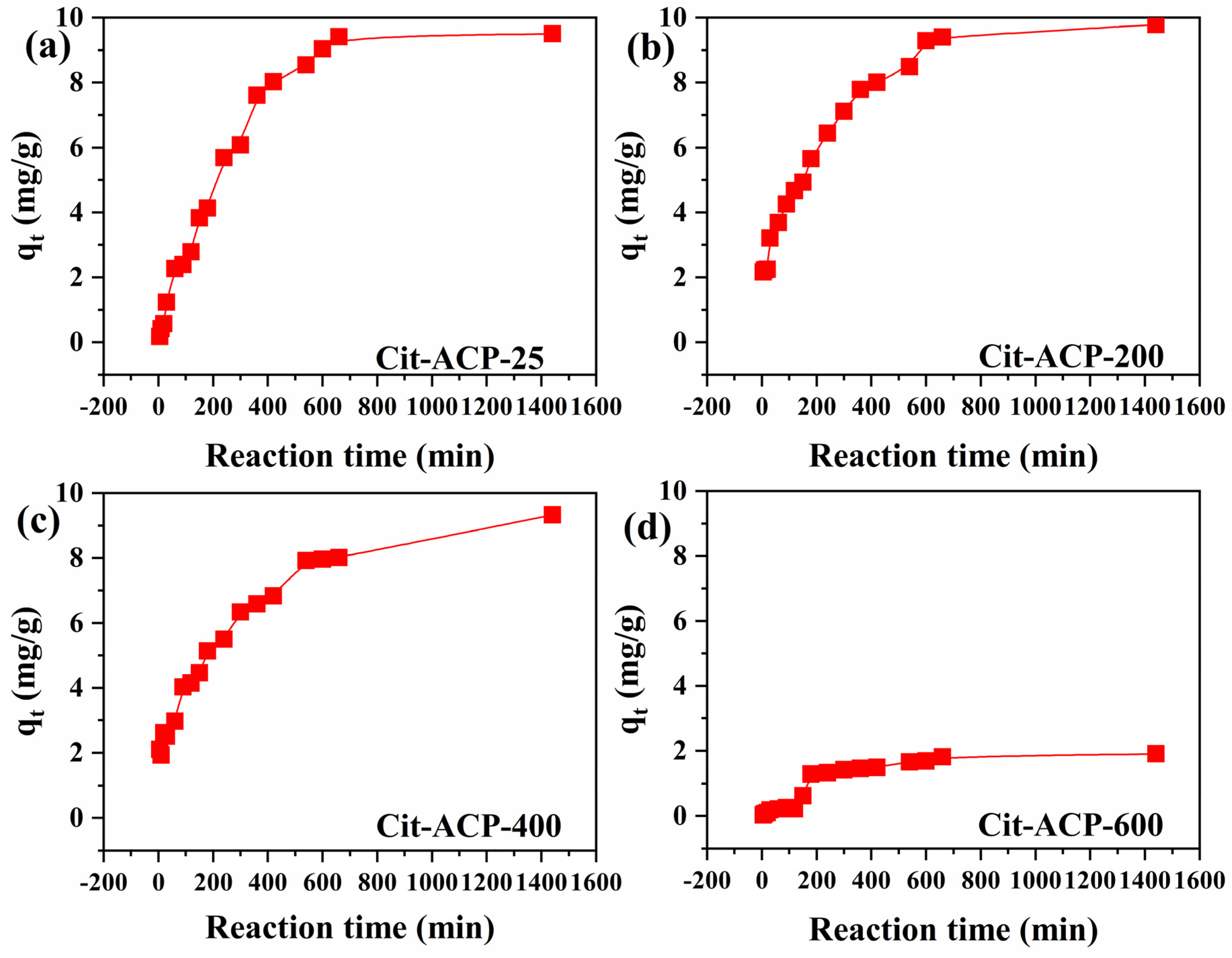


| Sample | C0 (mg/L) | qe(exp) (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| K1·10−3 | qe(cal) | R2 | K2·10−3 | qe(cal) | R2 | |||
| Cit-ACP-25 | 10 | 9.50 | 2.97 | 8.11 | 0.852 | 0.26 | 12.38 | 0.980 |
| Cit-ACP-200 | 9.78 | 2.48 | 6.78 | 0.903 | 0.92 | 10.38 | 0.993 | |
| Cit-ACP-400 | 9.32 | 1.69 | 7.19 | 0.952 | 0.84 | 9.70 | 0.990 | |
| Cit-ACP-600 | 1.71 | 0.39 | 9.28 | 0.661 | 0.63 | 2.94 | 0.771 | |
| Sample | T (°C) | Langmuir Constants | Freundlich Constants | |||||
|---|---|---|---|---|---|---|---|---|
| qm(cal) (mg/g) | qm(exp) (mg/g) | KL (L/mg) | R2 | KF (mg1n·Ln/g) | n | R2 | ||
| Cit-ACP-25 | 25 | 22.87 | 22.51 | 0.576 | 0.999 | 9.10 | 4.31 | 0.948 |
| 35 | 25.25 | 24.89 | 0.427 | 0.995 | 9.26 | 4.74 | 0.957 | |
| 45 | 27.69 | 27.48 | 0.400 | 0.988 | 11.22 | 4.82 | 0.983 | |
| Cit-ACP-200 | 25 | 32.03 | 30.99 | 0.285 | 0.996 | 10.18 | 3.66 | 0.947 |
| 35 | 33.55 | 33.80 | 0.541 | 0.995 | 14.26 | 4.56 | 0.934 | |
| 45 | 34.04 | 34.05 | 0.34 | 0.992 | 11.30 | 3.65 | 0.929 | |
| Cit-ACP-400 | 25 | 24.41 | 23.87 | 0.50 | 0.998 | 9.07 | 3.90 | 0.955 |
| 35 | 29.74 | 30.47 | 0.34 | 0.987 | 12.72 | 5.12 | 0.904 | |
| 45 | 25.91 | 25.86 | 0.41 | 0.996 | 9.64 | 4.00 | 0.939 | |
| Cit-ACP-600 | 25 | 8.13 | 7.35 | 0.03 | 0.846 | 0.84 | 2.39 | 0.843 |
| 35 | 12.40 | 9.08 | 0.02 | 0.802 | 0.76 | 1.96 | 0.803 | |
| 45 | 23.83 | 15.06 | 0.017 | 0.876 | 0.91 | 1.63 | 0.920 | |
| Adsorbents | qe (mg/g) | pH | Ref. |
|---|---|---|---|
| Sulfate-doped hydroxyapatite | 28.3 | 7 | [17] |
| Marble apatite | 7.2 | 7 | [51] |
| Cerium-containing bone char | 9.1 | 7 | [52] |
| Al-doped cow bone char | 18.5 | 7 | [53] |
| Non-calcined synthetic hydroxyapatite | 7.5 | 5 | [54] |
| Serpentine-loaded hydroxyapatite | 5.7 | 7 | [55] |
| Hydroxyapatite nanowires | 29.8 | 7 | [50] |
| Ag/MgO/HAP nanoparticles | 6.9 | 7 | [56] |
| Montmorillonite/hydroxyapatite nanocomposite | 16.7 | 5 | [57] |
| Hydroxyapatite decorated with carbon nanotube | 11.05 | 6 | [58] |
| Cubical-shaped rods of pectin–hydroxyapatite composite | 28.57 | 7 | [59] |
| Cit-ACP | 27.48 | 7 | This study |
| Sample | Temp. (K) | ∆G (kJ/mol) | ∆H (kJ/mol) | ∆S (J/mol·K) |
|---|---|---|---|---|
| Cit-ACP-25 | 298 | −4.32 | 24.87 | 97.18 |
| 308 | −4.56 | |||
| 318 | −6.29 | |||
| Cit-ACP-200 | 298 | −5.96 | 49.21 | 184.21 |
| 308 | −6.92 | |||
| 318 | −9.68 | |||
| Cit-ACP-400 | 298 | −5.49 | 50.24 | 185.69 |
| 308 | −6.32 | |||
| 318 | −8.96 | |||
| Cit-ACP-600 | 298 | 3.22 | 19.62 | 54.55 |
| 308 | 3.13 | |||
| 318 | 2.11 |
| Sample | Peak | BE (eV) | Percent (%) |
|---|---|---|---|
| Cit-ACP-25 | O2− | 530.5 | 49.1 |
| OH− | 531.3 | 30.8 | |
| H2O | 532.5 | 20.2 | |
| Cit-ACP-F | O2− | 530.3 | 49.2 |
| OH− | 531.3 | 32.3 | |
| H2O | 532.3 | 18.4 |
| Sample | Peak | BE (eV) | Percent (%) |
|---|---|---|---|
| Cit-ACP-25 | C-C | 284.8 | 79.6 |
| O-C=O | 288.64 | 20.4 | |
| Cit-ACP-F | C-C | 284.8 | 89.26 |
| O-C=O | 288.88 | 10.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, R.; Wang, M.; Jiang, Y.; Zhang, S.; Tan, J. Citrate-Stabilized Amorphous Calcium Phosphate Nanoparticles as an Effective Adsorbent for Defluorination. Nanomaterials 2025, 15, 621. https://doi.org/10.3390/nano15080621
Su R, Wang M, Jiang Y, Zhang S, Tan J. Citrate-Stabilized Amorphous Calcium Phosphate Nanoparticles as an Effective Adsorbent for Defluorination. Nanomaterials. 2025; 15(8):621. https://doi.org/10.3390/nano15080621
Chicago/Turabian StyleSu, Ruojiao, Miaomiao Wang, Yuwei Jiang, Shuang Zhang, and Junjun Tan. 2025. "Citrate-Stabilized Amorphous Calcium Phosphate Nanoparticles as an Effective Adsorbent for Defluorination" Nanomaterials 15, no. 8: 621. https://doi.org/10.3390/nano15080621
APA StyleSu, R., Wang, M., Jiang, Y., Zhang, S., & Tan, J. (2025). Citrate-Stabilized Amorphous Calcium Phosphate Nanoparticles as an Effective Adsorbent for Defluorination. Nanomaterials, 15(8), 621. https://doi.org/10.3390/nano15080621





