Scaling Amphiphilicity with Janus Nanoparticles: A New Frontier in Nanomaterials and Interface Science
Abstract
1. Introduction
2. Short Historical Account—Development of JNPs
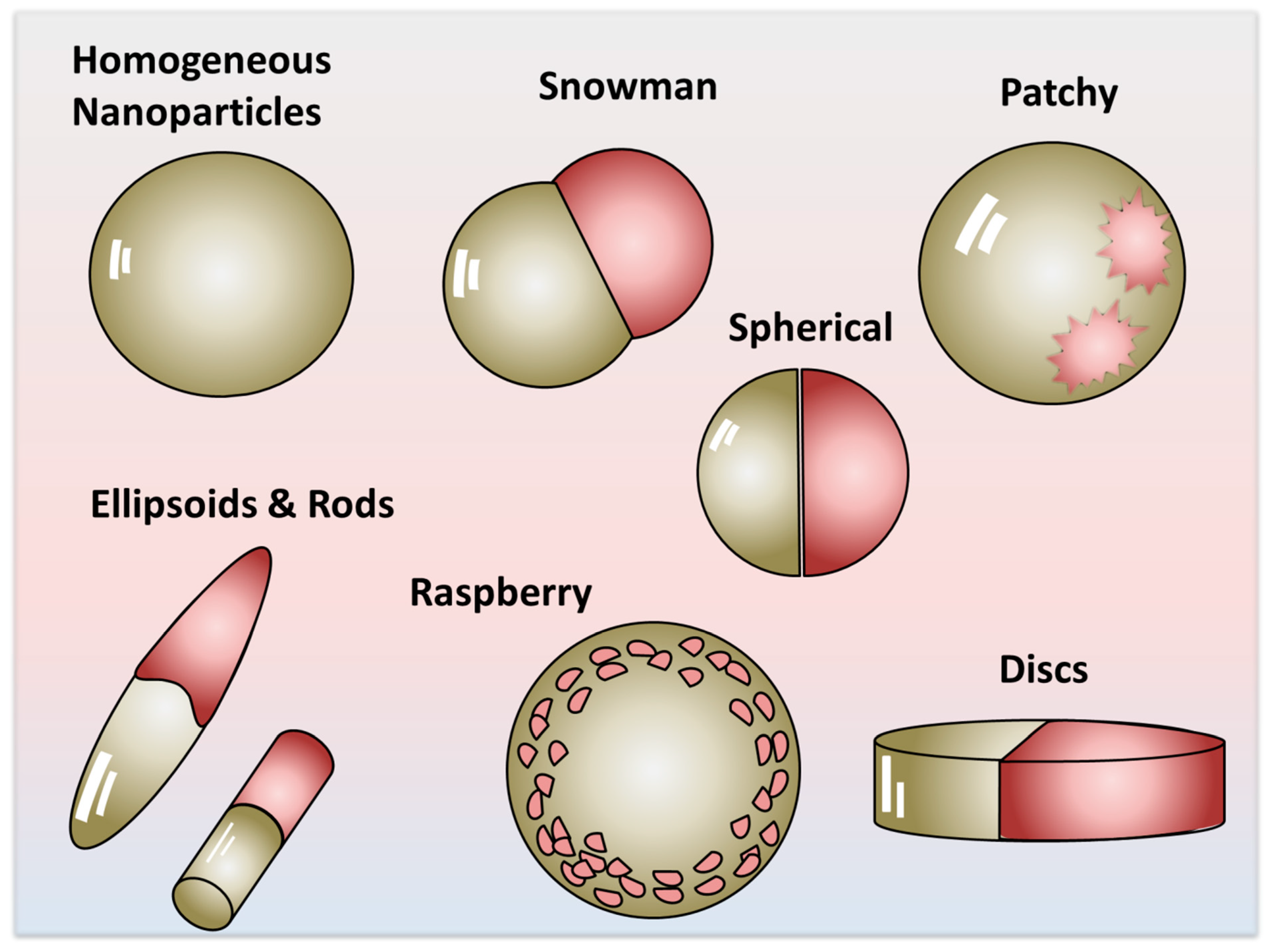
3. Definition of Amphiphilic Property and Its Manifestation
4. The Manifestation of Amphiphilicity—Scalability Beyond the Molecular Scale
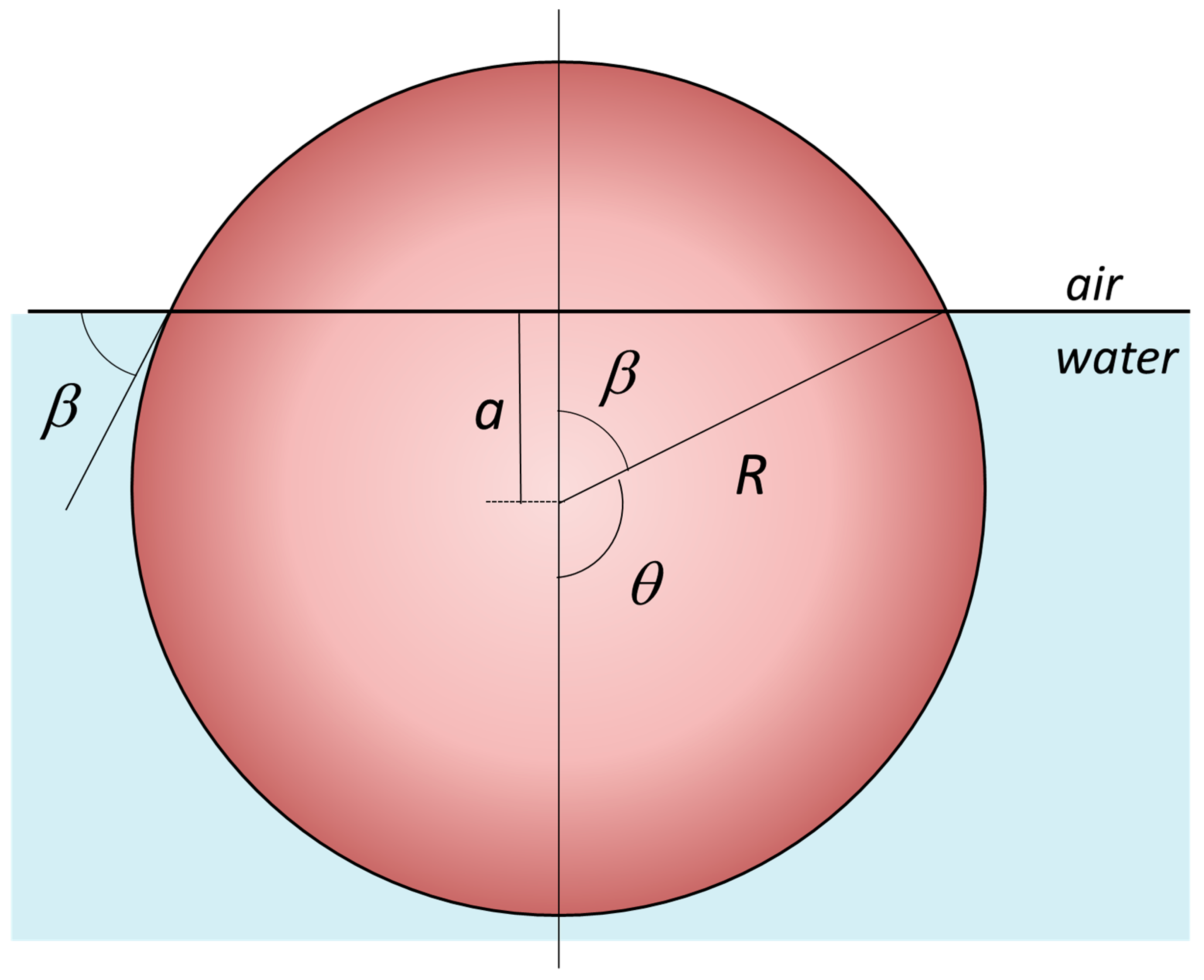
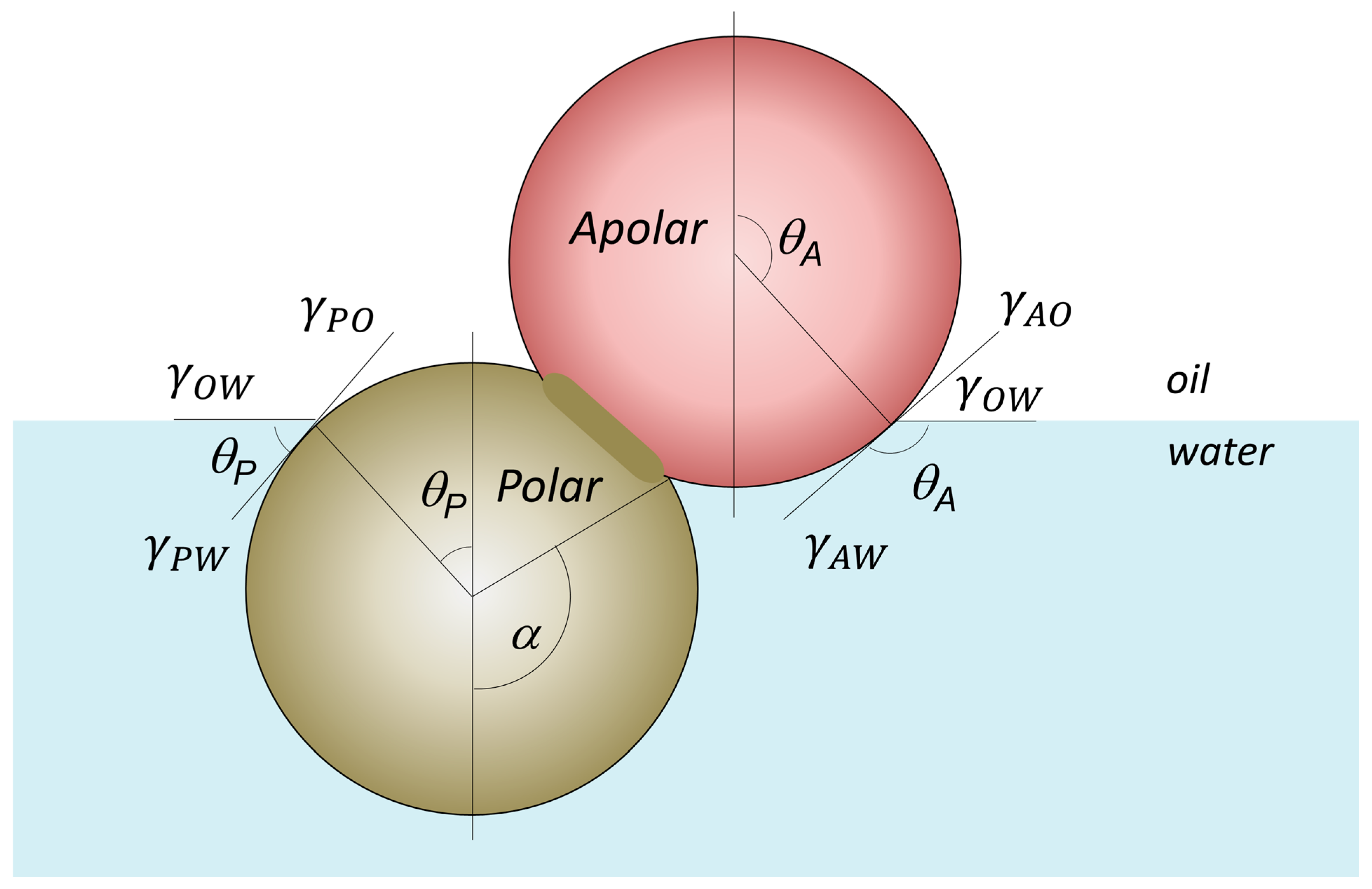
4.1. Amphiphilicity vs. Interfacial Attachment/Adsorption Energy of JNPs
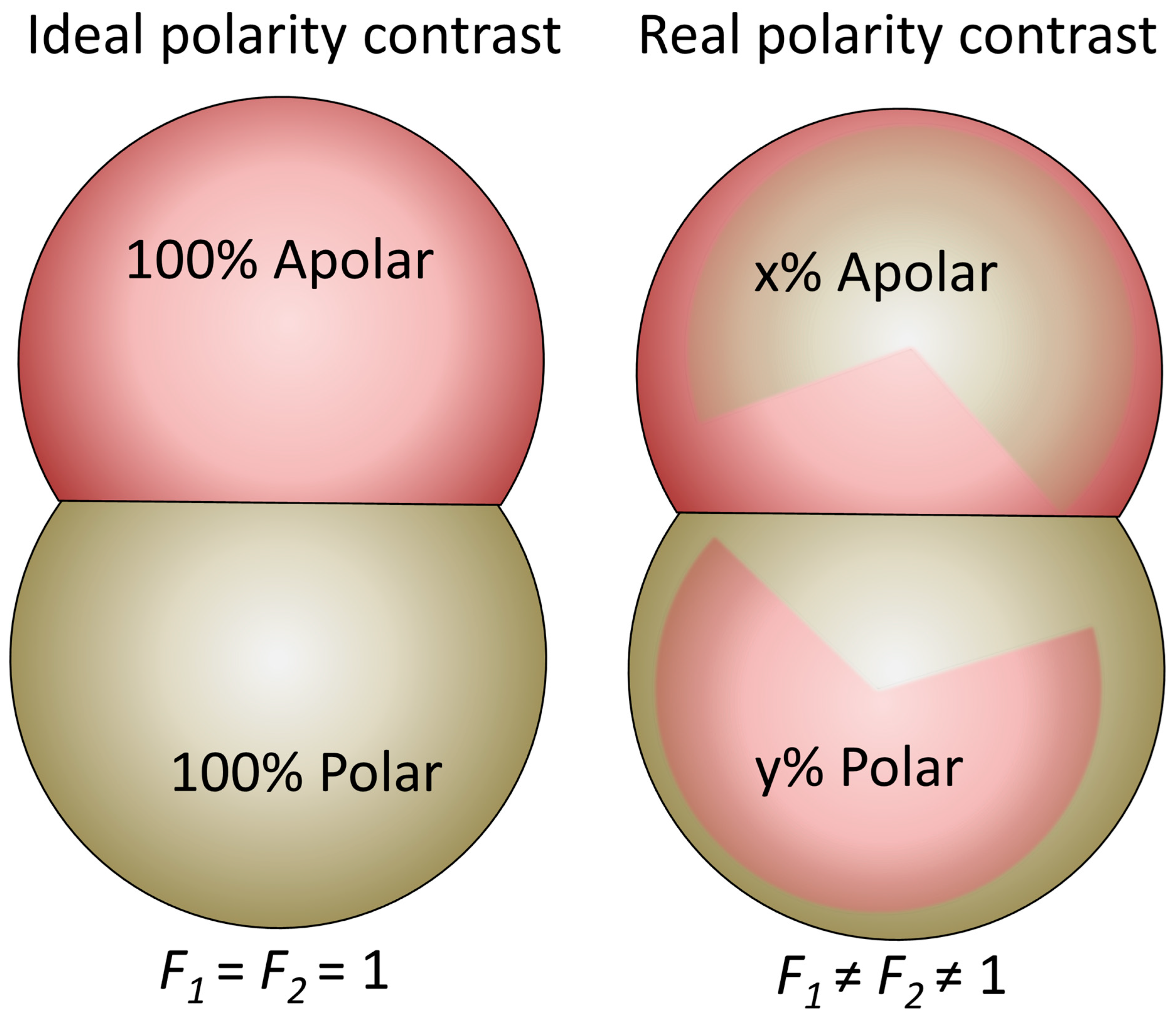
4.2. Amphiphilicity vs. Janus Balance and Polarity Contrast Between the Lobes
4.3. Amphiphilicity vs. Interfacial Tension Reduction
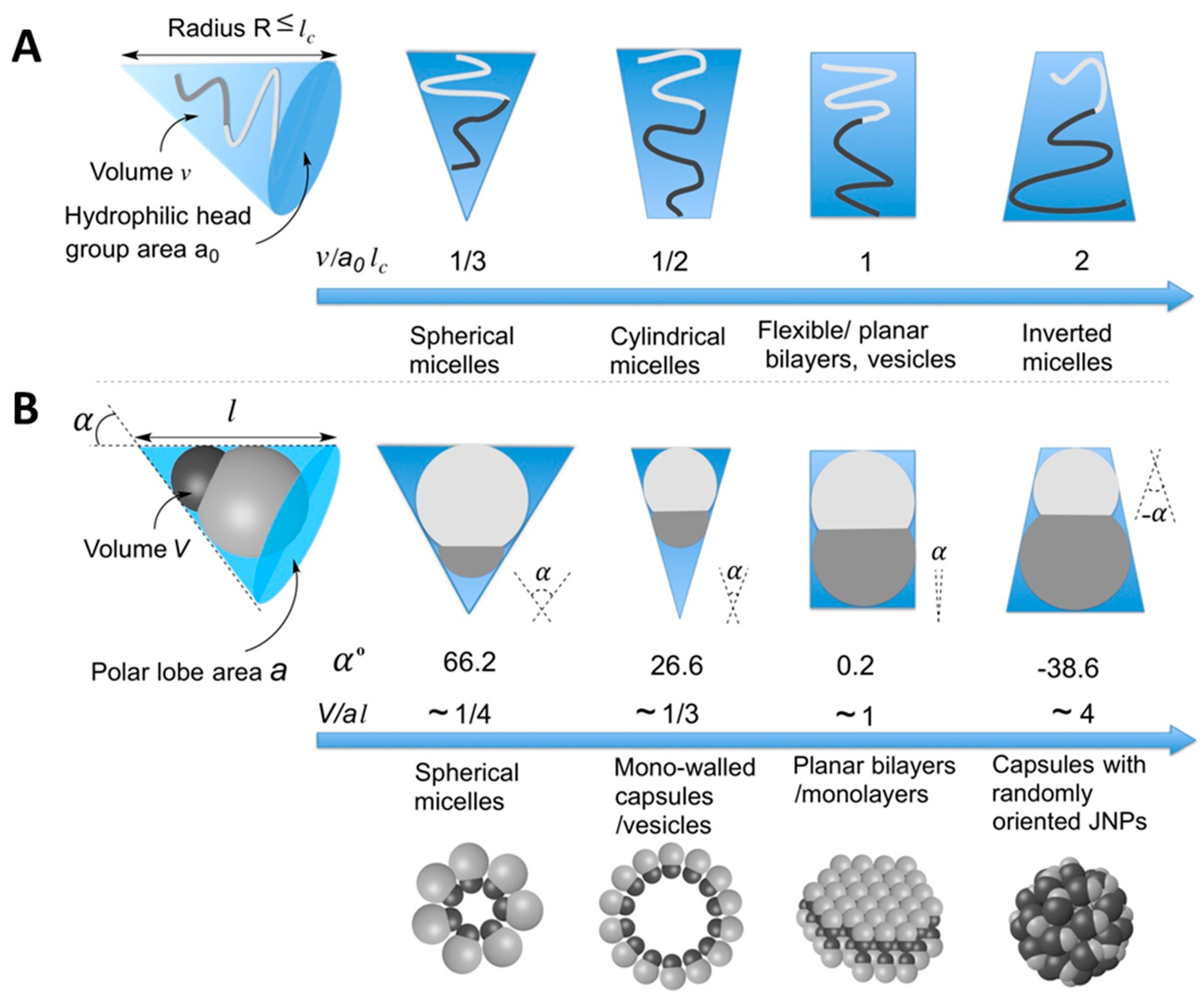
4.4. Amphiphilicity vs. Self-Assembly
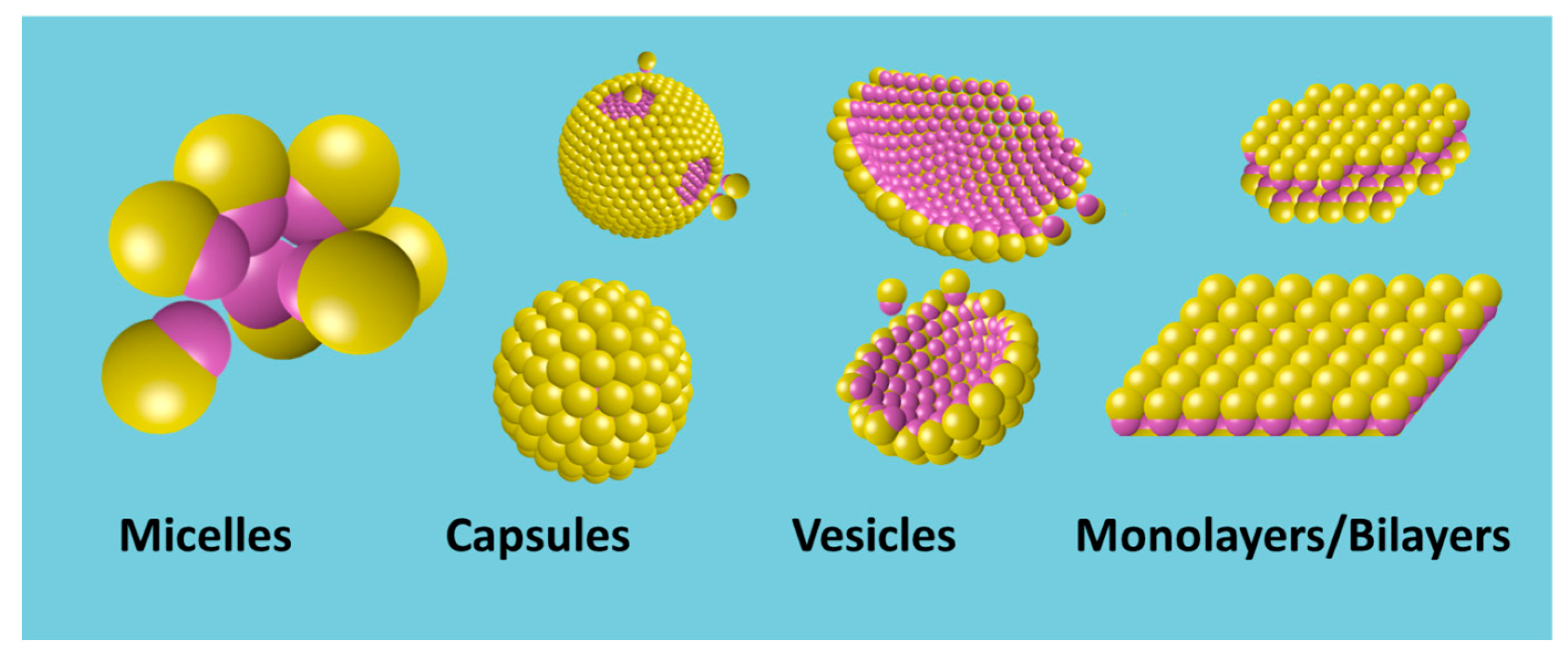
4.4.1. Formation of Supra-Structures in Homogeneous Media: From Micelles to Capsules and Vesicles
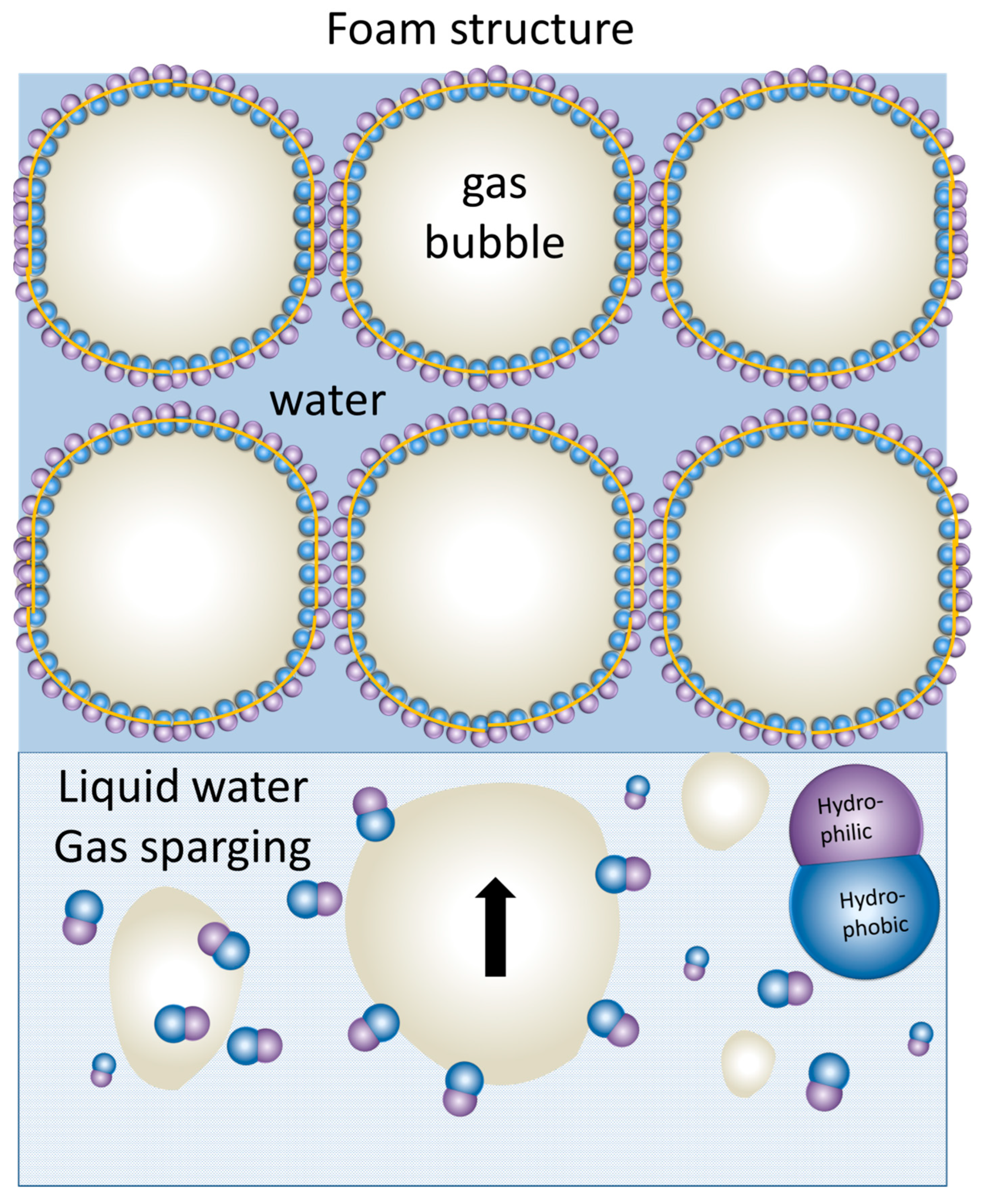
4.4.2. Templated Self-Assembly at Liquid/Air Interfaces: Foam Lamellae
4.4.3. Templated Self-Assembly at Liquid/Liquid Interfaces: Pickering Emulsions
4.4.4. Self-Assembly of JNPs in Langmuir–Blodgett Monolayers
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mihali, V.; Honciuc, A. Semiconductive Materials with Tunable Electrical Resistance and Surface Polarity Obtained by Asymmetric Functionalization of Janus Nanoparticles. Adv. Mater. Interfaces 2017, 4, 1700914. [Google Scholar] [CrossRef]
- Mihali, V.; Honciuc, A. Semiconductor–Insulator (Nano-)Couples with Tunable Properties Obtained from Asymmetric Modification of Janus Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 49206–49214. [Google Scholar] [CrossRef]
- Wu, D.; Chew, J.W.; Honciuc, A. Polarity Reversal in Homologous Series of Surfactant-Free Janus Nanoparticles: Toward the Next Generation of Amphiphiles. Langmuir 2016, 32, 6376–6386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Breed, D.R.; Manoharan, V.N.; Feng, L.; Hollingsworth, A.D.; Weck, M.; Pine, D.J. Colloids with Valence and Specific Directional Bonding. Nature 2012, 491, 51–55. [Google Scholar] [CrossRef]
- Passas-Lagos, E.; Schüth, F. Amphiphilic Pickering Emulsifiers Based on Mushroom-Type Janus Particles. Langmuir 2015, 31, 7749–7757. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; André, X.; Drechsler, M.; Abetz, V.; Müller, A.H.E. Janus Discs. J. Am. Chem. Soc. 2007, 129, 6187–6198. [Google Scholar] [CrossRef]
- Binks, B.P.; Fletcher, P.D.I. Particles Adsorbed at the Oil−Water Interface: A Theoretical Comparison between Spheres of Uniform Wettability and “Janus” Particles. Langmuir 2001, 17, 4708–4710. [Google Scholar] [CrossRef]
- Honciuc, A. Amphiphilic Janus Particles at Interfaces. In Flowing Matter; Toschi, F., Sega, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 95–136. ISBN 978-3-030-23369-3. [Google Scholar]
- Honciuc, A.; Solonaru, A.-M.; Honciuc, M. Pickering Emulsion Polymerization Technology─Toward Nanostructured Materials for Applications in Metal Ion Extractions from Wastewaters. ACS Appl. Polym. Mater. 2023, 5, 8012–8022. [Google Scholar] [CrossRef]
- Mihali, V.; Honciuc, A. Evolution of Self-Organized Microcapsules with Variable Conductivities from Self-Assembled Nanoparticles at Interfaces. ACS Nano 2019, 13, 3483–3491. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Demirci, S.; Dey, U.K.; Rawah, T.; Chaudary, A.; Ortega, R.; Yang, Z.; Pirhadi, E.; Huang, B.; et al. Two Sides of the Coin: Synthesis and Applications of Janus Particles. Nanoscale 2025, 17, 88–112. [Google Scholar] [CrossRef]
- Honciuc, A.; Solonaru, A.-M.; Honciuc, M. Water-Floating Hydrogel Polymer Microsphere Composites for Application in Hydrological Mining of Cu(II) Ions. Nanomaterials 2023, 13, 2619. [Google Scholar] [CrossRef] [PubMed]
- Honciuc, A.; Negru, O.-I. Role of Surface Energy of Nanoparticle Stabilizers in the Synthesis of Microspheres via Pickering Emulsion Polymerization. Nanomaterials 2022, 12, 995. [Google Scholar] [CrossRef]
- Fujii, S.; Nakamura, Y. Stimuli-Responsive Bubbles and Foams Stabilized with Solid Particles. Langmuir 2017, 33, 7365–7379. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Yokoyama, Y.; Nakayama, S.; Ito, M.; Yusa, S.; Nakamura, Y. Gas Bubbles Stabilized by Janus Particles with Varying Hydrophilic–Hydrophobic Surface Characteristics. Langmuir 2017, 34, 933–942. [Google Scholar] [CrossRef]
- Mihali, V.; Honciuc, A. Self-Assembly of Strongly Amphiphilic Janus Nanoparticles into Freestanding Membranes. Adv. Mater. Interfaces 2021, 9, 2101713. [Google Scholar] [CrossRef]
- Kang, C.; Honciuc, A. Self-Assembly of Janus Nanoparticles into Transformable Suprastructures. J. Phys. Chem. Lett. 2018, 9, 1415–1421. [Google Scholar] [CrossRef]
- Wu, D.; Mihali, V.; Honciuc, A. pH-Responsive Pickering Foams Generated by Surfactant-Free Soft Hydrogel Particles. Langmuir 2019, 35, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Honciuc, A. Contrasting Mechanisms of Spontaneous Adsorption at Liquid–Liquid Interfaces of Nanoparticles Constituted of and Grafted with pH-Responsive Polymers. Langmuir 2018, 34, 6170–6182. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Ma, B.; Ji, A.; Yao, H.; Chen, P.; Zhai, W.; Gao, S.; Shi, L.; Hu, H. Janus-Structured Micro/Nanomotors: Self-Propelled Mechanisms and Biomedical Applications. Biomater. Res. 2025, 29, 0155. [Google Scholar] [CrossRef]
- Asandulesa, M.; Solonaru, A.-M.; Honciuc, A. Effect of Asymmetric Lobe Size on the Molecular Dynamics, Glass Transition, and Dielectric Behavior in Janus Nanoparticles. ACS Appl. Nano Mater. 2024, 7, 3352–3360. [Google Scholar] [CrossRef]
- Mihali, V.; Jasko, P.; Skowicki, M.; Palivan, C.G. Controlled Enzymatic Reactions by Programmed Confinement in Clusters of Polymersomes and Janus Nanoparticles. Mater. Today 2024, 80, 201–217. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Y.; Zhang, Q.; Cao, J.; Lin, J.; Tong, X.; Wu, Y.; Zhu, L.; Gao, P.; Ma, J. Amphiphilic Janus Nanoparticles for a Superhydrophobic Coating on the Enamel Surface to Prevent Caries. Mater. Today Bio 2025, 32, 101627. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Fan, Q.; Liu, X.; Zhai, X.; Shi, X.; Li, W.; Hong, W. Amphiphilic Janus Nanoparticles for Effective Treatment of Bacterial Pneumonia by Attenuating Inflammation and Targeted Bactericidal Capability. IJN 2024, 19, 12039–12051. [Google Scholar] [CrossRef]
- Hao, M.; Chen, Y.; Leisen, J.; Whitworth, T.J.; Xia, Y. Multifunctional Janus Nanoparticles Capable of Anchoring to the Cell Membrane and Serving as “Cellular Backpacks” for Advanced Theranostics. J. Am. Chem. Soc. 2025, 147, 12973–12981. [Google Scholar] [CrossRef]
- He, H.; Zeng, Y.; Ma, Y.; Li, K.; Liu, H.; Yang, B.; Kan, Q.; Kang, G. Janus Nanoparticles Filled Elastomer Coating for the Improvement of the Low Velocity Impact Performance of Bio-Inspired Composite. Compos. Sci. Technol. 2025, 261, 111044. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Lv, Y.; Pei, Y.; Ding, L.; Zhang, Z.; Hu, N. Amphiphilic Janus Nanoparticles for Effective Foam Fractionation of Perfluorooctanoic Acid (PFOA): Effect of Modifier Type. Environ. Res. 2025, 283, 122186. [Google Scholar] [CrossRef] [PubMed]
- Ruan, P.; He, Y.; Yang, Q.; Luo, D. Backscattering Properties of Trigonal Ge-Au Janus Nanoparticles with Different Ambient Refractive Indices in the Visible Solar Spectrum. Optik 2025, 325, 172257. [Google Scholar] [CrossRef]
- Turpin, G.; Nguyen, D.; Sypkes, K.I.; Vega-Sánchez, C.; Davey, T.; Hawkett, B.S.; Neto, C. Encapsulation of Oil Droplets Using Film-Forming Janus Nanoparticles. Langmuir 2025, 41, 3166–3176. [Google Scholar] [CrossRef]
- Xu, K.; Yang, Z.; Li, X.; Jiang, B.; Shen, H.; Lin, M.; Dong, Z. Hydrophilic–Lipophilic Regulation by Amphiphilic Silicon-Based Janus Nanoparticles. ACS Appl. Nano Mater. 2024, 7, 23641–23651. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, J.; Sun, M.; Chen, B.; Zhou, X.; Ye, C.; Guan, Z.; Guo, W.; Wang, G.; Lu, S.; et al. Confined Growth of Silver–Copper Janus Nanostructures with {100} Facets for Highly Selective Tandem Electrocatalytic Carbon Dioxide Reduction. Adv. Mater. 2022, 34, 2110607. [Google Scholar] [CrossRef]
- Zhao, H.; Xing, Y.; Hao, W.; Fan, B.; Zhang, R. Gold–Silica Janus Nanoparticles for Multimodal Therapy against Tumors in Vitro. ChemistrySelect 2025, 10, e00231. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Li, G.; Yuan, Y.; Chang, J.; Kang, N.; Hou, J. Enhanced Oil Recovery Using Amphiphilic Nanomaterials with Tailored Functionalities: A Review. J. Mol. Liq. 2025, 425, 127190. [Google Scholar] [CrossRef]
- Tohidi, Z.; Teimouri, A.; Jafari, A.; Gharibshahi, R.; Omidkhah, M.R. Application of Janus Nanoparticles in Enhanced Oil Recovery Processes: Current Status and Future Opportunities. J. Pet. Sci. Eng. 2022, 208, 109602. [Google Scholar] [CrossRef]
- Rahiminezhad, Z.; Tamaddon, A.M.; Borandeh, S.; Abolmaali, S.S. Janus Nanoparticles: New Generation of Multifunctional Nanocarriers in Drug Delivery, Bioimaging and Theranostics. Appl. Mater. Today 2020, 18, 100513. [Google Scholar] [CrossRef]
- Rabanel, J.-M.; Adibnia, V.; Tehrani, S.F.; Sanche, S.; Hildgen, P.; Banquy, X.; Ramassamy, C. Nanoparticle Heterogeneity: An Emerging Structural Parameter Influencing Particle Fate in Biological Media? Nanoscale 2019, 11, 383–406. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, Q.; Duan, H.; Song, J.; Yang, H. Janus Nanoparticles: From Fabrication to (Bio)Applications. ACS Nano 2021, 15, 6147–6191. [Google Scholar] [CrossRef]
- Su, H.; Hurd Price, C.-A.; Jing, L.; Tian, Q.; Liu, J.; Qian, K. Janus Particles: Design, Preparation, and Biomedical Applications. Mater. Today Bio 2019, 4, 100033. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Chen, Z.; Liu, M.; Li, X.; Kou, Y.; Hou, M.; Wang, H.; Li, X.; Tian, B.; et al. Multifunctional Janus Nanoplatform for Efficiently Synergistic Theranostics of Rheumatoid Arthritis. ACS Nano 2023, 17, 8167–8182. [Google Scholar] [CrossRef]
- Duan, Z.; Han, J.; Liu, Y.; Zhao, X.; Wang, B.; Cao, S.; Wu, D. A Polymeric 1H/19F Dual-Modal MRI Contrast Agent with a Snowman-like Janus Nanostructure. J. Mater. Chem. B 2024, 12, 7090–7102. [Google Scholar] [CrossRef]
- Pauli, O.; Honciuc, A. Extraction of Metal Ions by Interfacially Active Janus Nanoparticles Supported by Wax Colloidosomes Obtained from Pickering Emulsions. Nanomaterials 2022, 12, 3738. [Google Scholar] [CrossRef]
- Kirillova, A.; Marschelke, C.; Synytska, A. Hybrid Janus Particles: Challenges and Opportunities for the Design of Active Functional Interfaces and Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 9643–9671. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Lee, K.-W. Morphology of Latex Particles Formed by Poly(Methyl Methacrylate)-Seeded Emulsion Polymerization of Styrene. J. Appl. Polym. Sci. 1985, 30, 1903–1926. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Dimonie, V.; El-Aasser, M.S. Interfacial Phenomena Controlling Particle Morphology of Composite Latexes. J. Appl. Polym. Sci. 1991, 42, 1049–1063. [Google Scholar] [CrossRef]
- Chen, Y.C.; Dimonie, V.; El-Aasser, M.S. Role of Surfactant in Composite Latex Particle Morphology. J. Appl. Polym. Sci. 1992, 45, 487–499. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Cui, D.; Jiang, W.; Han, L.; Niu, N. Preparation and Application of Janus Nanoparticles: Recent Development and Prospects. Coord. Chem. Rev. 2022, 454, 214318. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Shao, Y.; Deng, R.; Zhu, J.; Yang, Z. Recent Advances in Scalable Synthesis and Performance of Janus Polymer/Inorganic Nanocomposites. Prog. Mater. Sci. 2022, 124, 100888. [Google Scholar] [CrossRef]
- Casagrande, C.; Fabre, P.; Raphael, E.; Veyssie, M. Janus Beads—Realization and Behavior at Water Oil Interfaces. Europhys. Lett. 1989, 9, 251–255. [Google Scholar] [CrossRef]
- Casagrande, C.; Veyssie, M. «Grains Janus»: Réalisation et premières observations des propriétés interfaciales; Janus Beads: Realization and first observation of interfacial properties. Proc. Acad. Ser. II 1988, 306, 1423–1425. [Google Scholar]
- Erhardt, R.; Böker, A.; Zettl, H.; Kaya, H.; Pyckhout-Hintzen, W.; Krausch, G.; Abetz, V.; Müller, A.H.E. Janus Micelles. Macromolecules 2001, 34, 1069–1075. [Google Scholar] [CrossRef]
- Erhardt, R.; Zhang, M.; Böker, A.; Zettl, H.; Abetz, C.; Frederik, P.; Krausch, G.; Abetz, V.; Müller, A.H.E. Amphiphilic Janus Micelles with Polystyrene and Poly(Methacrylic Acid) Hemispheres. J. Am. Chem. Soc. 2003, 125, 3260–3267. [Google Scholar] [CrossRef]
- Gröschel, A.H.; Walther, A.; Löbling, T.I.; Schmelz, J.; Hanisch, A.; Schmalz, H.; Müller, A.H.E. Facile, Solution-Based Synthesis of Soft, Nanoscale Janus Particles with Tunable Janus Balance. J. Am. Chem. Soc. 2012, 134, 13850–13860. [Google Scholar] [CrossRef] [PubMed]
- Gröschel, A.H.; Walther, A.; Löbling, T.I.; Schacher, F.H.; Schmalz, H.; Müller, A.H.E. Guided Hierarchical Co-Assembly of Soft Patchy Nanoparticles. Nature 2013, 503, 247–251. [Google Scholar] [CrossRef]
- Walther, A.; Hoffmann, M.; Müller, A.H.E. Emulsion Polymerization Using Janus Particles as Stabilizers. Angew. Chem. Int. Ed. 2008, 47, 711–714. [Google Scholar] [CrossRef]
- Ruhland, T.M.; Gröschel, A.H.; Ballard, N.; Skelhon, T.S.; Walther, A.; Müller, A.H.E.; Bon, S.A.F. Influence of Janus Particle Shape on Their Interfacial Behavior at Liquid–Liquid Interfaces. Langmuir 2013, 29, 1388–1394. [Google Scholar] [CrossRef]
- Ruhland, T.M.; Gröschel, A.H.; Walther, A.; Müller, A.H.E. Janus Cylinders at Liquid–Liquid Interfaces. Langmuir 2011, 27, 9807–9814. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Synthesis, Properties and Applications of Janus Nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Walther, A.; Müller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef]
- Honciuc, A. Chemistry of Functional Materials Surfaces and Interfaces: Fundamentals and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-821059-8. [Google Scholar]
- Monnard, P.-A.; Deamer, D.W. Membrane Self-Assembly Processes: Steps Toward the First Cellular Life. In The Minimal Cell; Luisi, P.L., Stano, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 123–151. ISBN 978-90-481-9943-3. [Google Scholar]
- Israelachvili, J.N. 20-Soft and Biological Structures. In Intermolecular and Surface Forces, 3rd ed.; Academic Press: San Diego, CA, USA, 2011; pp. 535–576. [Google Scholar]
- Israelachvili, J. Self-Assembly in Two Dimensions: Surface Micelles and Domain Formation in Monolayers. Langmuir 1994, 10, 3774–3781. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhang, X. Amphiphilic Building Blocks for Self-Assembly: From Amphiphiles to Supra-Amphiphiles. Acc. Chem. Res. 2012, 45, 608–618. [Google Scholar] [CrossRef]
- Israelachvili, J.N. 17-Adhesion and Wetting Phenomena. In Intermolecular and Surface Forces: Revised; Academic Press: San Diego, CA, USA, 2011; pp. 415–467. [Google Scholar]
- Kang, C.; Honciuc, A. Influence of Geometries on the Assembly of Snowman-Shaped Janus Nanoparticles. ACS Nano 2018, 12, 3741–3750. [Google Scholar] [CrossRef]
- Griffin, C.W. Calculation of HLB Values of Non-Ionic Surfactants. J. Soc. Cosmet. Chem. 1955, 5, 249–257. [Google Scholar]
- Pasquali, R.C.; Taurozzi, M.P.; Bregni, C. Some Considerations about the Hydrophilic–Lipophilic Balance System. Int. J. Pharm. 2008, 356, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P. Particles as Surfactants—Similarities and Differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Pieranski, P. Two-Dimensional Interfacial Colloidal Crystals. Phys. Rev. Lett. 1980, 45, 569–572. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Effects of Oil Type and Aqueous Phase Composition on Oil–Water Mixtures Containing Particles of Intermediate Hydrophobicity. Phys. Chem. Chem. Phys. 2000, 2, 2959–2967. [Google Scholar] [CrossRef]
- Ondarçuhu, T.; Fabre, P.; Raphaël, E.; Veyssié, M. Specific Properties of Amphiphilic Particles at Fluid Interfaces. J. Phys. Fr. 1990, 51, 1527–1536. [Google Scholar] [CrossRef]
- Borówko, M.; Staszewski, T. Hybrid Nanoparticles at Fluid–Fluid Interfaces: Insight from Theory and Simulation. IJMS 2023, 24, 4564. [Google Scholar] [CrossRef]
- Jiang, S.; Granick, S. Controlling the Geometry (Janus Balance) of Amphiphilic Colloidal Particles. Langmuir 2008, 24, 2438–2445. [Google Scholar] [CrossRef]
- Lee, K.; Yu, Y. Lipid Bilayer Disruption by Amphiphilic Janus Nanoparticles: The Role of Janus Balance. Langmuir 2018, 34, 12387–12393. [Google Scholar] [CrossRef]
- Wu, D.; Binks, B.P.; Honciuc, A. Modeling the Interfacial Energy of Surfactant-Free Amphiphilic Janus Nanoparticles from Phase Inversion in Pickering Emulsions. Langmuir 2018, 34, 1225–1233. [Google Scholar] [CrossRef]
- Xie, S.; Chen, S.; Zhu, Q.; Li, X.; Wang, D.; Shen, S.; Jin, M.; Zhou, G.; Zhu, Y.; Shui, L. Janus Nanoparticles with Tunable Amphiphilicity for Stabilizing Pickering-Emulsion Droplets via Assembly Behavior at Oil–Water Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 26374–26383. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Cohen, A.W.; Dahanayake, M.; Hua, X.Y. Relationship of Structure to Properties in Surfactants. 10. Surface and Thermodynamic Properties of 2-Dodecyloxypoly (Ethenoxyethanol) s, C12H25 (OC2H4) xOH, in Aqueous Solution. J. Phys. Chem. 1982, 86, 541–545. [Google Scholar] [CrossRef]
- Rosen, M.J.; Li, F.; Morrall, S.W.; Versteeg, D.J. The Relationship between the Interfacial Properties of Surfactants and Their Toxicity to Aquatic Organisms. Environ. Sci. Technol. 2001, 35, 954–959. [Google Scholar] [CrossRef]
- Milton, J.R. Surfactants and Interfacial, Phenomena, 3rd ed. John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 978-0-471-67056-8.
- Inada, C.; Kobayashi, Y.; Yamakawa, M.; Kitagawa, A. Interfacial Assembly and Properties of Amphiphilic Polymer-Grafted Nanoparticles: Effect of Chemical Design and Density of Grafted Polymers. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 133921. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Nagayama, K. Capillary Interactions between Particles Bound to Interfaces, Liquid Films and Biomembranes. Adv. Colloid Interface Sci. 2000, 85, 145–192. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, N.D.; van den Ende, D.; Mugele, F.; Mellema, J. Capillary Forces between Spherical Particles Floating at a Liquid−Liquid Interface. Langmuir 2005, 21, 11190–11200. [Google Scholar] [CrossRef]
- Okubo, T. Surface Tension of Structured Colloidal Suspensions of Polystyrene and Silica Spheres at the Air-Water Interface. J. Colloid Interface Sci. 1995, 171, 55–62. [Google Scholar] [CrossRef]
- Wu, H.; Gao, K.; Lu, Y.; Meng, Z.; Gou, C.; Li, Z.; Yang, M.; Qu, M.; Liu, T.; Hou, J.; et al. Silica-Based Amphiphilic Janus Nanofluid with Improved Interfacial Properties for Enhanced Oil Recovery. Colloids Surf. A 2020, 586, 124162. [Google Scholar] [CrossRef]
- Giraldo, L.J.; Gallego, J.; Villegas, J.P.; Franco, C.A.; Cortés, F.B. Enhanced Waterflooding with NiO/SiO2 0-D Janus Nanoparticles at Low Concentration. J. Pet. Sci. Eng. 2019, 174, 40–48. [Google Scholar] [CrossRef]
- Cao, J.; Chen, Y.; Xu, G.; Wang, X.; Li, Y.; Zhao, S.; Liu, C.; Wang, X. Study on Interface Regulation Effects of Janus Nanofluid for Enhanced Oil Recovery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129880. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Yu, H.; Niu, L.; Yu, L.; Ni, D.; Zhang, Z. Functional Janus-SiO2 Nanoparticles Prepared by a Novel “Cut the Gordian Knot” Method and Their Potential Application for Enhanced Oil Recovery. ACS Appl. Mater. Interfaces 2020, 12, 24201–24208. [Google Scholar] [CrossRef] [PubMed]
- Saeedi Dehaghani, A.H.; Gharibshahi, R.; Mohammadi, M. Synthesis and Performance Analysis of Novel SiO2 Janus Nanoparticles for Enhancing Gas Foam Injection in Oil Reservoirs. Sci. Rep. 2025, 15, 2994. [Google Scholar] [CrossRef] [PubMed]
- Saeedi Dehaghani, A.H.; Gharibshahi, R.; Mohammadi, M. Utilization of Synthesized Silane-Based Silica Janus Nanoparticles to Improve Foam Stability Applicable in Oil Production: Static Study. Sci. Rep. 2023, 13, 18652. [Google Scholar] [CrossRef]
- Tang, S.; Sun, Z.; Dong, Y.; Zhu, Y.; Hu, H.; Wang, R.; Liao, H.; Dai, Q. Preparation of Amphiphilic Janus-SiO2 Nanoparticles and Evaluation of the Oil Displacement Effect. ACS Omega 2024, 9, 5838–5845. [Google Scholar] [CrossRef]
- McGlasson, A.; Morgenthaler, E.; Bradley, L.C.; Russell, T.P. On the Interfacial Assembly of Anisotropic Amphiphilic Janus Particles. Adv. Funct. Mater. 2024, 34, 2306651. [Google Scholar] [CrossRef]
- Wu, D.; Honciuc, A. Design of Janus Nanoparticles with pH-Triggered Switchable Amphiphilicity for Interfacial Applications. ACS Appl. Nano Mater. 2018, 34, 1225–1233. [Google Scholar] [CrossRef]
- Jiang, Y.; Löbling, T.I.; Huang, C.; Sun, Z.; Müller, A.H.E.; Russell, T.P. Interfacial Assembly and Jamming Behavior of Polymeric Janus Particles at Liquid Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 33327–33332. [Google Scholar] [CrossRef]
- Schröder, J.H.; Doroshenko, M.; Pirner, D.; Mauer, M.E.J.; Förster, B.; Boyko, V.; Reck, B.; Roschmann, K.J.; Müller, A.H.E.; Förster, S. Interfacial Stabilization by Soft Janus Nanoparticles. Polymer 2016, 106, 208–217. [Google Scholar] [CrossRef]
- Yang, F.; He, X.; Tan, W.; Liu, G.; Yi, T.; Lu, Q.; Wei, X.; Xie, H.; Long, Q.; Wang, G.; et al. Adhesion-Shielding Based Synthesis of Interfacially Active Magnetic Janus Nanoparticles. J. Colloid Interface Sci. 2022, 607, 1741–1753. [Google Scholar] [CrossRef]
- Sashuk, V.; Hołyst, R.; Wojciechowski, T.; Fiałkowski, M. Close-Packed Monolayers of Charged Janus-Type Nanoparticles at the Air–Water Interface. J. Colloid Interface Sci. 2012, 375, 180–186. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, M.A.; Ramos, J.; Isa, L.; Rodriguez-Valverde, M.A.; Cabrerizo-Vilchez, M.A.; Hidalgo-Alvarez, R. Interfacial Activity and Contact Angle of Homogeneous, Functionalized, and Janus Nanoparticles at the Water/Decane Interface. Langmuir 2015, 31, 8818–8823. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rodriguez, M.A.; Song, Y.; Rodríguez-Valverde, M.Á.; Chen, S.; Cabrerizo-Vilchez, M.A.; Hidalgo-Alvarez, R. Comparison of the Interfacial Activity between Homogeneous and Janus Gold Nanoparticles by Pendant Drop Tensiometry. Langmuir 2014, 30, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Correia, E.L.; Brown, N.; Razavi, S. Janus Particles at Fluid Interfaces: Stability and Interfacial Rheology. Nanomaterials 2021, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, F.; Zhu, J.; Cao, F.; Liu, Y.; Li, X.; Willson, R.C.; Yang, Z.; Chu, C.-W.; Ren, Z. Nanofluid of Graphene-Based Amphiphilic Janus Nanosheets for Tertiary or Enhanced Oil Recovery: High Performance at Low Concentration. Proc. Natl. Acad. Sci. USA 2016, 113, 7711–7716. [Google Scholar] [CrossRef]
- Deng, K.; Luo, Z.; Tan, L.; Quan, Z. Self-Assembly of Anisotropic Nanoparticles into Functional Superstructures. Chem. Soc. Rev. 2020, 49, 6002–6038. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Janus Nanoparticles: Recent Advances in Their Interfacial and Biomedical Applications. ACS Appl. Nano Mater. 2019, 2, 1738–1757. [Google Scholar] [CrossRef]
- Garbuzenko, O.B.; Winkler, J.; Tomassone, M.S.; Minko, T. Biodegradable Janus Nanoparticles for Local Pulmonary Delivery of Hydrophilic and Hydrophobic Molecules to the Lungs. Langmuir 2014, 30, 12941–12949. [Google Scholar] [CrossRef]
- Li, B.; Wang, M.; Chen, K.; Cheng, Z.; Chen, G.; Zhang, Z. Synthesis of Biofunctional Janus Particles. Macromol. Rapid Commun. 2015, 36, 1200–1204. Available online: http://onlinelibrary.wiley.com/doi/10.1002/marc.201500063/full (accessed on 21 September 2015). [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer Vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef]
- Jain, S.; Bates, F.S. On the Origins of Morphological Complexity in Block Copolymer Surfactants. Science 2003, 300, 460–464. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-Assembly and Mineralization of Peptide-Amphiphile Nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R. Molecular Packing Parameter and Surfactant Self-Assembly: The Neglected Role of the Surfactant Tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Hong, L.; Cacciuto, A.; Luijten, E.; Granick, S. Clusters of Amphiphilic Colloidal Spheres. Langmuir 2008, 24, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Borówko, M.; Staszewski, T.; Tomasik, J. Janus Ligand-Tethered Nanoparticles at Liquid–Liquid Interfaces. J. Phys. Chem. B 2023, 127, 5150–5161. [Google Scholar] [CrossRef]
- Baran, Ł.; Borówko, M.; Rżysko, W.; Smołka, J. Amphiphilic Janus Particles Confined in Symmetrical and Janus-Like Slits. ACS Omega 2023, 8, 18863–18873. [Google Scholar] [CrossRef]
- Baran, Ł.; Borówko, M.; Rżysko, W. Self-Assembly of Amphiphilic Janus Particles Confined between Two Solid Surfaces. J. Phys. Chem. C 2020, 124, 17556–17565. [Google Scholar] [CrossRef]
- Liu, F.; Goyal, S.; Forrester, M.; Ma, T.; Miller, K.; Mansoorieh, Y.; Henjum, J.; Zhou, L.; Cochran, E.; Jiang, S. Self-Assembly of Janus Dumbbell Nanocrystals and Their Enhanced Surface Plasmon Resonance. Nano Lett. 2019, 19, 1587–1594. [Google Scholar] [CrossRef]
- Hu, H.; Ji, F.; Xu, Y.; Yu, J.; Liu, Q.; Chen, L.; Chen, Q.; Wen, P.; Lifshitz, Y.; Wang, Y.; et al. Reversible and Precise Self-Assembly of Janus Metal-Organosilica Nanoparticles through a Linker-Free Approach. ACS Nano 2016, 10, 7323–7330. [Google Scholar] [CrossRef]
- Kirillova, A.; Stoychev, G.; Ionov, L.; Synytska, A. Self-Assembly Behavior of Hairy Colloidal Particles with Different Architectures: Mixed versus Janus. Langmuir 2014, 30, 12765–12774. [Google Scholar] [CrossRef]
- Gao, W.; Pei, A.; Feng, X.; Hennessy, C.; Wang, J. Organized Self-Assembly of Janus Micromotors with Hydrophobic Hemispheres. J. Am. Chem. Soc. 2013, 135, 998–1001. [Google Scholar] [CrossRef]
- Nie, Z.; Li, W.; Seo, M.; Xu, S.; Kumacheva, E. Janus and Ternary Particles Generated by Microfluidic Synthesis: Design, Synthesis, and Self-Assembly. J. Am. Chem. Soc. 2006, 128, 9408–9412. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Brett, G.; Gunton, J.D. Encapsulation by Janus Spheroids. Soft Matter 2012, 8, 6027–6032. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Perez, T.; Gunton, J.D.; Brett, G. Self Assembly of Janus Ellipsoids. Langmuir 2012, 28, 3–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, W.; Gunton, J.D. Self-Assembly of Janus Ellipsoids II: Janus Prolate Spheroids. Langmuir 2013, 29, 8517–8523. [Google Scholar] [CrossRef]
- Shin, H.; Schweizer, K.S. Theory of Two-Dimensional Self-Assembly of Janus Colloids: Crystallization and Orientational Ordering. Soft Matter 2013, 10, 262–274. [Google Scholar] [CrossRef]
- Whitelam, S.; Bon, S.A.F. Self-Assembly of Amphiphilic Peanut-Shaped Nanoparticles. J. Chem. Phys. 2010, 132, 074901. [Google Scholar] [CrossRef]
- Tsonchev, S.; Schatz, G.C.; Ratner, M.A. Hydrophobically-Driven Self-Assembly: A Geometric Packing Analysis. Nano Lett. 2003, 3, 623–626. [Google Scholar] [CrossRef]
- Kraft, D.J.; Ni, R.; Smallenburg, F.; Hermes, M.; Yoon, K.; Weitz, D.A.; van Blaaderen, A.; Groenewold, J.; Dijkstra, M.; Kegel, W.K. Surface Roughness Directed Self-Assembly of Patchy Particles into Colloidal Micelles. Proc. Natl. Acad. Sci. USA 2012, 109, 10787–10792. [Google Scholar] [CrossRef]
- Chen, Q.; Bae, S.C.; Granick, S. Directed Self-Assembly of a Colloidal Kagome Lattice. Nature 2011, 469, 381–384. [Google Scholar] [CrossRef]
- Chen, Q.; Diesel, E.; Whitmer, J.K.; Bae, S.C.; Luijten, E.; Granick, S. Triblock Colloids for Directed Self-Assembly. J. Am. Chem. Soc. 2011, 133, 7725–7727. [Google Scholar] [CrossRef]
- Kang, C.; Honciuc, A. Versatile Triblock Janus Nanoparticles: Synthesis and Self-Assembly. Chem. Mater. 2019, 31, 1688–1695. [Google Scholar] [CrossRef]
- Kraynik, A.M. Foam Structure: From Soap Froth to Solid Foams. MRS Bull. 2003, 28, 275–278. [Google Scholar] [CrossRef]
- Blute, I.; Pugh, R.J.; van de Pas, J.; Callaghan, I. Industrial Manufactured Silica Nanoparticle Sols. 2: Surface Tension, Particle Concentration, Foam Generation and Stability. Colloids Surf. A: Physicochem. Eng. Asp. 2009, 337, 127–135. [Google Scholar] [CrossRef]
- Fameau, A.-L.; Salonen, A. Effect of Particles and Aggregated Structures on the Foam Stability and Aging. Comptes Rendus. Phys. 2014, 15, 748–760. [Google Scholar] [CrossRef]
- Wang, K.; Wang, G.; Lu, C.; Wang, Y. Preparation of Amphiphilic Janus Particles and Their Application in Stabilising Foams. Micro. Nano Lett. 2018, 13, 397–402. [Google Scholar] [CrossRef]
- Finkle, P.; Draper, H.D.; Hildebrand, J.H. The Theory of Emulsification. J. Am. Chem. Soc. 1923, 45, 2780–2788. [Google Scholar] [CrossRef]
- Aveyard, R. Can Janus Particles Give Thermodynamically Stable Pickering Emulsions? Soft Matter 2012, 8, 5233–5240. [Google Scholar] [CrossRef]
- Honciuc, A.; Negru, O.-I. NanoTraPPED—A New Method for Determining the Surface Energy of Nanoparticles via Pickering Emulsion Polymerization. Nanomaterials 2021, 11, 3200. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Mori, T.; Hill, J.P. 25th Anniversary Article: What Can Be Done with the Langmuir-Blodgett Method? Recent Developments and Its Critical Role in Materials Science. Adv. Mater. 2013, 25, 6477–6512. [Google Scholar] [CrossRef]
- Bardosova, M.; Pemble, M.E.; Povey, I.M.; Tredgold, R.H. The Langmuir-Blodgett Approach to Making Colloidal Photonic Crystals from Silica Spheres. Adv. Mater. 2010, 22, 3104–3124. [Google Scholar] [CrossRef]
- Parchine, M.; McGrath, J.; Bardosova, M.; Pemble, M.E. Large Area 2D and 3D Colloidal Photonic Crystals Fabricated by a Roll-to-Roll Langmuir–Blodgett Method. Langmuir 2016, 32, 5862–5869. [Google Scholar] [CrossRef] [PubMed]
- Honciuc, A.; Negru, O.-I. Asymmetrically Nanostructured 2D Janus Films Obtained from Pickering Emulsions Polymerized in a Langmuir–Blodgett Trough. Micromachines 2023, 14, 1459. [Google Scholar] [CrossRef] [PubMed]
- Lenis, J.; Razavi, S.; Cao, K.D.; Lin, B.; Lee, K.Y.C.; Tu, R.S.; Kretzschmar, I. Mechanical Stability of Polystyrene and Janus Particle Monolayers at the Air/Water Interface. J. Am. Chem. Soc. 2015, 137, 15370–15373. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Song, L.; Lin, H.; Yang, Y.; Huang, Y.; Su, F.; Chen, T. Free-Standing 2D Janus Gold Nanoparticles Monolayer Film with Tunable Bifacial Morphologies via the Asymmetric Growth at Air–Liquid Interface. Langmuir 2020, 36, 250–256. [Google Scholar] [CrossRef]

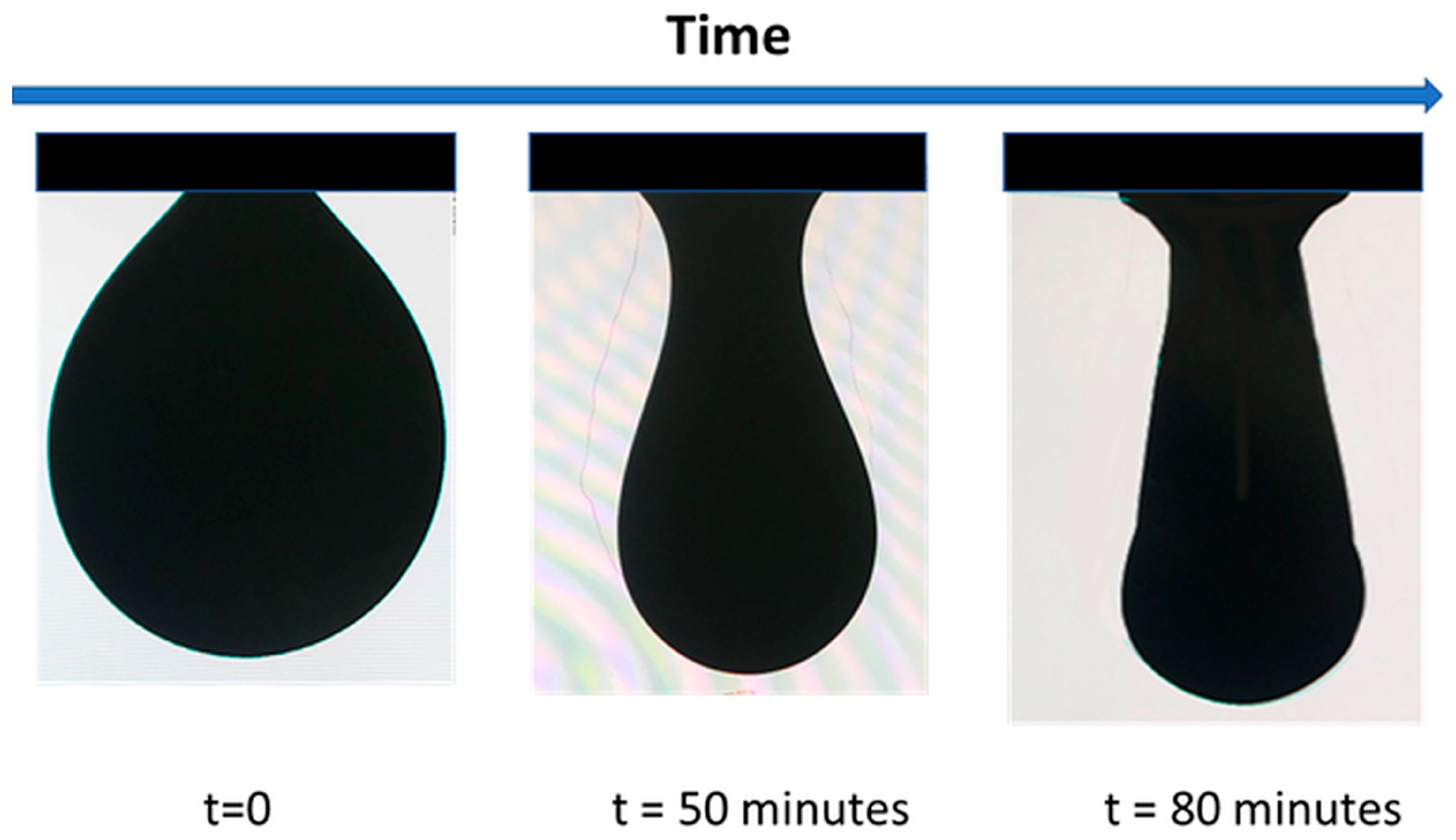


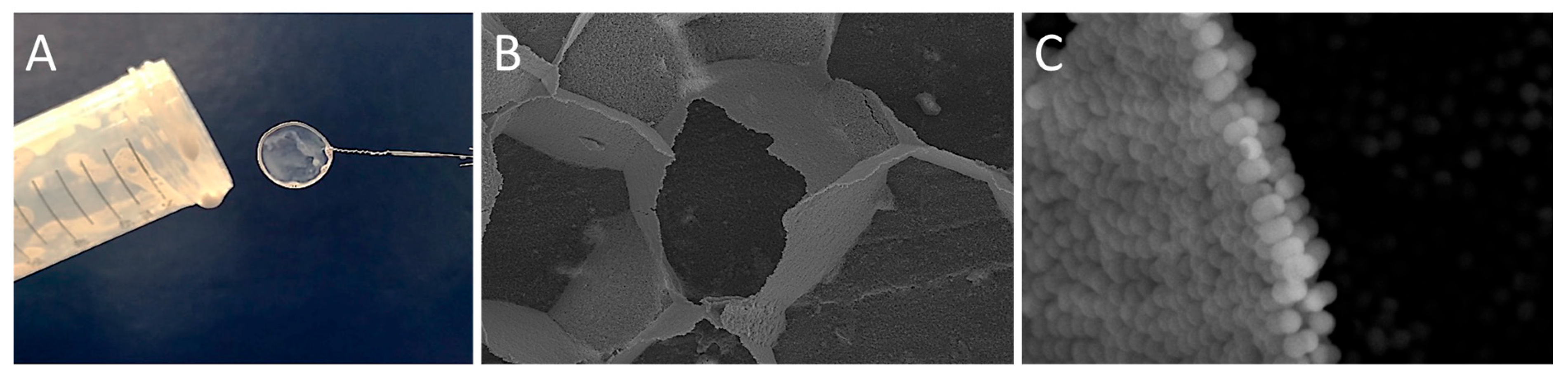

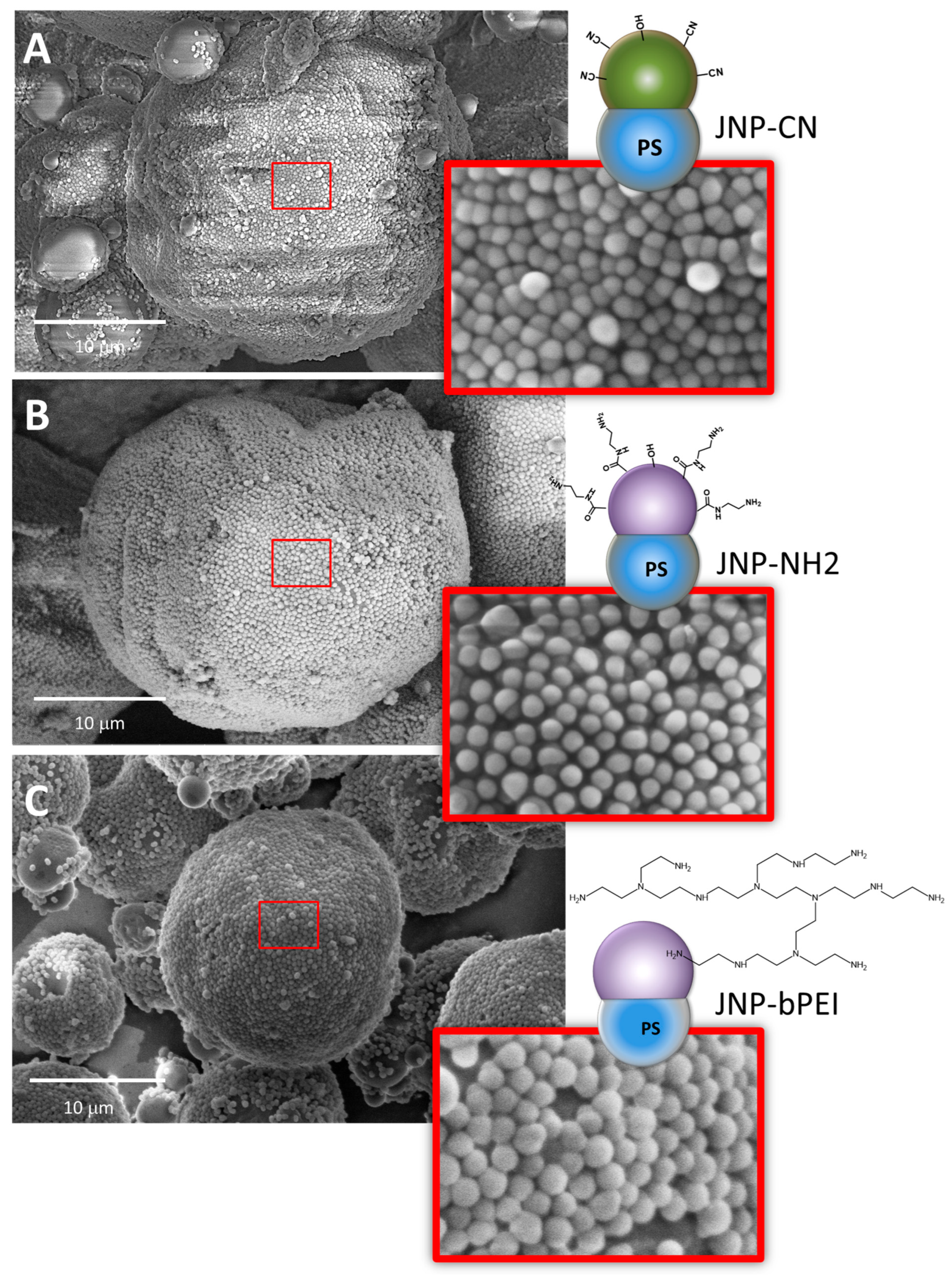

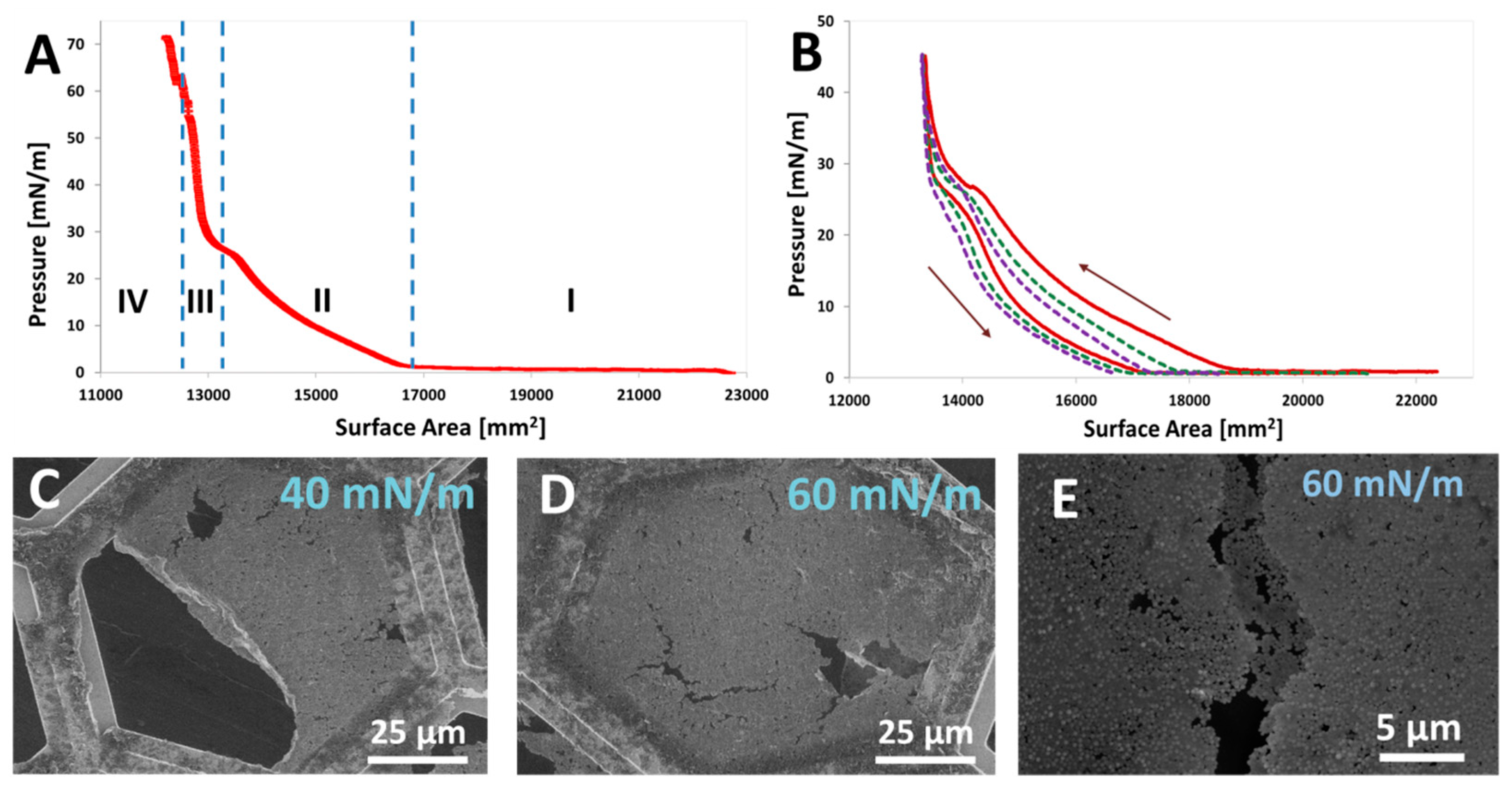
| * Mass Ratio Between the Lobes in (CPSAA/PS) JNPs | Area PS Lobe (×103 nm2) | Area CPSAA Lobe (×103 nm2) | APS/ACPSAA | a HLB | b F1 | b F2 | c HLB (Weighted) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 70.65 | 0 | 20 | 1 | 1 | 20 |
| 0.5 | 4.04 | 107.87 | 0.0314 | 19.3 | 1 | 1 | 19.3 |
| 1 | 14.02 | 143 | 0.0981 | 18.2 | 1 | 1 | 18.2 |
| 3 | 44.26 | 247.04 | 0.1791 | 16.9 | 1 | 1 | 16.9 |
| 5 | 108.37 | 232.31 | 0.4665 | 13.6 | 1 | 1 | 13.6 |
| 7 | 125.6 | 213.97 | 0.5871 | 12.5 | 1 | 1 | 12.5 |
| 10 | 138.7 | 197.83 | 0.6984 | 11.7 | 1 | 1 | 11.7 |
| ** JNP in the order of increasing of the P(3-TSPM) polar lobe | a Area PS lobe (×103 nm2) | a Area P(3-TSPM) lobe (×103 nm2) | JNPs aspect ratio P(3-TSPM)/PS | b HLB | b F1 | b F2 | c HLB (weighted) |
| PS/P(0.5 mL 3-TSPM) | 44.6 | 15.5 | 0.35 | 5 | 1 | 1 | 5 |
| PS/P(1 mL 3-TSPM) | 41.5 | 18.5 | 0.45 | 6 | 1 | 1 | 6 |
| PS/P(2 mL 3-TSPM) | 40.4 | 54.1 | 1.34 | 11 | 1 | 1 | 11 |
| PS/P(3 mL 3-TSPM) | 38.7 | 60.4 | 1.56 | 12 | 1 | 1 | 12 |
| PS/P(4 mL 3-TSPM) | 32.6 | 119.4 | 3.66 | 16 | 1 | 1 | 16 |
| *** JNPs in the order of increasing of the P(3-TSPM) polar lobe | a Area PPy-Lobe (×103 nm2) | a Area P(3-TSPM) Lobe (×103 nm2) | Aspect ratio P(3-TSPM)/PPy | a HLB | b F1 | b F2 | c HLB (weighted) |
| PPy/P(1 mL 3-TSPM) | 177.6 | 83.2 | 0.5 | 6 | 0.93 | 0.92 | 6 |
| PPy/P(2 mL 3-TSPM) | 211.8 | 172.9 | 0.8 | 9 | 0.93 | 0.92 | 9 |
| PPy/P(3 mL 3-TSPM) | 164.1 | 318.9 | 1.9 | 13 | 0.93 | 0.92 | 13 |
| PPy/P(4 mL 3-TSPM) | 152.7 | 348.7 | 2.3 | 14 | 0.93 | 0.92 | 14 |
| Janus Nanoparticle Type | Interface | Concentration | Interfacial Tension (IFT) Reduction | Reference |
|---|---|---|---|---|
| Silica JNPs obtained by functionalization of hemispheres | Oil/water | 0.05 wt% | Reduction of IFT to 2.28 mN/m | [84] |
| Silica JNPs obtained by functionalization of hemispheres with oleic acid | Water/air | ΔIFT = 25 mN/m, greater than bare SiO2 | [88] | |
| Silica JNPs obtained by functionalization of hemispheres with HMDS/APTS | Water/air | IFT reduced to 58–59 mN/m | [89] | |
| Silica Janus nanoparticles | Paraffin oil/water | IFT reduced from 31.3 to 10.17 mN/m | [30] | |
| Silica JNPs obtained by functionalization of hemispheres with octyl and amino groups | Water/air | 0.05 wt% | IFT reduced by JNPs to 36.4 mN/m, greater than that of silica HNPs of 69.8 mN/m | [90] |
| Silica JNPs obtained by functionalization of hemispheres with octyl and amino groups | Paraffin oil/water | 0.05 wt% | IFT reduced by JNPs to 0.067 mN/m, greater than that of silica HNPs of 28.4 mN/m | [90] |
| Solid polymer PS/PtBA JNPs later hydrolyzed to JNP–COOH by cleaving the tBA groups. | Toluene/water | 10 mg/mL | 27.9 mN/m | [91] |
| Solid polymer JNPs, PS− PDIPAEMA/P(3-TSPM) JNPs | Toluene/water | ΔIFT = 11.7 mN/m by JNPs, vs. ΔIFT = 4.0 for the PS− PDIPAEMA HNPs with the same composition as one of the JNP lobes; P(3-TSPM) HNPs not interfacially active | [92] | |
| Solid polymer JNPs, PS− PDIPAEMA/P(3-TSPM) JNPs | Heptane/water | ΔIFT = 15.3 mN/m by JNPs, vs. ΔIFT = 9.1 mN/m for the PS− PDIPAEMA HNPs with the same composition as one of the JNP lobes; P(3-TSPM) HNPs not interfacially active | [92] | |
| Solid polymer JNPs, PS− PDIPAEMA/P(3-TSPM) JNPs | Water/air | ΔIFT = 3.8 mN/m by JNPs, vs. 2.7 mN/m for the PS− PDIPAEMA HNPs with the same composition as one of the JNP lobes; P(3-TSPM) HNPs not interfacially active | [92] | |
| Solid polymer JNPs, obtained by seeded emulsion polymerization PS/P(3-TSPM)+P(3-TESPN) JNPs | Water/air | Variable reduction in the water surface tension with increase in the lobe size of the snowman-type JNPs | [16] | |
| Soft polymer JNPs, made by crosslinking polystyreneblock-polybutadiene-block-poly(methyl methacrylate) (PS-PB-PMMA) | Toluene/water | 1 mg/mL | Reduction in the IFT from 34 mN/m to ~19 mN/m. | [93] |
| Soft polymer JNPs from polystyrene-block-polybutadiene-block-poly(methyl methacrylate) | Toluene/water | 1 mg/mL | Reduction in the IFT as a function of the nanoparticle shape to 14 mN/m for cylinders, to 17.5 mN for spheres, to 19 mN/m for disks | [55] |
| Soft JNPs obtained by selective cross-linking of the polyisoprene domain in the triblock copolymer polystyrene-b-polyisoprene-b-poly(tert-butyl methacrylate) (PS-PI-PtBMA) triblock terpolymers | Water/air | 10 mg/mL | Reduction in the IFT from 72 to 63 mN/m | [94] |
| Soft JNPs obtained by selective cross-linking of the polyisoprene domain in the triblock copolymer polystyrene-b-polyisoprene-b-poly(tert-butyl methacrylate) (PS-PI-PtBMA) triblock terpolymers | Reduction in IFT depends on the HLB value; the largest IFT reduction is for JNPs with lowest HLB, namely ΔIFT = 26 mN/m for HLB = 6 vs. ΔIFT = 10 mN/m for the JNPs with HLB = 6.5 | [94] | ||
| Metal oxide Fe3O4 JNPs, capped with hydrophobic C18, C16, C12 alkyl capped magnetic on one side. | Toluene/water | Reduction in the IFT function of the chain length of the capping layer 26.52 ± 0.13 mN/m, in comparison with 27.02 ± 0.07 mN/m and 30.38 ± 0.49 mN/m | [95] | |
| Metallic Au and Ag JNPs obtained by ligand exchange of dodecylamine and decanoic acid with 11-mercaptoundecanoic acid and 1-undecanthiol | Water/air | Strong reduction in the water/air surface tension observed | [96] | |
| Metallic Ag JNPs with 11mercaptoundecanoic acid and 1-undecanthiol capping agents | Water/air Decane/water | DIFTwater/air = 8 mN/m DIFTdecane/water = 9.1 mN/m The interfacial activity of Ag-JNPs was significantly higher compared to silica and PMMA-HNPs of the same size | [97] | |
| Metallic Au JNPs of 3.5 nm diameter (capped with hydrophobic hexanethiolates on one hemisphere and hydrophilic 2-(2mercapto-ethoxy)ethanol on the other) | Water/air | n = 17 × 1012/5 μL | IFT decreased from 72 to 59.50 mN/m | [98] |
| Gold–iron oxide Janus nanoparticles | Hexane/water | Review listing findings on greater reduction in IFT by JNPs than HNPs | [99] | |
| Graphene-based amphiphilic Janus nanosheets | Oil/water | Significant changes in IFT observed | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honciuc, M.; Honciuc, A. Scaling Amphiphilicity with Janus Nanoparticles: A New Frontier in Nanomaterials and Interface Science. Nanomaterials 2025, 15, 1079. https://doi.org/10.3390/nano15141079
Honciuc M, Honciuc A. Scaling Amphiphilicity with Janus Nanoparticles: A New Frontier in Nanomaterials and Interface Science. Nanomaterials. 2025; 15(14):1079. https://doi.org/10.3390/nano15141079
Chicago/Turabian StyleHonciuc, Mirela, and Andrei Honciuc. 2025. "Scaling Amphiphilicity with Janus Nanoparticles: A New Frontier in Nanomaterials and Interface Science" Nanomaterials 15, no. 14: 1079. https://doi.org/10.3390/nano15141079
APA StyleHonciuc, M., & Honciuc, A. (2025). Scaling Amphiphilicity with Janus Nanoparticles: A New Frontier in Nanomaterials and Interface Science. Nanomaterials, 15(14), 1079. https://doi.org/10.3390/nano15141079








