Adverse Outcome Pathways (AOPs) Oriented Approach to Assess In Vitro Hazard of Silica and Lignin Nanomaterials Derived from Biomass Residues
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Literature Search and AOP-Wiki Database Examination
2.3. SiNPs and LigNPs: Synthesis, Preparation of Suspensions and Physico-Chemical Characterization
2.3.1. Synthesis of SiNPs and LigNPs
2.3.2. Preparation of SiNPs and LigNPs Suspensions
2.3.3. Physico-Chemical (p-Chem) Characterization of SiNPs and LigNPs
2.4. B-NMs In Vitro Hazard Assessment in Cell Monocultures
2.4.1. Cell Maintenance and Treatments of Monocultures
2.4.2. Cell Viability (MTT, Alamar Blue)
2.4.3. Intracellular Reactive Oxygen Species Detection
2.4.4. Release of Inflammatory Mediators (IL-8, IL-6 and IL-1β)
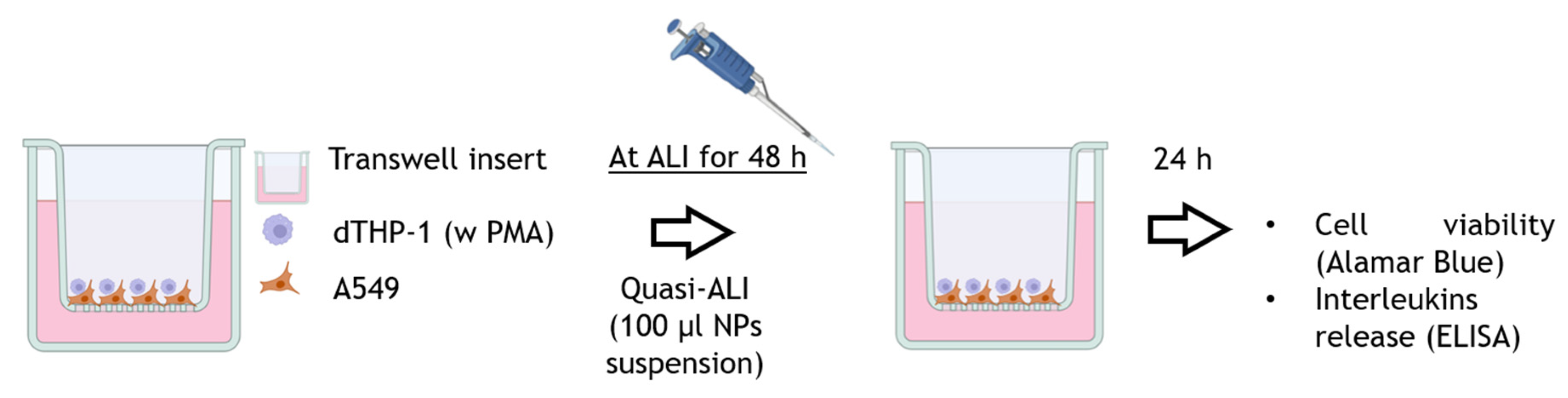
2.5. B-NMs In Vitro Hazard Assessment in Cell Co-Cultures
2.5.1. Co-Culture Set-Up and Quasi-ALI Exposure
2.5.2. Cell Viability and Inflammatory Responses of Co-Culture Models
2.6. Statistical Analysis
3. Results
3.1. Literature and AOP-Wiki Research for the Definition of the Biological Endpoints
3.2. NPs Characterization
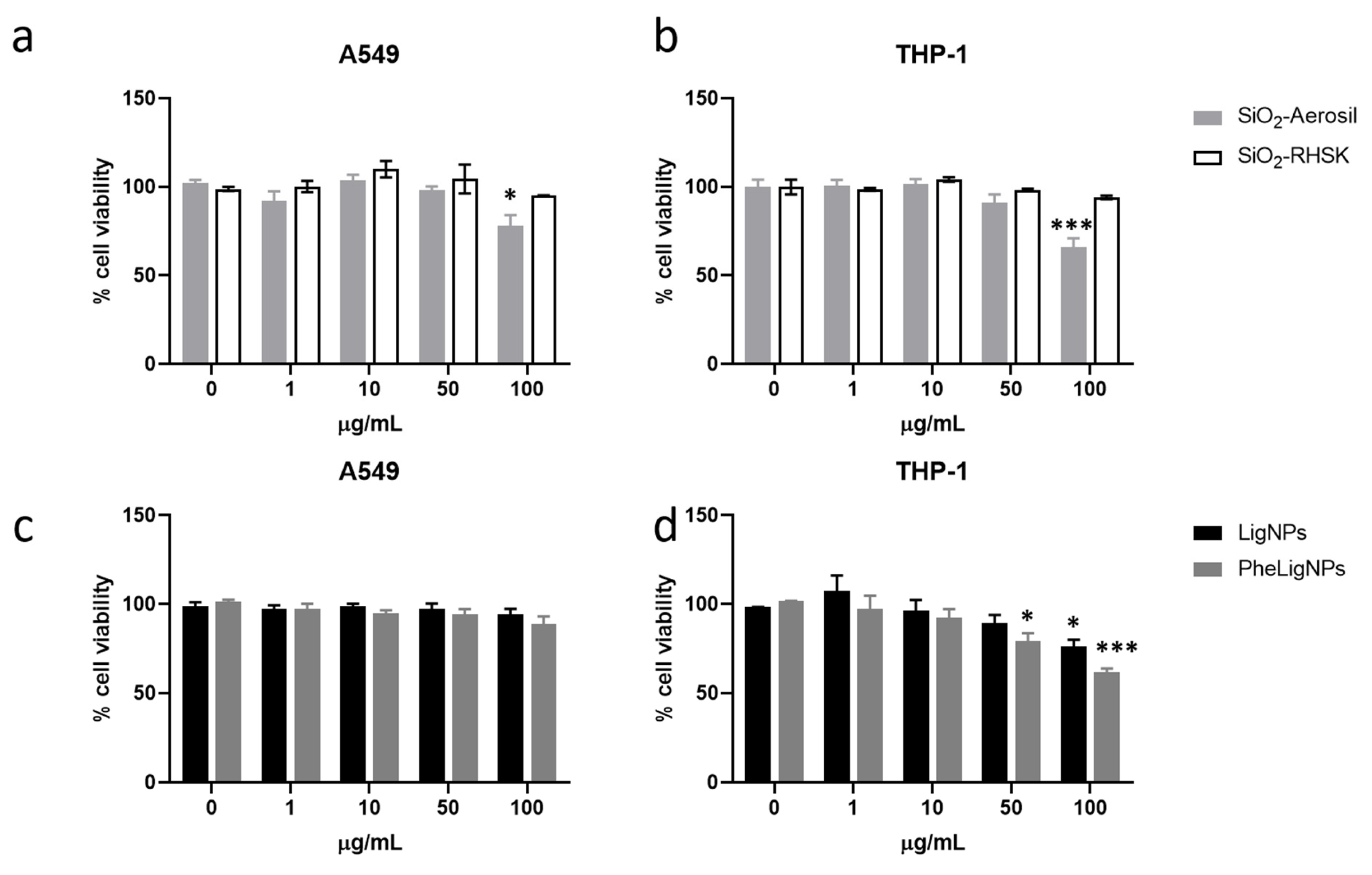
3.3. In Vitro Cellular Responses in Monocultures
3.3.1. Cell Viability
3.3.2. ROS Formation
3.3.3. Release of Inflammatory Mediators
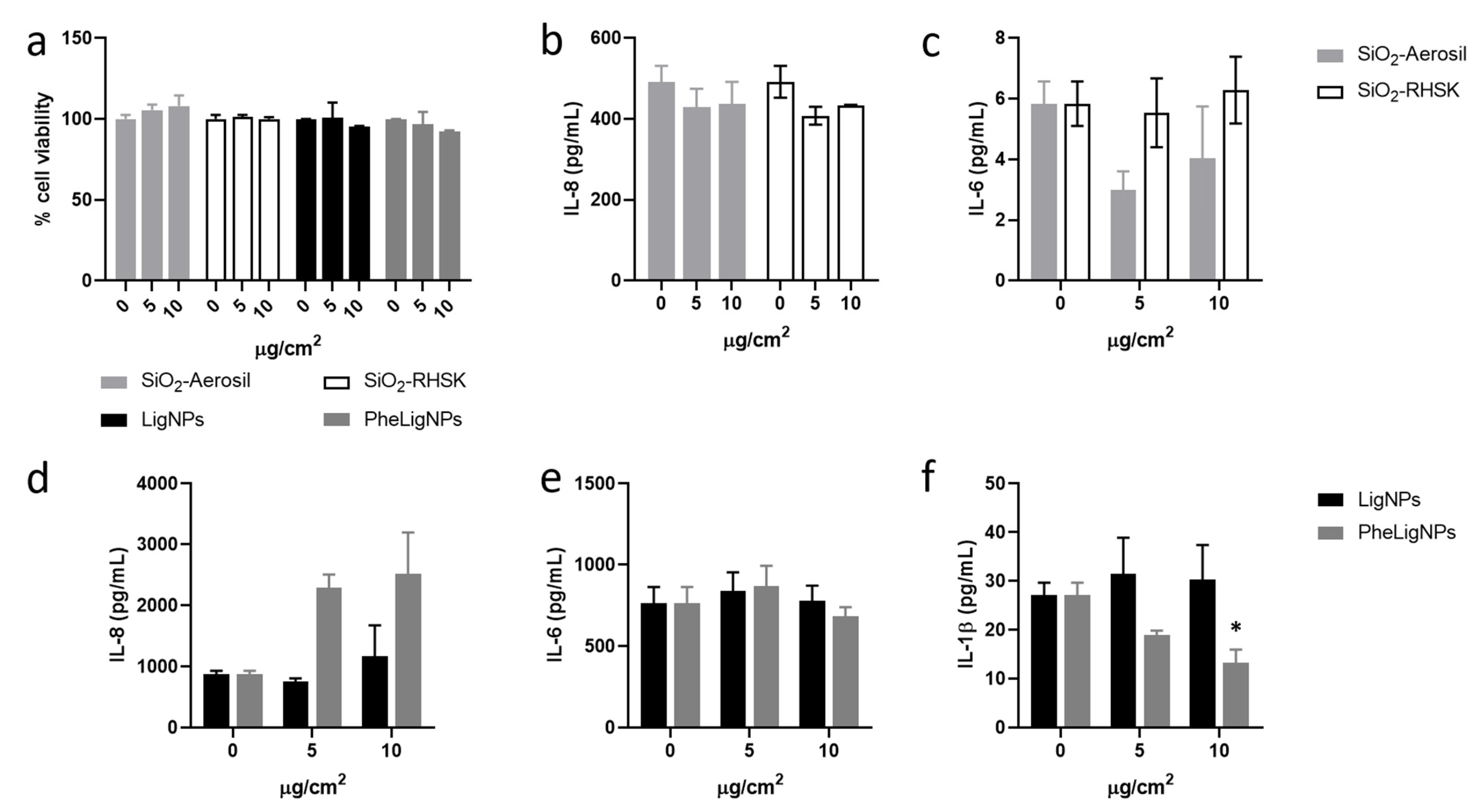
3.4. In Vitro Responses in Co-Culture Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
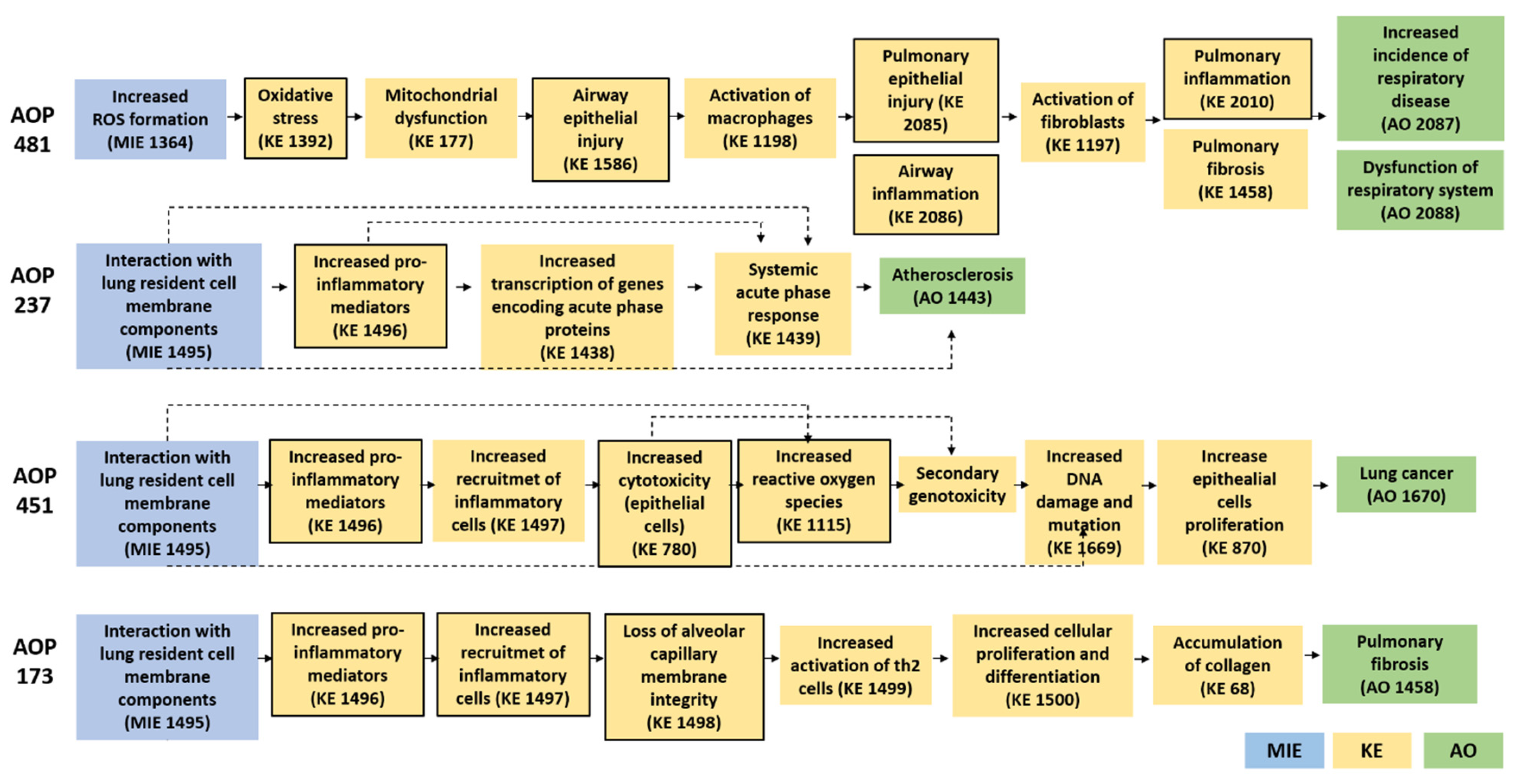
| Stressor (ID: Title) | AOP (ID: Title) | KEs (ID: Title) | AO (IDr: Title) |
|---|---|---|---|
| 224: nanoparticles | 144: Endocytic lysosomal uptake leading to liver fibrosis | 898: Disruption, Lysosome 177: Mitochondrial dysfunction 55: Cell injury/death 1493: Increased Pro-inflammatory mediators 1494: Leukocyte recruitment/activation 265: Activation, Stellate cells 68: Accumulation, Collagen | 344: Liver Fibrosis |
| 392: Decreased fibrinolysis and activated bradykinin system leading to hyperinflammation | 1496: Increased, secretion of proinflammatory mediators 1497: Increased, recruitment of inflammatory cells | 1868: Hyperinflammation | |
| 451: Interaction with lung resident cell membrane components leads to lung cancer | 1496: Increased, secretion of proinflammatory mediators 1497: Increased, recruitment of inflammatory cells 780: Increase, Cytotoxicity (epithelial cells) 1115: Increased, Reactive oxygen species 2006: Secondary genotoxicity 1669: Increased, DNA damage and mutation 870: Increase, Cell Proliferation Increase | 1670: Lung cancer | |
| 254: silica nanoparticles | 209: Perturbation of cholesterol and glutathione homeostasis leading to hepatotoxicity: Integrated multi-OMICS approach for building AOP | 1284: Up Regulation, SREBF2 1285: Up Regulation, Unsaturated fatty acid Up Regulation, 1286: Down Regulation, GSS and GSTs gene 1287: Glutathione synthesis 1288: Activation, 3-hydroxy-3-methylglutaryl-CoA reductase gene 1289: Perturbation of cholesterol 1290: Glutathione homeostasis | 1291: Hepatoxicity |
| 481: AOPs of amorphous silica nanoparticles: ROS-mediated oxidative stress increased respiratory dysfunction and diseases. | 1392: Oxidative Stress; 1816: Mitochondrial dysfunction; 1586: Airway epithelial injury 1198: Activation, Macrophages 2085: Pulmonary epithelial injury 1197: Activation, Fibroblasts 2086: Airway inflammation 2010: Pulmonary inflammation 1458: Pulmonary fibrosis | 2087: Increased incidence of respiratory disease 2088—Respiratory dysfunction | |
| 377: Insoluble nanosized particles | 237: Substance interaction with lung resident cell membrane components leading to atherosclerosis | 1496: Increased, secretion of proinflammatory mediators 1438: Transcription of genes encoding acute phase proteins, Increased 1439: Systemic acute phase response | 1443: Atherosclerosis |
References
- Saraswat, P.; Singh, S.; Prasad, M.; Misra, R.; Rajput, V.D.; Ranjan, R. Applications of bio-based nanomaterials in environment and agriculture: A review on recent progresses. Hybrid Adv. 2023, 4, 100097. [Google Scholar] [CrossRef]
- Akhter, F.; Rao, A.A.; Abbasi, M.N.; Wahocho, S.A.; Mallah, M.A.; Anees-ur-Rehman, H.; Chandio, Z.A. A Comprehensive Review of Synthesis, Applications and Future Prospects for Silica Nanoparticles (SNPs). Silicon 2022, 14, 8295–8310. [Google Scholar] [CrossRef]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin nanoparticles enter the scene: A promising versatile green tool for multiple applications. Biotechnol. Adv. 2021, 47, 107685. [Google Scholar] [CrossRef]
- Solihat, N.N.; Hidayat, A.F.; Ilyas, R.A.; Thiagamani, S.M.K.; Azeele, N.I.W.; Sari, F.P.; Ismayati, M.; Bakshi, M.I.; Garba, Z.N.; Hussin, M.H.; et al. Recent antibacterial agents from biomass derivatives: Characteristics and applications. J. Bioresour. Bioprod. 2024, 9, 283–309. [Google Scholar] [CrossRef]
- Morena, A.G.; Tzanov, T. Antibacterial lignin-based nanoparticles and their use in composite materials. Nanoscale Adv. 2022, 4, 4447–4469. [Google Scholar] [CrossRef]
- Sienkiewicz, N.; Członka, S. Natural Additives Improving Polyurethane Antimicrobial Activity. Polymers 2022, 14, 2533. [Google Scholar] [CrossRef] [PubMed]
- Alhadhrami, A.; Mohamed, G.G.; Sadek, A.H.; Ismail, S.H.; Ebnalwaled, A.A.; Almalki, A.S.A. Behavior of Silica Nanoparticles Synthesized from Rice Husk Ash by the Sol–Gel Method as a Photocatalytic and Antibacterial Agent. Materials 2022, 15, 8211. [Google Scholar] [CrossRef]
- Augaitis, N.; Vaitkus, S.; Członka, S.; Kairytė, A. Research of Wood Waste as a Potential Filler for Loose-Fill Building Insulation: Appropriate Selection and Incorporation into Polyurethane Biocomposite Foams. Materials 2020, 13, 5336. [Google Scholar] [CrossRef]
- Wurster, S.; Ladu, L. Bio-Based Products in the Automotive Industry: The Need for Ecolabels, Standards, and Regulations. Sustainability 2020, 12, 1623. [Google Scholar] [CrossRef]
- Faruk, O.; Sain, M.; Farnood, R.; Pan, Y.; Xiao, H. Development of Lignin and Nanocellulose Enhanced Bio PU Foams for Automotive Parts. J. Polym. Environ. 2014, 22, 279–288. [Google Scholar] [CrossRef]
- Hossain, S.K.S.; Mathur, L.; Roy, P.K. Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. J. Asian Ceram. Soc. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Bragato, C.; Mazzotta, R.; Persico, A.; Bengalli, R.; Ornelas, M.; Gomes, F.; Bonfanti, P.; Mantecca, P. Biocompatibility Analysis of Bio-Based and Synthetic Silica Nanoparticles during Early Zebrafish Development. Int. J. Mol. Sci. 2024, 25, 5530. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.Y.; Gu, Y.M.; Jang, I.S.; Park, H.; Oh, K.K.; Lee, J.H.; Chun, J. Eco-friendly and facile synthesis of size-controlled spherical silica particles from rice husk. Nanoscale Adv. 2021, 3, 6965–6973. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y. Rice husk silica derived nanomaterials for sustainable applications. Renew. Sustain. Energy Rev. 2017, 80, 453–466. [Google Scholar] [CrossRef]
- Chun, J.; Lee, J.H. Recent Progress on the Development of Engineered Silica Particles Derived from Rice Husk. Sustainability 2020, 12, 10683. [Google Scholar] [CrossRef]
- Steven, S.; Restiawaty, E.; Bindar, Y. Routes for energy and bio-silica production from rice husk: A comprehensive review and emerging prospect. Renew. Sustain. Energy Rev. 2021, 149, 111329. [Google Scholar] [CrossRef]
- Lee, D.I.; Ha, Y.H.; Jeon, H.; Kim, S.H. Preparation and Properties of Polyurethane Composite Foams with Silica-Based Fillers. Appl. Sci. 2022, 12, 7418. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Zhang, F.; Jin, S.; Xiao, Y.; Xin, B.; Zheng, Y. Preparation of Waterproof and Breathable Polyurethane Fiber Membrane Modified by Fluorosilane-modified Silica. Fibers Polym. 2020, 21, 954–964. [Google Scholar] [CrossRef]
- Grossman, A.; Wilfred, V. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Cavallo, E.; He, X.; Luzi, F.; Dominici, F.; Cerrutti, P.; Bernal, C.; Foresti, M.L.; Torre, L.; Puglia, D. UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles. Molecules 2020, 26, 126. [Google Scholar] [CrossRef]
- Tortora, M.; Cavalieri, F.; Mosesso, P.; Ciaffardini, F.; Melone, F.; Crestini, C. Ultrasound driven assembly of lignin into microcapsules for storage and delivery of hydrophobic molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Bragato, C.; Persico, A.; Ferreres, G.; Tzanov, T.; Mantecca, P. Exploring the Effects of Lignin Nanoparticles in Different Zebrafish Inflammatory Models. Int. J. Nanomed. 2024, 19, 7731–7750. [Google Scholar] [CrossRef]
- Catalán, J.; Norppa, H. Safety aspects of bio-based nanomaterials. Bioengineering 2017, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, C.; Farcal, L.R.; Garmendia Aguirre, I.; Mancini, L.; Tosches, D.; Amelio, A.; Rasmussen, K.; Rauscher, H.; Riego Sintes, J.; Sala, S.; et al. Safe and Sustainable by Design Chemicals and Materials—Framework for the Definition of Criteria and Evaluation Procedure for Chemicals and Materials; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liang, Q.; Wang, F.; Yan, K.; Sun, M.; Lin, L.; Li, T.; Duan, J.; Sun, Z. Silica nanoparticles induce pulmonary autophagy dysfunction and epithelial-to-mesenchymal transition via p62/NF-κB signaling pathway. Ecotoxicol. Environ. Saf. 2022, 232, 113303. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Yang, M.; Li, Y.; Cui, S.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles induce malignant transformation and tumorigenesis of human lung epithelial cells via P53 signaling. Nanotoxicology 2017, 11, 1176–1194. [Google Scholar] [CrossRef]
- AOP-Wiki. Available online: https://aopwiki.org/aops/481 (accessed on 27 August 2024).
- Halappanavar, S.; Nymark, P.; Krug, H.F.; Clift, M.J.D.; Rothen-Rutishauser, B.; Vogel, U. Non-Animal Strategies for Toxicity Assessment of Nanoscale Materials: Role of Adverse Outcome Pathways in the Selection of Endpoints. Small 2021, 17, e2007628. [Google Scholar] [CrossRef]
- Lee, J.H.; Im, J.S.; Jin, X.; Kim, T.M.; Choi, J.W. In Vitro and In Vivo Evaluation of Drug-Encapsulated Lignin Nanoparticles for Release Control. ACS Sustain. Chem. Eng. 2022, 10, 5792–5802. [Google Scholar] [CrossRef]
- Pérez-Rafael, S.; Ivanova, K.; Stefanov, I.; Puiggalí, J.; del Valle, L.J.; Todorova, K.; Dimitrov, P.; Hinojosa-Caballero, D.; Tzanov, T. Nanoparticle-driven self-assembling injectable hydrogels provide a multi-factorial approach for chronic wound treatment. Acta Biomater. 2021, 134, 131–143. [Google Scholar] [CrossRef]
- Morena, A.G.; Pérez-Rafael, S.; Tzanov, T. Lignin-Based Nanoparticles as Both Structural and Active Elements in Self-Assembling and Self-Healing Multifunctional Hydrogels for Chronic Wound Management. Pharmaceutics 2022, 14, 2658. [Google Scholar] [CrossRef]
- Kose, O.; Béal, D.; Motellier, S.; Pelissier, N.; Collin-Faure, V.; Blosi, M.; Bengalli, R.; Costa, A.; Furxhi, I.; Mantecca, P.; et al. Physicochemical Transformations of Silver Nanoparticles in the Oro-Gastrointestinal Tract Mildly Affect Their Toxicity to Intestinal Cells In Vitro: An AOP-Oriented Testing Approach. Toxics 2023, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, Y.; Li, Y. Adverse effects of amorphous silica nanoparticles: Focus on human cardiovascular health. J. Hazard. Mater. 2021, 406, 124626. [Google Scholar] [CrossRef]
- McLean, P.; Mueller, W.; Gosens, I.; Cassee, F.R.; Rothen-Rutishauser, B.; Boyles, M.; Tran, L. Establishing relationships between particle-induced in vitro and in vivo inflammation endpoints to better extrapolate between in vitro markers and in vivo fibrosis. Part. Fibre Toxicol. 2023, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, H.; Barros, A.; Ornelas, M.; Malgueiro, R.; Carvalho, A.; Portela, A.; Silva, C.; Grishchuk, S.; Gryshchuk, L.; Gilberg, M.; et al. Use of biogenic silica nanoparticles derived from biomass in polymeric formulations and their applications. Rev. Mater. Compuestos 2020, 4, 22–28. [Google Scholar]
- Pérez-Rafael, S.; Ferreres, G.; Kessler, R.W.; Kessler, W.; Blair, J.; Rathee, G.; Morena, A.G.; Tzanov, T. Continuous sonochemical nanotransformation of lignin—Process design and control. Ultrason. Sonochem. 2023, 98, 106499. [Google Scholar] [CrossRef] [PubMed]
- Morena, A.G.; Bassegoda, A.; Natan, M.; Jacobi, G.; Banin, E.; Tzanov, T. Antibacterial Properties and Mechanisms of Action of Sonoenzymatically Synthesized Lignin-Based Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 37270–37279. [Google Scholar] [CrossRef]
- Motta, G.; Gualtieri, M.; Bengalli, R.; Saibene, M.; Belosi, F.; Nicosia, A.; Cabellos, J.; Mantecca, P. An integrated new approach methodology for inhalation risk assessment of safe and sustainable by design nanomaterials. Environ. Int. 2024, 183, 108420. [Google Scholar] [CrossRef]
- Meldrum, K.; Moura, J.A.; Doak, S.H.; Clift, M.J.D. Dynamic Fluid Flow Exacerbates the (Pro-)Inflammatory Effects of Aerosolised Engineered Nanomaterials In Vitro. Nanomaterials 2022, 12, 3431. [Google Scholar] [CrossRef]
- Nymark, P.; Karlsson, H.L.; Halappanavar, S.; Vogel, U. Adverse Outcome Pathway Development for Assessment of Lung Carcinogenicity by Nanoparticles. Front. Toxicol. 2021, 3, 653386. [Google Scholar] [CrossRef]
- Gutierrez, C.T.; Loizides, C.; Hafez, I.; Brostrøm, A.; Wolff, H.; Szarek, J.; Berthing, T.; Mortensen, A.; Jensen, K.A.; Roursgaard, M.; et al. Acute phase response following pulmonary exposure to soluble and insoluble metal oxide nanomaterials in mice. Part. Fibre Toxicol. 2023, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Lv, S.; Xu, H.; Ma, R.; Sun, Z.; Li, Y.; Guo, C. Long-term respiratory exposure to amorphous silica nanoparticles promoted systemic inflammation and progression of fibrosis in a susceptible mouse model. Chemosphere 2022, 300, 134633. [Google Scholar] [CrossRef]
- Vietti, G.; Lison, D.; van den Brule, S. Mechanisms of lung fibrosis induced by carbon nanotubes: Towards an Adverse Outcome Pathway (AOP). Part. Fibre Toxicol. 2016, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Chen, F.; Shi, X.; Yucesoy, B.; Mossman, B.; Vallyathan, V. Diseases caused by silica: Mechanisms of injury and disease development. Int. Immunopharmacol. 2002, 2, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Li, K.M.; Jiang, J.G.; Tian, S.C.; Chen, X.J.; Yan, F. Influence of silica types on synthesis and performance of amine-silica hybrid materials used for CO2 capture. J. Phys. Chem. C 2014, 118, 2454–2462. [Google Scholar] [CrossRef]
- Ullah, R.; Deb, B.K.; Mollah, M.Y.A. Synthesis and Characterization of Silica Coated Iron-Oxide Composites of Different Ratios. Int. J. Compos. Mater. 2014, 4, 135–145. [Google Scholar] [CrossRef]
- Fontana, D.; Recupido, F.; Lama, G.C.; Liu, J.; Boggioni, L.; Silvano, S.; Lavorgna, M.; Verdolotti, L. Effect of Different Methods to Synthesize Polyol-Grafted-Cellulose Nanocrystals as Inter-Active Filler in Bio-Based Polyurethane Foams. Polymers 2023, 15, 923. [Google Scholar] [CrossRef]
- Gómez, D.M.; Urcuqui-Inchima, S.; Hernandez, J.C. Silica nanoparticles induce NLRP3 inflammasome activation in human primary immune cells. Innate Immun. 2017, 23, 697–708. [Google Scholar] [CrossRef]
- Skuland, T.; Låg, M.; Gutleb, A.C.; Brinchmann, B.C.; Serchi, T.; Øvrevik, J.; Holme, J.A.; Refsnes, M. Pro-inflammatory effects of crystalline- And nano-sized non-crystalline silica particles in a 3D alveolar model. Part. Fibre Toxicol. 2020, 17, 13. [Google Scholar] [CrossRef]
- Gualtieri, M.; Skuland, T.; Iversen, T.G.; Låg, M.; Schwarze, P.; Bilaničová, D.; Pojana, G.; Refsnes, M. Importance of agglomeration state and exposure conditions for uptake and pro-inflammatory responses to amorphous silica nanoparticles in bronchial epithelial cells. Nanotoxicology 2012, 6, 700–712. [Google Scholar] [CrossRef]
- Peivandi, Z.; Shirazi, F.H.; Teimourian, S.; Farnam, G.; Babaei, V.; Mehrparvar, N.; Koohsari, N.; Ashtarinezhad, A. Silica nanoparticles-induced cytotoxicity and genotoxicity in A549 cell lines. Sci. Rep. 2024, 14, 14484. [Google Scholar] [CrossRef]
- Inoue, M.; Sakamoto, K.; Suzuki, A.; Nakai, S.; Ando, A.; Shiraki, Y.; Nakahara, Y.; Omura, M.; Enomoto, A.; Nakase, I.; et al. Size and surface modification of silica nanoparticles affect the severity of lung toxicity by modulating endosomal ROS generation in macrophages. Part. Fibre Toxicol. 2021, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.E.; Morris, A.S.; Mueller, P.S.; Salem, A.K.; Grassian, V.H.; Larsen, S.C. Silica nanoparticle-generated ROS as a predictor of cellular toxicity: Mechanistic insights and safety by design. Environ. Sci. Nano 2016, 3, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, L.; Jin, M.; Du, Z.; Liu, X.; Guo, C.; Li, Y.; Huang, P.; Sun, Z. Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cells. Toxicol. Vitr. 2011, 25, 1343–1352. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Athinarayanan, J.; Periasamy, V.S. Biocompatibility assessment of rice husk-derived biogenic silica nanoparticles for biomedical applications. Mater. Sci. Eng. C 2015, 47, 8–16. [Google Scholar] [CrossRef]
- Murugadoss, S.; Van Den Brule, S.; Brassinne, F.; Sebaihi, N.; Mejia, J.; Lucas, S.; Petry, J.; Godderis, L.; Mast, J.; Lison, D.; et al. Is aggregated synthetic amorphous silica toxicologically relevant? Part. Fibre Toxicol. 2020, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, J.; Xu, X.; Wang, Z.; Mao, L.; Zhang, S.; Zhang, H.; Li, Y.; Yu, Q.; Jiang, N.; et al. Lignin-Based Nanoparticles for Combination of Tumor Oxidative Stress Amplification and Reactive Oxygen Species Responsive Drug Release. Bioconjug. Chem. 2024, 35, 1207–1217. [Google Scholar] [CrossRef]
- Poulsen, K.M.; Payne, C.K. Concentration and composition of the protein corona as a function of incubation time and serum concentration: An automated approach to the protein corona. Anal. Bioanal. Chem. 2022, 414, 7265–7275. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. The toxicological mode of action and the safety of synthetic amorphous silica-a nanostructured material. Toxicology 2012, 294, 61–79. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Hoet, P.H. The nanosilica hazard: Another variable entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef]
- Stella, J.; Abdelaal, M.A.M.E.; Kamal, M.A.M.; Shehu, K.; Alhayek, A.; Haupenthal, J.; Hirsch, A.K.; Schneider, M. Spray drying of a zinc complexing agent for inhalation therapy of pulmonary fibrosis. Eur. J. Pharm. Sci. 2024, 202, 106891. [Google Scholar] [CrossRef]
- Siddiqui, L.; Bag, J.; Seetha; Mittal, D.; Leekha, A.; Mishra, H.; Mishra, M.; Verma, A.K.; Mishra, P.K.; Ekielski, A.; et al. Assessing the potential of lignin nanoparticles as drug carrier: Synthesis, cytotoxicity and genotoxicity studies. Int. J. Biol. Macromol. 2020, 152, 786–802. [Google Scholar] [CrossRef]
- Castaneda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive Investigation of the Antioxidant and Pro-oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B 2018, 122, 6198–6214. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Zhang, H.; Gobe, V.; Asante-Donyinah, D.; Duan, Y. Anti- and pro-oxidant properties of polyphenols and their role in modulating glutathione synthesis, activity and cellular redox potential: Potential synergies for disease management. Adv. Redox Res. 2024, 11, 100099. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Alqahtani, A.; Kazi, M.; Ahmad, M.Z.; Alahmari, A.; Alsenaidy, M.A.; Syed, R. Wound-healing potential of curcumin loaded lignin nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 60, 102020. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.M.; Choi, I.-G.; Choi, J.W. Phenolic Hydroxyl Groups in the Lignin Polymer Affect the Formation of Lignin Nanoparticles. Nanomaterials 2021, 11, 1790. [Google Scholar] [CrossRef]
- Kim, I.-B.; Kim, D.-Y.; Lee, S.-J.; Sun, M.-J.; Lee, M.-S.; Li, H.; Cho, J.-J.; Park, C.-S. Inhibition of IL-8 Production by Green Tea Polyphenols in Human Nasal Fibroblasts and A549 Epithelial Cells. Biol. Pharm. Bull. 1120, 29, 1120–1125. [Google Scholar] [CrossRef]
- Panas, A.; Comouth, A.; Saathoff, H.; Leisner, T.; Al-Rawi, M.; Simon, M.; Seemann, G.; Dössel, O.; Mülhopt, S.; Paur, H.R.; et al. Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein J. Nanotechnol. 2014, 5, 1590–1602. [Google Scholar] [CrossRef]
- Muto, E.; Dell’Agli, M.; Sangiovanni, E.; Mitro, N.; Fumagalli, M.; Crestani, M.; De Fabiani, E.; Caruso, D. Olive oil phenolic extract regulates interleukin-8 expression by transcriptional and posttranscriptional mechanisms in Caco-2 cells. Mol. Nutr. Food Res. 2015, 59, 1217–1221. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef]




| NPs (100 µg/L) | Medium | Time (h) | Zeta-Average (nm) ± SD | PdI ± SD | ζ-Potential (mV) |
|---|---|---|---|---|---|
| SiO2-Aerosil | mQ water | 0 | 246.7 ± 40.5 | 0.225 ± 0.0002 | −21.03 ± 0.62 |
| mQ water | 24 | 218.7 ± 20.7 | 0.225 ± 0.022 | ||
| DMEM 1% FBS | 0 | 625.5 ± 344.7 | 0.59 ± 0.23 | ||
| DMEM 1% FBS | 24 | 185.7 ± 4.12 | 0.168 ± 0.068 | ||
| Opti-MEM 1% FBS | 0 | 702.1 ± 261.6 | 0.588 ± 0.12 | ||
| Opti-MEM 1% FBS | 24 | 629.1 ± 190.6 | 0.771 ± 0.139 | ||
| SiO2-RHSK | mQ water | 0 | 507. 9 ± 104.8 | 0.693 ± 0.037 | −22.80 ± 0.14 |
| mQ water | 24 | 726.9 ± 208.66 | 0.818 ± 0.052 | ||
| DMEM 1% FBS | 0 | 1022.7 ± 510.04 | 0.888 ± 0.096 | ||
| DMEM 1% FBS | 24 | 154.9 ± 62.52 | 0.226 ± 0.241 | ||
| Opti-MEM 1% FBS | 0 | 1359.1 ± 821.5 | 0.925 ± 0.110 | ||
| Opti-MEM 1% FBS | 24 | 177.9 ± 29.6 | 0.296 ± 0.166 | ||
| LigNPs | mQ water | 0 | 355.5 ± 7.366 | 0.197 ± 0.017 | −33.2 ± 2.5 |
| mQ water | 24 | 293.0 ± 4.456 | 0.172 ± 0.043 | ||
| DMEM 1% FBS | 0 | 528.8 ± 2.41 | 0.162 ± 0.122 | ||
| DMEM 1% FBS | 24 | 210.9 ± 4.15 | 0.16 ± 0.011 | ||
| Opti-MEM 1% FBS | 0 | 533.3 ± 9,00 | 0.241 ± 0.006 | ||
| Opti-MEM 1% FBS | 24 | 366.1 ± 5.16 | 0.103 ± 0.094 | ||
| PheLigNPs | mQ water | 0 | 525.4 ± 23.290 | 0.345 ± 0.016 | −32.7 ± 0.451 |
| mQ water | 24 | 568.9 ± 10.060 | 0.099 ± 0.015 | ||
| DMEM 1% FBS | 0 | 871.2 ± 107.1 | 0.367 ± 0.08 | ||
| DMEM 1% FBS | 24 | 196.6 ± 4.64 | 0.113 ± 0.046 | ||
| Opti-MEM 1% FBS | 0 | 918.7 ± 42.48 | 0.398 ± 0.103 | ||
| Opti-MEM 1% FBS | 24 | 396.6 ± 11.28 | 0.262 ± 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bengalli, R.D.; Gualtieri, M.; Ornelas, M.; Tzanov, T.; Mantecca, P. Adverse Outcome Pathways (AOPs) Oriented Approach to Assess In Vitro Hazard of Silica and Lignin Nanomaterials Derived from Biomass Residues. Nanomaterials 2025, 15, 549. https://doi.org/10.3390/nano15070549
Bengalli RD, Gualtieri M, Ornelas M, Tzanov T, Mantecca P. Adverse Outcome Pathways (AOPs) Oriented Approach to Assess In Vitro Hazard of Silica and Lignin Nanomaterials Derived from Biomass Residues. Nanomaterials. 2025; 15(7):549. https://doi.org/10.3390/nano15070549
Chicago/Turabian StyleBengalli, Rossella Daniela, Maurizio Gualtieri, Mariana Ornelas, Tzanko Tzanov, and Paride Mantecca. 2025. "Adverse Outcome Pathways (AOPs) Oriented Approach to Assess In Vitro Hazard of Silica and Lignin Nanomaterials Derived from Biomass Residues" Nanomaterials 15, no. 7: 549. https://doi.org/10.3390/nano15070549
APA StyleBengalli, R. D., Gualtieri, M., Ornelas, M., Tzanov, T., & Mantecca, P. (2025). Adverse Outcome Pathways (AOPs) Oriented Approach to Assess In Vitro Hazard of Silica and Lignin Nanomaterials Derived from Biomass Residues. Nanomaterials, 15(7), 549. https://doi.org/10.3390/nano15070549










