Encapsulation of Sulforaphane from Cruciferous Vegetables in mPEG-PLGA Nanoparticles Enhances Cadmium’s Inhibitory Effect on HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Cd-γ-PGA Conjugate
2.3. Preparation of NP-Cd-SFN
2.4. Drug Release In Vitro
2.5. Cytotoxicity Studies in HepG2 Cells
2.6. Cellular Uptake
2.7. Cell Apoptosis Study in HepG2 Cells
Incubation of RAW 264.7 Cells
2.8. Western Blot Assay
2.9. In Vitro Metallothionein Detection
2.10. Statistics
3. Results and Discussion
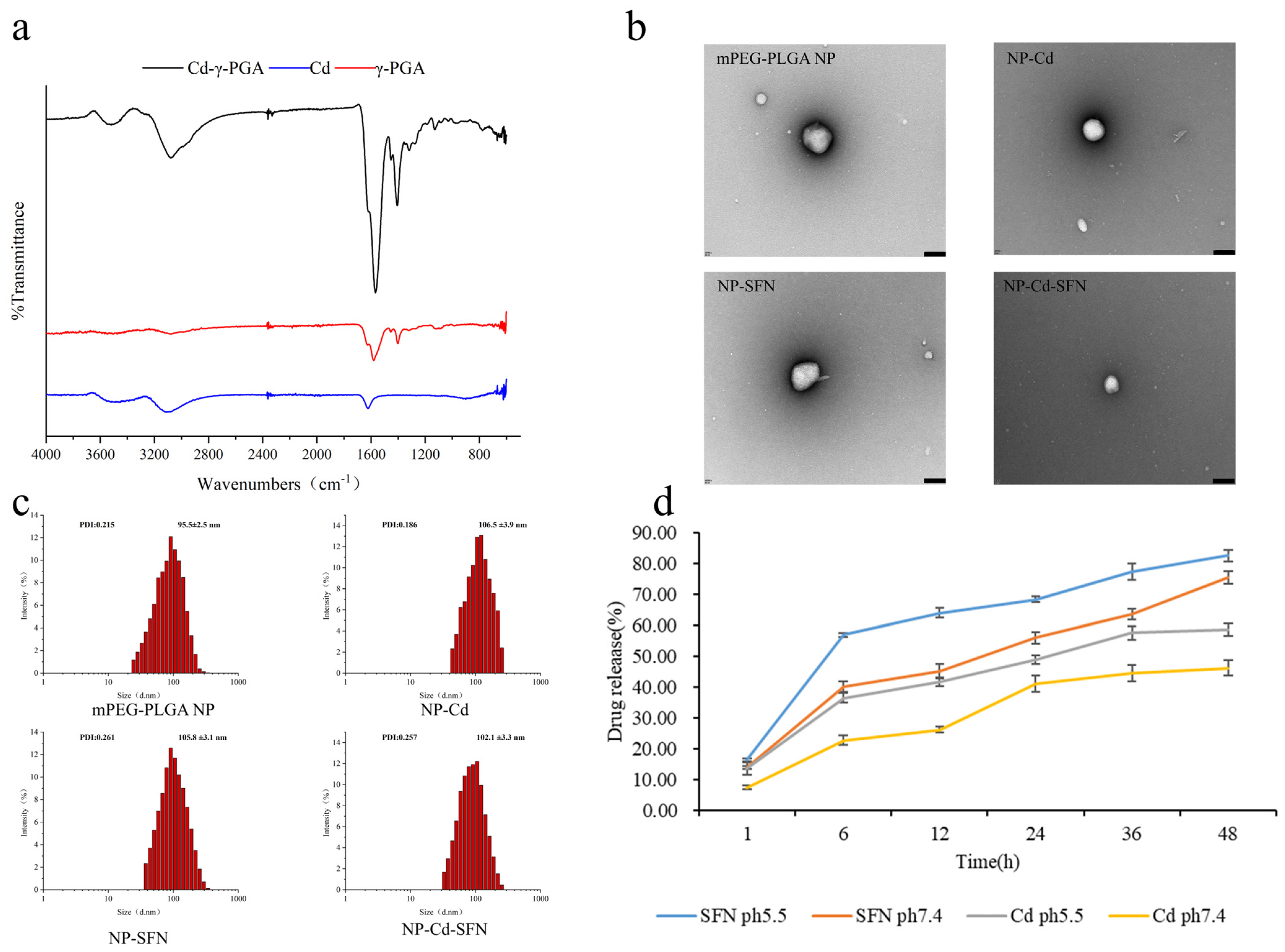
3.1. Characterization of NP-Cd-SFN
3.2. In Vitro Drug Release
3.3. Stability and Safety of NP-Cd-SFN
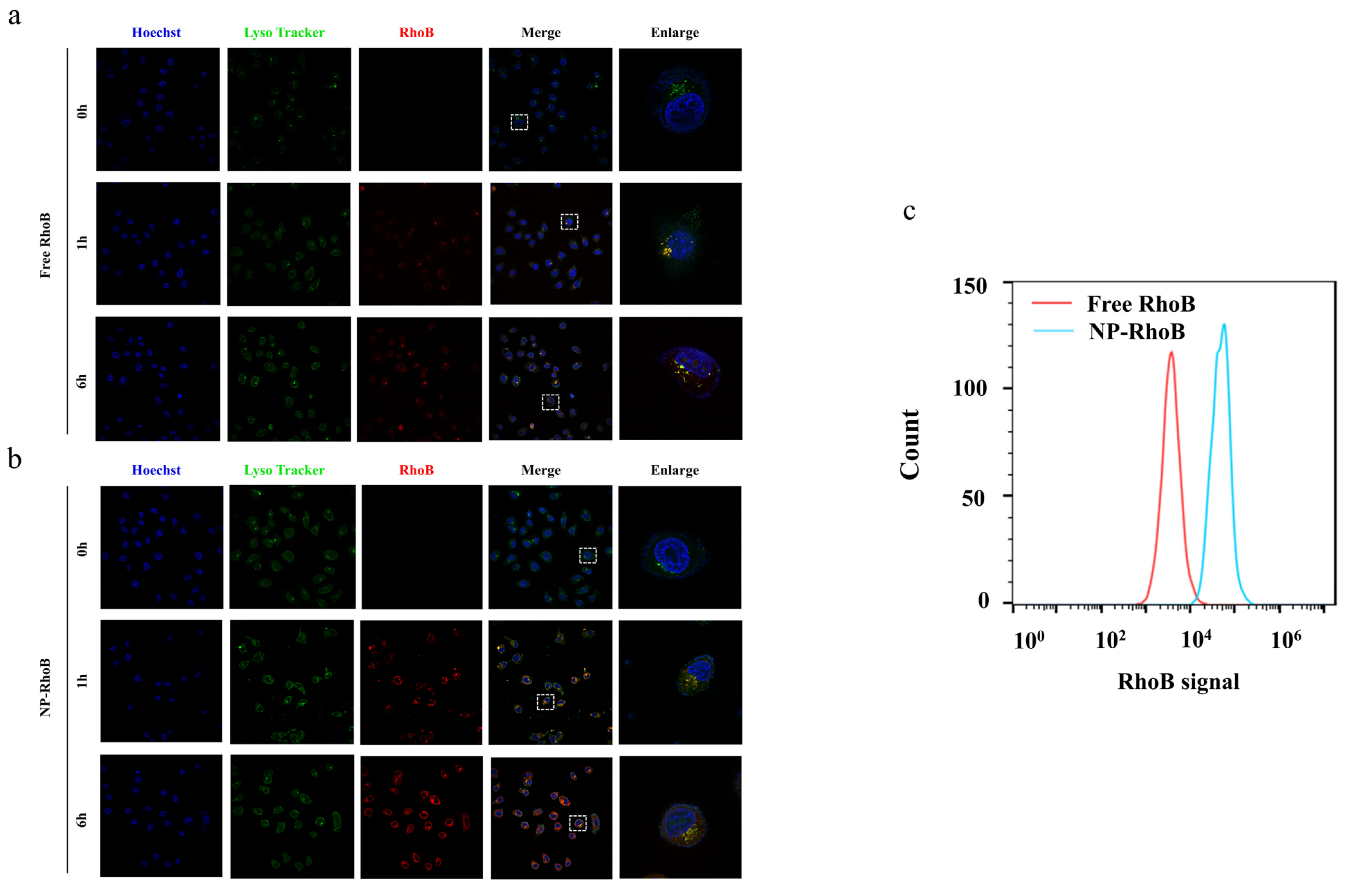
3.4. Cellular Uptake and Intracellular Localization
3.5. Evaluation of Cytotoxicity in HepG2 Cells
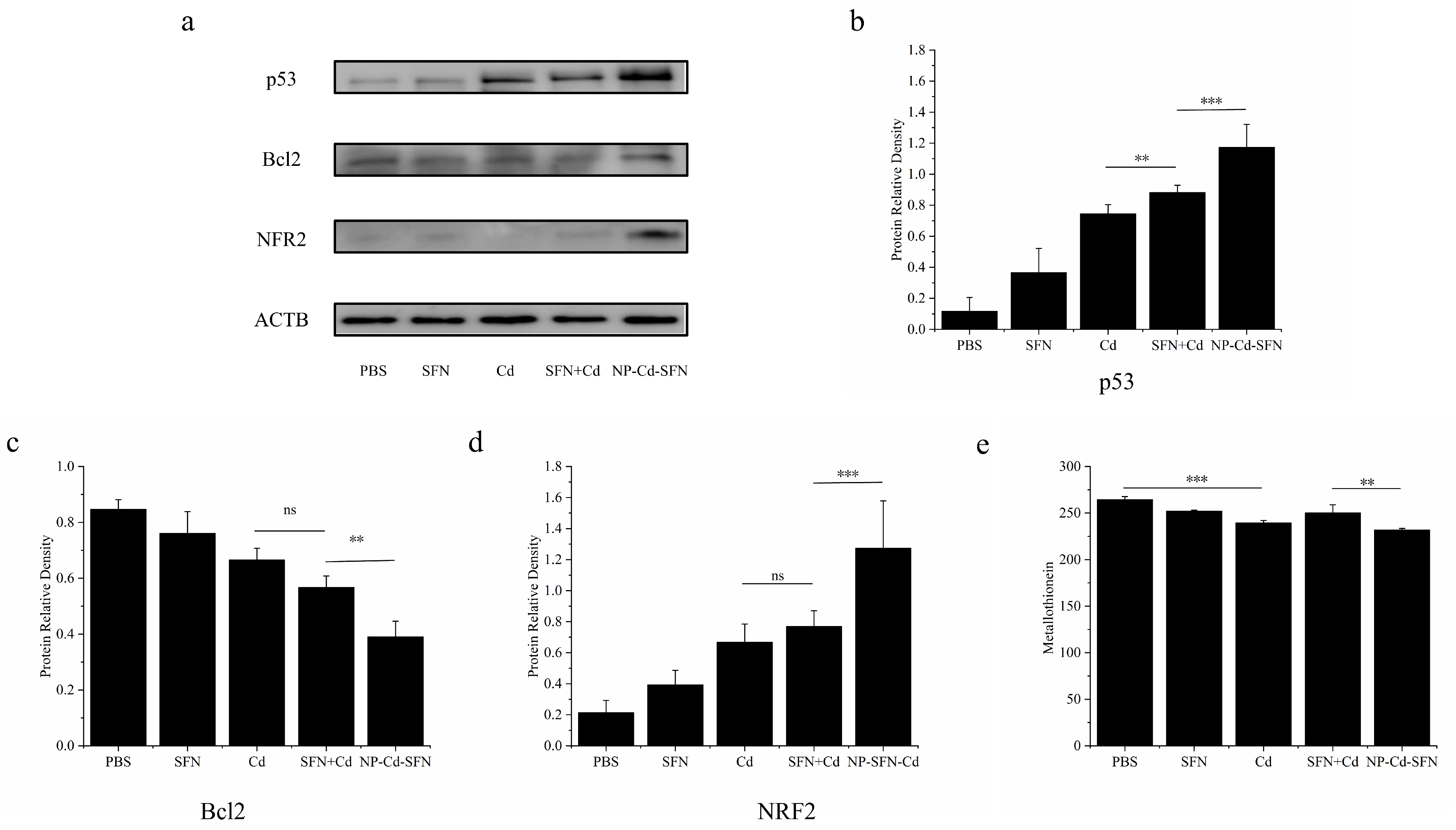
3.6. Mechanistic Analysis of Apoptosis Induced by NP-Cd-SFN
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alzamami, A.; Radwan, E.M.; Abo-Elabass, E.; Behery, M.E.; Alshwyeh, H.A.; Al-Olayan, E.; Altamimi, A.S.; Attallah, N.G.M.; Altwaijry, N.; Jaremko, M.; et al. Novel 8-Methoxycoumarin-3-Carboxamides with Potent Anticancer Activity against Liver Cancer via Targeting Caspase-3/7 and β-Tubulin Polymerization. BMC Chem. 2023, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.J.; Alirezaei, M.; Soltani, M.; Es-haghi, A.; Minai-Tehrani, D. Synthesis and Characterization of Albumin Nanoparticles Loaded by Ellagic Acid and Chitosan and Investigation of Its Anticancer Activity Against Liver Cancer Cells. BioNanoScience 2025, 15, 200. [Google Scholar] [CrossRef]
- Solanki, R.; Rawat, L.; Tabasum, S.; Pal, S.; Patel, S.; Sabarwal, A. A Comprehensive Review of Anti-Cancer Mechanisms of Polyphenol Honokiol and Nano Carrier-Based Approaches to Enhance Its Therapeutic Potential. Phytochem. Rev. 2025, 1–27. [Google Scholar] [CrossRef]
- Maleki, H.; Hosseini Najafabadi, M.R.; Webster, T.J.; Hadjighassem, M.R.; Sadroddiny, E.; Ghanbari, H.; Khosravani, M.; Adabi, M. Effect of Paclitaxel/Etoposide Co-Loaded Polymeric Nanoparticles on Tumor Size and Survival Rate in a Rat Model of Glioblastoma. Int. J. Pharm. 2021, 604, 120722. [Google Scholar] [CrossRef]
- Malekpour, M.R.; Hosseindoost, S.; Madani, F.; Kamali, M.; khosravani, M.; Adabi, M. Combination Nanochemotherapy of Brain Tumor Using Polymeric Nanoparticles Loaded with Doxorubicin and Paclitaxel: An in Vitro and in Vivo Study. Eur. J. Pharm. Biopharm. 2023, 193, 175–186. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, M.; He, C.; Chen, H.; Li, J.; Zhang, L.; Zhang, M.; Choi, M.M.F.; Jia, H.; Bian, W. Application of pH and Hyaluronidase Dual-Responsive Mesoporous Carbon Nitride Nano-Drug Delivery System for Chemodynamic Therapy and Chemotherapy Combination Therapy of Non-Small Cell Lung Cancer. Appl. Mater. Today 2024, 41, 102469. [Google Scholar] [CrossRef]
- Ai, Z.; Liu, B.; Chen, J.; Zeng, X.; Wang, K.; Tao, C.; Chen, J.; Yang, L.; Ding, Q.; Zhou, M. Advances in Nano Drug Delivery Systems for Enhanced Efficacy of Emodin in Cancer Therapy. Int. J. Pharm. X 2025, 9, 100314. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Q.; Shi, X.; Shen, J.; Li, R.; Zhou, J. Construction and Synergistic Anti-Tumor Study of a Tumor Microenvironment-Based Multifunctional Nano-Drug Delivery System. J. Photochem. Photobiol. B 2024, 258, 112977. [Google Scholar] [CrossRef]
- Katoch, O.; Kumar, A.; Adhikari, J.S.; Dwarakanath, B.S.; Agrawala, P.K. Sulforaphane Mitigates Genotoxicity Induced by Radiation and Anticancer Drugs in Human Lymphocytes. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 758, 29–34. [Google Scholar] [CrossRef]
- Mielczarek, L.; Krug, P.; Mazur, M.; Milczarek, M.; Chilmonczyk, Z.; Wiktorska, K. In the Triple-Negative Breast Cancer MDA-MB-231 Cell Line, Sulforaphane Enhances the Intracellular Accumulation and Anticancer Action of Doxorubicin Encapsulated in Liposomes. Int. J. Pharm. 2019, 558, 311–318. [Google Scholar] [CrossRef]
- Negrette-Guzmán, M. Combinations of the Antioxidants Sulforaphane or Curcumin and the Conventional Antineoplastics Cisplatin or Doxorubicin as Prospects for Anticancer Chemotherapy. Eur. J. Pharmacol. 2019, 859, 172513. [Google Scholar] [CrossRef]
- Alansari, W.S. Sulforaphane (4-Methylsulfnylbutyl Isothiocyanate) Mitigates Gold Nanoparticle Induced Brain Toxicity in Male Albino Rats. J. King Saud Univ. Sci. 2024, 36, 103257. [Google Scholar] [CrossRef]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Sulforaphane Alleviates Cadmium-Induced Toxicity in Human Mesenchymal Stem Cells through POR and TNFSF10 Genes Expression. Biomed. Pharmacother. 2019, 115, 108896. [Google Scholar] [CrossRef]
- Cascajosa-Lira, A.; Prieto, A.I.; Pichardo, S.; Jos, A.; Cameán, A.M. Protective Effects of Sulforaphane against Toxic Substances and Contaminants: A Systematic Review. Phytomedicine 2024, 130, 155731. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T. Targeting Cancer Stem Cells with Sulforaphane, A Dietary Component from Broccoli and Broccoli Sprouts. Future Oncol. 2013, 9, 1097–1103. [Google Scholar] [CrossRef]
- Wang, S.; Wu, X.; Tan, M.; Gong, J.; Tan, W.; Bian, B.; Chen, M.; Wang, Y. Fighting Fire with Fire: Poisonous Chinese Herbal Medicine for Cancer Therapy. J. Ethnopharmacol. 2012, 140, 33–45. [Google Scholar] [CrossRef]
- Niu, C.; Yan, H.; Yu, T.; Sun, H.-P.; Liu, J.-X.; Li, X.-S.; Wu, W.; Zhang, F.-Q.; Chen, Y.; Zhou, L.; et al. Studies on Treatment of Acute Promyelocytic Leukemia With Arsenic Trioxide: Remission Induction, Follow-Up, and Molecular Monitoring in 11 Newly Diagnosed and 47 Relapsed Acute Promyelocytic Leukemia Patients. Blood 1999, 94, 3315–3324. [Google Scholar] [CrossRef]
- Fillman, T.; Shimizu-Furusawa, H.; Ng, C.F.S.; Parajuli, R.P.; Watanabe, C. Association of Cadmium and Arsenic Exposure with Salivary Telomere Length in Adolescents in Terai, Nepal. Environ. Res. 2016, 149, 8–14. [Google Scholar] [CrossRef]
- Liu, J.; Wang, E.; Cheng, Z.; Gao, Y.; Chen, C.; Jia, R.; Luo, Z.; Wang, L. Zinc Alleviates Cadmium-Induced Reproductive Toxicity via Regulating Ion Homeostasis, Metallothionein Expression, and Inhibiting Mitochondria-Mediated Apoptosis in the Freshwater Crab Sinopotamon Henanense. Ecotoxicol. Environ. Saf. 2023, 262, 115188. [Google Scholar] [CrossRef]
- Wahyudi, L.D.; Yu, S.H.; Cho, M.K. The Effect of Curcumin on the Cadmium-Induced Mitochondrial Apoptosis Pathway by Metallothionein 2A Regulation. Life Sci. 2022, 310, 121076. [Google Scholar] [CrossRef]
- Wen, S.H.; Li, L.B.; Huang, H.D.; Xie, Y.; Luo, L. Metallothionein 1E Alleviates Cadmium-Induced Renal Cytotoxicity through Promoting Mitochondrial Functional Recovery. Biomed. Environ. Sci. 2024, 37, 117–121. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, Y.; Liu, X.; Ma, N.; Du, H.; Jin, M.; Liu, Y.; Zhang, L.; Xu, Y.; Huang, P.; et al. Block Ionomer Complex Micelles Based on the Self-Assembly of Poly(Ethylene Glycol)-Block-Poly(Acrylic Acid) and CdCl2 for Anti-Tumor Drug Delivery. Chem. Pharm. Bull. 2011, 59, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Saedi, Z.; Hoveizi, E.; Roushani, M.; Massahi, S.; Hadian, M.; Salehi, K. Synthesis, Characterization, Anticancer Properties and Theoretical Study of Asymmetrical Cd(II)N2-Schiff Base Complexes. J. Mol. Struct. 2019, 1176, 207–216. [Google Scholar] [CrossRef]
- Yu, H.-N.; Shen, S.-R.; Yin, J.-J. Effects of Interactions of EGCG and Cd2+ on the Growth of PC-3 Cells and Their Mechanisms. Food Chem. Toxicol. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Sharifyrad, M.; Gohari, S.; Fathi, M.; Danafar, H.; Hosseini, M.-J.; Mostafavi, H.; Manjili, H.K. The Efficacy and Neuroprotective Effects of Edaravone-Loaded mPEG-b-PLGA Polymeric Nanoparticles on Human Neuroblastoma SH-SY5Y Cell Line as in Vitro Model of Ischemia. J. Drug Deliv. Sci. Technol. 2022, 73, 103378. [Google Scholar] [CrossRef]
- Yao, H.; Zhao, J.; Wang, Z.; Lv, J.; Du, G.; Jin, Y.; Zhang, Y.; Song, S.; Han, G. Enhanced Anticancer Efficacy of Cantharidin by mPEG-PLGA Micellar Encapsulation: An Effective Strategy for Application of a Poisonous Traditional Chinese Medicine. Colloids Surf. B Biointerfaces 2020, 196, 111285. [Google Scholar] [CrossRef]
- Hasanpour, A.; Esmaeili, F.; Hosseini, H.; Amani, A. Use of mPEG-PLGA Nanoparticles to Improve Bioactivity and Hemocompatibility of Streptokinase: In-Vitro and in-Vivo Studies. Mater. Sci. Eng. C 2021, 118, 111427. [Google Scholar] [CrossRef]
- Liu, P.; Yu, H.; Sun, Y.; Zhu, M.; Duan, Y. A mPEG-PLGA-b-PLL Copolymer Carrier for Adriamycin and siRNA Delivery. Biomaterials 2012, 33, 4403–4412. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hyun, H.; Youn, J.Y.; Kim, B.S.; Song, I.B.; Kim, M.S.; Lee, B.; Khang, G.; Lee, H.B. Preparation of Nano-Emulsified Paclitaxel Using MPEG–PLGA Diblock Copolymers. Colloids Surf. Physicochem. Eng. Asp. 2008, 313–314, 126–130. [Google Scholar] [CrossRef]
- Qureshi, W.A.; Zhao, R.; Wang, H.; Ji, T.; Ding, Y.; Ihsan, A.; Mujeeb, A.; Nie, G.; Zhao, Y. Co-Delivery of Doxorubicin and Quercetin via mPEG–PLGA Copolymer Assembly for Synergistic Anti-Tumor Efficacy and Reducing Cardio-Toxicity. Sci. Bull. 2016, 61, 1689–1698. [Google Scholar] [CrossRef]
- Xu, Y.; Han, X.; Li, Y.; Min, H.; Zhao, X.; Zhang, Y.; Qi, Y.; Shi, J.; Qi, S.; Bao, Y.; et al. Sulforaphane Mediates Glutathione Depletion via Polymeric Nanoparticles to Restore Cisplatin Chemosensitivity. ACS Nano 2019, 13, 13445–13455. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sun, Y.; Wang, Q.; Sun, Y.; Li, H.; Duan, Y. Intracellular Trafficking and Cellular Uptake Mechanism of mPEG-PLGA-PLL and mPEG-PLGA-PLL-Gal Nanoparticles for Targeted Delivery to Hepatomas. Biomaterials 2014, 35, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, K.; Zhao, R.; Ji, T.; Wang, X.; Yang, X.; Zhang, Y.; Cheng, K.; Liu, S.; Hao, J.; et al. Inducing Enhanced Immunogenic Cell Death with Nanocarrier-Based Drug Delivery Systems for Pancreatic Cancer Therapy. Biomaterials 2016, 102, 187–197. [Google Scholar] [CrossRef]
- Krishnakumar, N.; Prabu, S.M.; Sulfikkarali, N.K. Quercetin Protects against Cadmium-Induced Biochemical and Structural Changes in Rat Liver Revealed by FT-IR Spectroscopy. Biomed. Prev. Nutr. 2012, 2, 179–185. [Google Scholar] [CrossRef]
- Patra, P.; Katke, S.; Singh, S.; Panchal, K.; Johari, A.; Pawar, A.V.; Paliwal, R.; Chaurasiya, A. Development and Evaluation of Stable Long Circulating Decitabine-Loaded Copolymeric Nanoparticles: Harnessing QbD Approach and Lyophilization Technique. J. Pharm. Innov. 2024, 19, 38. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Li, C.; Xiao, Y.; Zhou, J.; Liu, S.; Zhang, L.; Song, X.; Guo, X.; Song, Q.; Zhao, J.; Deng, N. Knockout of Onecut2 Inhibits Proliferation and Promotes Apoptosis of Tumor Cells through SKP2-Mediated P53 Acetylation in Hepatocellular Carcinoma. Cell. Mol. Life Sci. 2024, 81, 469. [Google Scholar] [CrossRef]
- Nabih, H.K.; Hamed, A.R.; Yahya, S.M.M. Anti-Proliferative Effect of Melatonin in Human Hepatoma HepG2 Cells Occurs Mainly through Cell Cycle Arrest and Inflammation Inhibition. Sci. Rep. 2023, 13, 4396. [Google Scholar] [CrossRef]
- Nasr, S.A.; Saad, A.A.E.-M. Evaluation of the Cytotoxic Anticancer Effect of Polysaccharide of Nepeta Septemcrenata. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 53. [Google Scholar] [CrossRef]
- Tabbasam, R.; Khursid, S.; Ishaq, Y.; Farrukh, S.Y. Synergistic Cytotoxic Effects of Doxorubicin Loaded Silver, Gold and Zinc Oxide Nanoparticles in HepG2 Liver Cancer Cells. BioNanoScience 2024, 15, 105. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, L. Chebulinic Acid Ameliorates Diethylnitrosamine-Induced Hepatocarcinogenesis in Rats by Inhibiting AKT/mTOR Activation and Inhibits Liver Cancer Cell (HepG2) Proliferation. Rev. Bras. Farmacogn. 2025, 35, 101–113. [Google Scholar] [CrossRef]
- Chu, X.; Wang, X.; Feng, K.; Bi, Y.; Xin, Y.; Liu, S. Fucoidan Ameliorates Lipid Accumulation, Oxidative Stress, and NF-κB-Mediated Inflammation by Regulating the PI3K/AKT/Nrf2 Signaling Pathway in a Free Fatty Acid-Induced NAFLD Spheroid Model. Lipids Health Dis. 2025, 24, 55. [Google Scholar] [CrossRef] [PubMed]
- Hajimohammadi, S.; Rameshrad, M.; Karimi, G. Exploring the Therapeutic Effects of Sulforaphane: An in-Depth Review on Endoplasmic Reticulum Stress Modulation across Different Disease Contexts. Inflammopharmacology 2024, 32, 2185–2201. [Google Scholar] [CrossRef]
- Vijiyakumar, N.; Prince, S.E. A Comprehensive Review of Cadmium-Induced Toxicity, Signalling Pathways, and Potential Mitigation Strategies. Toxicol. Environ. Health Sci. 2024, 17, 79–94. [Google Scholar] [CrossRef]
- Yan, L.; Sun, C.; Sun, L.; Cao, C. Role of Metallothionein Gene in Cd and Pb Detoxification in Chironomus kiiensis. Ecotoxicology 2025. [Google Scholar] [CrossRef]



| Group | Cd (μg/mL) | SFN (μg/mL) |
|---|---|---|
| 1 | 0.1 | 2 |
| 2 | 0.2 | 2 |
| 3 | 0.6 | 4 |
| 4 | 0.8 | 8 |
| SFN (mg) | Cd (mg) | mPEG-PLGA (mg) | Cd Encapsulation Efficiency (%) | SFN Encapsulation Efficiency (%) | Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|---|---|---|---|
| 0.25 | 0.2 | 10 | 86.5 ± 2.4% | 61.2 ± 4.6 | 100.5 ± 3.8 | −13.24 ± 1.8 | 0.203 |
| 0.5 | 0.2 | 10 | 63.6 ± 2.8 | 102.1 ± 3.3 | −14.48 ± 1.4 | 0.257 | |
| 1 | 0.2 | 10 | 55.3 ± 5.2 | 106.3 ± 4.3 | −15.57 ± 2.6 | 0.175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhu, Y. Encapsulation of Sulforaphane from Cruciferous Vegetables in mPEG-PLGA Nanoparticles Enhances Cadmium’s Inhibitory Effect on HepG2 Cells. Nanomaterials 2025, 15, 615. https://doi.org/10.3390/nano15080615
Li R, Zhu Y. Encapsulation of Sulforaphane from Cruciferous Vegetables in mPEG-PLGA Nanoparticles Enhances Cadmium’s Inhibitory Effect on HepG2 Cells. Nanomaterials. 2025; 15(8):615. https://doi.org/10.3390/nano15080615
Chicago/Turabian StyleLi, Ren, and Yi Zhu. 2025. "Encapsulation of Sulforaphane from Cruciferous Vegetables in mPEG-PLGA Nanoparticles Enhances Cadmium’s Inhibitory Effect on HepG2 Cells" Nanomaterials 15, no. 8: 615. https://doi.org/10.3390/nano15080615
APA StyleLi, R., & Zhu, Y. (2025). Encapsulation of Sulforaphane from Cruciferous Vegetables in mPEG-PLGA Nanoparticles Enhances Cadmium’s Inhibitory Effect on HepG2 Cells. Nanomaterials, 15(8), 615. https://doi.org/10.3390/nano15080615






