Rational Design of Core–Shell MoS2@ZIF-67 Nanocomposites for Enhanced Photocatalytic Degradation of Tetracycline
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Core–Shell MoS2@ZIF-67 Composites
2.3. Photocatalytic Degradation Analysis
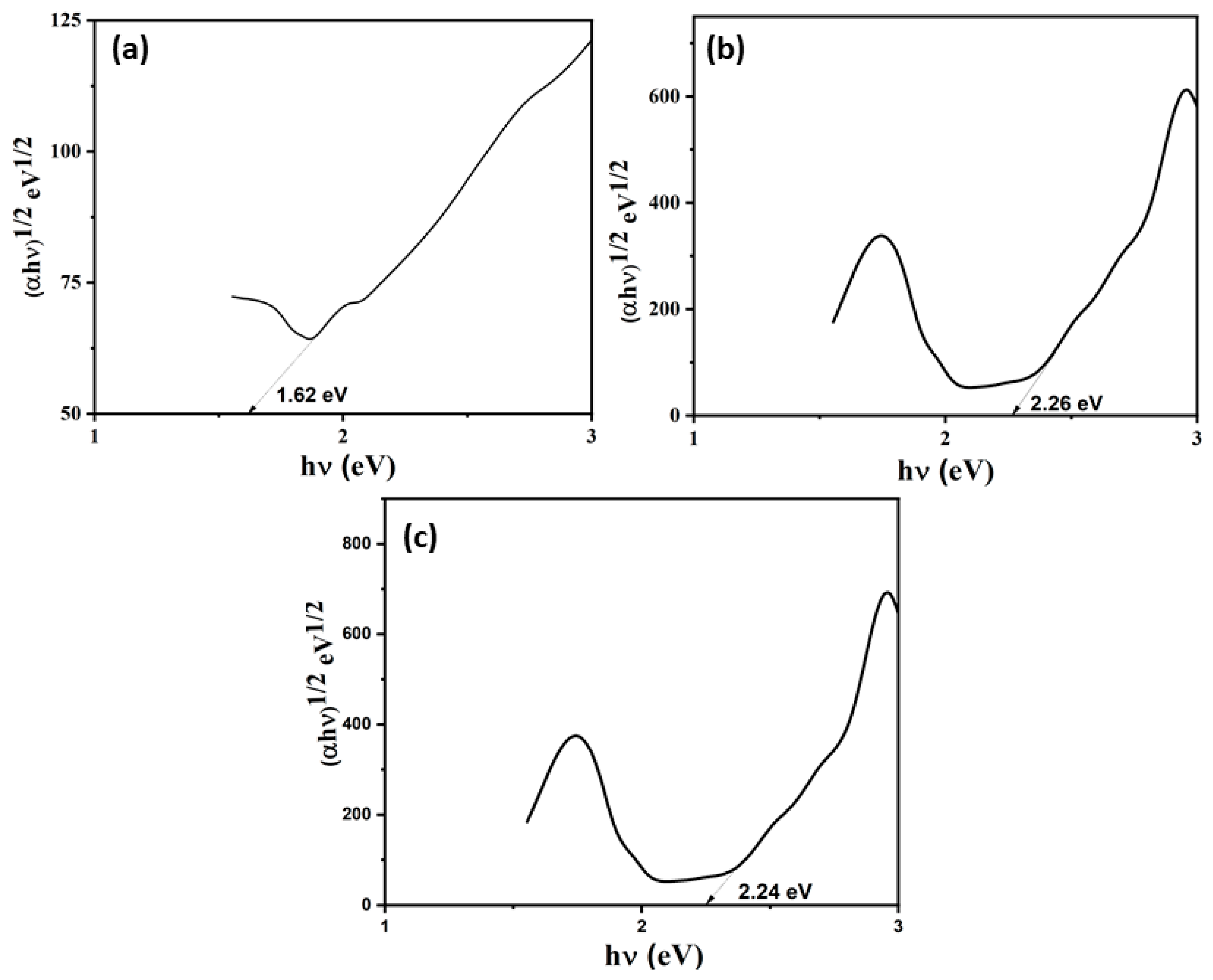
2.4. Band Gap Calculation
2.5. Band Potential Calculation
2.6. Characterization Details
3. Results and Discussion
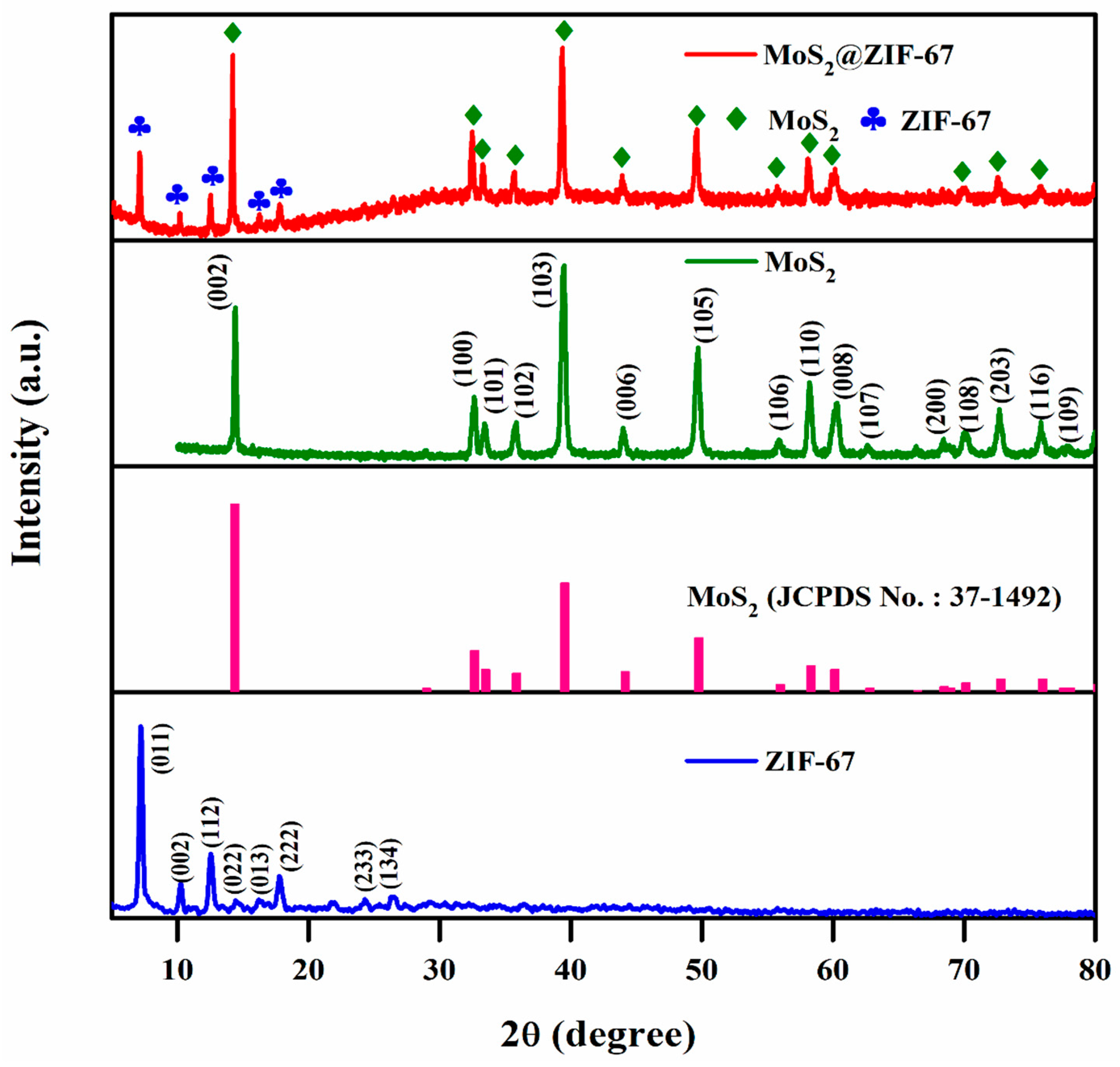
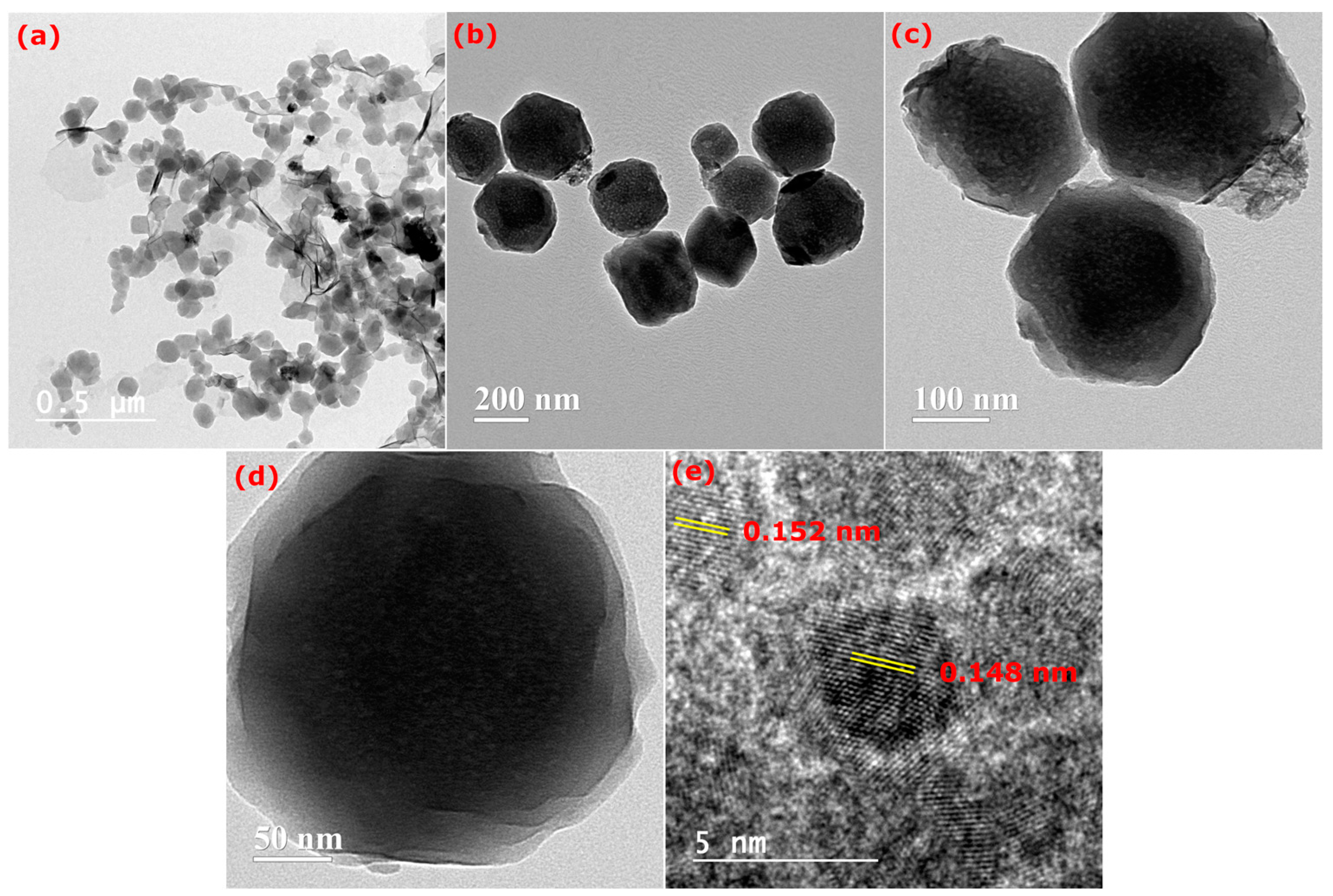
3.1. Structural and Surface Morphology
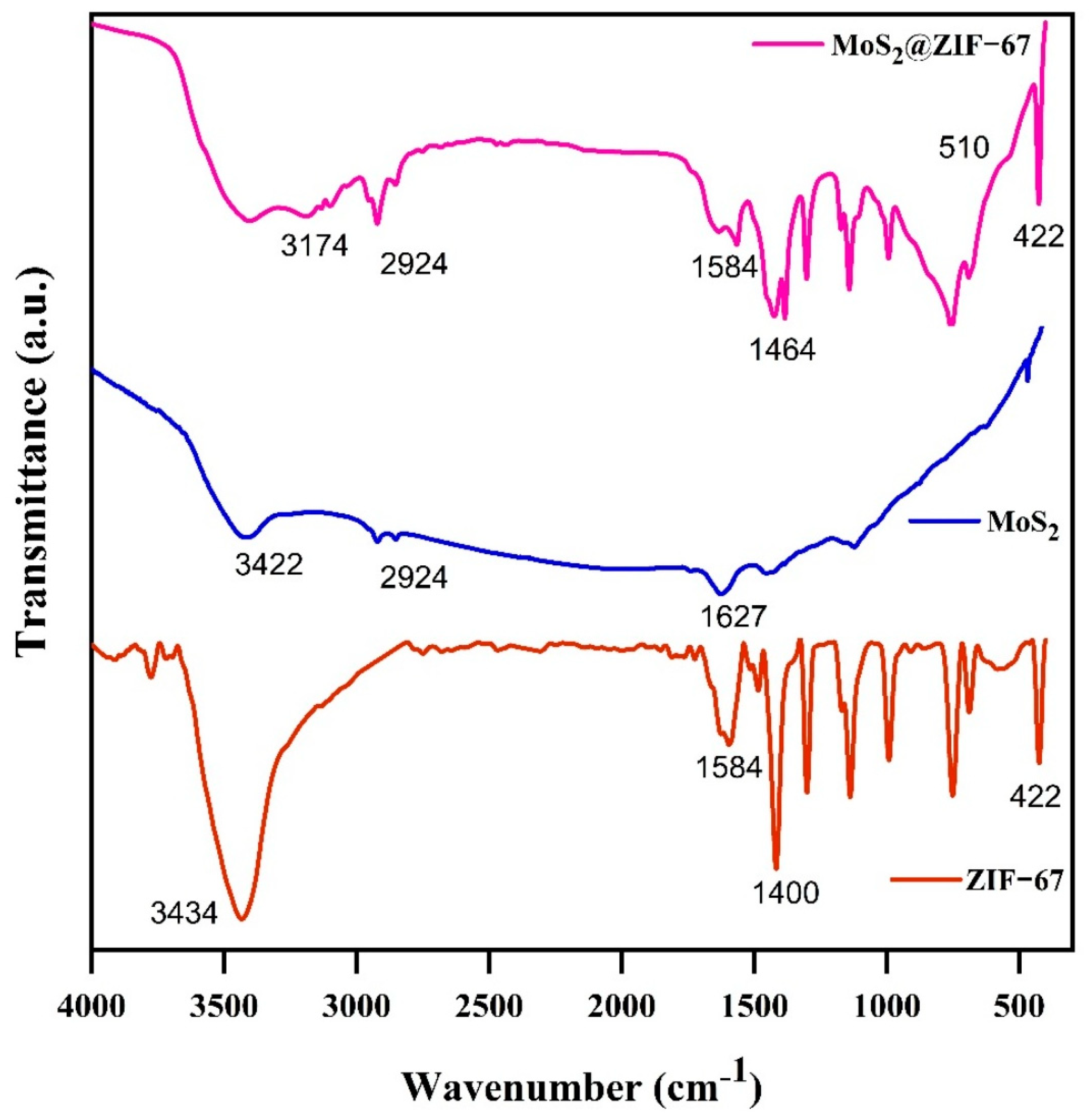
3.2. FT-IR Analysis
3.3. X-Ray Photoelectron Spectral Analysis
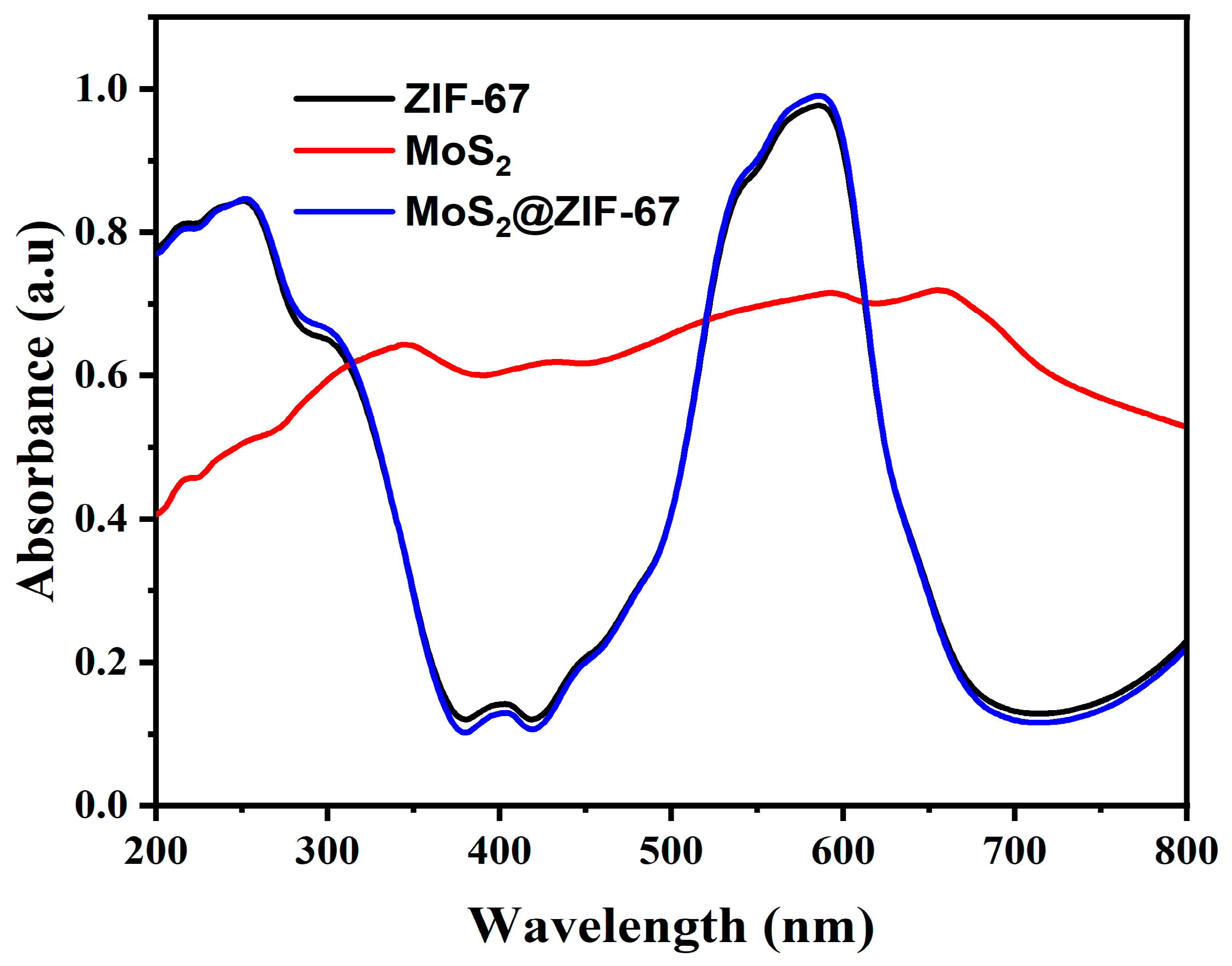
3.4. Optical Absorption Studies
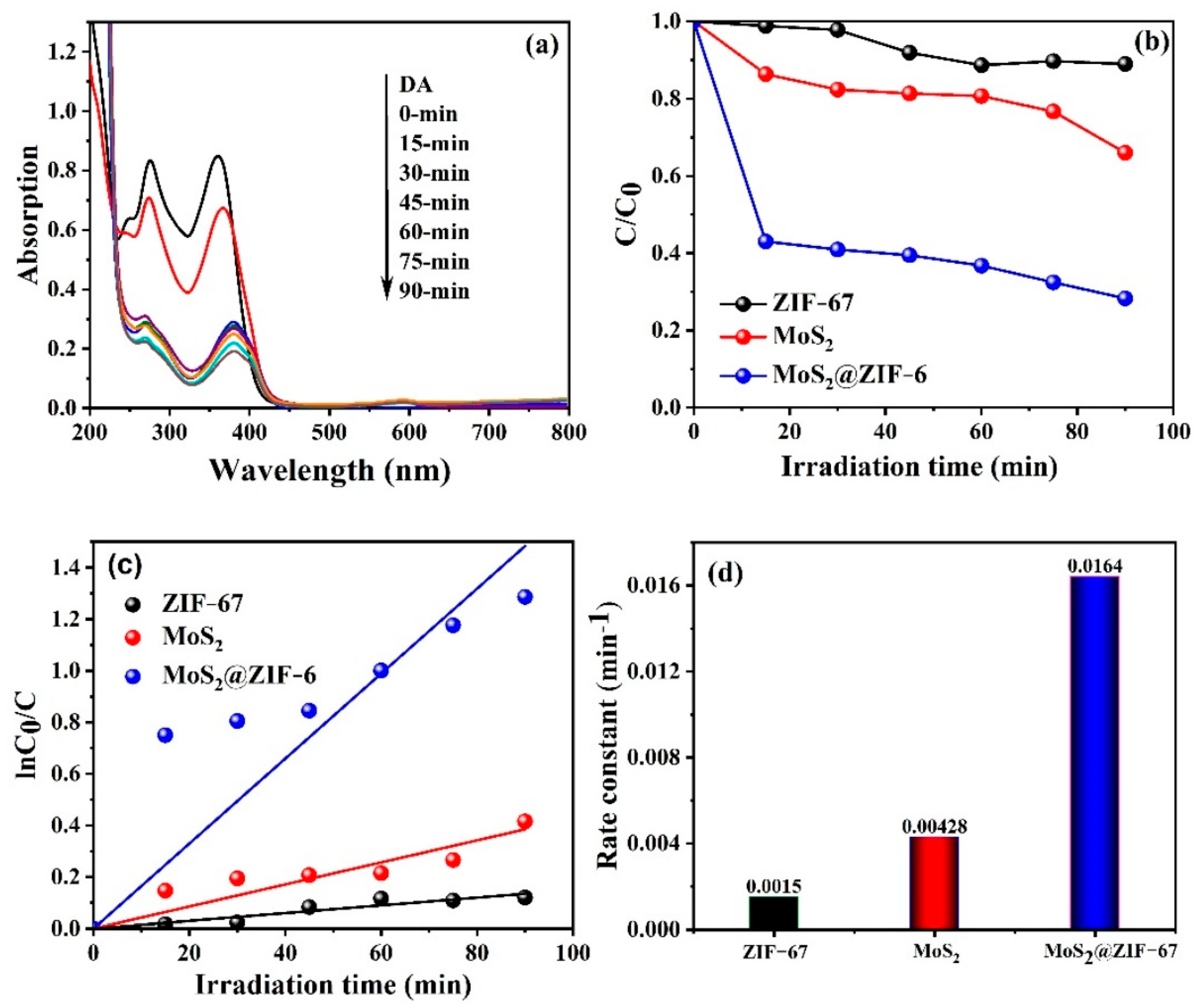
3.5. Photocatalytic Performance Analysis
3.6. Electrochemical Performance
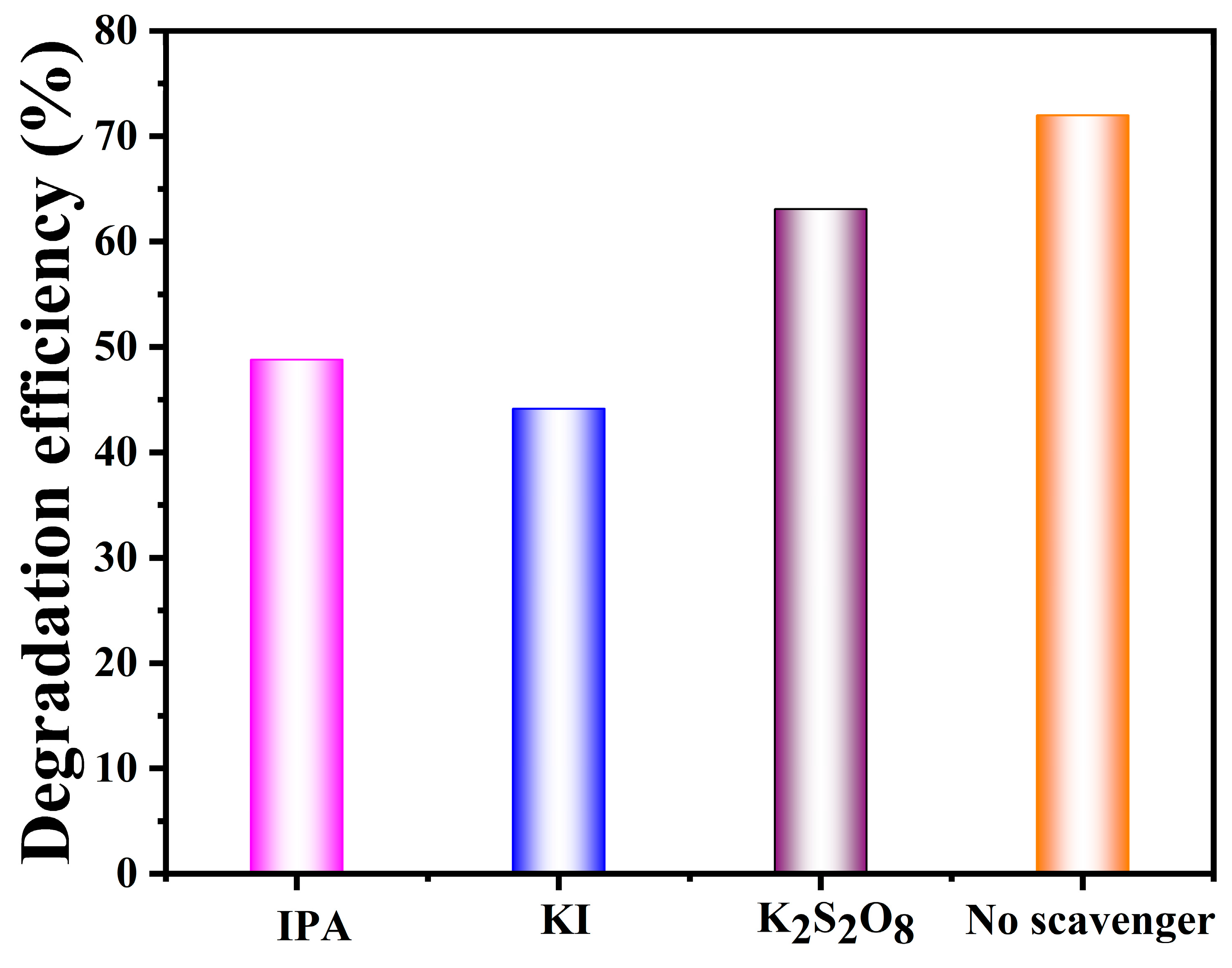
3.7. Charge Transfer Mechanism for Degradation of Tetracycline
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Samrot, A.V.; Wilson, S.; Sanjay Preeth, R.S.; Prakash, P.; Sathiyasree, M.; Saigeetha, S.; Shobana, N.; Pachiyappan, S.; Rajesh, V.V. Sources of Antibiotic Contamination in Wastewater and Approaches to Their Removal—An Overview. Sustainability 2023, 15, 12639. [Google Scholar] [CrossRef]
- Lu, S.; You, S.; Hu, J.; Li, X.; Li, L. Magnetic MnFe2O4/ZIF-67 nanocomposites with high activation of peroxymonosulfate for the degradation of tetracycline hydrochloride in wastewater. RSC Adv. 2024, 14, 7528–7539. [Google Scholar] [CrossRef]
- Madhogaria, B.; Banerjee, S.; Kundu, A.; Dhak, P. Efficacy of new generation biosorbents for the sustainable treatment of antibiotic residues and antibiotic resistance genes from polluted waste effluent. Infect. Med. 2024, 3, 100092. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Zhao, Y.; Zhang, J. Performance of Traditional and Emerging Water-Treatment Technologies in the Removal of Tetracycline Antibiotics. Catalysts 2024, 14, 269. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Ali, M.H. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, M.; Gao, X.; Zhang, J.; Wang, E.; Gao, Z. The application of MOFs-based materials for antibacterials adsorption. Coord. Chem. Rev. 2021, 440, 213970. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Schumacher, L.; Marschall, R. Recent Advances in Semiconductor Heterojunctions and Z-Schemes for Photocatalytic Hydrogen Generation. Top. Curr. Chem. (Z) 2022, 380, 53. [Google Scholar] [CrossRef]
- Abhishek, B.; Jayarama, A.; Rao, A.S.; Nagarkar, S.S.; Dutta, A.; Duttagupta, S.P.; Prabhu, S.S.; Pinto, R. Challenges in photocatalytic hydrogen evolution: Importance of photocatalysts and photocatalytic reactors. Int. J. Hydrogen Energy 2024, 81, 1442–1466. [Google Scholar] [CrossRef]
- Yu, Z.; Qian, L.; Zhong, T.; Ran, Q.; Huang, J.; Hou, Y.; Li, F.; Li, M.; Sun, Q.; Zhang, H. Enhanced visible light photocatalytic activity of CdS through controllable self-assembly compositing with ZIF-67. Mol. Catal. 2020, 485, 110797. [Google Scholar] [CrossRef]
- Shameem, A.; Devendran, P.; Siva, V.; Raja, M.; Bahadur, S.A.; Manikandan, A. Preparation and Characterization Studies of Nanostructured CdO Thin Films by SILAR Method for Photocatalytic Applications. J. Inorg. Organomet. Polym. Mater. 2017, 27, 692–699. [Google Scholar] [CrossRef]
- Murugan, A.; Thirumal, V.; Kim, J.; Siva, V.; Kasinathan, P.; Alothman, A.A.; Mohammad, S.; Selvakumar, K. Effective visible light-driven binary ZnO catalyst decorated on layered double hydroxides for simultaneous photocatalytic degradation of Ciprofloxacin and Ampicillin. J. Taiwan Inst. Chem. Eng. 2024, 161, 105495. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Vijayan, P.; Krishnan, P.S.; Raja, A.; Kumaravel, S.; Venthan, S.M.; Siva, V.; Palanisamy, G.; Lee, J.; Afzal, M. Design of bifunctional synergistic NiMoO4/g-C3N4 nanocomposite for the augmentation of electrochemical water splitting and photocatalytic antibiotic degradation performances. J. Alloys Compd. 2024, 987, 174128. [Google Scholar] [CrossRef]
- Chakravorty, A.; Roy, S. A review of photocatalysis, basic principles, processes, and materials. Sustain. Chem. Environ. 2024, 8, 100155. [Google Scholar] [CrossRef]
- Nadolska, M.; Szkoda, M.; Trzciński, K.; Ryl, J.; Lewkowicz, A.; Sadowska, K.; Smalc-Koziorowska, J.; Prześniak-Welenc, M. New light on the photocatalytic performance of NH4V4O10 and its composite with rGO. Sci. Rep. 2023, 13, 3946. [Google Scholar] [CrossRef]
- Vizza, M.; Giurlani, W.; Cerri, L.; Calisi, N.; Leonardi, A.A.; Faro, M.J.L.; Irrera, A.; Berretti, E.; Perales-Rondón, J.V.; Colina, A.; et al. Electrodeposition of Molybdenum Disulfide (MoS2) Nanoparticles on Monocrystalline Silicon. Molecules 2022, 27, 5416. [Google Scholar] [CrossRef]
- Rahman, A.; Jennings, J.R.; Tan, A.L.; Khan, M.M. Molybdenum disulfide-based nanomaterials for visible-light-induced photocatalysis. ACS Omega 2022, 7, 22089–22110. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Asiri, A.M.; Singh, P. An overview of strategies for enhancement in photocatalytic oxidative ability of MoS2 for water purification. J. Environ. Chem. Eng. 2020, 8, 104307. [Google Scholar] [CrossRef]
- Thomas, N.; Mathew, S.; Nair, K.M.; O’Dowd, K.; Forouzandeh, P.; Goswami, A.; McGranaghan, G.; Pillai, S.C. 2D MoS2: Structure, mechanisms, and photocatalytic applications. Mater. Today Sustain. 2021, 13, 100073. [Google Scholar] [CrossRef]
- Luo, M.; Xu, J.; Xu, W.; Zheng, Y.; Wu, G.; Jeong, T. Photocatalytic Activity of MoS2 Nanoflower-Modified CaTiO3 Composites for Degradation of RhB under Visible Light. Nanomaterials 2023, 13, 636. [Google Scholar] [CrossRef]
- Kiteto, M.; Vidija, B.; Mecha, C.A.; Mrosso, R.; Chollom, M.N. Advances in metal–organic frameworks as adsorbents, photocatalysts and membranes: A new frontier in water purification. Discov. Water 2024, 4, 54. [Google Scholar] [CrossRef]
- Chen, J.; Abazari, R.; Adegoke, K.A.; Maxakato, N.W.; Bello, O.S.; Tahir, M.; Tasleem, S.; Sanati, S.; Kirillov, A.M.; Zhou, Y. Metal–organic frameworks and derived materials as photocatalysts for water splitting and carbon dioxide reduction. Coord. Chem. Rev. 2022, 469, 214664. [Google Scholar] [CrossRef]
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent advances in application of metal-organic frameworks (MOFs) as adsorbent and catalyst in removal of persistent organic pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127. [Google Scholar] [CrossRef]
- Luo, T.; Gilmanova, L.; Kaskel, S. Advances of MOFs and COFs for photocatalytic CO2 reduction, H2 evolution and organic redox transformations. Coord. Chem. Rev. 2023, 490, 215210. [Google Scholar] [CrossRef]
- Santos, W.D.; Teixeira, M.M.; Campos, I.R.; de Lima, R.B.; Mantilla, A.; Osajima, J.A.; de Menezes, A.S.; Manzani, D.; Rojas, A.; Alcântara, A.C. Photocatalytic degradation of ciprofloxacin using semiconductor derived from heterostructured ZIF-8-based materials. Microporous Mesoporous Mater. 2023, 359, 112657. [Google Scholar] [CrossRef]
- Vadivel, S.; Muthuraj, A.; Anbazhagan, M.; Arumugam, R. A novel CoMoO4 enwrapped ZIF-8 nanocomposite with enhanced visible light photocatalytic activity. Environ. Pollut. 2023, 336, 122450. [Google Scholar] [CrossRef]
- Elaouni, A.; El Ouardi, M.; Zbair, M.; BaQais, A.; Saadi, M.; Ahsaine, H.A. ZIF-8 metal organic framework materials as a superb platform for the removal and photocatalytic degradation of organic pollutants, a review. RSC Adv. 2022, 12, 31801–31817. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, H.; Ouyang, T.; Jiang, Y.; Mu, M.; Yin, X. Cobalt-based ZIF coordinated hybrids with defective TiO2-x for boosting visible light-driven photo-Fenton-like degradation of bisphenol A. Chemosphere 2020, 259, 127431. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Keshavarzi, S.; Oveisi, M.; Rahimi, S.; Hayati, B. Metal-organic framework (ZIF-8)/inorganic nanofiber (Fe2O3) nanocomposite: Green synthesis and photocatalytic degradation using LED irradiation. J. Mol. Liq. 2019, 291, 111333. [Google Scholar] [CrossRef]
- Sanjana, S.; Siva, V.; Murugan, A.; Shameem, A.S. Core-shell structured Zn3(VO4)2@ZIF-67 nanocrystallites with excellent cycling stability for energy storage systems. J. Energy Storage 2025, 106, 114882. [Google Scholar] [CrossRef]
- Zabihi, M.; Motavalizadehkakhky, A. PbS/ZIF-67 nanocomposite: Novel material for photocatalytic degradation of basic yellow 28 and direct blue 199 dyes. J. Taiwan Inst. Chem. Eng. 2022, 140, 104572. [Google Scholar] [CrossRef]
- Arabian, S.; Gordanshekan, A.; Farhadian, M.; Nazar, A.R.S.; Tangestaninejad, S.; Sabzyan, H. Adsorption/photocatalytic degradation of Cefixime by the green Bi2WO6/g-C3N4/ZIF-67 dual S-scheme heterojunction: Artificial neural network, genetic algorithm, density functional theory, and toxicity assessments. Chem. Eng. J. 2024, 488, 150686. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, J.; Wang, M.; Zhang, X.; Xu, Y.; Wei, Q.; Lan, F.; Gao, P.; Liu, Y.; Wang, R. The double heterostructure photocatalyst MoS2@ ZIF-67/TiO2: Triumphant modification of TiO2 and efficient removal of Karenia mikimotoi. Chem. Eng. J. 2024, 479, 147954. [Google Scholar]
- Siva, V.; Sanjana, S.; Murugan, A.; Shameem, A.; Jauhar, R.M.; Babu, S. Facile synthesis and asymmetric device fabrication of zeolite like Co-MOF as a promising electrode material with improved cyclic stability. J. Mater. Sci. Mater. Electron. 2024, 35, 75. [Google Scholar] [CrossRef]
- Jubu, P.R.; Obaseki, O.S.; Ajayi, D.I.; Danladi, E.; Chahrour, K.M.; Muhammad, A.; Landi, S., Jr.; Igbawua, T.; Chahul, H.F.; Yam, F.K. Considerations about the determination of optical bandgap from diffuse reflectance spectroscopy using the Tauc plot. J. Opt. 2024, 53, 5054–5064. [Google Scholar] [CrossRef]
- Abdul-wahid, I.K.; Ammar, S.H.; Elaibi, A.I.; Jabbar, Z.H. Enhanced synergistic photocatalytic degradation of oxytetracycline antibiotic using novel Ag2MoO4/Co-zeolitic imidazolate framework (ZIF-67) Z-type heterojunction. Inorg. Chem. Commun. 2023, 156, 111277. [Google Scholar] [CrossRef]
- Teymourinia, H.; Alshamsi, H.A.; Al-nayili, A.; Sohouli, E.; Gholami, M. Synthesis of new photocatalyst based on g-C3N4/N, P CQD/ZIF-67 nanocomposite for ciprofloxacin degradation under visible light irradiation. J. Ind. Eng. Chem. 2023, 125, 259–268. [Google Scholar] [CrossRef]
- Davoodi, M.; Davar, F.; Rezayat, M.R.; Jafari, M.T.; Bazarganipour, M.; Shalan, A.E. Synthesis and characterization of a new ZIF-67@ MgAl2O4 nanocomposite and its adsorption behaviour. RSC Adv. 2021, 11, 13245–13255. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.H.; Chuang, K.S.; Catherine, H.N.; Huang, J.H.; Hsu, H.J.; Shen, Y.C.; Hu, C. MoS2-coupled coniferous ZnO for photocatalytic degradation of dyes. J. Taiwan Inst. Chem. Eng. 2023, 142, 104638. [Google Scholar] [CrossRef]
- Kshirsagar, S.D.; Shelake, S.P.; Biswas, B.; Singh, A.; Pakhira, S.; Sainath, A.V.S.; Pal, U. In situ decoration of 2D-MoS2/ZIF-67 type II heterojunction for enhanced hydrogen production under simulated sunlight. Catal. Today 2024, 445, 115056. [Google Scholar] [CrossRef]
- Sanjana, S.; Siva, V.; Sharmila, S.; Murugan, A.; Shameem, A. Fabrication of V2O5@Co-MOF as a cathode material with excellent rate capability. Sustain. Mater. Technol. 2024, 41, e01072. [Google Scholar] [CrossRef]
- Lalithambika, K.C.; Shanmugapriya, K.; Sriram, S. Photocatalytic activity of MoS2 nanoparticles: An experimental and DFT analysis. Appl. Phys. A 2019, 125, 817. [Google Scholar] [CrossRef]
- Perkins, C.L.; Lee, S.H.; Li, X.; Asher, S.E.; Coutts, T.J. Identification of nitrogen chemical states in N-doped ZnO via x-ray photoelectron spectroscopy. J. Appl. Phys. 2005, 97, 034907. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, M.; Hao, X.; Zhu, P. ZIF-67 dodecahedron coupled with CoAl-layered double hydroxide as S-scheme heterojunction for efficient visible-light-driven hydrogen evolution. Appl. Surf. Sci. 2022, 592, 153300. [Google Scholar] [CrossRef]
- Zhuang, S.; Lee, E.S.; Lei, L.; Nunna, B.B.; Kuang, L.; Zhang, W. Synthesis of nitrogen-doped graphene catalyst by high-energy wet ball milling for electrochemical systems. Int. J. Energy Res. 2016, 40, 2136–2149. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, J.; Tian, Y.; Zhang, B.; Wu, Y. Preparation and performances of ZIF-67-derived FeCo bimetallic catalysts for CO2 hydrogenation to light olefins. Catalysts 2020, 10, 455. [Google Scholar] [CrossRef]
- Devarayapalli, K.C.; Vattikuti, S.V.P.; Sreekanth, T.V.M.; Yoo, K.S.; Nagajyothi, P.C.; Shim, J. Hydrogen production and photocatalytic activity of g-C3N4/Co-MOF (ZIF-67) nanocomposite under visible light irradiation. Appl. Organomet. Chem. 2020, 34, e5376. [Google Scholar] [CrossRef]
- Ngo, C.M.; Nguyen, H.D.; Saito, N.; Do, T.M.D.; Nakayama, T.; Niihara, K.; Suematsu, H. Synthesis of β-MoO3 whiskers by the thermal evaporation method with flowing oxygen gas. J. Am. Ceram. Soc. 2022, 105, 1622–1628. [Google Scholar] [CrossRef]
- Ngo, M.T.T.; Ueyama, T.; Makabe, R.; Bui, X.T.; Nghiem, L.D.; Nga, T.T.V.; Fujioka, T. Fouling behavior and performance of a submerged flat-sheet nanofiltration membrane system for direct treatment of secondary wastewater effluent. J. Water Process Eng. 2021, 41, 101991. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, T.; Fang, Y. High-efficient preparation of gasoline-ranged C5–C6 alkanes from biomass-derived sugar polyols of sorbitol over Ru-MoO3−x/C catalyst. Fuel Process. Technol. 2019, 183, 19–26. [Google Scholar] [CrossRef]
- Mei, P.; Yang, M.; Bai, Y.; Fei, S.; Dong, Y.; Cheng, H. Facile hydrothermal synthesis of nanorod-structured Mo0.6W0.4O3 catalyst for olefin hydrogenation with high activity. J. Catal. 2018, 360, 213–220. [Google Scholar] [CrossRef]
- Mao, Y.; Fang, Y.; Pan, J.; Wang, D.; Bu, K.; Che, X.; Zhao, W.; Huang, F. Synthesis, crystal structures and physical properties of Ax(H2O)yMoS2 (A = K, Rb, Cs). J. Solid State Chem. 2019, 279, 120937. [Google Scholar] [CrossRef]
- Amin, R.; Hossain, M.A.; Zakaria, Y. Interfacial kinetics and ionic diffusivity of the electrodeposited MoS2 film. ACS Appl. Mater. Interfaces 2018, 10, 13509–13518. [Google Scholar] [CrossRef]
- Veerasubramani, G.K.; Park, M.S.; Nagaraju, G.; Kim, D.W. Unraveling the Na-ion storage performance of a vertically aligned interlayer-expanded two-dimensional MoS2@C@MoS2 heterostructure. J. Mater. Chem. A Mater. 2019, 7, 24557–24568. [Google Scholar] [CrossRef]
- Luo, S.; Dong, S.; Lu, C.; Yu, C.; Ou, Y.; Luo, J.; Sun, J.; Sun, J. Rational and green synthesis of novel two-dimensional WS2/MoS2 heterojunction via direct exfoliation in ethanol-water targeting advanced visible-light-responsive photocatalytic performance. J. Colloid Interface Sci. 2018, 513, 389–399. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, J.; Xing, Z.; Guo, X.; Yu, X.; Tang, B.; Zou, J. Enhanced generation of H2O2 and radicals on Co9S8/partly-graphitized carbon cathode for degradation of bio-refractory organic wastewater. Electrochim. Acta 2016, 213, 341–350. [Google Scholar] [CrossRef]
- Zhu, R.; Ding, J.; Yang, J.; Pang, H.; Xu, Q.; Zhang, D.; Braunstein, P. Quasi-ZIF-67 for boosted oxygen evolution reaction catalytic activity via a low temperature calcination. ACS Appl. Mater. Interfaces 2020, 12, 25037–25041. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, X.; Wang, G.; Liu, Y.; Li, Y.; Jin, Z. Dodecahedron ZIF-67 anchoring ZnCdS particles for photocatalytic hydrogen evolution. Mol. Catal. 2020, 485, 110832. [Google Scholar] [CrossRef]
- Sahadevan, J.; Sivaprakash, P.; Esakki Muthu, S.; Kim, I.; Padmanathan, N.; Eswaramoorthi, V. Influence of Te-incorporated LaCoO3 on structural, morphology and magnetic properties for multifunctional device applications. Int. J. Mol. Sci. 2023, 24, 10107. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Wang, C.C.; Wang, P.; Liu, W.; Fu, H.; Zhao, C. Superior removal of inorganic and organic arsenic pollutants from water with MIL-88A(Fe) decorated on cotton fibers. Chemosphere 2020, 254, 126829. [Google Scholar] [CrossRef]
- Ding, K.; Wang, W.; Yu, D.; Wang, W.; Gao, P.; Liu, B. Facile formation of flexible Ag/AgCl/polydopamine/cotton fabric composite photocatalysts as an efficient visi-ble-light photocatalysts. Appl. Surf. Sci. 2018, 454, 101–111. [Google Scholar] [CrossRef]
- Ao, K.; Daoud, W.A. Facile controlled formation of CoNi alloy and CoO em-bedded in N-doped carbon as advanced electrocatalysts for oxygen evolution and zinc-air battery. Electrochim. Acta 2021, 395, 139204. [Google Scholar] [CrossRef]
- Varodi, C.; Pogăcean, F.; Cioriță, A.; Pană, O.; Leoștean, C.; Cozar, B.; Radu, T.; Coroș, M.; Staden, R.I.Ș.-V.; Pruneanu, S.M. Nitrogen and sulfur co-doped graphene as efficient electrode material for l-cysteine detection. Chemosensors 2021, 9, 146. [Google Scholar] [CrossRef]
- Lin, S.; Luo, J.; Xiao, C.; Zheng, Y.; Gao, B.; Lin, B. Facile one-step synthesis of porous hybrid material fabricated by 2D nanosheets of molybdenum di-sulfide and reduced graphene oxide for efficient electrocatalytic hydrogen evolution. J. Porous Mater. 2020, 27, 123–131. [Google Scholar] [CrossRef]
- Sahadevan, J.; Radhakrishnan, M.; Padmanathan, N.; Muthu, S.E.; Sivaprakash, P.; Kadiresan, M. Effect of Mn substitution on magnetic behaviour of oxygen defective LaCoO3 perovskite oxide. Mater. Sci. Eng. B 2022, 284, 115875. [Google Scholar] [CrossRef]
- Aravinthkumar, K.; Das, B.; Chen, S.S.; Mohan, C.R. Synergistic effect in ZIF-67/MoS2 entrenched MWCNT photo (electro) catalyst for degradation of tetracycline and alternative to Pt counter electrode in dye-sensitized solar cells. J. Environ. Chem. Eng. 2024, 12, 113604. [Google Scholar] [CrossRef]
- Li, Y.; Niu, J.; Yin, L.; Wang, W.; Bao, Y.; Chen, J.; Duan, Y. Photocatalytic degradation kinetics and mechanism of pentachlorophenol based on superoxide radicals. J. Environ. Sci. 2011, 23, 1911–1918. [Google Scholar]
- Xia, S.; Zhang, L.; Pan, G.; Qian, P.; Ni, Z. Photocatalytic degradation of methylene blue with a nanocomposite system: Synthesis, photocatalysis and degradation pathways. Phys. Chem. Chem. Phys. 2015, 17, 5345–5351. [Google Scholar]
- Jin, C.; Kang, J.; Li, Z.; Wang, M.; Wu, Z.; Xie, Y. Enhanced visible light photocatalytic degradation of tetracycline by MoS2/Ag/g-C3N4 Z-scheme composites with peroxymonosulfate. Appl. Surf. Sci. 2020, 514, 146076. [Google Scholar] [CrossRef]
- Li, Y.; Lai, Z.; Huang, Z.; Wang, H.; Zhao, C.; Ruan, G.; Du, F. Fabrication of BiOBr/MoS2/graphene oxide composites for efficient adsorption and photocatalytic removal of tetracycline antibiotics. Appl. Surf. Sci. 2021, 550, 149342. [Google Scholar] [CrossRef]
- Liu, J.; Lin, H.; He, Y.; Dong, Y.; Menzembere, E.R.G.Y. Novel CoS2/MoS2@Zeolite with excellent adsorption and photocatalytic performance for tetracycline removal in simulated wastewater. J. Clean. Prod. 2020, 260, 121047. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Lim, S.J.; Kim, H. Visible light activated MoS2/ZnO composites for photocatalytic degradation of ciprofloxacin antibiotic and hydrogen production. J. Photochem. Photobiol. A Chem. 2023, 434, 114250. [Google Scholar] [CrossRef]
- Sundriyal, S.; Shrivastav, V.; Kaur, H.; Mishra, S.; Deep, A. High-performance symmetrical supercapacitor with a combination of a ZIF-67/rGO composite electrode and a redox additive electrolyte. ACS Omega 2018, 3, 17348–17358. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Guo, R.; Cui, C.; Ren, E.; Xiao, H.; Qin, Q.; Jiang, S.; Zhang, Y. Co–N-codoped carbon/Co@ carbon cloth hybrid derived from ZIF-67 for the oxygen evolution reaction and supercapacitors. Energy Fuels 2020, 34, 13023–13031. [Google Scholar] [CrossRef]
- Wei, X.; Chai, Y.; Liu, N.; Qiao, S.; Fu, Y.; Chong, S. ZIF67@ MoO3 NSs@ NF composite electrocatalysts reinforced by chemical bonds and oxygen vacancy for efficient oxygen evolution reaction and overall water-splitting. Int. J. Hydrogen Energy 2022, 47, 9606–9615. [Google Scholar]
- Abdelkader-Fernández, V.K.; Fernandes, D.M.; Balula, S.S.; Cunha-Silva, L.; Freire, C. Advanced framework-modified POM@ ZIF-67 nanocomposites as enhanced oxygen evolution reaction electrocatalysts. J. Mater. Chem. A 2020, 8, 13509–13521. [Google Scholar] [CrossRef]
- Li, X.; You, S.; Du, J.; Dai, Y.; Chen, H.; Cai, Z.; Ren, N.; Zou, J. ZIF-67-derived Co3O4@ carbon protected by oxygen-buffering CeO2 as an efficient catalyst for boosting oxygen reduction/evolution reactions. J. Mater. Chem. A 2019, 7, 25853–25864. [Google Scholar] [CrossRef]
- Ângelo, J.; Magalhães, P.; Andrade, L.; Mendes, A. Characterization of TiO2-based semiconductors for photocatalysis by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2016, 387, 183–189. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Feng, P.; Chai, H.; Huang, Y. ZIF-67 derived hollow cobalt sulfide as superior adsorbent for effective adsorption removal of ciprofloxacin antibiotics. Chem. Eng. J. 2018, 344, 95–104. [Google Scholar] [CrossRef]






| S. No | Element | Atomic % |
|---|---|---|
| 1. | S 2p | 5.67 |
| 2. | Mo 3d | 2.47 |
| 3. | C 1s | 62.32 |
| 4. | N 1s | 21.04 |
| 5. | Co 2p | 4.11 |
| 6. | O 1s | 4.39 |
| S. No | Photocatalyst | Pollutants | Photocatalytic Efficiency (%) | Irradiation Time (min) | Light Source | Morphology | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | MoS2/Ag/g-C3N4 | Tetracycline | 98.9 | 50 | 300 W Xe lamp equipped with a UV cutoff filter (λ > 420 nm). | Flower-like shape | [72] |
| 2. | BiOBr/MoS2/GO | Tetracycline | 98 | 40 | Visible 300 W Xe lamp with a 380 nm cut-off filter was used to simulate visible light source. | Flower-like lamellar clusters | [73] |
| 3. | CoS2/MoS2@Zeolite | Tetracycline | 96.71 | 160 | A 300 W Xe-lamp was used as the visible light source and the UV light was filtered by a filter (420 nm). | Spherical hydrangea-like | [74] |
| 4. | Ag2MoO4/ZIF-67 | Tetracycline | 98.2 | 75 | under a LED type visible light 50 W illumination with a cut-off filter (420 nm). | Rhombic-like | [39] |
| 5. | C3N4/N, P CQD/ZIF-67 | Ciprofloxacin | 98 | 90 | Under Visible Light | - | [40] |
| 6. | ZIF-67/MoS2/MWCNT | Tetracycline | 96.1 | 80 | Visible light 25 W LED lamp. | Rhombic dodecahedron shape | [69] |
| 7. | MoS2/ZnO | Ciprofloxacin | 89 | 120 | UV light 250 W metal halide lamp | Rod-shaped ZnO microstructure and flakes of MoS2. | [75] |
| 8. | MoS2@ZIF-67 | Tetracycline | 72 | 90 | Visible 160 W tungsten lamb | Polyhedral morphology | Present work |
| S. No | Photocatalyst | Pollutants | Trapping Agent | Active Radicals | Irradiation Time (min) | Light Source | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | MoS2/Ag/g-C3N4 | TC | IPA BQ EDTA-2Na MeOH | BQ (O2−) | 50 | 300 W Xe lamp equipped with a UV cutoff filter (λ > 420 nm). | [72] |
| 2. | BiOBr/MoS2/GO | TC | IPA KI Ascorbic acid | KI (h+) Ascorbic acid (O2−) | 40 | Visible 300 W Xe lamp with a 380 nm cut-off filter was used to simulate visible light source. | [73] |
| 3. | Ag2MoO4/ZIF-67 | TC | IPA Na2C2O4 BQ AO | BQ (O2−) AO (h+) | 75 | Under a LED type visible light 50 W illumination with a cut-off filter (420 nm). | [39] |
| 4. | C3N4/N, P CQD/ZIF-67 | TC | TEA IPA BQ | TEA (h+) IPA (OH−) | 90 | Under Visible Light | [40] |
| 5. | ZIF-67/MoS2/MWCNT | TC | IPA EDTA-2Na BQ | IPA (OH−) BQ (O2−) | 80 | Visible light 25 W LED lamp. | [69] |
| 6. | MoS2@ZIF-67 | TC | IPA KI K2S2O8 | KI (h+) IPA (OH−) | 90 | Visible 160 W tungsten lamb | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannerselvam, M.; Siva, V.; Murugan, A.; Shameem, A.S.; Bavani, T.; Jhelai, S.; Shanmugan, S.; Ali, I.H.S.; Kannan, K. Rational Design of Core–Shell MoS2@ZIF-67 Nanocomposites for Enhanced Photocatalytic Degradation of Tetracycline. Nanomaterials 2025, 15, 545. https://doi.org/10.3390/nano15070545
Pannerselvam M, Siva V, Murugan A, Shameem AS, Bavani T, Jhelai S, Shanmugan S, Ali IHS, Kannan K. Rational Design of Core–Shell MoS2@ZIF-67 Nanocomposites for Enhanced Photocatalytic Degradation of Tetracycline. Nanomaterials. 2025; 15(7):545. https://doi.org/10.3390/nano15070545
Chicago/Turabian StylePannerselvam, Maruthasalam, Vadivel Siva, Anbazhagan Murugan, Abdul Samad Shameem, Thirugnanam Bavani, Sahadevan Jhelai, Sengottaiyan Shanmugan, Imran Hussain Showkath Ali, and Karthik Kannan. 2025. "Rational Design of Core–Shell MoS2@ZIF-67 Nanocomposites for Enhanced Photocatalytic Degradation of Tetracycline" Nanomaterials 15, no. 7: 545. https://doi.org/10.3390/nano15070545
APA StylePannerselvam, M., Siva, V., Murugan, A., Shameem, A. S., Bavani, T., Jhelai, S., Shanmugan, S., Ali, I. H. S., & Kannan, K. (2025). Rational Design of Core–Shell MoS2@ZIF-67 Nanocomposites for Enhanced Photocatalytic Degradation of Tetracycline. Nanomaterials, 15(7), 545. https://doi.org/10.3390/nano15070545







