Hydrophobic Silicon Quantum Dots for Potential Imaging of Tear Film Lipid Layer
Abstract
1. Introduction
2. Results
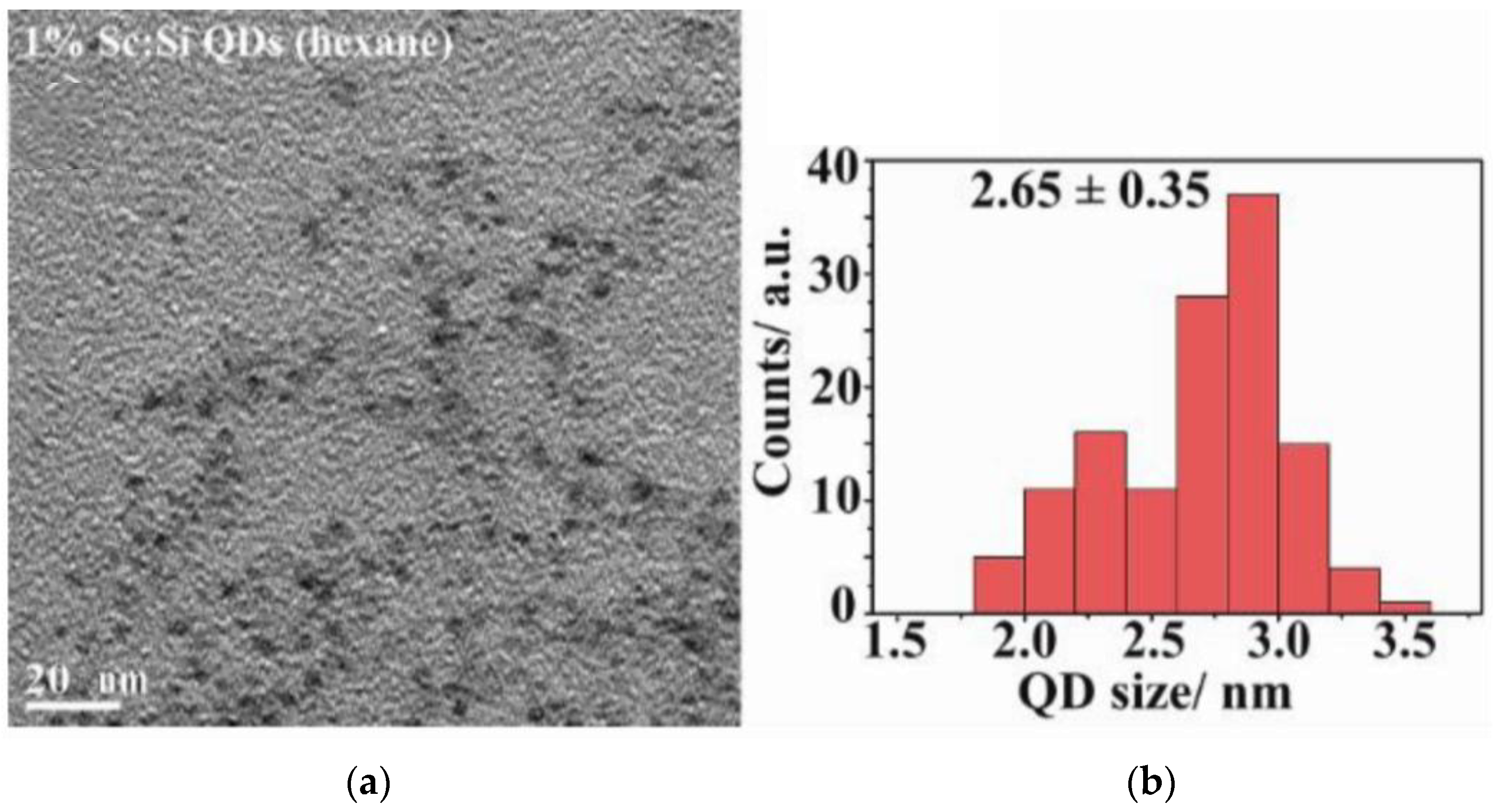
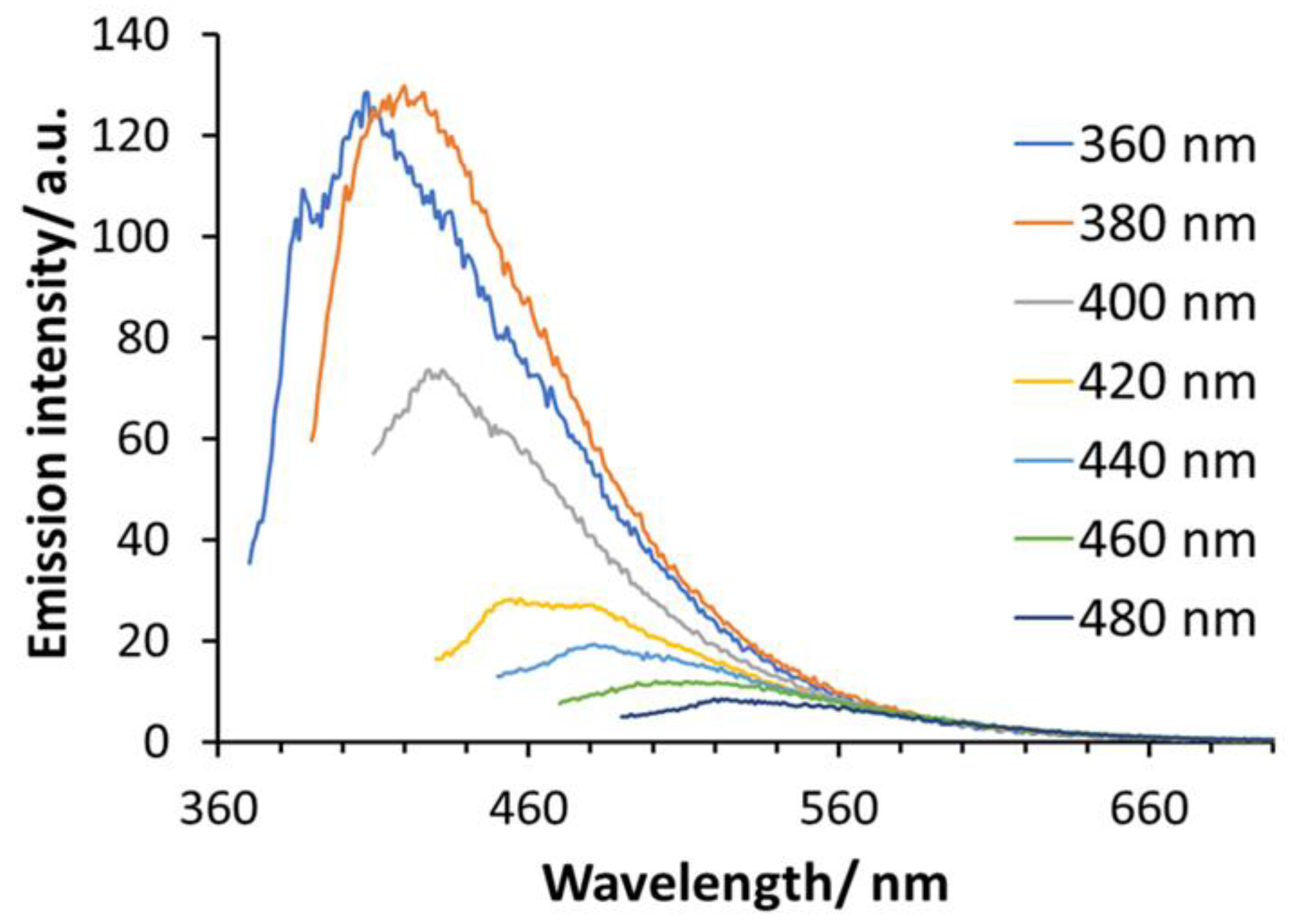
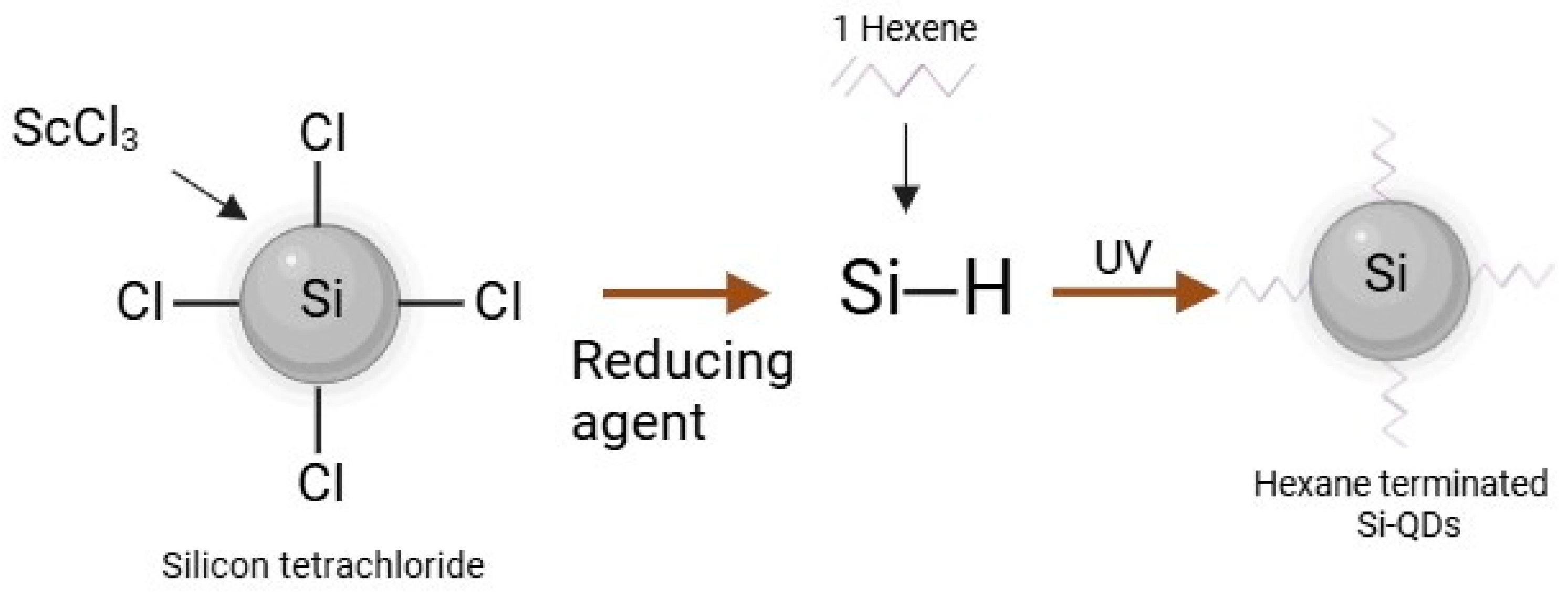
2.1. Characteristics of Si-QDs
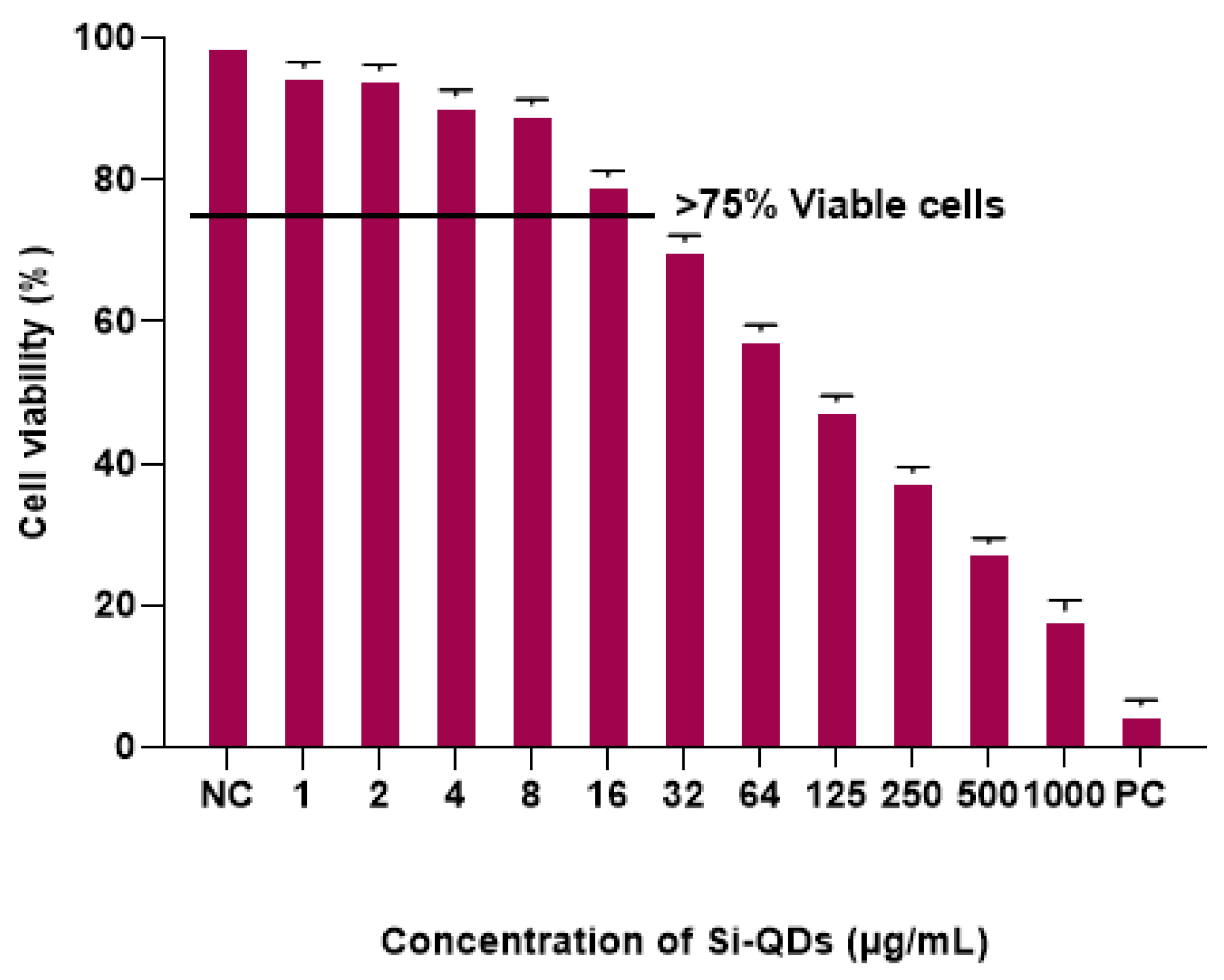
2.2. Cytotoxicity of Si-QDs
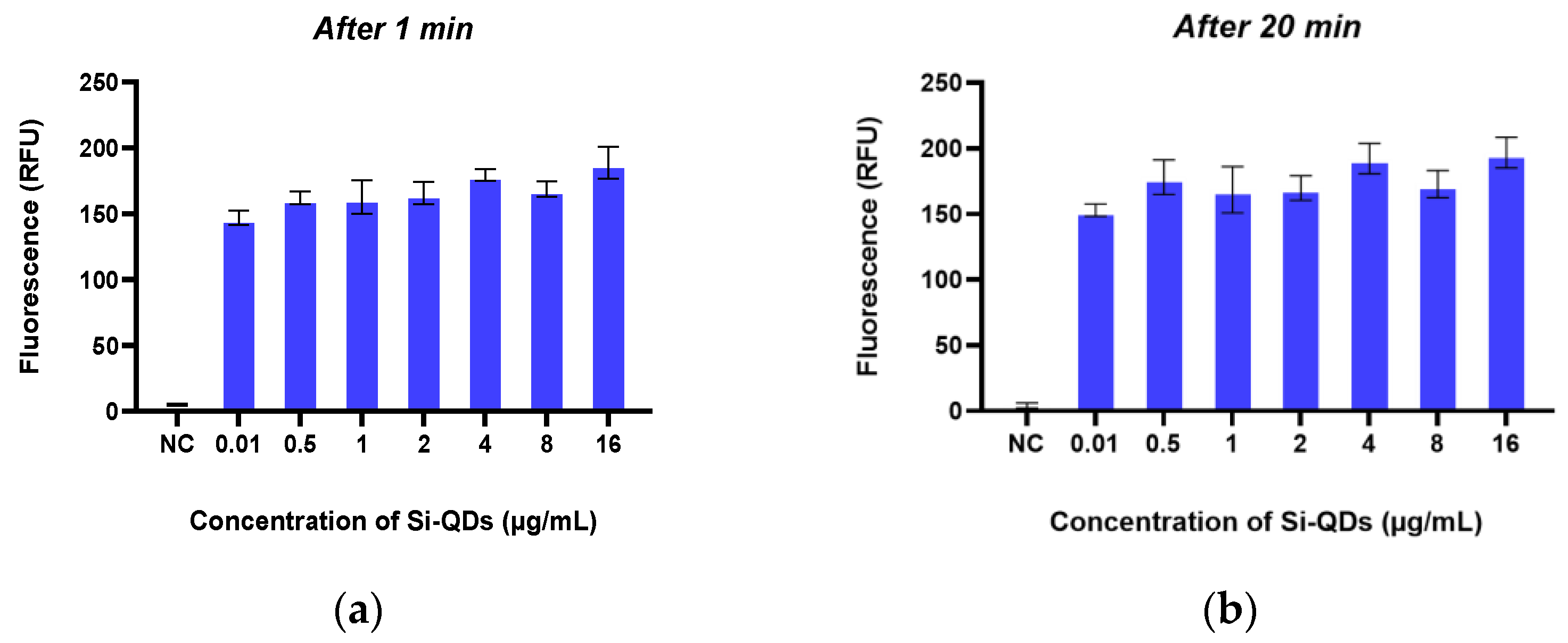
2.3. In Vitro Fluorescence Imaging of TheraTears® with Si-QDs
2.4. Solubility of Si-QDs
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterisation of Hydrophobic Sc-Si-QDs
4.2. MTT Assay
4.3. In Vitro Imaging of Si-QDs
4.4. Assessment of Si-QDs Solubility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Pflugfelder, S.C.; Liu, Z.; Baudouin, C.; Kim, H.M.; Messmer, E.M.; Kruse, F.; Liang, L.; Carreno-Galeano, J.T.; Rolando, M.; et al. Defining dry eye from a clinical perspective. Int. J. Mol. Sci. 2020, 21, 9271. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Zierhut, M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv. Ophthalmol. 2001, 45, 203–210. [Google Scholar] [CrossRef]
- Farrand, K.F.; Fridman, M.; Stillman, I.O.; Schaumberg, D.A. Prevalence of diagnosed dry eye disease in the united states among adults aged 18 years and older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II tear film report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Craig, J.P.; Alves, M.; Wolffsohn, J.S.; Downie, L.E.; Efron, N.; Galor, A.; Gomes, J.A.P.; Jones, L.; Markoulli, M.; Stapleton, F.; et al. TFOS lifestyle report executive summary: A lifestyle epidemic-ocular surface disease. Ocul. Surf. 2023, 30, 240–253. [Google Scholar] [CrossRef]
- Potvin, R.; Makari, S.; Rapuano, C.J. Tear Film osmolarity and dry eye disease: A review of the literature. Clin. Ophthalmol. 2015, 2015, 2039. [Google Scholar] [CrossRef]
- Rouen, P.A.; White, M.L. Dry eye disease: Prevalence, assessment, and management. Home Healthc. Now 2018, 36, 74–83. [Google Scholar]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Shapiro, A.; Merin, S. Schirmer test and break-up time of tear film in normal subjects. Am. J. Ophthalmol. 1979, 88, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Millar, T.J. Nanoscale phase dynamics of the normal tear film. Nanomed. Nanotechnol. 2010, 6, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763. [Google Scholar] [CrossRef] [PubMed]
- Serruya, L.G.; Nogueira, D.C.; Hida, R.Y. Schirmer test performed with open and closed eyes: Variations in normal individuals. Arq. Bras. Oftalmol. 2009, 72, 65–67. [Google Scholar] [CrossRef]
- Doane, M.G. An instrument for in vivo tear film interferometry. Optom. Vis. Sci. 1989, 66, 383–388. [Google Scholar] [CrossRef]
- Wang, J.; Aquavella, J.; Palakuru, J.; Chung, S.; Feng, C. Relationships between central tear film thickness and tear menisci of the upper and lower eyelids. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4349–4355. [Google Scholar] [CrossRef]
- Holly, F.J. Physical chemistry of the normal and disordered tear film. Trans. Ophthalmol. Soc. UK 1985, 104, 374–380. [Google Scholar]
- Papas, E.B. Diagnosing dry-eye: Which tests are most accurate? Contactlens Anterior Eye 2023, 46, 102048. [Google Scholar] [CrossRef]
- Walling, M.A.; Novak, J.A.; Shepard, J.R.E. Quantum dots for live cell and in vivo imaging. Int. J. Mol. Sci. 2009, 10, 441–491. [Google Scholar] [CrossRef]
- Arya, H.; Kaul, Z.; Wadhwa, R.; Taira, K.; Hirano, T.; Kaul, S.C. Quantum dots in bio-imaging: Revolution by the small. Biochem. Biophys. Res. Commun. 2005, 329, 1173–1177. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; RodriguezViejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Sarwat, S.; Stapleton, F.J.; Willcox, M.D.P.; O’Mara, P.B.; Tilley, R.D.; Gooding, J.J.; Roy, M. Feasibility of silicon quantum dots as a biomarker for the bioimaging of tear film. Nanomaterials 2022, 12, 1965. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Jeong, H.; Hong, J.; Chang, M.; Kim, M.; Chuck, R.S.; Lee, J.K.; Park, C.-Y. The effect of silica nanoparticles on human corneal epithelial cells. Sci. Rep. 2016, 6, 37762. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.H.; Kim, M.; Jeong, H.; Hong, J.; Chuck, R.S.; Park, C.Y. Safety of nonporous silica nanoparticles in human corneal endothelial cells. Sci. Rep. 2017, 7, 14566. [Google Scholar] [CrossRef]
- Yim, B.; Park, J.H.; Jeong, H.; Hong, J.; Shin, Y.J.; Chuck, R.S.; Park, C.Y. The effects of nonporous silica nanoparticles on cultured human keratocytes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 362–371. [Google Scholar] [CrossRef]
- Korhonen, E.; Rönkkö, S.; Hillebrand, S.; Riikonen, J.; Xu, W.; Järvinen, K.; Lehto, V.-P.; Kauppinen, A. Cytotoxicity assessment of porous silicon microparticles for ocular drug delivery. Eur. J. Pharm. Biopharm. 2016, 100, 1–8. [Google Scholar] [CrossRef]
- Fan, J.-W.; Vankayala, R.; Chang, C.-L.; Chang, C.-H.; Chiang, C.-S.; Hwang, K.C. Preparation, cytotoxicity and in vivo bioimaging of highly luminescent water-soluble silicon quantum dots. Nanotechnology 2015, 26, 215703. [Google Scholar] [CrossRef]
- McVey, B.F.P.; König, D.; Cheng, X.; O’Mara, P.B.; Seal, P.; Tan, X.; Tahini, H.A.; Smith, S.C.; Gooding, J.J.; Tilley, R.D. Synthesis, optical properties and theoretical modelling of discrete emitting states in doped silicon nanocrystals for bioimaging. Nanoscale 2018, 10, 15600–15607. [Google Scholar] [CrossRef]
- McVey, B.F.P.; Butkus, J.; Halpert, J.E.; Hodgkiss, J.M.; Tilley, R.D. Solution synthesis and optical properties of transition-metal-doped silicon nanocrystals. J. Phys. Chem. Lett. 2015, 6, 1573–1576. [Google Scholar] [CrossRef]
- McVey, B.F.P.; Tilley, R.D. Solution synthesis, optical properties, and bioimaging applications of silicon nanocrystals. Acc. Mater. Res. 2014, 47, 3045–3051. [Google Scholar] [CrossRef]
- Shiohara, A.; Prabakar, S.; Faramus, A.; Hsu, C.-Y.; Lai, P.-S.; Northcote, P.T.; Tilley, R.D. Sized controlled synthesis, purification, and cell studies with silicon quantum dots. Nanoscale 2011, 3, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.H.; Hoshino, A.; Yamamoto, K.; Tilley, R.D. Water-soluble photoluminescent silicon quantum dots. Angew. Chem. Int. Ed. 2005, 44, 4550–4554. [Google Scholar] [CrossRef] [PubMed]
- King-Smith, P.E.; Ramamoorthy, P.; Braun, R.; Nichols, J.J. Tear film images and breakup analyzed using fluorescent quenching. Investig. Opthalmol. Vis. Sci. 2013, 54, 6003. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, G.; Padma, M. Clinical profile of patients with aqueous deficient dry eye. Indian J. Clin. Exp. Ophthalmol. 2022, 8, 340–344. [Google Scholar] [CrossRef]
- Matvienko, O.O.; Savin, Y.N.; Kryzhanovska, A.S.; Vovk, O.M.; Dobrotvorska, M.V.; Pogorelova, N.V.; Vashchenko, V.V. Dispersion and aggregation of quantum dots in polymer–inorganic hybrid films. Thin Solid Film. 2013, 537, 226–230. [Google Scholar] [CrossRef]
- Poderys, V.; Matulionytė, M.; Selskis, A.; Rotomskis, R. Interaction of water-soluble CdTe quantum dots with bovine serum albumin. Nanoscale Res. Lett. 2010, 6, 9. [Google Scholar] [CrossRef]
- Xie, J.; Mei, L.; Sun, Y.; Yong, X.; Han, N.; Dai, J.; Yang, X.; Ruan, G. Direct and noninvasive penetration of bare hydrophobic quantum dots through live cell membranes. ACS Biomater. Sci. Eng. 2019, 5, 468–477. [Google Scholar] [CrossRef]
- Zrazhevskiy, P.; Dave, S.R.; Gao, X. Addressing key technical aspects of quantum dot probe preparation for bioassays. Part. Part. Syst. Charact. 2014, 31, 1291–1299. [Google Scholar] [CrossRef]
- Singh, N.P.; Charan, S.; Sanjiv, K.; Huang, S.-H.; Hsiao, Y.C.; Kuo, C.-N.; Chien, F.C.; Lee, T.C.; Chen, P. Synthesis of tunable and multifunctional ni-doped near-infrared qds for cancer cell targeting and cellular sorting. Bioconjugate Chem. 2012, 23, 421–430. [Google Scholar] [CrossRef]
- Chen, N.; He, Y.; Su, Y.; Li, X.; Huang, Q.; Wang, H.; Zhang, X.; Tai, R.; Fan, C. The cytotoxicity of cadmium-based quantum dots. Biomaterials 2012, 33, 1238–1244. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Interaction of Mesoporous Silica Nanoparticles with Human Red Blood Cell Membranes: Size and Surface Effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, D.; Wu, P. One-pot, facile, and versatile synthesis of monolayer MoS quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Stanković, N.K.; Bodík, M.; Šiffalovič, P.; Kotlár, M.; Mičušík, M.; Špitálský, Z.; Danko, M.; Milivojević, D.; Kleinová, A.; Kubát, P.; et al. Antibacterial and antibiofouling properties of light triggered fluorescent hydrophobic carbon quantum dots langmuir–blodgett thin films. Acs Sustain. Chem. Eng. 2018, 6, 4154–4163. [Google Scholar] [CrossRef]
- Amna, T.; Ba, H.V.; Vaseem, M.; Hassan, M.S.; Khil, M.-S.; Hahn, Y.B.; Lee, H.-K.; Hwang, I. Apoptosis induced by copper oxide quantum dots in cultured C2C12 cells via caspase 3 and caspase 7: A study on cytotoxicity assessment. Appl. Microbiol. Biotechnol. 2013, 97, 5545–5553. [Google Scholar] [CrossRef]
- Bai, C.; Wei, T.; Zou, L.; Liu, N.; Huang, X.; Tang, M. The apoptosis induced by CdTe quantum dots through the mitochondrial pathway in dorsal root ganglion cell line ND7/23. J. Appl. Toxicol. 2022, 42, 1218–1229. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Lee, C.-F.; Lin, S.-J.; Dong, C.-Y.; Chen, C.-C.; Tan, H.-Y. Studies of intracorneal distribution and cytotoxicity of quantum dots: Risk assessment of eye exposure. Chem. Res. Toxicol. 2011, 24, 253–261. [Google Scholar] [CrossRef]
- Parak, W.J.; Pellegrino, T.; Plank, C. Labelling of cells with quantum dots. Nanotechnology 2005, 16, R9–R25. [Google Scholar] [CrossRef]
- Tilley, R.D.; Yamamoto, K. The microemulsion synthesis of hydrophobic and hydrophilic silicon nanocrystals. Adv. Mater. 2006, 18, 2053–2056. [Google Scholar] [CrossRef]
- Akerman, M.E.; Chan, W.C.W.; Laakkonen, P.; Bhatia, S.N.; Ruoslahti, E. Nanocrystal targeting in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 12617–12621. [Google Scholar]
- Sarwat, S.; Stapleton, F.; Willcox, M.; Roy, M. Quantum dots in ophthalmology: A literature review. Curr. Eye Res. 2019, 44, 1037–1046. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwat, S.; Stapleton, F.; Willcox, M.D.P.; O’Mara, P.B.; Roy, M. Hydrophobic Silicon Quantum Dots for Potential Imaging of Tear Film Lipid Layer. Nanomaterials 2025, 15, 552. https://doi.org/10.3390/nano15070552
Sarwat S, Stapleton F, Willcox MDP, O’Mara PB, Roy M. Hydrophobic Silicon Quantum Dots for Potential Imaging of Tear Film Lipid Layer. Nanomaterials. 2025; 15(7):552. https://doi.org/10.3390/nano15070552
Chicago/Turabian StyleSarwat, Sidra, Fiona Stapleton, Mark D. P. Willcox, Peter B. O’Mara, and Maitreyee Roy. 2025. "Hydrophobic Silicon Quantum Dots for Potential Imaging of Tear Film Lipid Layer" Nanomaterials 15, no. 7: 552. https://doi.org/10.3390/nano15070552
APA StyleSarwat, S., Stapleton, F., Willcox, M. D. P., O’Mara, P. B., & Roy, M. (2025). Hydrophobic Silicon Quantum Dots for Potential Imaging of Tear Film Lipid Layer. Nanomaterials, 15(7), 552. https://doi.org/10.3390/nano15070552







