Thin Films of Tungsten Disulfide Grown by Sulfurization of Sputtered Metal for Ultra-Low Detection of Nitrogen Dioxide Gas
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis and Characterization
2.2. Sensors Fabrication and Tests
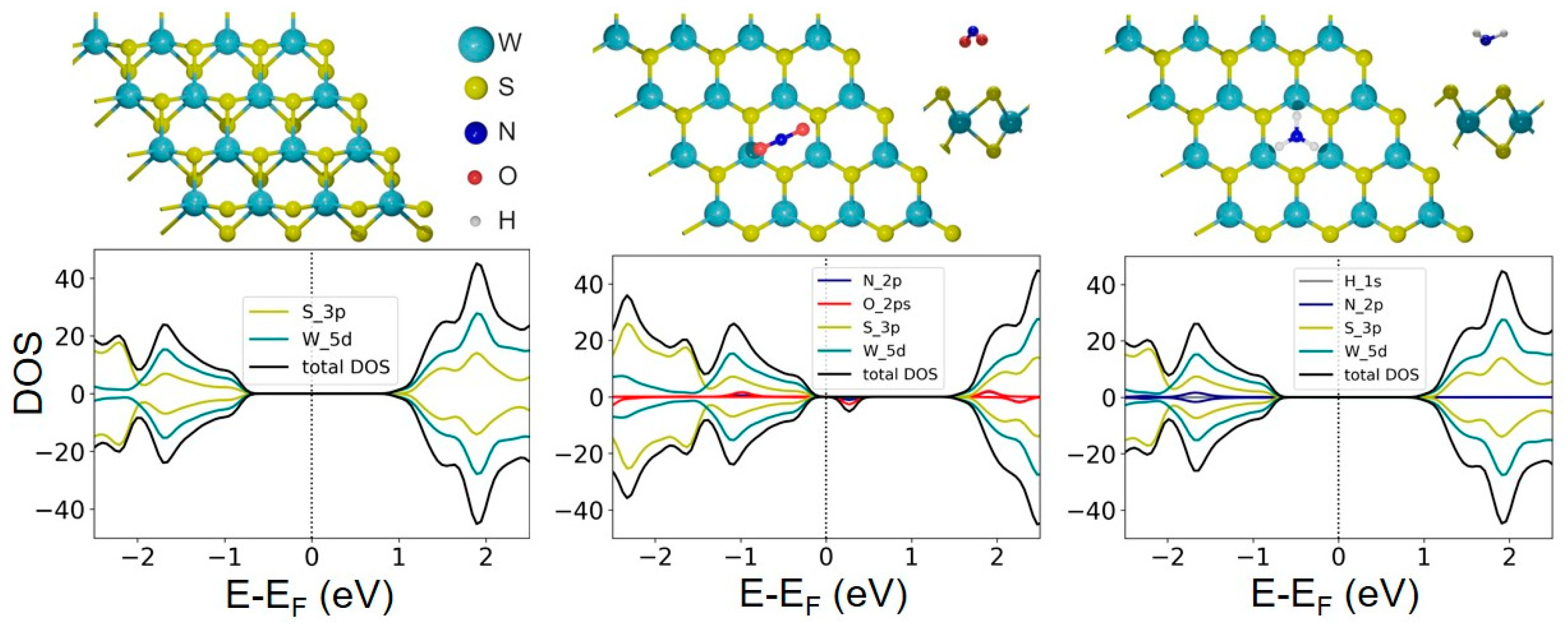
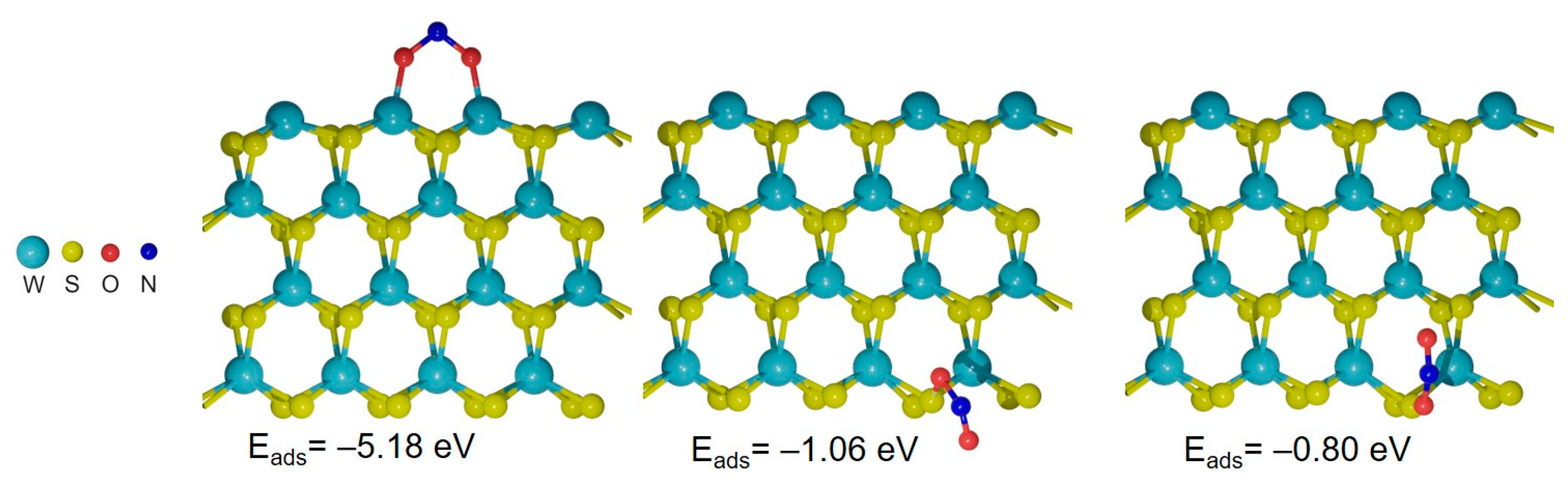
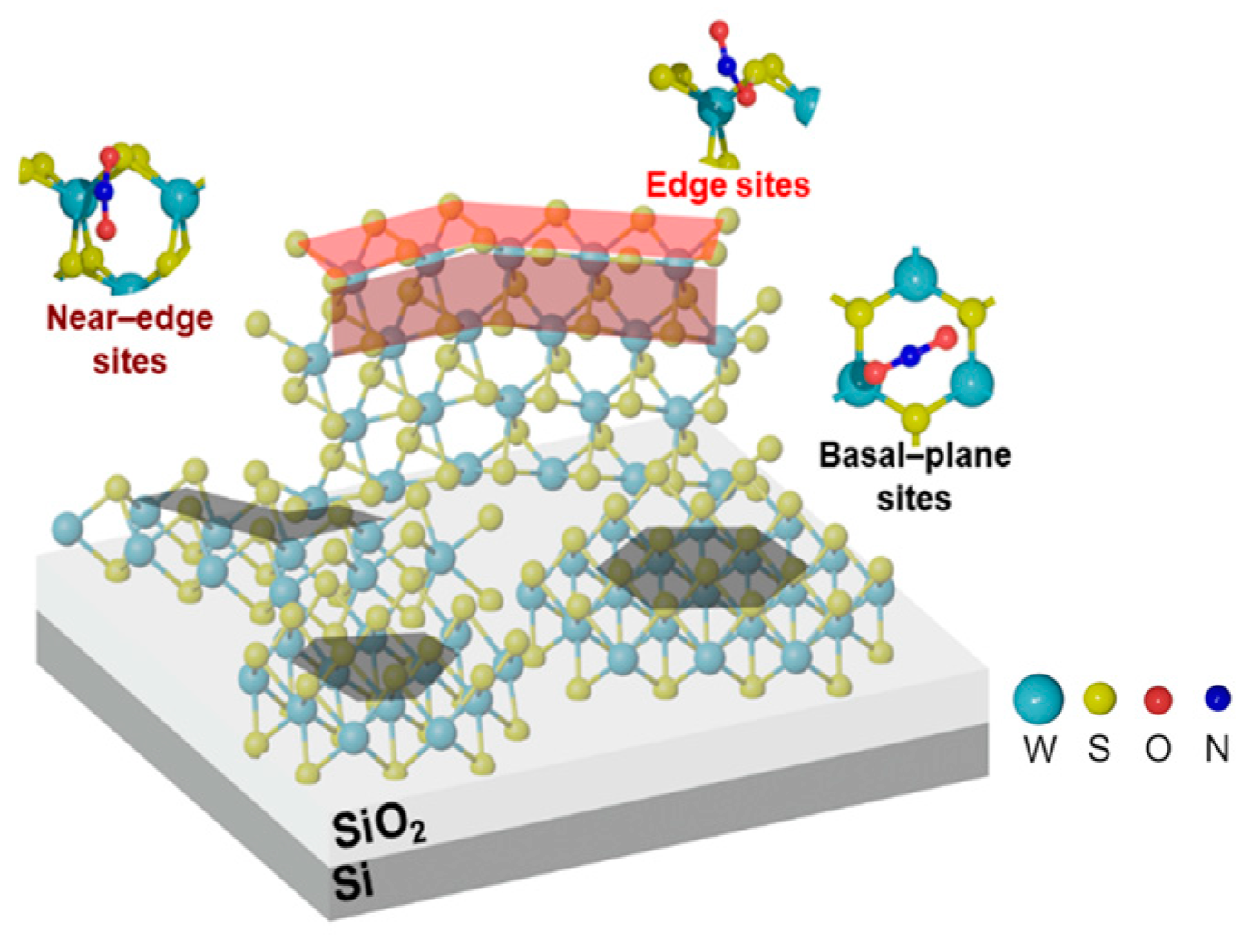
2.3. DFT Calculations
3. Results
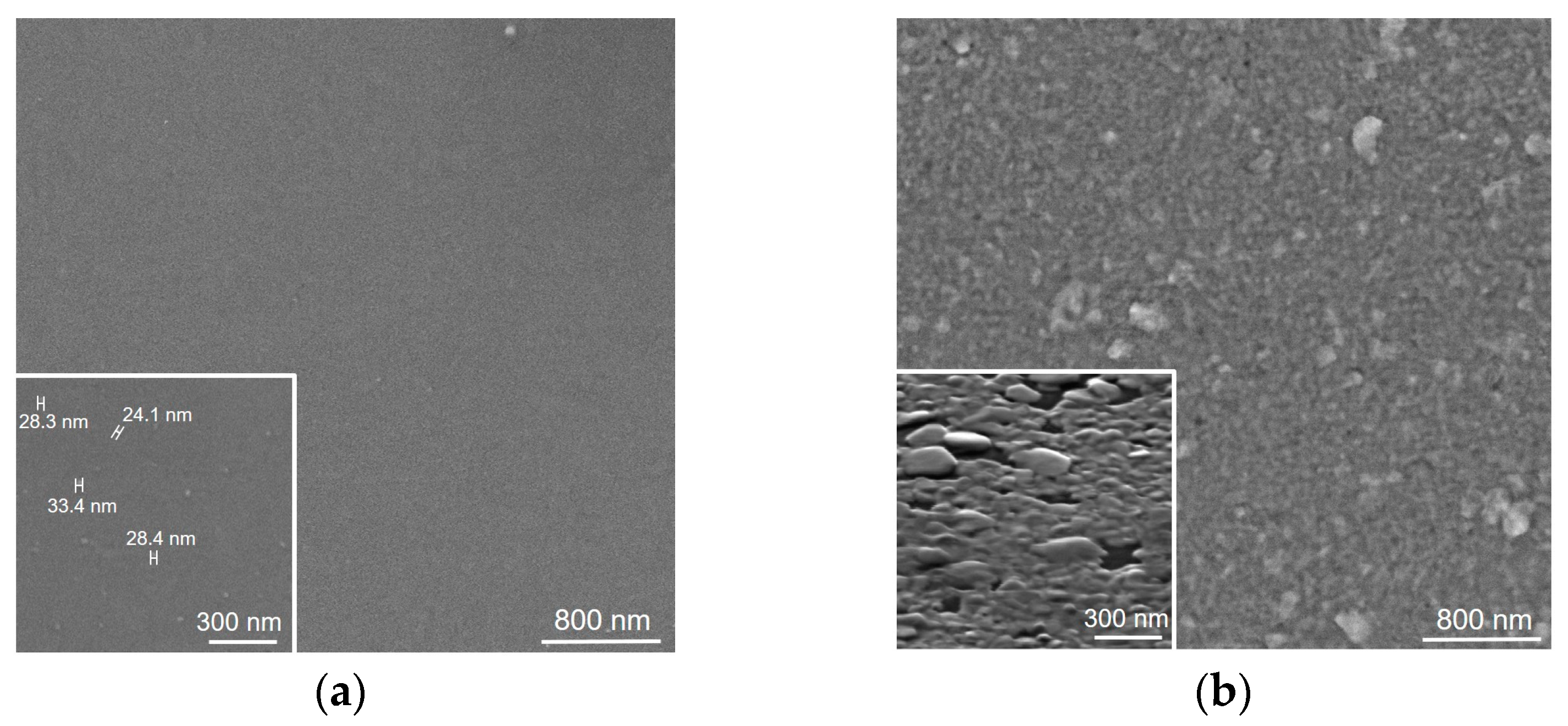
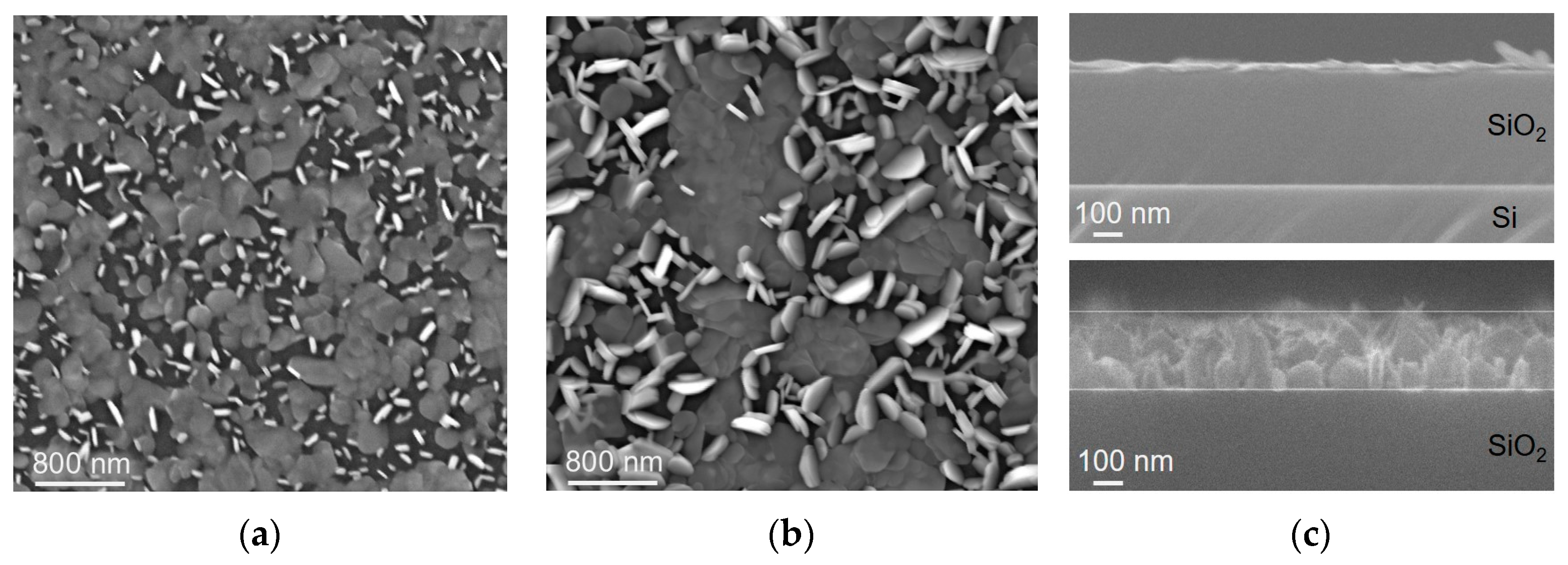
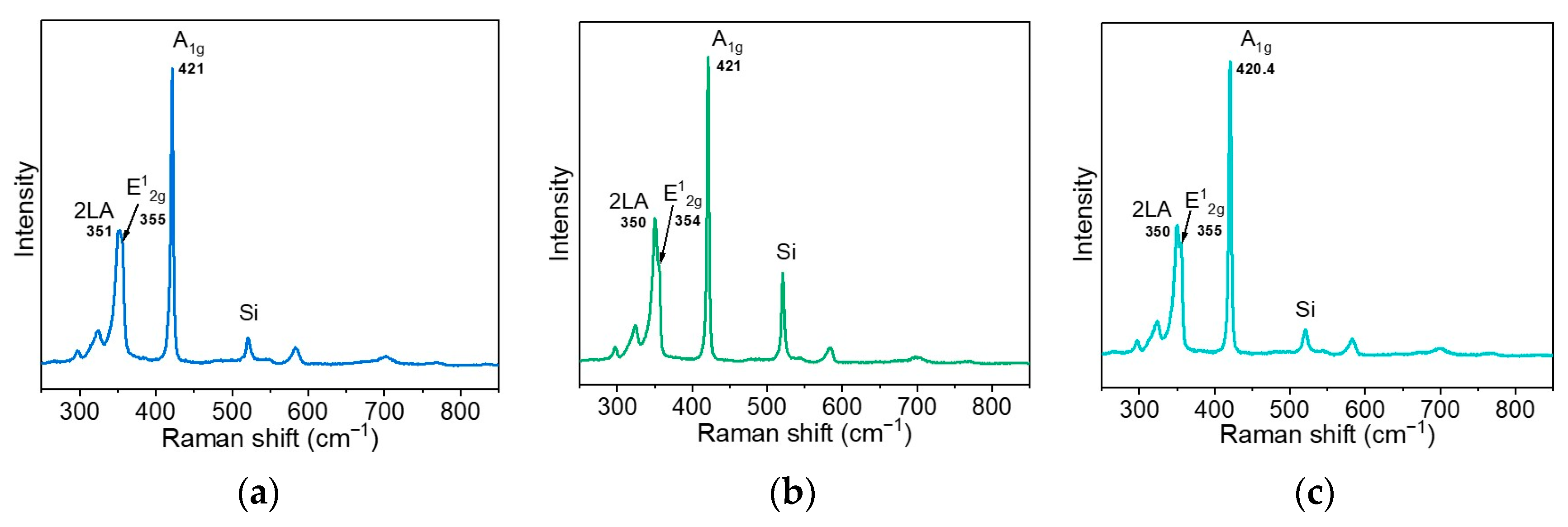
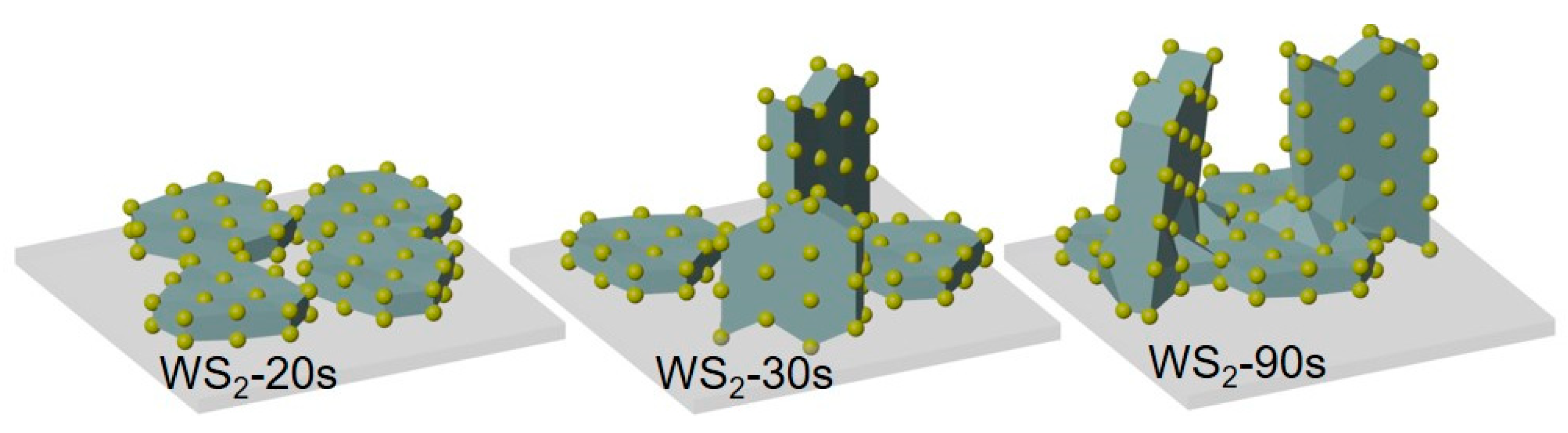
3.1. Structure and Composition of WS2 Films
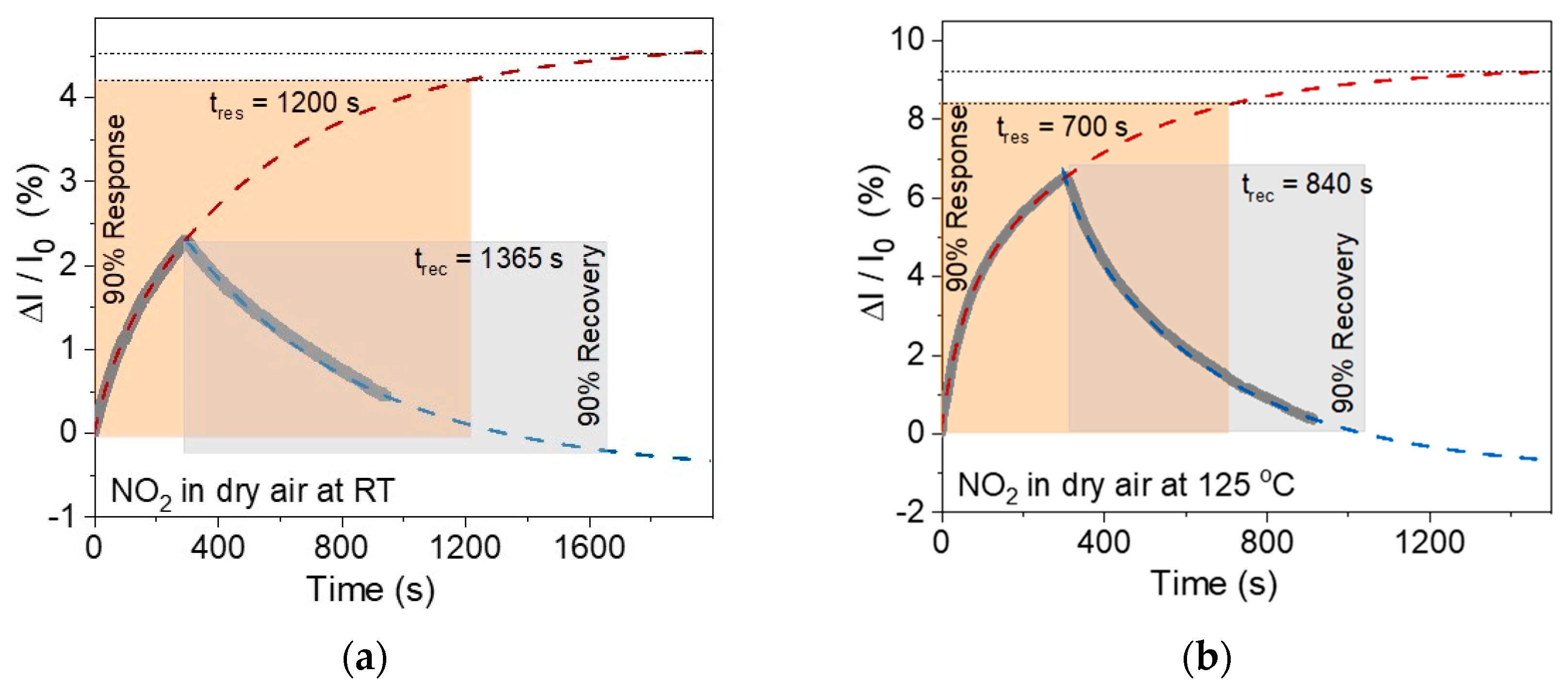
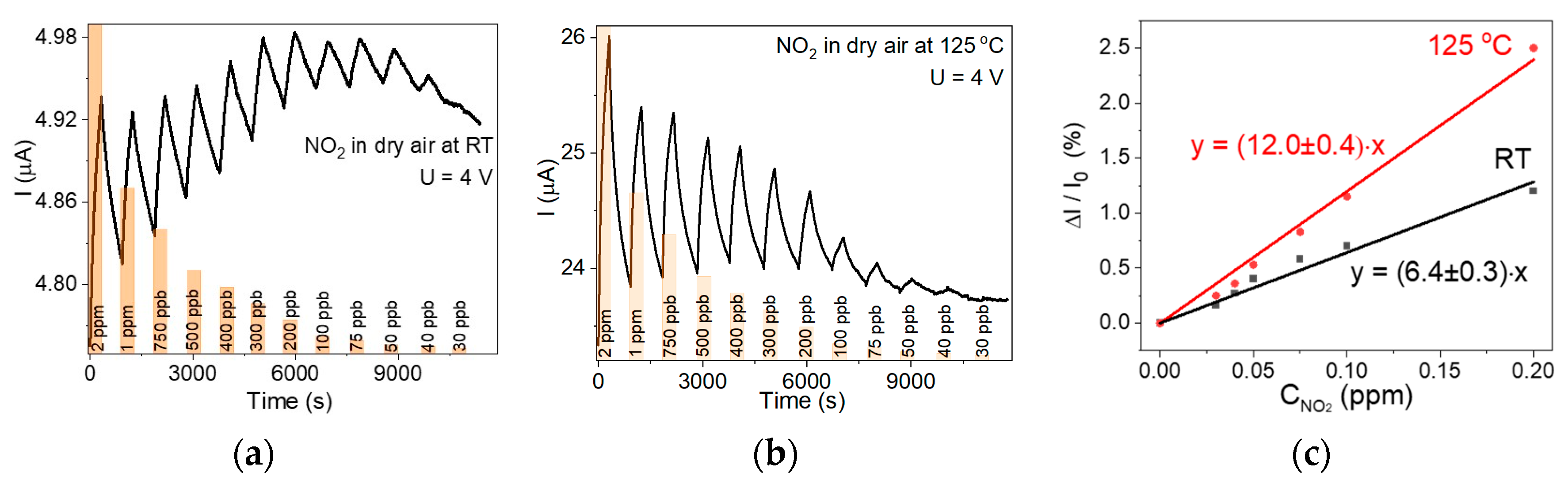
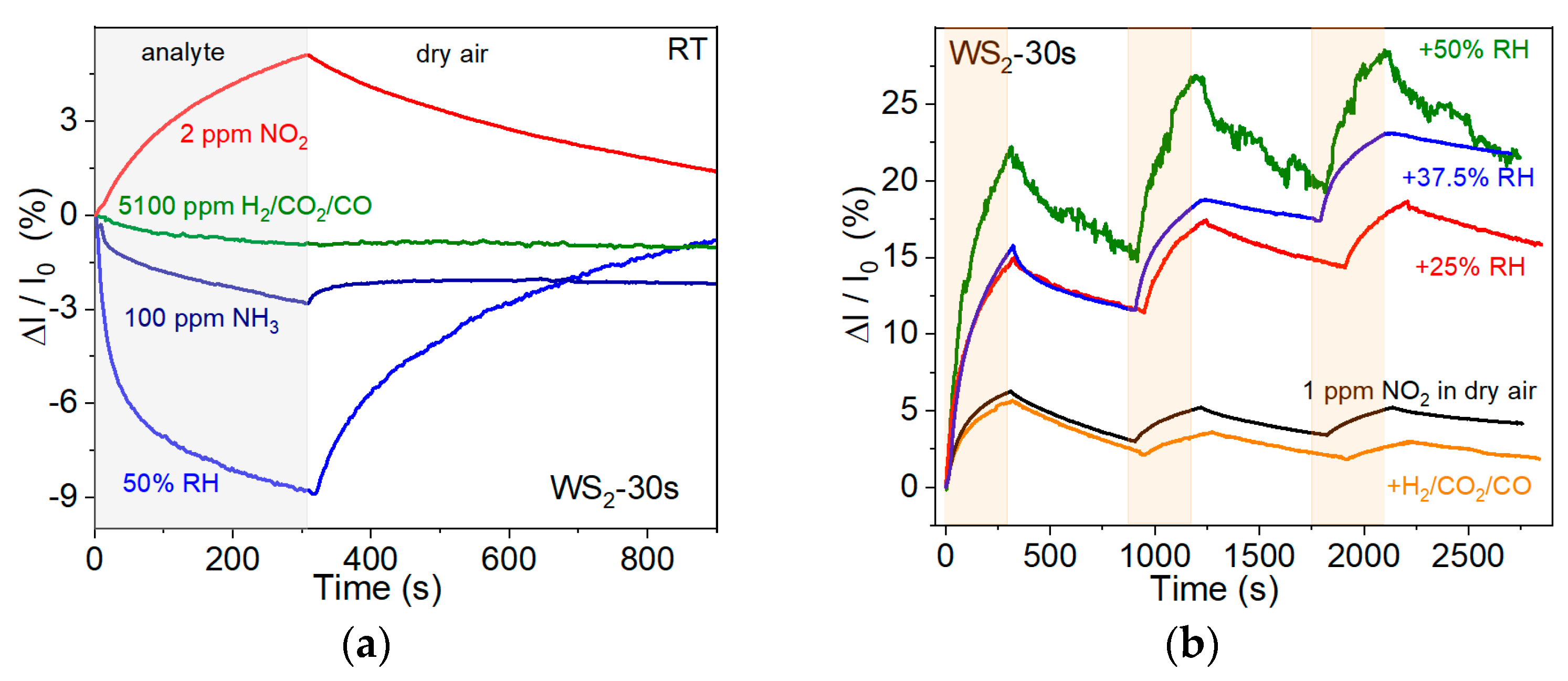
3.2. Sensor Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanmugam, V.; Mensah, R.A.; Babu, K.; Gawusu, S.; Chanda, A.; Tu, Y.; Neisiany, R.E.; Försth, M.; Sas, G.; Das, O. A Review of the Synthesis, Properties, and Applications of 2D Materials. Part. Part. Syst. Charact. 2022, 39, 2200031. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Semushkina, G.I.; Fedorenko, A.D. Heteroatom-Doped Molybdenum Disulfide Nanomaterials for Gas Sensors, Alkali Metal-Ion Batteries and Supercapacitors. Nanomaterials 2023, 13, 2182. [Google Scholar] [CrossRef] [PubMed]
- Pawbake, A.S.; Waykar, R.G.; Late, D.J.; Jadkar, S.R. Highly Transparent Wafer-Scale Synthesis of Crystalline WS2 Nanoparticle Thin Film for Photodetector and Humidity-Sensing Applications. ACS Appl. Mater. Interfaces 2016, 8, 3359–3365. [Google Scholar] [CrossRef] [PubMed]
- Singh Rana, D.; Thakur, N.; Singh, D.; Sonia, P. Molybdenum and Tungsten Disulfide Based Nanocomposites as Chemical Sensor: A Review. Mater. Today 2022, 62, 2755–2761. [Google Scholar] [CrossRef]
- Wu, R.; Hao, J.; Wang, Y. Recent Advances in Engineering of 2D Layered Metal Chalcogenides for Resistive-Type Gas Sensor. Small 2024, 20, 2404821. [Google Scholar] [CrossRef]
- Zhou, C.J.; Yang, W.H.; Wu, Y.P.; Lin, W.; Zhu, H.L. Theoretical Study of the Interaction of Electron Donor and Acceptor Molecules with Monolayer WS2. J. Phys. D Appl. Phys. 2015, 48, 285303. [Google Scholar] [CrossRef]
- Sanyal, G.; Vaidyanathan, A.; Rout, C.S.; Chakraborty, B. Recent Developments in Two-Dimensional Layered Tungsten Dichalcogenides Based Materials for Gas Sensing Applications. Mater. Today Commun. 2021, 28, 102717. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S. Gas Sensing in 2D Materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Fu, X.; Qiao, Z.; Zhou, H.; Xie, D. Defect Engineering in Transition Metal Dichalcogenide-Based Gas Sensors. Chemosensors 2024, 12, 85. [Google Scholar] [CrossRef]
- Victorin, K. Review of the Genotoxicity of Nitrogen Oxides. Mutat. Res. 1994, 317, 43–55. [Google Scholar] [CrossRef]
- Khomarloo, N.; Mohsenzadeh, E.; Gidik, H.; Bagherzadeh, R.; Latifi, M. Overall Perspective of Electrospun Semiconductor Metal Oxides as High-Performance Gas Sensor Materials for NOx Detection. RSC Adv. 2024, 14, 7806–7824. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, W.; Hong, Y.; Lee, G.; Yoon, D.S. Recent Advances in Carbon Material-Based NO2 Gas Sensors. Sens. Actuators B Chem. 2018, 255, 1788–1804. [Google Scholar] [CrossRef]
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas Sensing Performance of 2D Nanomaterials/Metal Oxide Nanocomposites: A Review. J. Mater. Chem. C 2021, 9, 8776–8808. [Google Scholar] [CrossRef]
- Cao, P.J.; Chen, R.G.; Cai, Y.Z.; Pawar, D.; Rao, C.N.; Han, S.; Xu, W.Y.; Fang, M.; Liu, X.K.; Zeng, Y.Z.; et al. Ultra-High Sensitive and Ultra-Low NO2 Detection at Low Temperature Based on Ultrathin In2O3 Nanosheets. J. Mater. Sci. Mater. Electron. 2021, 32, 19487–19498. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Zhang, Y.; Tian, F.H.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Edge-enriched WS2 Nanosheets on Carbon Nanofibers Boosts NO2 Detection at Room Temperature. J. Hazard. Mater. 2021, 411, 125120. [Google Scholar] [CrossRef]
- Kumar, S.; Meng, G.; Mishra, P.; Tripathi, N.; Bannov, A.G. A Systematic Review on 2D MoS2 for Nitrogen Dioxide (NO2) Sensing at Room Temperature. Mater. Today Commun. 2023, 34, 105045. [Google Scholar] [CrossRef]
- Ovchinnikov, D.; Allain, A.; Huang, Y.-S.; Dumcenco, D.; Kis, A. Electrical Transport Properties of Single-Layer WS2. ACS Nano 2014, 8, 8174–8181. [Google Scholar] [CrossRef]
- Sihag, S.; Dahiya, R.; Rani, S.; Berwal, P.; Jatrana, A.; Sisodiya, A.K.; Sharma, A.; Kumar, V. Low ppm NO2 Detection through Advanced Ultrasensitive Copper Oxide Gas Sensor. Discov. Nano 2024, 19, 107. [Google Scholar] [CrossRef]
- Walleni, C.; Ali, M.B.; Ncib, M.F.; Llobet, E. Synergistic Enhancement of Chemiresistive NO2 Gas Sensors Using Nitrogn-Doped Reduced Graohene Oxide (N-rGO) Decorated with Nickel Oxide (NiO) Nanoparticles: Achieving sub-ppm DetectionLimit. Sensors 2025, 25, 1631. [Google Scholar] [CrossRef]
- Sysoev, V.I.; Sedelnikova, O.V.; Vorfolomeeva, A.A.; Geraseva, T.A.; Bulusheva, L.G.; Okotrub, A.V. Single-Walled Carbon Nanotubes Filled with Sulfur and Phosphorus Compounds for Real-Time Detection of NO2 in Air. Carbon 2025, 233, 119915. [Google Scholar] [CrossRef]
- Urs, K.; Carey, T.; Tsetseris, L.; Liu, S.; Synnatschke, K.; Sofer, Z.; Coleman, J.N.; Wenger, J.C.; Biswas, S.; Holmes, J.D. Exfoliated Molybdenum Disulfide Nanosheet Networks as Sensing Materials for Nitrogen Dioxide Detection. ACS Appl. Nano Mater. 2025, 8, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.S.; Archana, J.; Navaneethan, M.; Mohan, M.K. Boosting the Response Characteristics towards Rapid Detection of NO2 Gas Molecules Utilizing 2D—WS2/rGO Hybrid Nanocomposites at Ambient Environment. J. Environ. Chem. Eng. 2024, 12, 113367. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Fedoseeva, Y.; Lavrukhina, S.A.; Sysoev, V.I.; Maksimovskii, E.A.; Makarova, A.A.; Okotrub, A.V. Role of Graphene Substrate in the Formation of MoS2-Based Nanoparticles with Improved Sensitivity to NO2 Gas. Appl. Surf. Sci. 2025, 679, 161104. [Google Scholar] [CrossRef]
- Guo, P.; He, X.; An, X.; Zhang, X.; Liang, S.; Zhang, J.; Fu, Y. N-doped Carbon Dots/SnS2 Heterostructures for Ultra-Fast and Sub-ppm-Level Detection of NO2 in Multiple Enviroments. Chem. Eng. J. 2024, 500, 156882. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Jiang, Y.; Duan, Z.; Tuan, Z.; Liu, Z.; Huang, Q.; Zhao, Q.; Yang, Y.; Tai, H. Synergistic Effect of Charge Transfer and Interlayer Swelling in V2CTx/SnS2 Driving Ultrafast and Highly Sensitive NO2 Detection at Room Temperature. Sens. Actuators B Chem. 2024, 411, 135788. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, W.; Zhang, M.; Wang, Y.; Duan, Z.; Tan, C.; Liu, B.; Ouyang, F.; Yuan, Z.; Tai, H.; et al. Edge-Enriched Mo2TiC2Tx/MoS2 Heterostructure with Coupling Interface for Selective NO2 Monitoring. Adv. Funct. Mater. 2022, 32, 2203528. [Google Scholar] [CrossRef]
- Kodan, S.; Kumar, A.; Sanger, A.; Arora, A.; Malik, A.A.; Chandra, R. Vertically Aligned MoSe2-WS2 Nanoworms Heterojunction Towads Room Temparature NO2 Gas Sensors. Sens. Actuators B Chem. 2024, 407, 135481. [Google Scholar] [CrossRef]
- O’Brien, M.; Lee, K.; Morrish, R.; Berner, N.C.; McEvoy, N.; Wolden, C.A.; Duesberg, G.S. Plasma Assisted Synthesis of WS2 for Gas Sensing Applications. Chem. Phys. Lett. 2014, 615, 6–10. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Llobet, E. Single-Step CVD Synthesis of Layered WS2 Films for NO2 Gas Sensing. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Kundu, S.; Gorthala, G.; Ghosh, R. Room Temperature Detection of H2S by Two Dimensional WS2 Based Chemiresistive Sensors. Sens. Actuators B Chem. 2024, 416, 136018. [Google Scholar] [CrossRef]
- Ko, K.Y.; Song, J.-G.; Kim, Y.; Choi, T.; Shin, S.; Lee, C.W.; Lee, K.; Koo, J.; Lee, H.; Kim, J.; et al. Improvement of Gas-Sensing Performance of Large-Area Tungsten Disulfide Nanosheets by Surface Functionalization. ACS Nano 2016, 10, 9287–9296. [Google Scholar] [CrossRef]
- Manoharan, M.; Govindharaj, K.; Muthumalai, K.; Pandian, R.; Haldorai, Y.; Rajendra Kumar, R.T. Highly Selective Room Temperature Detection of NH3 and NOx Using Oxygen-Deficient W18 O49 -Supported WS2 Heterojunctions. ACS Appl. Mater. Interfaces 2023, 15, 4703–4712. [Google Scholar] [CrossRef] [PubMed]
- Alagh, A.; Annanouch, F.E.; Youssef, K.A.; Bittencourt, C.; Güell, F.; Martínez-Alanis, P.R.; Reguant, M.; Llobet, E. PdO and PtO Loaded WS2 Boosts NO2 Gas Sensing Characteristics at Room Temperature. Sens. Actuators B Chem. 2022, 364, 131905. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, W.; Zhu, H. Mechanism of Charge Transfer and Its Impacts on Fermi-Level Pinning for Gas Molecules Adsorbed on Monolayer WS2. J. Chem. Phys. 2015, 142, 214704. [Google Scholar] [CrossRef]
- Hou, S.; Wang, Z.; Yang, H.; Jiang, J.; Gao, C.; Liu, Y.; Tang, X.; Ye, H. A Theoretical Study of Atmospheric Pollutant NO2 on as-Doped Monolayer WS2 Based on DFT Method. Phys. E Low-Dimens. Syst. Nanostructures 2022, 144, 115446. [Google Scholar] [CrossRef]
- Moumen, A.; Konar, R.; Zappa, D.; Teblum, E.; Nessim, G.D.; Comini, E. Energy-Efficient NO2 Sensors Based on Two-Dimensional Layered 2H-WS2 Nanosheets. Sens. Actuators B Chem. 2024, 406, 135379. [Google Scholar] [CrossRef]
- Qin, Z.; Zeng, D.; Zhang, J.; Wu, C.; Wen, Y.; Shan, B.; Xie, C. Effect of Layer Number on Recovery Rate of WS2 Nanosheets for Ammonia Detection at Room Temperature. Appl. Surf. Sci. 2017, 414, 244–250. [Google Scholar] [CrossRef]
- Alagh, A.; Annanouch, F.E.; Umek, P.; Bittencourt, C.; Sierra-Castillo, A.; Haye, E.; Colomer, J.F.; Llobet, E. CVD Growth of Self-Assembled 2D and 1D WS2 Nanomaterials for the Ultrasensitive Detection of NO2. Sens. Actuators B Chem. 2021, 326, 128813. [Google Scholar] [CrossRef]
- Duan, X.; Xu, D.; Jia, W.; Li, R.; Sun, B.; Yan, R.; Zhao, W. WO3/WS2 Nanoflowers Fabricated by Low-Temperature in-Situ Oxidation for Rapid Detection of Nitrogen Dioxide. Sens. Actuators A Phys. 2024, 365, 114854. [Google Scholar] [CrossRef]
- Perrozzi, F.; Emamjomeh, S.M.; Paolucci, V.; Taglieri, G.; Ottaviano, L.; Cantalini, C. Thermal Stability of WS2 Flakes and Gas Sensing Properties of WS2/WO3 Composite to H2, NH3 and NO2. Sens. Actuators B Chem. 2017, 243, 812–822. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, K.; Shen, Y.; Yuan, Z.; Dong, Y.; Yuan, P.; Li, E. Toxic Gas Molecules Adsorbed on Intrinsic and Defective WS2: Gas Sensing and Detection. Appl. Surf. Sci. 2023, 613, 155978. [Google Scholar] [CrossRef]
- Suh, J.M.; Kwon, K.C.; Lee, T.H.; Kim, C.; Lee, C.W.; Song, Y.G.; Choi, M.-J.; Choi, S.; Cho, S.H.; Kim, S.; et al. Edge-Exposed WS2 on 1D Nanostructures for Highly Selective NO2 Sensor at Room Temperature. Sens. Actuators B Chem. 2021, 333, 129566. [Google Scholar] [CrossRef]
- Cha, J.-H.; Choi, S.-J.; Yu, S.; Kim, I.-D. 2D WS2 -Edge Functionalized Multi-Channel Carbon Nanofibers: Effect of WS2 Edge-Abundant Structure on Room Temperature NO2 Sensing. J. Mater. Chem. A 2017, 5, 8725–8732. [Google Scholar] [CrossRef]
- Bisht, P.; Kumar, A.; Ghosh, A.; Vullum, P.E.; Sunding, M.F.; Belle, B.D.; Mehta, B.R. Tailoring the Vertical and Planar Growth of 2D WS2 Thin Films Using Pulsed Laser Deposition for Enhanced Gas Sensing Properties. ACS Appl. Mater. Interfaces 2022, 14, 36789–36800. [Google Scholar] [CrossRef]
- Jung, Y.; Shen, J.; Liu, Y.; Woods, J.M.; Sun, Y.; Cha, J.J. Metal Seed Layer Thickness-Induced Transition From Vertical to Horizontal Growth of MoS2 and WS2. Nano Lett. 2014, 14, 6842–6849. [Google Scholar] [CrossRef]
- Hotovy, I.; Spiess, L.; Sojkova, M.; Kostic, I.; Mikolasek, M.; Predanocy, M.; Romanus, H.; Hulman, M.; Rehacek, V. Structural and Optical Properties of WS2 Prepared Using Sulfurization of Different Thick Sputtered Tungsten Films. Appl. Surf. Sci. 2018, 461, 133–138. [Google Scholar] [CrossRef]
- Yadav, P.V.K.; Ashok Kumar Reddy, Y. Controlled Two-Step Synthesis of Nanostructured WS2 Thin Films for Enhanced UV–Visible Photodetector Applications. Sens. Actuators A Phys. 2022, 345, 113780. [Google Scholar] [CrossRef]
- Rao, R.; Kim, H.; Perea-López, N.; Terrones, M.; Maruyama, B. Interaction of Gases with Monolayer WS2: An in Situ Spectroscopy Study. Nanoscale 2021, 13, 11470–11477. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas-Sensor Applications: A Review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Galvani, M.; Freddi, S.; Sangaletti, L. Disclising Fast Detection Opportunities with Nanostructures Chemiresistor Gas Sensirs Based on Metal Oxides, Carbon, and Transition Metal Dichalcogenides. Sensors 2024, 24, 584. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A Grid-Based Bader Analysis Algorithm without Lattice Bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Berkdemir, A.; Gutiérrez, H.R.; Botello-Méndez, A.R.; Perea-López, N.; Elías, A.L.; Chia, C.-I.; Wang, B.; Crespi, V.H.; López-Urías, F.; Charlier, J.-C.; et al. Identification of Individual and Few Layers of WS2 Using Raman Spectroscopy. Sci. Rep. 2013, 3, 1755. [Google Scholar] [CrossRef]
- Moses Badlyan, N.; Quincke, M.; Kaiser, U.; Maultzsch, J. TEM-Processed Defect Densities in Single-Layer TMDCs and Their Substrate-Dependent Signature in PL and Raman Spectroscopy. Nanotechnology 2024, 35, 435001. [Google Scholar] [CrossRef]
- Hossain, E.; Rahman, A.A.; Shah, A.P.; Chalke, B.A.; Bhattacharya, A. Large-Area, Thermally-Sulfurized WS2 Thin Films: Control of Growth Direction and Use as a Substrate for GaN Epitaxy. Semicond. Sci. Technol. 2020, 35, 035011. [Google Scholar] [CrossRef]
- Jain, R.K.; Khanna, A. CuO-Doped WO3 Thin Film H2S Sensors. Sens. Actuators B Chem. 2021, 343, 130153. [Google Scholar] [CrossRef]
- Nevolin, V.N.; Fominski, D.V.; Romanov, R.I.; Rubinkovskaya, O.V.; Soloviev, A.A.; Shvets, P.V.; Maznitsyna, E.A.; Fominski, V.Y. Influence of Sulfidation Conditions of WO3 Nanocrystalline Film on Photoelectrocatalytic Activity of WS2/WO3 Hybrid Structure in Production of Hydrogen. Inorg. Mater. Appl. Res. 2021, 12, 1139–1147. [Google Scholar] [CrossRef]
- Scarfiello, R.; Mazzotta, E.; Altamura, D.; Nobile, C.; Mastria, R.; Rella, S.; Giannini, C.; Cozzoli, P.D.; Rizzo, A.; Malitesta, C. An Insight into Chemistry and Structure of Colloidal 2D-WS2 Nanoflakes: Combined XPS and XRD Study. Nanomaterials 2021, 11, 1969. [Google Scholar] [CrossRef]
- Stolyarova, S.G.; Kotsun, A.A.; Shubin, Y.V.; Koroteev, V.O.; Plyusnin, P.E.; Mikhlin, Y.L.; Mel’gunov, M.S.; Okotrub, A.V.; Bulusheva, L.G. Synthesis of Porous Nanostructured MoS2 Materials in Thermal Shock Conditions and Their Performance in Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 10802–10813. [Google Scholar] [CrossRef]
- Qin, F.; Gao, J.; Jiang, L.; Fan, J.; Sun, B.; Fan, Y.; Lv, H.; Shi, K. Biomorphic WO3@WS2 Heterojunction Composites for Enhanced NO2 Gas-Sensing Performance at Room Temperature. Appl. Surf. Sci. 2023, 615, 156338. [Google Scholar] [CrossRef]
- Simon Patrick, D.; Bharathi, P.; Kamalakannan, S.; Archana, J.; Navaneethan, M.; Krishna Mohan, M. Confined Oxidation of 2D WS2 Nanosheets Forming WO3/WS2 Nanocomposites for Room Temperature NO2 Gas Sensing Application. Appl. Surf. Sci. 2024, 642, 158554. [Google Scholar] [CrossRef]
- Cumpson, P.J. The Thickogram: A Method for Easy Film Thickness Measurement in XPS. Surf. Interface Anal. 2000, 29, 403–406. [Google Scholar] [CrossRef]
- Scardamaglia, M.; Casanova-Cháfer, J.; Temperton, R.; Annanouch, F.E.; Mohammadpour, A.; Malandra, G.; Das, A.; Alagh, A.; Arbouch, I.; Montoisy, L.; et al. Operando Investigation of WS2 Gas Sensors: Simultaneous Ambient Pressure X-Ray Photoelectron Spectroscopy and Electrical Characterization in Unveiling Sensing Mechanisms during Toxic Gas Exposure. ACS Sens. 2024, 9, 4079–4088. [Google Scholar] [CrossRef]
- Rathod, U.P.; Jha, J.K.; Voevodin, A.A.; Shepherd, N.D. A Photoelectron Study of Annealing Induced Changes to Workfunction and Majority Carrier Type in Pulsed Laser Deposited Few Layer WS2 Films. J. Mater. Sci Mater. Electron. 2018, 29, 20051–20056. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Su, C.; Wang, S.; Li, H.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; et al. Interface Engineered WS2/ZnS Heterostructures for Sensitive and Reversible NO2 Room Temperature Sensing. Sens. Actuators B Chem. 2019, 296, 126666. [Google Scholar] [CrossRef]



| Material | Temperature | LOD | τres/τrec (s) | Gases in ST | Reference |
|---|---|---|---|---|---|
| CuO | 100 °C | <300 ppb | 64/274 at 5 ppm | NH3, CO, CO2, H2S | [18] |
| In2O3 nanosheets | 50 °C | 5 ppb | 690/1951 at 2 ppm | NH3, CO2, H2S | [14] |
| NiO/N-rGO | 100 °C | <1 ppb | 660/2700 at 0.8 ppm | NH3, CO2 | [19] |
| N-rGO | 100 °C | 39 ppb | 600/2640 at 0.8 ppm | NH3, CO2 | [19] |
| SWCNTs | 150 °C | 12 ppb | 208/193 at 1 ppm | not studied | [20] |
| MoS2 exfoliated | RT | 100 ppb | 600/1200 at 1 ppm | NH3, CH4, SO2 | [21] |
| WS2/rGO | RT | 363 ppb | 58/627 at 10 ppm | NH3, CO2, H2S, SO2, N2O | [22] |
| WS2@Carbon fibers | RT | 31 ppb | 54/305 at 10 ppm | NH3, CO, CO2, H2S, SO2, H2 | [16] |
| MoS2/Graphene | RT | 18 ppb | 254/1262 at 1 ppm | NH3, CO, CO2, H2S, H2 | [23] |
| N-Carbon dots/SnS2 | RT | 10 ppb | 9/132 at 1 ppm | NH3, CO, CO2, SO2, H2 | [24] |
| V2CTx/SnS2 | RT | 300 ppb | 4.8/4.7 at 5 ppm | NH3, CO2, H2S, CH4, NO | [25] |
| Mo2TiC2Tx/MoS2 | RT | <3 ppb | 61/154 at 10 ppm | NH3, CO2, H2S, CH4, NO | [26] |
| MoSe2-WS2@Si | RT | <50 ppb | 69/66 at 50 ppb | NH3, CO, H2S | [27] |
| Material | Concentration | Response (%) | LOD | Reference |
|---|---|---|---|---|
| multilayered WS2 | 50 ppb | 17.5 | <20 ppb | [29] |
| four-layered WS2 | 25 ppm | 8.7 | <25 ppm | [31] |
| WS2 nanotriangles | 200 ppb | 4.0 | N/A | [32] |
| two layered WS2 | 1 ppm | 419 | N/A | [36] |
| WS2 nanoflakes | 300 ppb | 0.5 | N/A | [38] |
| 800 ppb | 26.6 | ~50 ppb (150 °C) 1 | ||
| WS2 nanosheets | 10 ppm | 1.13 | N/A | [15] |
| WS2 nanorods | 5 ppm | 151.2 | 13.7 ppb (theor) 2 | [42] |
| vertical WS2 flakes | 50 ppb | 4 | <50 ppb (40 °C) 1 | [44] |
| WS2 nanosheets | 100 ppm | 14 | <50 ppb | [62] |
| WS2 nanosheets | 2 ppm | 8 | 3.16 ppm (theor) 2 | [63] |
| WS2 nanosheets | 2 ppm | 7 | 523 ppb (theor) 2 | [22] |
| thin WS2 film | 40 ppb | 0.27 | 15 ppb(theor) 2 | this work |
| 40 ppb | 0.36 | 8 ppb (theor 2, 125 °C) 1 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorenko, A.D.; Lavrukhina, S.A.; Alekseev, V.A.; Sysoev, V.I.; Sulyaeva, V.S.; Okotrub, A.V.; Bulusheva, L.G. Thin Films of Tungsten Disulfide Grown by Sulfurization of Sputtered Metal for Ultra-Low Detection of Nitrogen Dioxide Gas. Nanomaterials 2025, 15, 594. https://doi.org/10.3390/nano15080594
Fedorenko AD, Lavrukhina SA, Alekseev VA, Sysoev VI, Sulyaeva VS, Okotrub AV, Bulusheva LG. Thin Films of Tungsten Disulfide Grown by Sulfurization of Sputtered Metal for Ultra-Low Detection of Nitrogen Dioxide Gas. Nanomaterials. 2025; 15(8):594. https://doi.org/10.3390/nano15080594
Chicago/Turabian StyleFedorenko, Anastasiya D., Svetlana A. Lavrukhina, Victor A. Alekseev, Vitalii I. Sysoev, Veronica S. Sulyaeva, Alexander V. Okotrub, and Lyubov G. Bulusheva. 2025. "Thin Films of Tungsten Disulfide Grown by Sulfurization of Sputtered Metal for Ultra-Low Detection of Nitrogen Dioxide Gas" Nanomaterials 15, no. 8: 594. https://doi.org/10.3390/nano15080594
APA StyleFedorenko, A. D., Lavrukhina, S. A., Alekseev, V. A., Sysoev, V. I., Sulyaeva, V. S., Okotrub, A. V., & Bulusheva, L. G. (2025). Thin Films of Tungsten Disulfide Grown by Sulfurization of Sputtered Metal for Ultra-Low Detection of Nitrogen Dioxide Gas. Nanomaterials, 15(8), 594. https://doi.org/10.3390/nano15080594









