Apple Extract as an Effective Silver Reducer in the Synthesis of Ag Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Silver Nanoparticles
- Apple extract preparation
- Synthesis of silver nanoparticles with apple extract
- pH influence
2.2. Physicochemical Characterization of Nanoparticles
2.3. Antimicrobial Activity
3. Results and Discussion
3.1. Nanoparticle Characterization
Optical Characterization
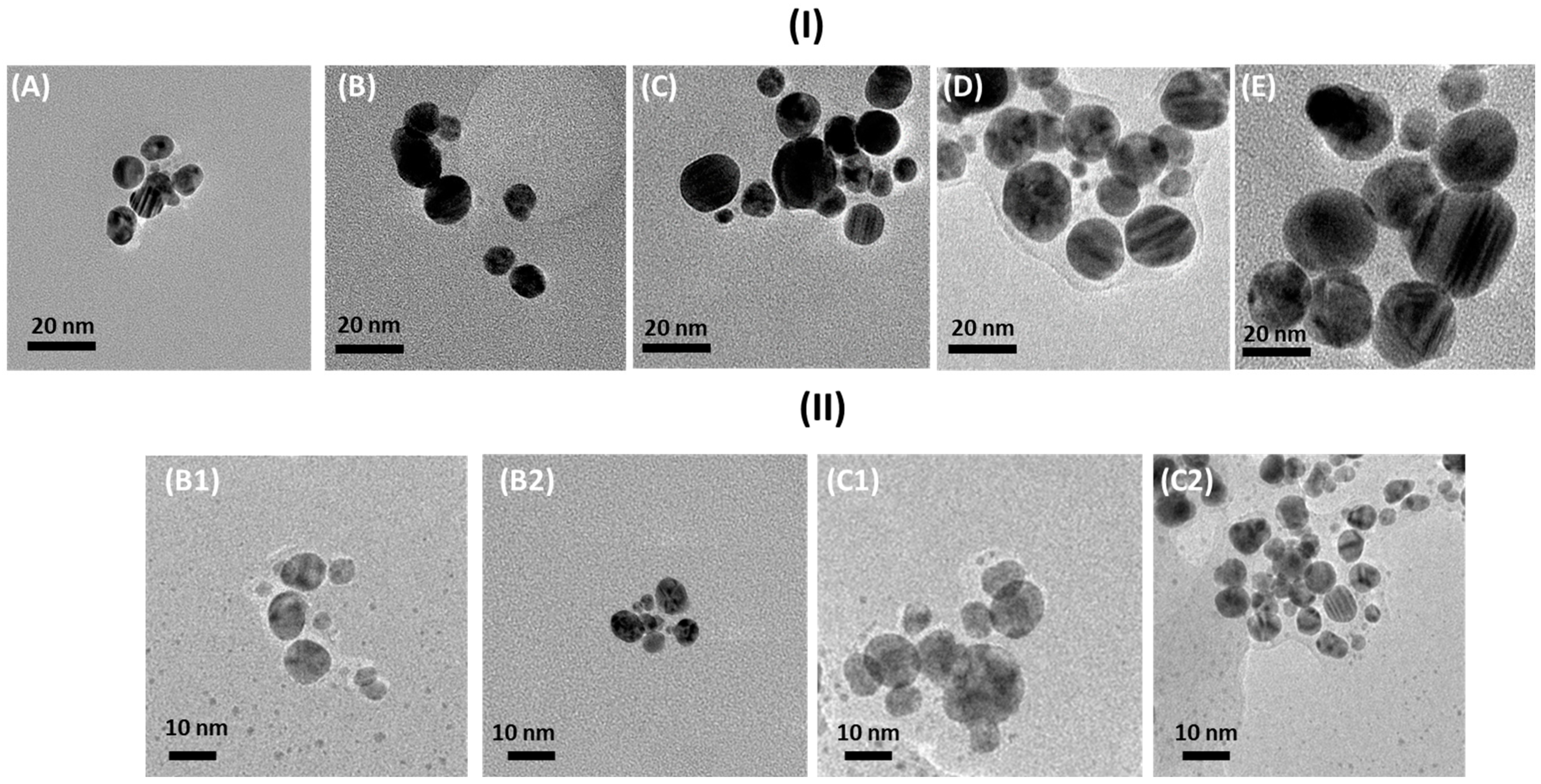
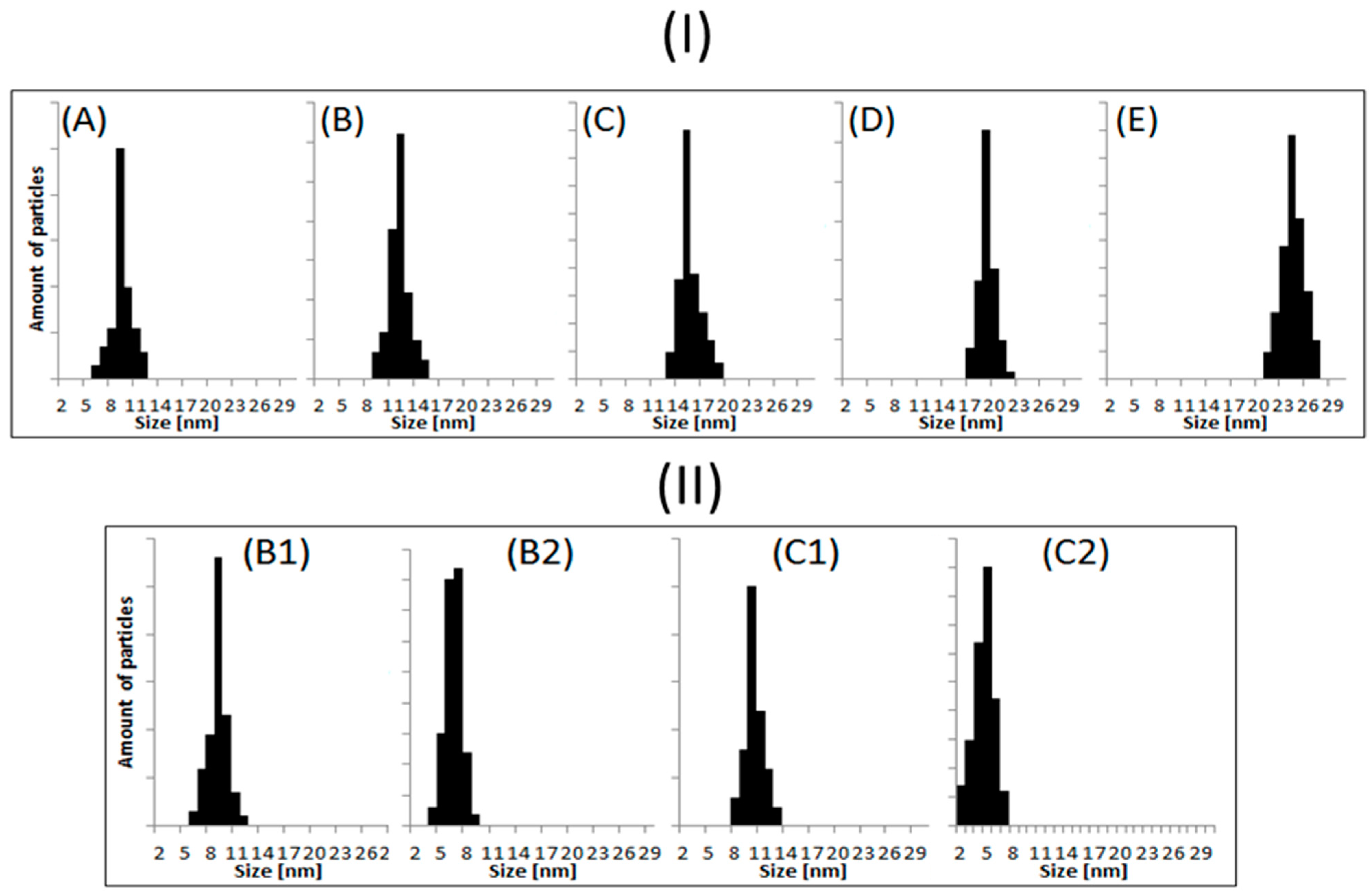
3.2. Transmission Electron Microscopy (TEM)
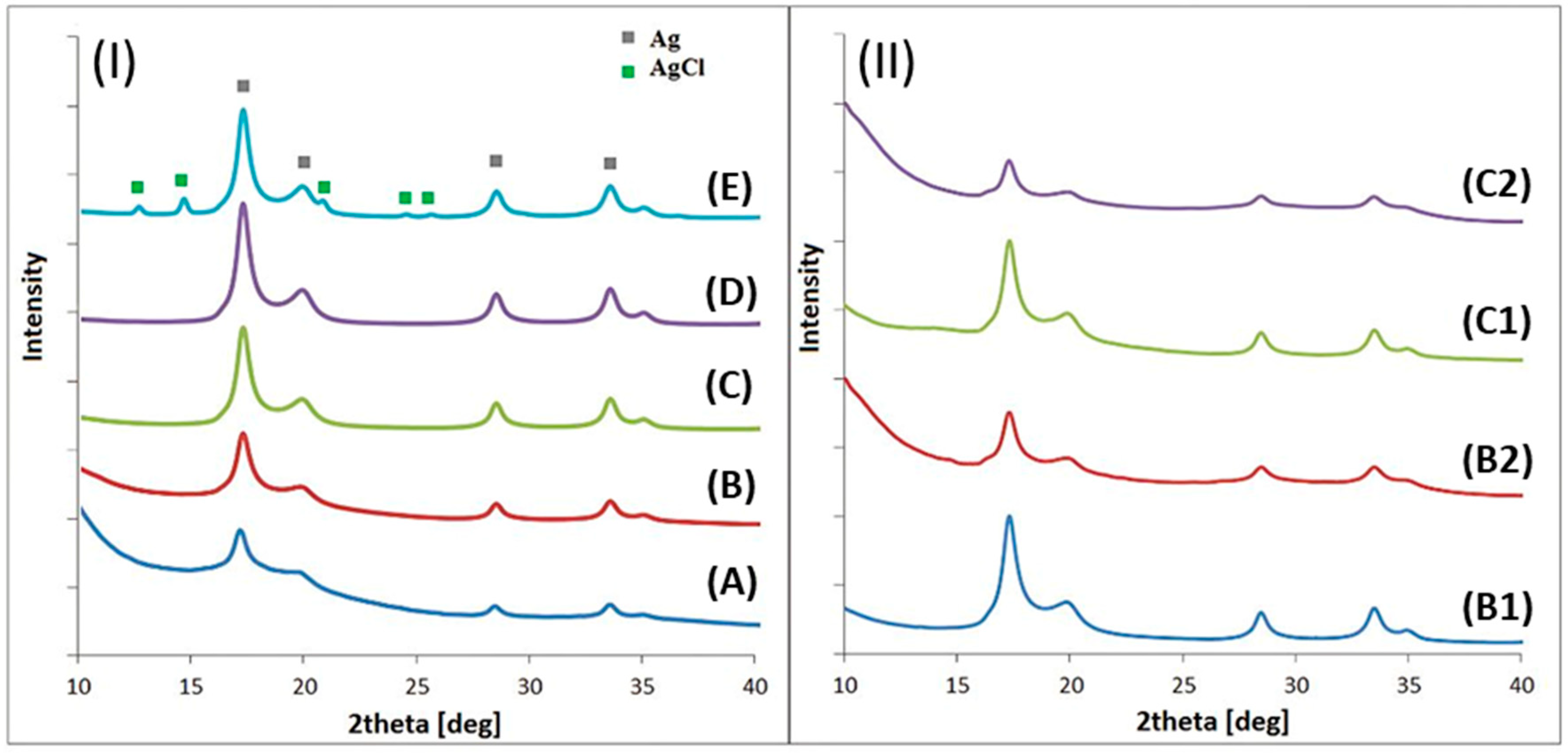
3.3. X-Ray Diffraction (XRD)
- D—grain size [Å];
- λ—wavelength (for Mo source, this is 0.7136 Å);
- β—full width at half maximum intensity of the peak [rad];
- ε—strain;
- Ɵ—diffraction angle [rad].
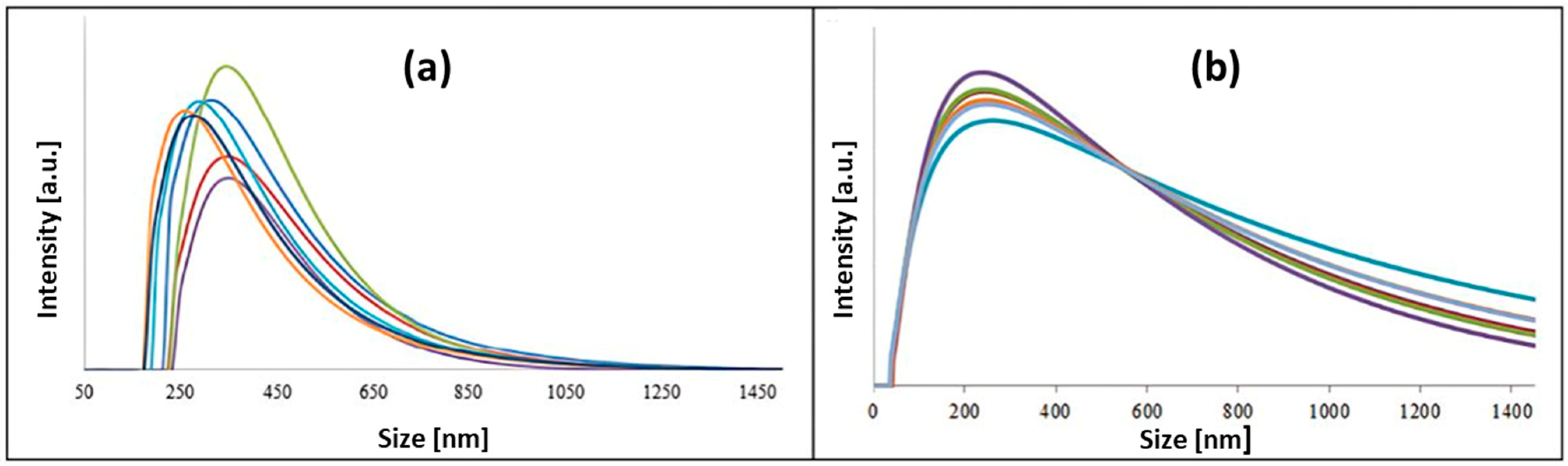
3.4. Dynamic Light Scattering (DLS)
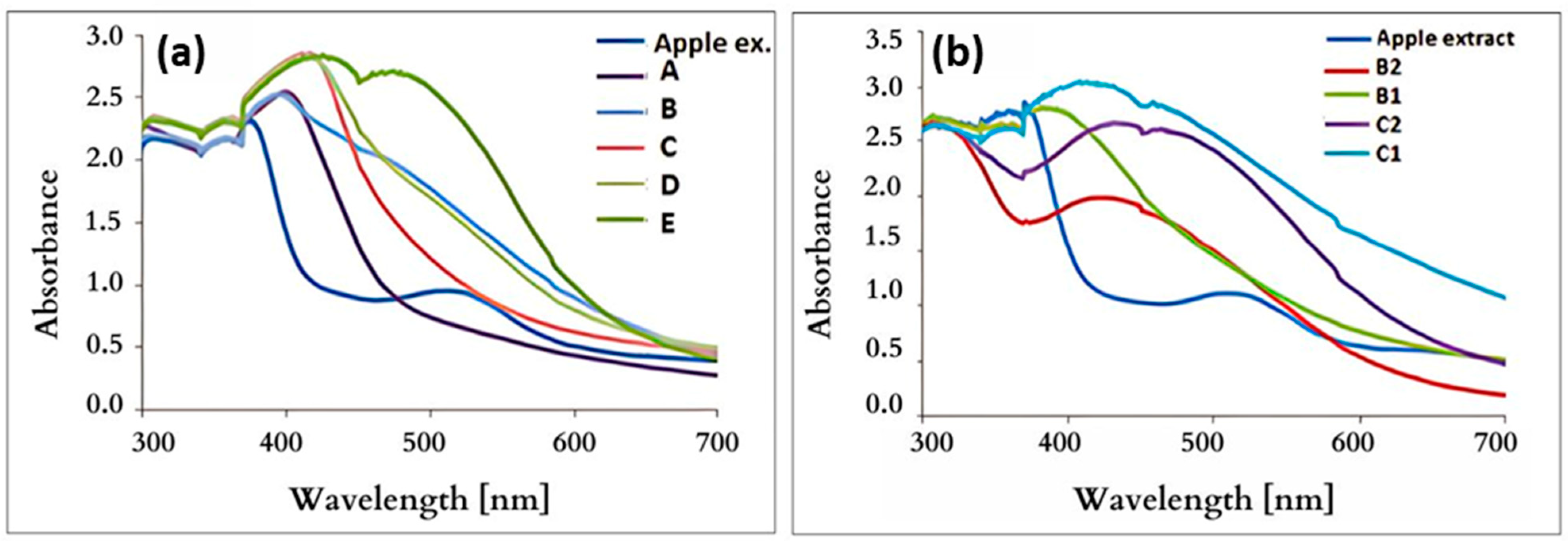
3.5. UV–Vis Spectroscopy
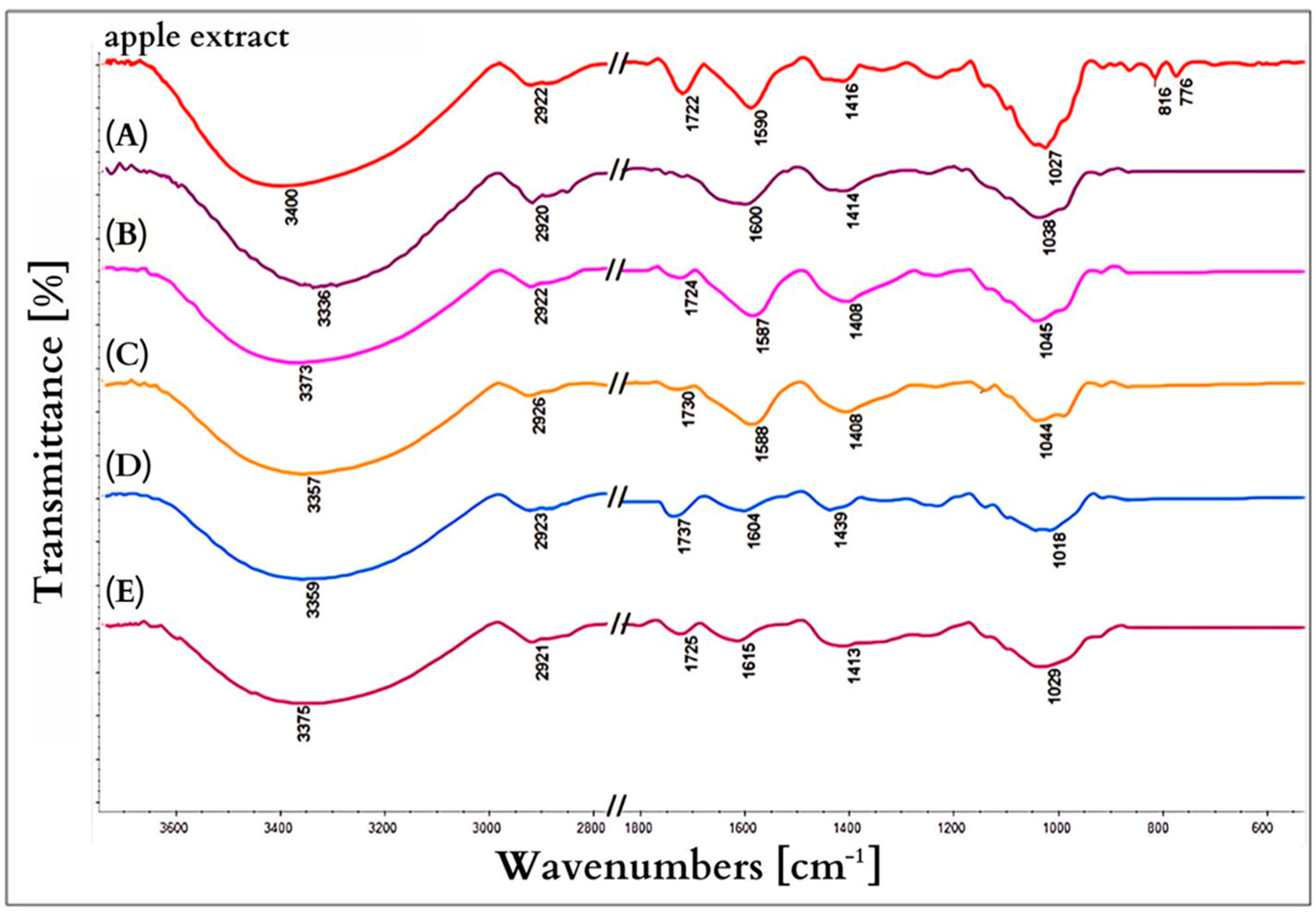
3.6. Infrared Spectroscopy
3.7. Antimicrobial Activity of Silver Nanoparticles Obtained with Apple Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Klekotka, U.; Rogacz, D.; Szymanek, I.; Malejko, J.; Rychter, P.; Kalska-Szostko, B. Ecotoxicological Assessment of Magnetite and Magnetite/Ag Nanoparticles on Terrestrial and Aquatic Biota from Different Trophic Levels. Chemosphere 2022, 308, 136207. [Google Scholar] [CrossRef]
- Siciliano, G.; Alsadig, A.; Chiriacò, M.S.; Turco, A.; Foscarini, A.; Ferrara, F.; Primiceri, E. Beyond Traditional Biosensors: Recent Advances in Gold Nanoparticles Modified Electrodes for Biosensing Applications. Talanta 2023, 253, 125280. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Venkatkarthick, R.; SenthilKannan, K.; Kuppam, C.; Stephy, G.M.; Ngamcharussrivichai, C. Advanced Technologies on the Sustainable Approaches for Conversion of Organic Waste to Valuable Bioproducts: Emerging Circular Bioeconomy Perspective. Fuel 2022, 324, 124313. [Google Scholar] [CrossRef]
- Chakraborty, D.; Yurdusen, A.; Mouchaham, G.; Nouar, F.; Serre, C. Large-Scale Production of Metal–Organic Frameworks. Adv. Funct. Mater. 2024, 34, 2309089. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.; Abreu, R.M.; Santo-Buelga, C.; Ferreira, I.C.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Plaskova, A.; Mlcek, J. New Insights into the Application of Water or Ethanol-Water Plant Extracts Rich in Active Compounds in Food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and Biodegradability of Pure and Aqueous Mixtures of Deep Eutectic Solvents: Glyceline, Ethaline, and Reline. Environ. Sci. Pollut. Res. 2021, 28, 8812–8821. [Google Scholar] [CrossRef]
- Lin, T.C.; Yang, Y.R.; Nguyen, Q.K.; Chiu, M.C.; Yen, H.M.; Chen, K.H.; Chuang, S.H. The Significance of Slowly Biodegradable COD on Denitrification in the OAO Process. J. Environ. Manag. 2025, 378, 124805. [Google Scholar] [CrossRef]
- Madani, M.; Hosny, S.; Alshangiti, D.M.; Nady, N.; Alkhursani, S.A.; Alkhaldi, H.; Gaber, G.A. Green Synthesis of Nanoparticles for Varied Applications: Green Renewable Resources and Energy-Efficient Synthetic Routes. Nanotechnol. Rev. 2022, 11, 731–759. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.U.; Khan, M.K.I.; Riaz, S.; Nazir, A.; Maan, A.A.; Amin, U.; Afzaal, M. Role of Fruits in Aging and Age-Related Disorders. Exp. Gerontol. 2022, 162, 111763. [Google Scholar] [CrossRef]
- Arnold, M.; Gramza-Michalowska, A. Recent Development on the Chemical Composition and Phenolic Extraction Methods of Apple (Malus domestica)—A Review. Food Bioprocess Technol. 2024, 17, 2519–2560. [Google Scholar] [CrossRef]
- Tiwari, S.; Jha, N.K.; Kalaivany. Nutraceuticals from Apple Waste. In Nutraceuticals from Fruit and Vegetable Waste; Elsevier: Amsterdam, The Netherlands, 2024; pp. 97–119. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Singh, I.; Hasan, M.M.; Khan, M.S.; Yousafi, Q.; Cavalu, S. Green Metallic Nanoparticles: Biosynthesis to Applications. Front. Bioeng. Biotechnol. 2022, 10, 548. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2024, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. The Use of Capping Agents in the Stabilization and Functionalization of Metallic Nanoparticles for Biomedical Applications. Part. Part. Syst. Charact. 2023, 40, 2200146. [Google Scholar] [CrossRef]
- El Shafey, A.M. Green Synthesis of Metal and Metal Oxide Nanoparticles from Plant Leaf Extracts and Their Applications: A Review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from Fruits: Relationships between Extraction Methods, Structural Characteristics, and Functional Properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Maťátková, O.; Michailidu, J.; Miškovská, A.; Kolouchová, I.; Masák, J.; Čejková, A. Antimicrobial Properties and Applications of Metal Nanoparticles Biosynthesized by Green Methods. Biotechnol. Adv. 2022, 58, 107905. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, Translocation, and Transformation of Silver Nanoparticles in Plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Gökşen Tosun, N.; Kaplan, Ö.; Türkekul, İ.; Gökçe, İ.; Özgür, A. Green Synthesis of Silver Nanoparticles Using Schizophyllum commune and Geopora sumneriana Extracts and Evaluation of Their Anticancer and Antimicrobial Activities. Part. Sci. Technol. 2022, 40, 801–811. [Google Scholar] [CrossRef]
- Shreyash, N.; Bajpai, S.; Khan, M.A.; Vijay, Y.; Tiwary, S.K.; Sonker, M. Green Synthesis of Nanoparticles and Their Biomedical Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 11428–11457. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A.; Al-Sabri, A.; Almansob, A.; AlNAdhari, S. Anti-Oxidant, Anti-Fungal, and Cytotoxic Effects of Silver Nanoparticles Synthesized Using Marine Fungus Cladosporium Halotolerans. Appl. Nanosci. 2023, 13, 623–631. [Google Scholar] [CrossRef]
- Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E. Investigation of Nanoparticle Metallic Core Antibacterial Activity: Gold and Silver Nanoparticles Against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 1905. [Google Scholar] [CrossRef]
- Ali, Z.A.; Yahya, R.; Sekaran, S.D.; Puteh, R. Green Synthesis of Silver Nanoparticles Using Apple Extract and Its Antibacterial Properties. Adv. Mater. Sci. Eng. 2016, 2016, 4102196. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Bhuvaneshwari, S.; Jayashree, M.; BoopathiRaja, R. Green Synthesis of Silver (Ag) Nanoparticles Using Extract of Apple and Grape and with Enhanced Visible Light Photocatalytic Activity. BioNanoScience 2019, 9, 423–432. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Yang, H.; Wang, T.; Wang, C. Bio-Synthesis of Silver Nanoparticles with Antibacterial Activity. Mater. Chem. Phys. 2019, 235, 121746. [Google Scholar] [CrossRef]
- Kazlagić, A.; Abud, O.A.; Ćibo, M.; Hamidović, S.; Borovac, B.; Omanović-Mikličanin, E. Green Synthesis of Silver Nanoparticles Using Apple Extract and Its Antimicrobial Properties. Health Technol. 2020, 10, 147–150. [Google Scholar] [CrossRef]
- Wasilewska, A.; Klekotka, U.; Zambrzycka, M.; Zambrowski, G.; Święcicka, I.; Kalska-Szostko, B. Physico-Chemical Properties and Antimicrobial Activity of Silver Nanoparticles Fabricated by Green Synthesis. Food Chem. 2023, 400, 133960. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.A. Performance Standards for Antimicrobial Susceptibility Testing: 20th Informational Supplement; CLSI Document M100-S20; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2010. [Google Scholar]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A Critical Review of Synthesis Parameters Affecting the Properties of Zinc Oxide Nanoparticle and Its Application in Wastewater Treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Abdelaziz, O.A.; Abdallah, R.M.; Khater, R.A.; Abo Dena, A.S.; El-Sherbiny, I.M. Optical Ammonia-Sensing Probe Based on Surface-Plasmon Resonance of Silver-Nanoparticle-Decorated Superparamagnetic Dendritic Nanoparticles. Plasmonics 2023, 18, 201–212. [Google Scholar] [CrossRef]
- Gunjal, P.R.; Gaikwad, S.D. Green Synthesis and Characterization of Silver Nanoparticles Using Banana Peel Extract and Their Biological Activity against Representative Microorganism. World J. Biol. Pharm. Health Sci. 2023, 13, 425–439. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Wykowska, U.; Satuła, D. Core–Shell and Multilayered Magnetite Nanoparticles—Structural and Mössbauer Studies. Appl. Surf. Sci. 2014, 306, 7–15. [Google Scholar] [CrossRef]
- El-Khawaga, A.M.; Zidan, A.; Abd El-Mageed, A.I. Preparation Methods of Different Nanomaterials for Various Potential Applications: A Review. J. Mol. Struct. 2023, 1281, 135148. [Google Scholar] [CrossRef]
- Marciniak, L.; Nowak, M.; Trojanowska, A.; Tylkowski, B.; Jastrząb, R. The Effect of pH on the Size of Silver Nanoparticles Obtained in the Reduction Reaction with Citric and Malic Acids. Materials 2020, 13, 5444. [Google Scholar] [CrossRef]
- Harvey, D. Analytical Chemistry 2.0—An open-access digital textbook. Anal. Bioanal. Chem. 2011, 399, 149–152. [Google Scholar] [CrossRef]
- Lanje, A.S.; Sharma, S.J.; Pode, R.B. Synthesis of Silver Nanoparticles: A Safer Alternative to Conventional Antimicrobial and Antibacterial Agents. J. Chem. Pharm. Res. 2010, 2, 478–483. [Google Scholar]
- Asefian, S.; Ghavam, M. Green and Environmentally Friendly Synthesis of Silver Nanoparticles with Antibacterial Properties from Some Medicinal Plants. BMC Biotechnol. 2024, 24, 5. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, X.; Xu, X.; Jing, Y.; Lai, Y.; Su, S.; Zhu, J. Controlled Synthesis of Silver/Silver Chloride Composite Crystals from [AgCl2]− Complex and Its Photocatalysis Properties on Organic Pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 128984. [Google Scholar] [CrossRef]
- Shaik, K.; Cole, S. Comparative Study of Crystallite Size from XRD and TEM Results for Pure and V2O5 Doped CdO-FePO4 Composite Nanopowders. Phys. Chem. Res. 2023, 11, 241–251. [Google Scholar] [CrossRef]
- Kawsar, M.; Hossain, M.S.; Bahadur, N.M.; Ahmed, S. Synthesis of Nano-Crystallite Hydroxyapatites in Different Media and a Comparative Study for Estimation of Crystallite Size Using Scherrer Method, Halder-Wagner Method Size-Strain Plot, and Williamson-Hall Model. Heliyon 2024, 10, e08431. [Google Scholar] [CrossRef]
- Alam, M.K.; Hossain, M.S.; Bahadur, N.M.; Ahmed, S. A Comparative Study in Estimating of Crystallite Sizes of Synthesized and Natural Hydroxyapatites Using Scherrer Method, Williamson-Hall Model, Size-Strain Plot and Halder-Wagner Method. J. Mol. Struct. 2024, 1306, 137820. [Google Scholar] [CrossRef]
- Al Aboody, M.S. Silver/Silver Chloride (Ag/AgCl) Nanoparticles Synthesized from Azadirachta indica Latex and Its Antibiofilm Activity against Fluconazole Resistant Candida Tropicalis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Kalska-Szostko, B.; Hilgendorff, M.; Giersig, M.; Fumagalli, P. Magneto-Optical Properties of Co Nanoparticles/(Cu, Ag, Au) Sandwich Systems. Sci. Adv. Mater. 2015, 7, 540–545. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Gorshkova, Y.; Ungureanu, C.; Badea, N.; Bokuchava, G.; Lazea-Stoyanova, A.; Petrovic, S. Characterization and Antitumoral Activity of Biohybrids Based on Turmeric and Silver/Silver Chloride Nanoparticles. Materials 2021, 14, 4726. [Google Scholar] [CrossRef]
- Velidandi, A.; Pabbathi, N.P.P.; Dahariya, S.; Kagithoju, S.; Baadhe, R.R. Bio-Fabrication of Silver-Silver Chloride Nanoparticles Using Annona Muricata Leaf Extract: Characterization, Biological, Dye Degradation and Eco-Toxicity Studies. Int. J. Environ. Sci. Technol. 2022, 19, 6555–6572. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS Zeta Potential–What They Are What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A.; Doroshkevich, A.S.; Chicea, L.M.; Ozkendir, O.M. Comparative Synthesis of Silver Nanoparticles: Evaluation of Chemical Reduction Procedures, AFM and DLS Size Analysis. Materials 2023, 16, 5244. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Yan, H.; Wang, X.; Liu, G.; Wang, J.; Han, Y.; Zhao, G. A Flexible and Wearable PET-Based Chemiresistive H2S Gas Sensor Modified with MoS2–AgCl@AgNPs Nanocomposite for the Dynamic Monitoring of Egg Spoilage. Anal. Chim. Acta 2023, 1234, 341836. [Google Scholar] [CrossRef]
- Ding, H.; Chen, Z.; Ponce, C.; Zheng, Y. Optothermal Rotation of Micro-/Nano-Objects. Chem. Commun. 2023, 59, 2208–2221. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Sabah, A.; Shafaqat, I.; Naifar, A.; Albalawi, H.; Alqahtani, M.S.; Ashiq, M.G.B.; Shabbir, S.A. Investigation of Band Parameters and Electrochemical Analysis of Multi Core-Shell CdSe/CdS/ZnS Quantum Dots. Opt. Mater. 2023, 142, 114065. [Google Scholar] [CrossRef]
- Nandhini, S.N.; Sisubalan, N.; Vijayan, A.; Karthikeyan, C.; Gnanaraj, M.; Gideon, D.A.M.; Sadiku, R. Recent Advances in Green Synthesized Nanoparticles for Bactericidal and Wound Healing Applications. Heliyon 2023, 9, e12345. [Google Scholar] [CrossRef]
- Jadoun, S.; Chauhan, N.P.S.; Zarrintaj, P.; Barani, M.; Varma, R.S.; Chinnam, S.; Rahdar, A. Synthesis of Nanoparticles Using Microorganisms and Their Applications: A Review. Environ. Chem. Lett. 2022, 20, 3153–3197. [Google Scholar] [CrossRef]
- Soni, V.; Raizada, P.; Singh, P.; Cuong, H.N.; Rangabhashiyam, S.; Saini, A.; Nguyen, V.H. Sustainable and Green Trends in Using Plant Extracts for the Synthesis of Biogenic Metal Nanoparticles Toward Environmental and Pharmaceutical Advances: A Review. Environ. Res. 2021, 202, 111622. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ahmed, S. Easy and Green Synthesis of TiO2 (Anatase and Rutile): Estimation of Crystallite Size Using Scherrer Equation, Williamson-Hall Plot, Monshi-Scherrer Model, Size-Strain Plot, Halder-Wagner Model. Results Mater. 2023, 20, 100492. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Fitriani, I.; Wyantuti, S.; Hartati, Y.W.; Khaydarov, R.; McAlister, J.A.; Gamo, T. Colorimetric Detection of Mercury (II) Ion in Aqueous Solution Using Silver Nanoparticles. Anal. Sci. 2017, 33, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple By-Products: Antioxidant Properties and Food Applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Kadeřábková, N.; Mahmood, A.J.; Mavridou, D.A. Antibiotic Susceptibility Testing Using Minimum Inhibitory Concentration (MIC) Assays. NPJ Antimicrob. Resist. 2024, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.; Rodrigues, F.A.; dos Santos Barbosa, C.R.; dos Santos, J.F.S.; Macêdo, N.S.; de Sousa Silveira, Z.; da Cunha, F.A.B. Antibacterial Activity of the Pyrogallol Against Staphylococcus Aureus Evaluated by Optical Image. Biologics 2022, 2, 139–150. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Wang, X.; Liu, D. Release Strategies of Silver Ions from Materials for Bacterial Killing. ACS Appl. Bio Mater. 2021, 4, 3985–3999. [Google Scholar] [CrossRef]
- Sorinolu, A.J.; Godakhindi, V.; Siano, P.; Vivero-Escoto, J.L.; Munir, M. Influence of Silver Ion Release on the Inactivation of Antibiotic Resistant Bacteria Using Light-Activated Silver Nanoparticles. Mater. Adv. 2022, 3, 9090–9102. [Google Scholar] [CrossRef]
- Gupta, P.C.; Sharma, N.; Rai, S.; Mishra, P. Use of Smart Silver Nanoparticles in Drug Delivery System. In Metal and Metal-Oxide Based Nanomaterials: Synthesis, Agricultural, Biomedical and Environmental Interventions; Springer Nature: Singapore, 2024; pp. 213–241. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Bold, B.E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial Activity and Characteristics of Silver Nanoparticles Biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef]
- Draviana, H.T.; Fitriannisa, I.; Khafid, M.; Krisnawati, D.I.; Widodo; Lai, C.H.; Kuo, T.R. Size and Charge Effects of Metal Nanoclusters on Antibacterial Mechanisms. J. Nanobiotechnol. 2023, 21, 428. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Frias Batista, L.M.; John, M.G.; Rodrigues, C.J.; Tibbetts, K.M. Mechanism of Gold–Silver Alloy Nanoparticle Formation by Laser Coreduction of Gold and Silver Ions in Solution. J. Phys. Chem. B 2021, 125, 907–917. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E.; Moya, M.; Castro, E. Biocidal and Synergistic Effect of Three Types of Biologically Synthesised Silver/Silver Chloride Nanoparticles. World J. Microbiol. Biotechnol. 2024, 40, 18. [Google Scholar] [CrossRef] [PubMed]
- Kaabipour, S.; Hemmati, S. A Review on the Green and Sustainable Synthesis of Silver Nanoparticles and One-Dimensional Silver Nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef] [PubMed]
- Castiello, C.; Junghanns, P.; Mergel, A.; Jacob, C.; Ducho, C.; Valente, S.; Mai, A. GreenMedChem—The Challenge in the Next Decade toward Eco-Friendly Compounds and Processes in Drug Design. Green Chem. 2023, 25, 1234–1256. [Google Scholar] [CrossRef]
- Soni, R.A.; Rizwan, M.A.; Singh, S. Opportunities and Potential of Green Chemistry in Nanotechnology. Nanotechnol. Environ. Eng. 2022, 7, 661–673. [Google Scholar] [CrossRef]


| Sample | Concentration of AgNO3 [mmol/L] | Amount of NaOH [mL] | pH of the Solution |
|---|---|---|---|
| B1 | 0.5 | 3 | 6.2 |
| B2 | 0.5 | 6 | 9.6 |
| C1 | 1 | 3 | 6.2 |
| C2 | 1 | 6 | 9.6 |
| Sample | Size ± 2 nm | Shape |
|---|---|---|
| A | 9 | Spherical |
| B | 12 | Spherical |
| B1 | 9 | Ellipsoidal |
| B2 | 7 | Ellipsoidal or spherical |
| C | 15 | Spherical |
| C1 | 11 | Ellipsoidal |
| C2 | 6 | Spherical |
| D | 19 | Ellipsoidal or spherical |
| E | 24 | Ellipsoidal or spherical |
| Sample | Size ± 2 [nm] | Lattice Parameter Ag ± 0.002 [Å] | % Ag /%AgCl ± 1% |
|---|---|---|---|
| A | 8 | 4.091 | 100 |
| B | 13 | 4.092 | 100 |
| B1 | 10 | 4.072 | 100 |
| B2 | 7 | 4.091 | 100 |
| C | 16 | 4.090 | 100 |
| C1 | 12 | 4.072 | 100 |
| C2 | 8 | 4.090 | 100 |
| D | 19 | 4.091 | 100 |
| E | 20 | 4.062 /5.490 | 89 /11% |
| Sample | A | B | B1 | B2 | C | C1 | C2 | D | E |
|---|---|---|---|---|---|---|---|---|---|
| Maximum absorption [nm] | 411 | 412 | 400 | 417 | 419 | 420 | 428 | 420 | 425 |
| Microorganism | Specimen | ||||||
|---|---|---|---|---|---|---|---|
| Extract Apple | AgNO3 | Sample A | Sample B | Sample C | Sample D | Sample E | |
| B. cereus ATCC 10987 | - | 1:128 | 1:128 | 1:128 | 1:512 | 1:512 | 1:1024 |
| S. aureus ATCC 6538 | - | 1:32 | 1:128 | 1:32 | 1:64 | 1:256 | 1:256 |
| E. coli ATCC 11229 | - | - | 1:8 | 1:32 | 1:64 | 1:8 | 1:4 |
| C. krusei ATCC 30135 | - | 1:32 | 1:32 | 1:64 | 1:256 | 1:512 | 1:512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewska, A.; Basa, A.; Zambrzycka, M.; Swiecicka, I.; Kalska-Szostko, B. Apple Extract as an Effective Silver Reducer in the Synthesis of Ag Nanoparticles. Nanomaterials 2025, 15, 595. https://doi.org/10.3390/nano15080595
Wasilewska A, Basa A, Zambrzycka M, Swiecicka I, Kalska-Szostko B. Apple Extract as an Effective Silver Reducer in the Synthesis of Ag Nanoparticles. Nanomaterials. 2025; 15(8):595. https://doi.org/10.3390/nano15080595
Chicago/Turabian StyleWasilewska, Anna, Anna Basa, Monika Zambrzycka, Izabela Swiecicka, and Beata Kalska-Szostko. 2025. "Apple Extract as an Effective Silver Reducer in the Synthesis of Ag Nanoparticles" Nanomaterials 15, no. 8: 595. https://doi.org/10.3390/nano15080595
APA StyleWasilewska, A., Basa, A., Zambrzycka, M., Swiecicka, I., & Kalska-Szostko, B. (2025). Apple Extract as an Effective Silver Reducer in the Synthesis of Ag Nanoparticles. Nanomaterials, 15(8), 595. https://doi.org/10.3390/nano15080595







