Biomass-Derived Activated Porous Carbon from Foxtail Millet Husk to Utilizing High-Performance Symmetric Supercapacitor Applications
Abstract
1. Introduction
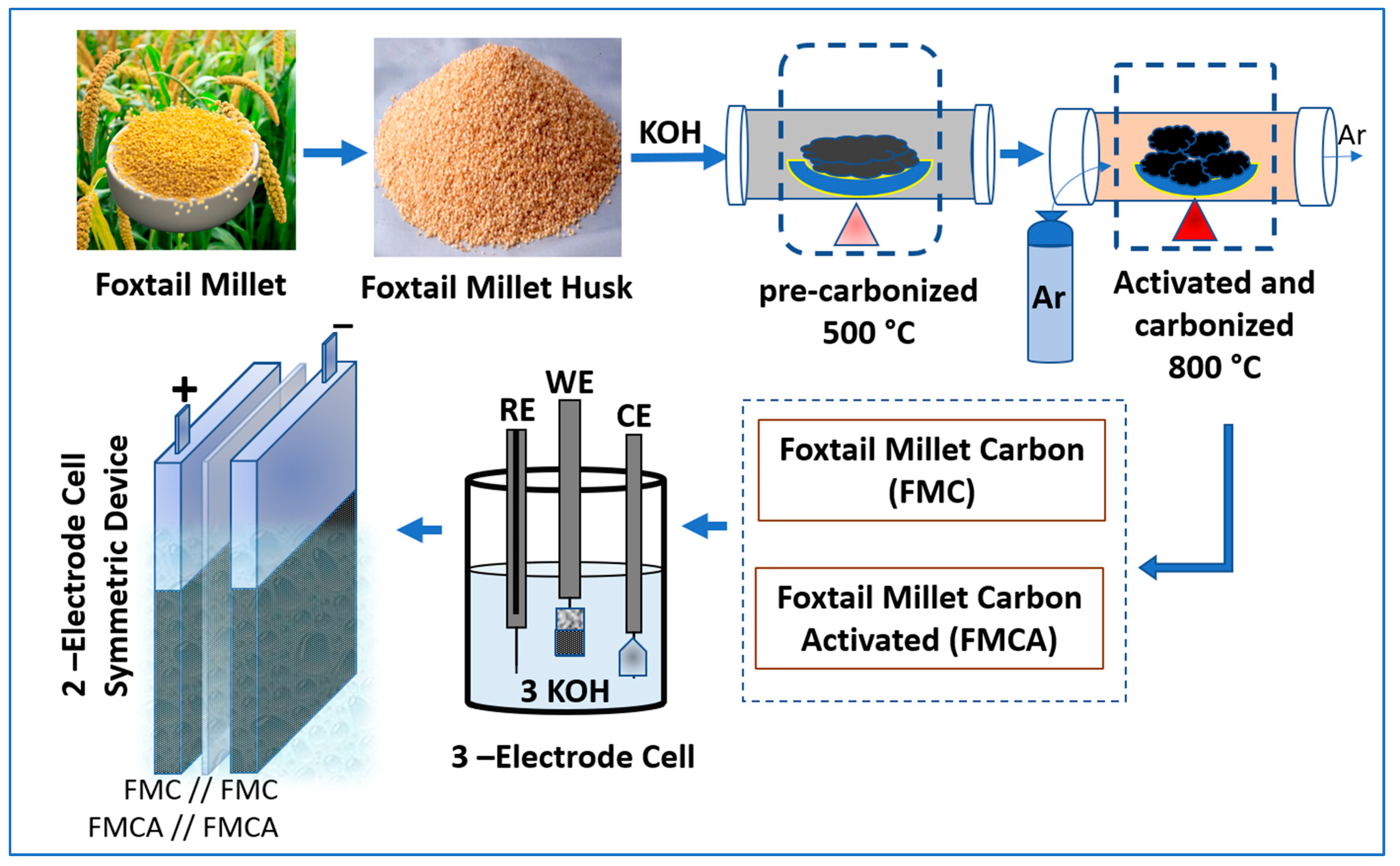
2. Materials and Methods
2.1. Materials Characterization
2.2. Electrode Fabrications and Electrochemical Characterization
3. Results and Discussion
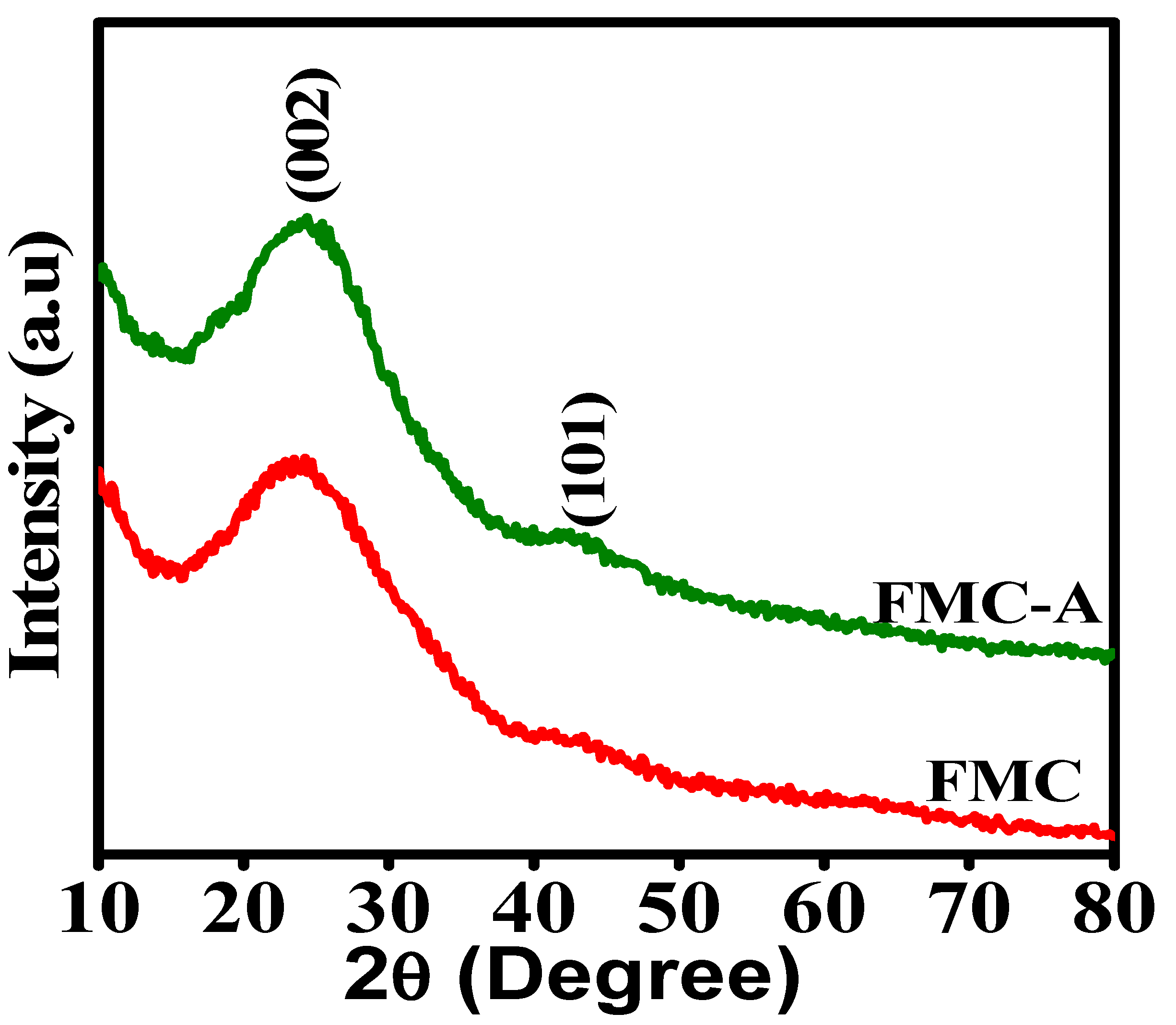
3.1. X-Ray Diffraction Analysis
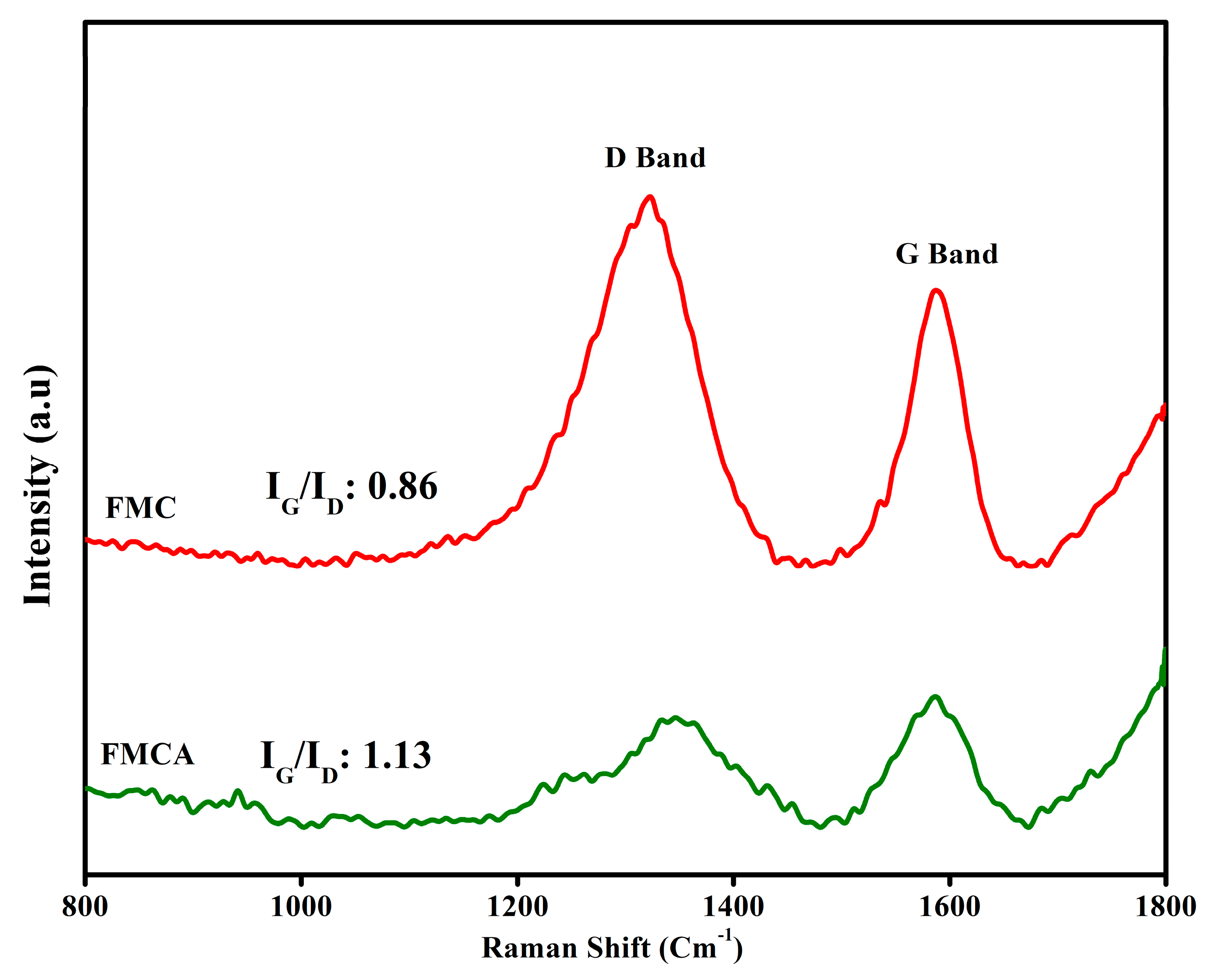
3.2. RAMAN Analysis
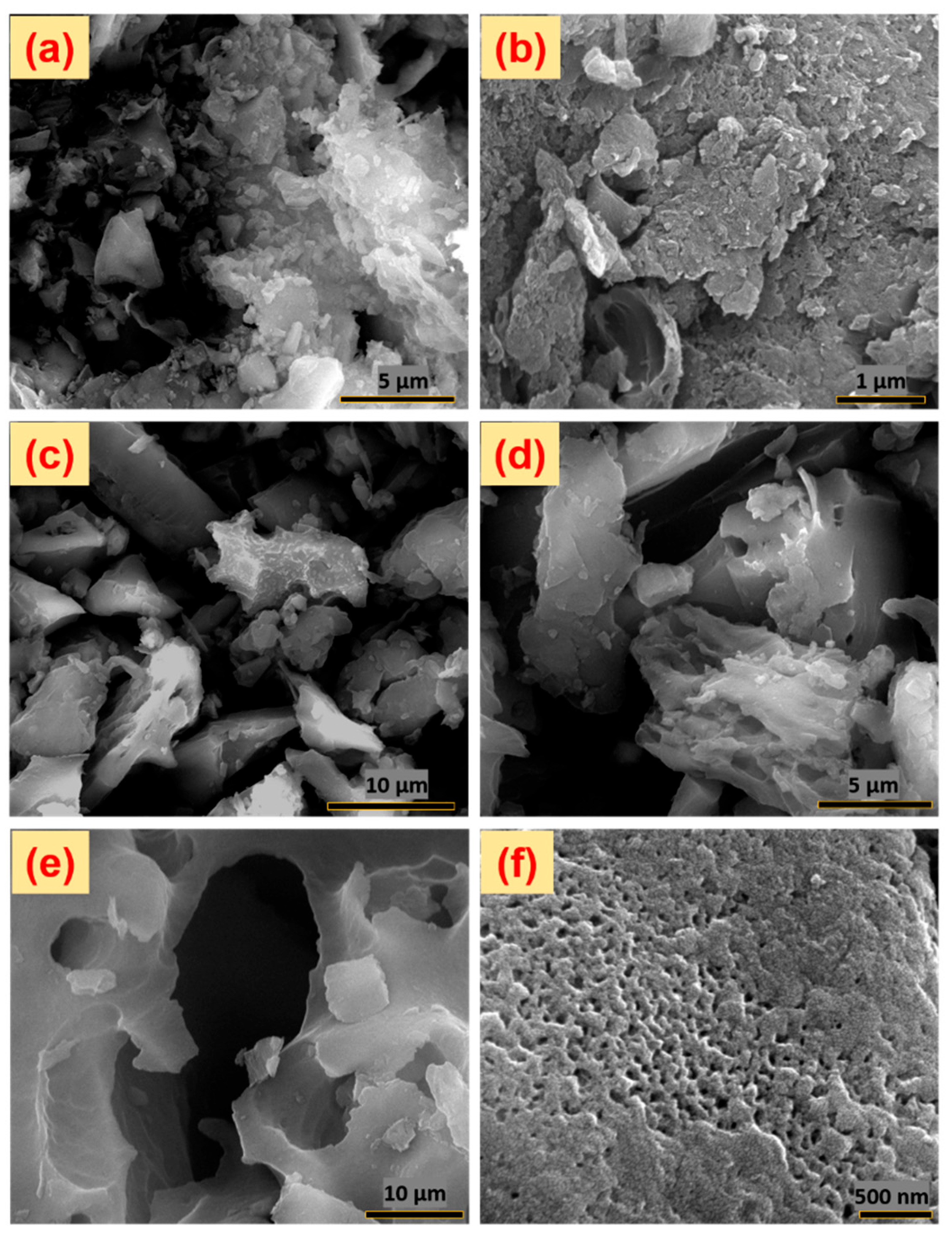
3.3. FE-SEM Surface Morphological Analysis
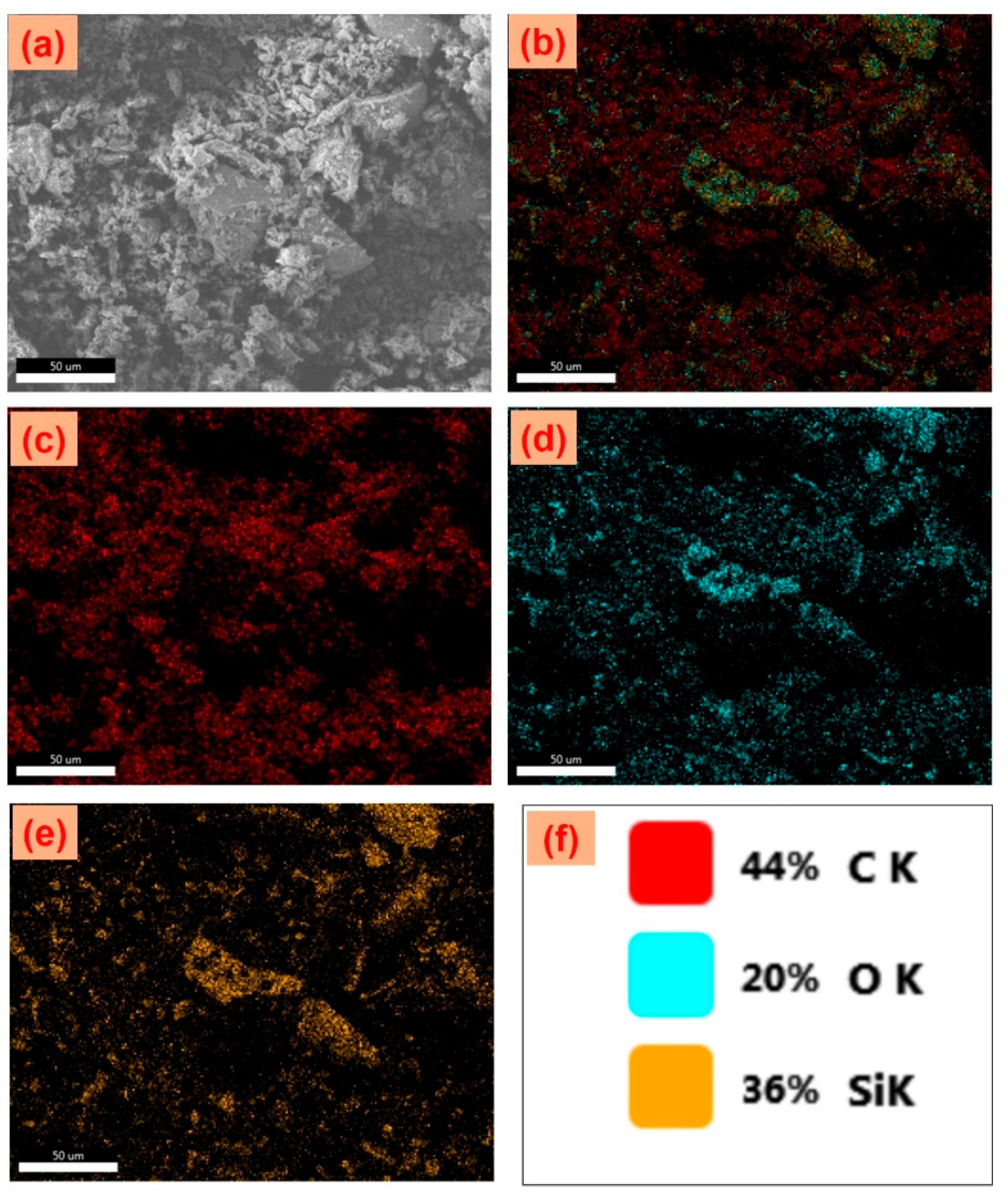
3.4. FE-SEM, EDS Elemental Mapping Analysis
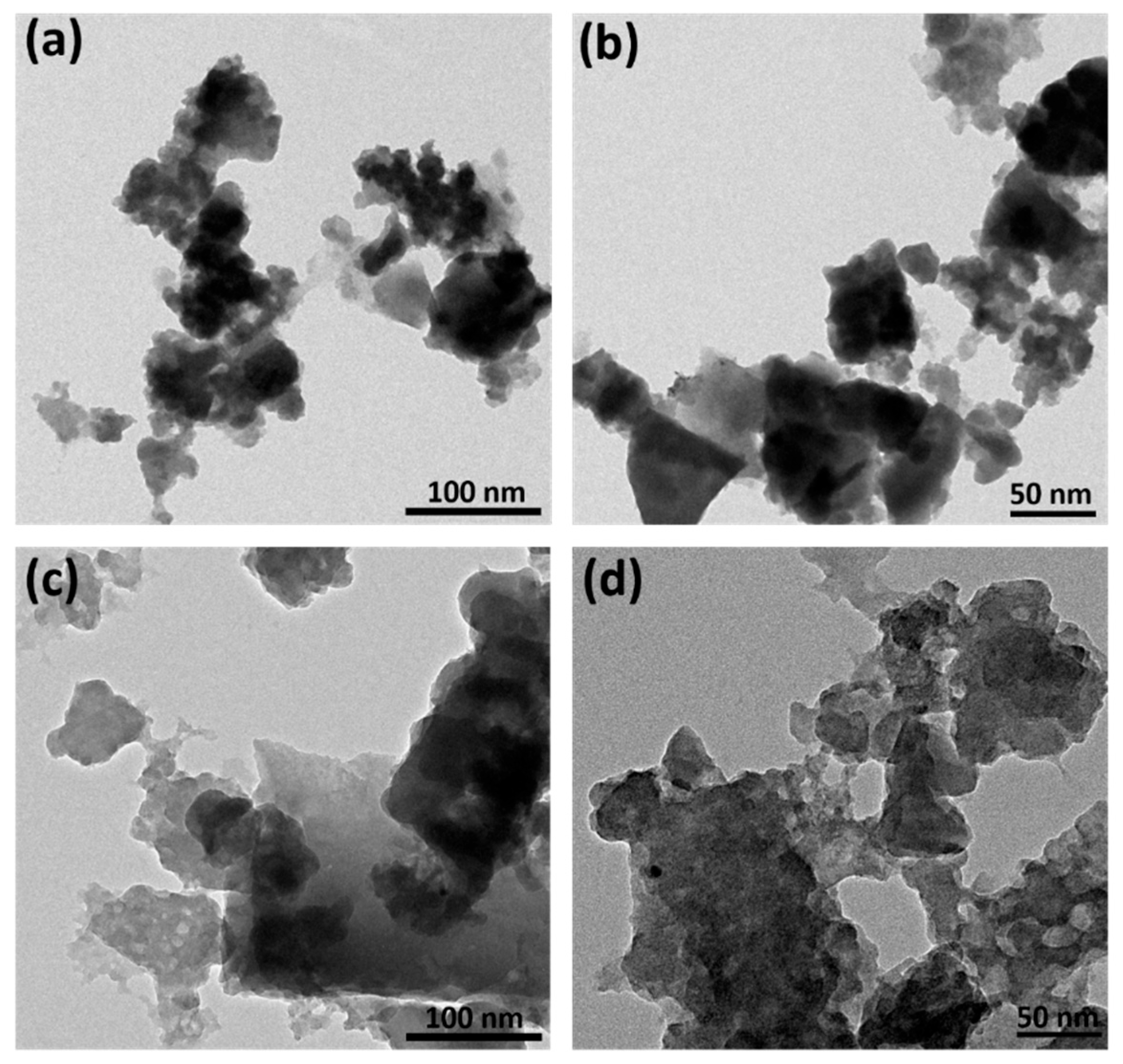
3.5. FE-TEM Analysis
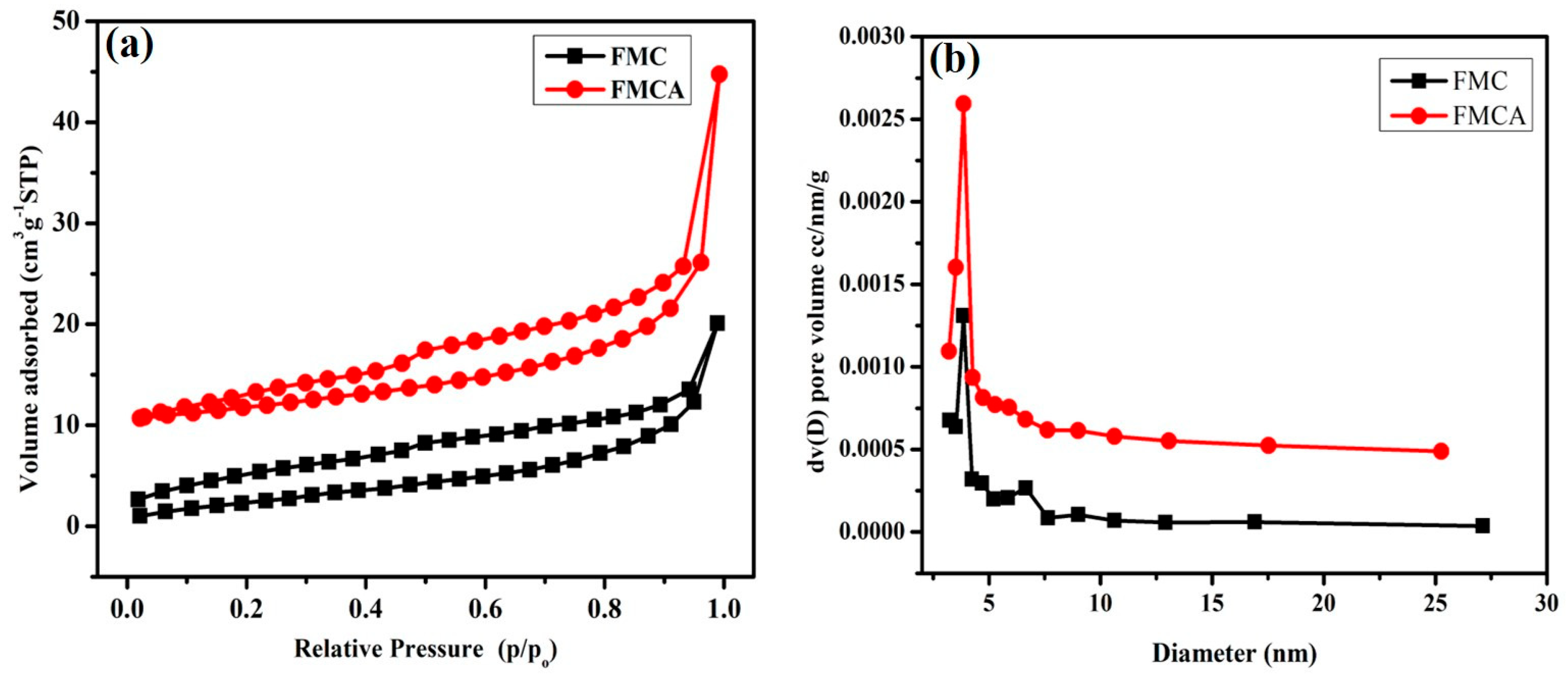
3.6. BET Surface Analysis
4. Electrochemical Analysis
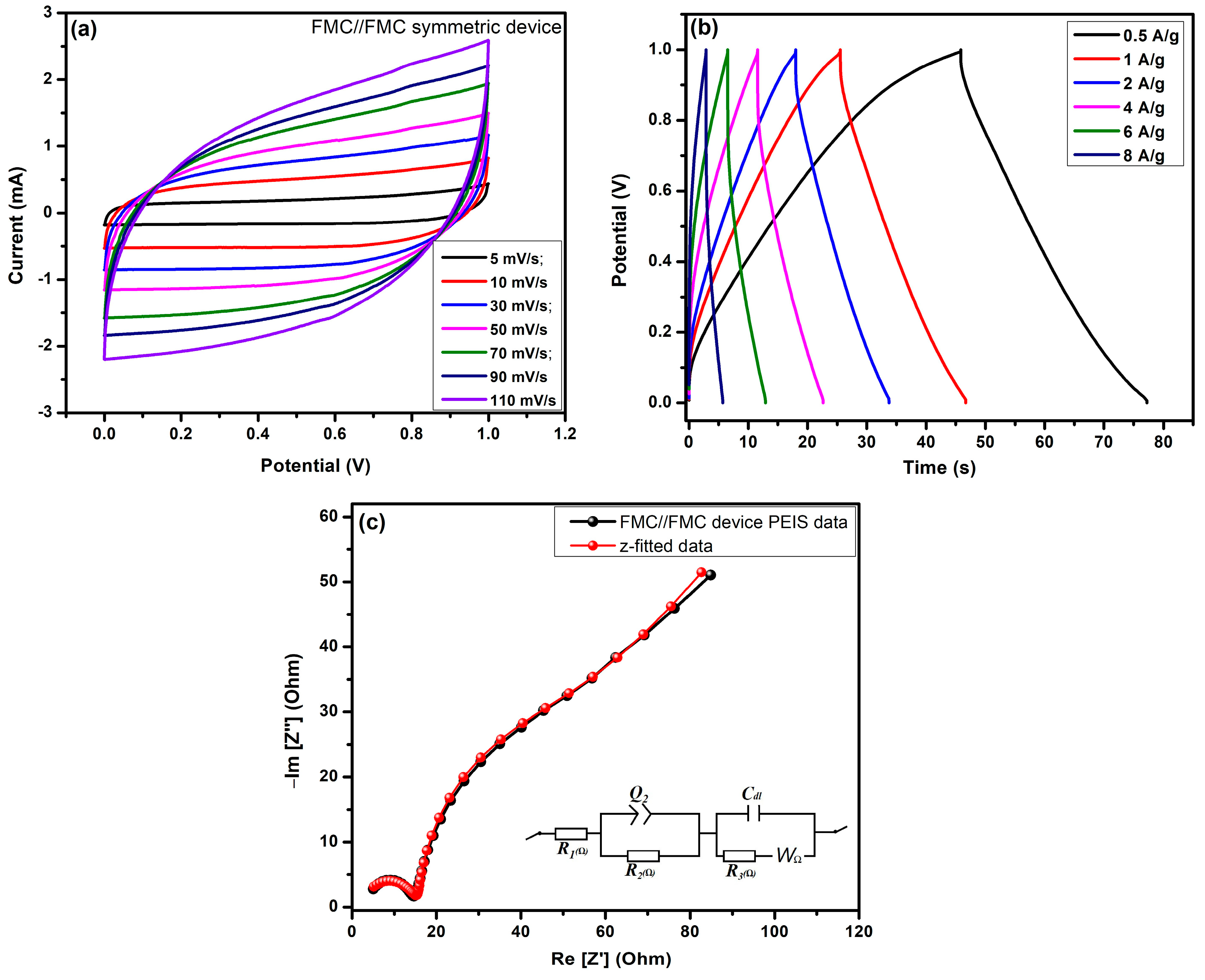
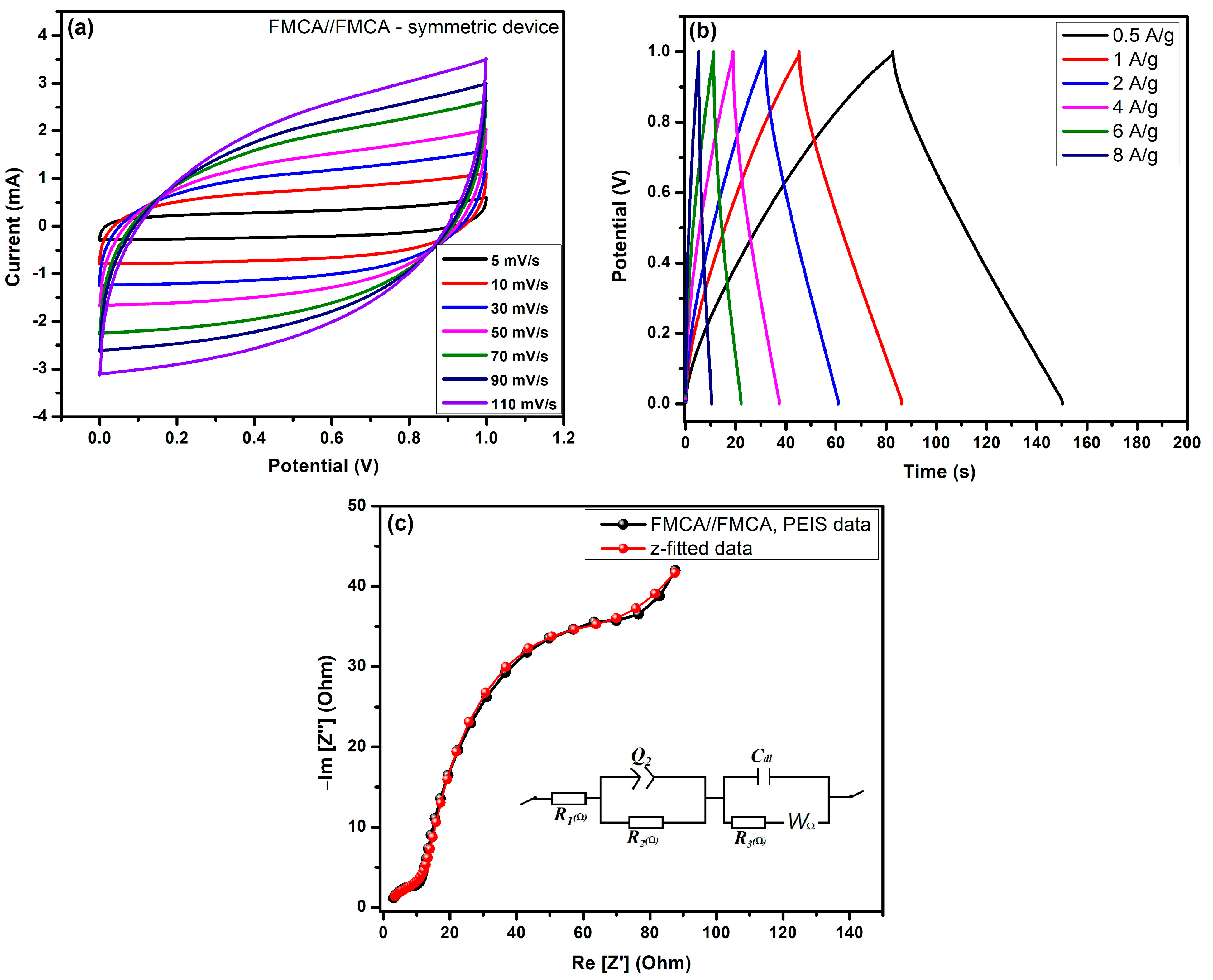
4.1. Two-Electrode Symmetry Device Performance
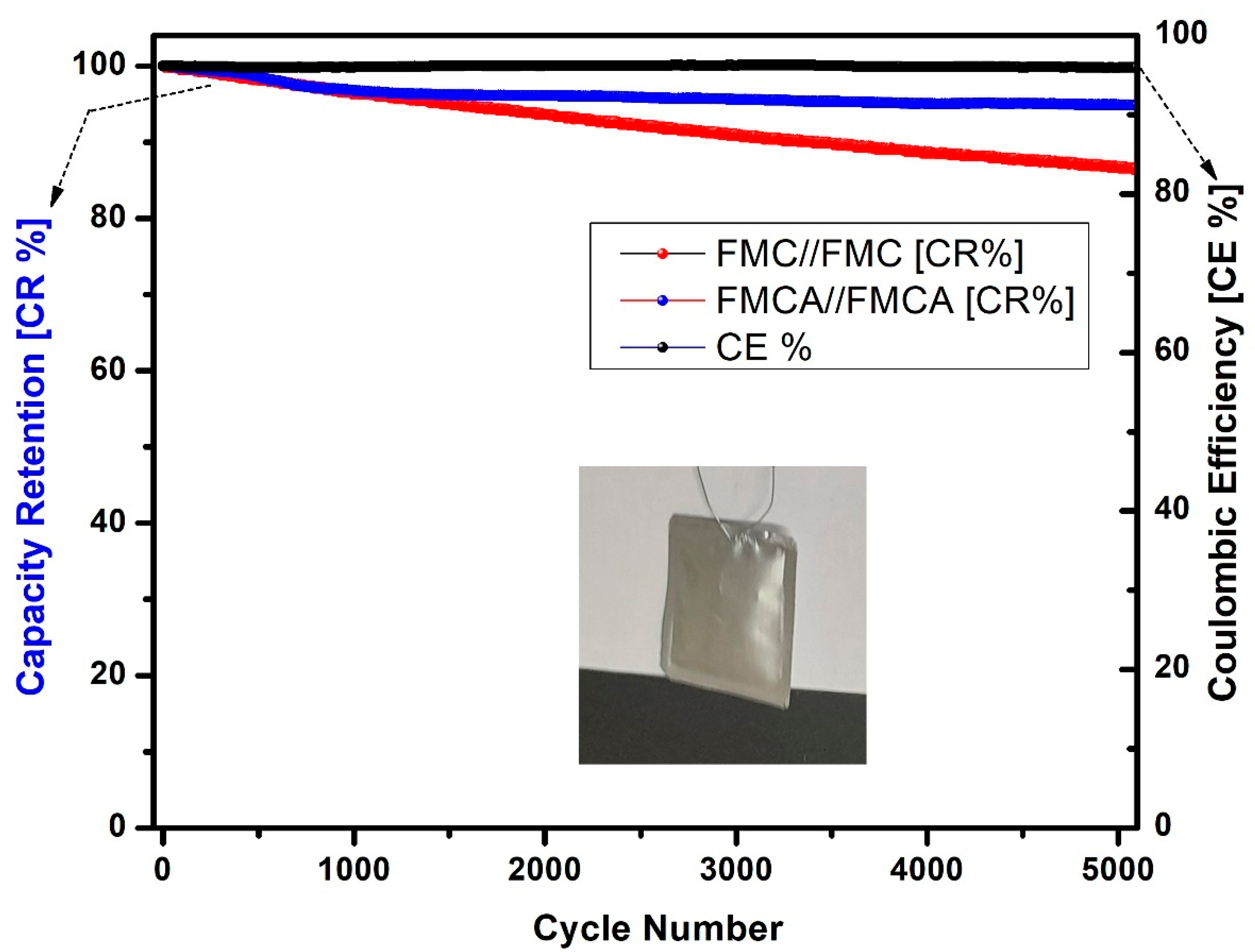
4.2. Cycling Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Jalal, N.I.; Ibrahim, R.I.; Oudah, M.K. A review on supercapacitors: Types and components. J. Phys. Conf. Ser. 2021, 1973, 012015. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Env. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, F.-G.; Liu, L.-N.; Xu, Z.-W.; Xie, G.; Li, J.; Gao, T.; Li, W.; Li, W.-S. Carbon Nanomaterials-Enabled High-Performance Supercapacitors: A Review, Adv. Energy Sustain. Res. 2023, 4, 2200152. [Google Scholar] [CrossRef]
- You, X.; Misra, M.; Gregori, S.; Mohanty, A.K. Preparation of an Electric Double Layer Capacitor (EDLC) Using Miscanthus-Derived Biocarbon. ACS Sustain. Chem. Eng. 2018, 6, 318–324. [Google Scholar] [CrossRef]
- Patel, A.; Patel, S.K.; Singh, R.S.; Patel, R.P. Review on recent advancements in the role of electrolytes and electrode materials on supercapacitor performances. Discov. Nano 2024, 19, 188. [Google Scholar] [CrossRef]
- Shaheen, I.; Hussain, I.; Zahra, T.; Javed, M.S.; Shah, S.S.A.; Khan, K.; Hanif, M.B.; Assiri, M.A.; Said, Z.; Arifeen, W.U.; et al. Recent advancements in metal oxides for energy storage materials: Design, classification, and electrodes configuration of supercapacitor. J. Energy Storage 2023, 72 Pt E, 108719. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwo, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Iqbal, S.; Khatoon, H.; Pandit, A.H.; Ahmad, S. Recent development of carbon based materials for energy storage devices. Mater. Sci. Energy Technol. 2019, 2, 417–428. [Google Scholar] [CrossRef]
- Jayaraman, T.; Murthy, A.P.; Elakkiya, V.; Chandrasekaran, S.; Nithyadharseni, P.; Khan, Z.; Senthil, R.A.; Shanker, R.; Raghavender, M.; Kuppusami, P.; et al. Recent development on carbon based heterostructures for their applications in energy and environment: A review. J. Ind. Eng. Chem. 2018, 64, 16–59. [Google Scholar] [CrossRef]
- Luo, L.; Lan, Y.; Zhang, Q.; Deng, J.; Luo, L.; Zeng, Q.; Gao, H.; Zhao, W. A review on biomass-derived activated carbon as electrode materials for energy storage supercapacitors. J. Energy Storage 2022, 55 Pt D, 105839. [Google Scholar] [CrossRef]
- Chaudhary, P.; Bansal, S.; Sharma, B.B.; Saini, S.; Joshi, A. Waste biomass-derived activated carbons for various energy storage device applications: A review. J. Energy Storage 2024, 78, 109996. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, S. Acid/base-treated activated carbons: Characterization of functional groups and metal adsorptive properties. Langmuir 2004, 20, 2233–2242. [Google Scholar] [CrossRef]
- Le Van, K.; Thu, T.L.T.; Thu, H.N.T.; Van Hoang, H. Activated Carbon by KOH and NaOH Activation: Preparation and Electrochemical Performance in K2SO4 and Na2SO4 Electrolytes. Russ. J. Electrochem. 2019, 55, 900–907. [Google Scholar] [CrossRef]
- Qu, Q.T.; Wang, B.; Yang, L.C.; Shi, Y. Study on electrochemical performance of activated carbon in aqueous Li2SO4, Na2SO4 and K2SO4 electrolytes. Electrochem. Commun. 2008, 10, 1652–1655. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Si, H.; Wang, B. Preparation and electrochemical performance of coconut shell activated carbon produced by the H3PO4 activation with rapid cooling method. Int. J. Electrochem. Sci. 2017, 12, 7844–7852. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Belver, C.; Bedia, J. Effect of synthesis conditions on the porous texture of activated carbons obtained from Tara Rubber by FeCl3 activation. Sci. Rep. 2024, 14, 2266. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Adsorption of Cr(VI) from aqueous phase by high surface area activated carbon prepared by chemical activation with ZnCl2. Process Saf. Environ. Prot. 2017, 109, 63–71. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Rash, T.; Firlej, L.; Kuchta, B.; Yu, P.; Suppes, G.; Wexler, C.; Pfeifer, P. Nanospace engineering of KOH activated carbon. Nanotechnology 2011, 23, 015401. [Google Scholar] [CrossRef]
- Yagmur, E.; Ozmak, M.; Aktas, Z. A novel method for production of activated carbon from waste tea by chemical activation with microwave energy. Fuel 2008, 87, 3278–3285. [Google Scholar] [CrossRef]
- De Castro, P.M.; Ferrarini, S.R.; Rimoli, M.D.S.F.; Merlo, A.A.; Nogueira, R.M.; Pires, E.M. Preparation and characterization of steam and CO2 activated carbon from Brazil nut shell. Biosci. J. 2023, 39, 39054. [Google Scholar] [CrossRef]
- Mai, N.T.; Nguyen, M.N.; Tsubota, T.; Nguyen, P.L.T.; Nguyen, N.H. Evolution of physico-chemical properties of Dicranopteris linearis-derived activated carbon under various physical activation atmospheres. Sci. Rep. 2021, 11, 14430. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Larsson, S.H.; de Oliveira, H.P.; Thyrel, M.; Claudio Lima, E. Sustainable Biomass Activated Carbons as Electrodes for Battery and Supercapacitors—A Mini-Review. Nanomaterials 2020, 10, 1398. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I.; Kaare, K.; Locs, J.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Biomass based activated carbons for fuel cells. Renew. Energy 2019, 141, 40–45. [Google Scholar] [CrossRef]
- Choudhary, R.; Pandey, O.P.; Brar, L.K. Influence of thermal treatment atmosphere on N-doped carbon spheres for wastewater treatment and supercapacitor applications. Mater. Chem. Phys. 2022, 284, 126037. [Google Scholar] [CrossRef]
- Selva, R.K.; Zhu, P.; Yan, C.I.; Zhu, J.D.; Dirican, M.; Shanmugavani, A.; Lee, Y.S.; Zhang, X.W. Biomass-derived porous carbon modified glass fiber separator as polysulfide reservoir for Li-S batteries. J. Colloid Interf. Sci. 2018, 513, 231–239. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.; Yang, Y.; Suo, G.; Zhang, L.; Lu, S.; Chen, Z. Biomass-Derived Carbon for High-Performance Batteries: From Structure to Properties. Adv. Funct. Mater. 2022, 32, 2201584. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, S.; Li, L.; Dou, S. Bio-nanotechnology in high-performance supercapacitors. Adv. Energy Mater. 2017, 7, 1700592. [Google Scholar] [CrossRef]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. C 2021, 7, 39. [Google Scholar] [CrossRef]
- Dobele, G.; Vervikishko, D.; Volperts, A.; Bogdanovich, N.; Shkolnikov, E. Characterization of the pore structure of nanoporous activated carbons produced from wood waste. Holzforschung 2013, 67, 587–594. [Google Scholar] [CrossRef]
- Li, S.; Tan, X.; Li, H.; Gao, Y.; Wang, Q.; Li, G.; Guo, M. Investigation on pore structure regulation of activated carbon derived from sargassum and its application in supercapacitor. Sci. Rep. 2022, 12, 10106. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Sun, L.; Li, N.; Wang, X.; Wang, Q.; Zhang, D.; Wang, B. Hydrothermal synthesis of 3D hierarchical ordered porous carbon from yam biowastes for enhanced supercapacitor performance. Chem. Eng. Sci. 2022, 252, 117514. [Google Scholar] [CrossRef]
- Mondal, A.K.; Kretschmer, K.; Zhao, Y.F.; Liu, H.; Wang, C.Y.; Sun, B.; Wang, G.X. Nitrogen-doped porous carbon nanosheets from eco-friendly eucalyptus leaves as high performance electrode materials for supercapacitors and lithium ion batteries. Chem. A Eur. J. 2017, 23, 3683–3690. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Zapata-Benabithe, Z.; Carrasco-Marín, F.; Moreno-Castilla, C. Activated carbons from KOH-activation of argan (Argania spinosa) seed shells as supercapacitor electrodes. Bioresour. Technol. 2012, 111, 185–190. [Google Scholar] [CrossRef]
- Baig, M.M.; Gul, I.H. Conversion of wheat husk to high surface area activated carbon for energy storage in high-performance supercapacitors. Biomass Bioenergy 2021, 144, 105909. [Google Scholar] [CrossRef]
- Saleh, A.S.; Zhang, Q.; Chen, J.; Shen, Q. Millet grains: Nutritional quality, processing, and potential health henefits Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Hutabarat, D.J.C.; Bowie, V.A. Bioactive compounds in foxtail millet (Setaria italica)-extraction, biochemical activity, and health functional: A Review. IOP Conf. Ser. Earth Environ. Sci. 2022, 998, 012060. [Google Scholar] [CrossRef]
- Darshitha, P.P.; Ravi, A.; Lasya, P.; Menon, M.; Sivasubramanian, G.; Sreekanth, K.M.; Sreedhar, K.M. Foxtail millet husk as an innovative biomass in the preparation of silica-silver composite with antimicrobial and free radicle scavenging activities. Mater. Today Proc. 2022, 66 Pt 4, 1830–1836. [Google Scholar] [CrossRef]
- Babu, A.; Kumar, A.R.; Amrutha, N.R.; Madhurya, S.; Kumar, H.N.P.; Reddy, J.P.; Murthy, P.S.K.; Varaprasad, K. Utilizing foxtail millet husk waste for sustainable new bioplastic composites with enhanced thermal stability and biodegradability. Int. J. Biol. Macromol. 2024, 282, 137283. [Google Scholar] [CrossRef]
- Tagade, A.; Sawarkar, A.N. Valorization of millet agro-residues for bioenergy production through pyrolysis: Recent inroads, technological bottlenecks, possible remedies, and future directions. Bioresour. Technol. 2023, 384, 129335. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Xiang, J.; Johnson, J.B.; Zheng, B.; Luo, L.; Beta, T. From Foxtail Millet Husk (Waste) to Bioactive Phenolic Extracts Using Deep Eutectic Solvent Extraction and Evaluation of Antioxidant, Acetylcholinesterase, and α-Glucosidase Inhibitory Activities. Foods 2023, 12, 1144. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Gairola, S.; Sinha, S.; Singh, I. Novel millet husk crop-residue based thermoplastic composites: Waste to value creation. Ind. Crops Prod. 2022, 182, 114891. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Insight into biochar properties and its cost analysis. Biomass Bioenergy 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Kuhe, A.; Terhemba, A.V.; Iortyer, H. Biomass valorization for energy applications: A preliminary study on millet husk. Heliyon 2021, 7, e07802. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Oyewusi, T.F.; Osunbitan, J.A.; Ogunjimi, L.A.O. Effect of binder type, binder concentration and compacting pressure on some physical properties of carbonized corncob briquette. Energy Rep. 2019, 5, 909–918. [Google Scholar] [CrossRef]
- Gairola, S.; Sinha, S.; Singh, I. Thermal stability of extracted lignin from novel millet husk crop residue. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124725. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.P.; Qiu, L.L.; Xiao, J.; Wu, F.P.; Cao, J.P.; Bai, Y.H.; Liu, F.J. Insights into the KOH activation parameters in the preparation of corncob-based microporous carbon for high-performance supercapacitors. Diam. Relat. Mater. 2022, 129, 109331. [Google Scholar] [CrossRef]
- Jafari, M.; Botte, G.G. Sustainable green route for activated carbon synthesis from biomass waste for high-performance supercapacitors. ACS Omega 2024, 9, 13134–13147. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chang, C.Y.; Tsai, S.W.; Lin, S.C.; Hsu, T.C.; Hsieh, T.H. Activated carbon for supercapacitor electrodes produced by the carbonation and activation of glucose with potassium nitrate. ACS Appl. Energy Mater. 2024, 7, 6873–6886. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Zhang, K.; Feng, J.; Sun, X.; Li, C.; Wang, K.; Zhang, X.; Ma, Y. Effects of activated carbon types on the CO2 supercapacitive swing adsorption performances. J. Solid State Electrochem. 2025, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Chi, Y.; Li, Z.; Sun, X.; Gu, H.; Zhang, H.; Chen, G.Z. Effects of Pore Widening vs oxygenation on capacitance of activated carbon in aqueous sodium sulfate electrolyte. J. Electrochem. Soc. 2020, 167, 040524. [Google Scholar] [CrossRef]
- Rajasekaran, S.J.; Grace, A.N.; Jacob, G.; Alodhayb, A.; Pandiaraj, S.; Raghavan, V. Investigation of Different Aqueous Electrolytes for Biomass-Derived Activated Carbon-Based Supercapacitors. Catalysts 2023, 13, 286. [Google Scholar] [CrossRef]
- Thirumal, V.; Yuvakkumar, R.; Ravi, G.; Dineshkumar, G.; Ganesan, M.; Alotaibi, S.H.; Velauthapillai, D. Characterization of activated biomass carbon from tea leaf for supercapacitor applications. Chemosphere 2022, 291 Pt 2, 132931. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajivgandhi, P.; Thirumal, V.; Sekar, A.; Kim, J. Biomass-Derived Activated Porous Carbon from Foxtail Millet Husk to Utilizing High-Performance Symmetric Supercapacitor Applications. Nanomaterials 2025, 15, 575. https://doi.org/10.3390/nano15080575
Rajivgandhi P, Thirumal V, Sekar A, Kim J. Biomass-Derived Activated Porous Carbon from Foxtail Millet Husk to Utilizing High-Performance Symmetric Supercapacitor Applications. Nanomaterials. 2025; 15(8):575. https://doi.org/10.3390/nano15080575
Chicago/Turabian StyleRajivgandhi, Perumal, Vediyappan Thirumal, Alagan Sekar, and Jinho Kim. 2025. "Biomass-Derived Activated Porous Carbon from Foxtail Millet Husk to Utilizing High-Performance Symmetric Supercapacitor Applications" Nanomaterials 15, no. 8: 575. https://doi.org/10.3390/nano15080575
APA StyleRajivgandhi, P., Thirumal, V., Sekar, A., & Kim, J. (2025). Biomass-Derived Activated Porous Carbon from Foxtail Millet Husk to Utilizing High-Performance Symmetric Supercapacitor Applications. Nanomaterials, 15(8), 575. https://doi.org/10.3390/nano15080575





