Precisely Targeted Nanoparticles for CRISPR-Cas9 Delivery in Clinical Applications
Abstract
1. Introduction
2. The Strategies to Improve CRISPR-Cas9 Delivery Efficiency
2.1. Improve CRISPR-Cas9 Encapsulation Efficiency
2.1.1. Based on Electrostatic Interaction
2.1.2. Based on Base-Pair
2.1.3. Based on Protein–Ligand Interactions
2.1.4. Other Influencing Factors
2.2. Improve CRISPR-Cas9 Delivery Efficiency to the Cytoplasm/Nucleus
2.2.1. Improve the Cellular Uptake Efficiency
2.2.2. Improve Endosome/Lysosome Escape Efficiency
2.2.3. Targeting the Cell Nucleus
3. The Strategies for Targeted Delivery to Specific Tissues or Cells
3.1. Ligand-Mediated Targeting
3.1.1. Low-Density Lipoprotein Receptor-Related Protein-1 (LRP-1)-Mediated Targeting
3.1.2. CD44 Receptor-Mediated Targeting
3.1.3. Asialoglycoprotein Receptor-Mediated Targeting
3.1.4. Transferrin Receptor-Mediated Targeting
3.1.5. Folate Receptor-Mediated Targeting
3.1.6. Sialic Acid (SA)-Mediated Targeting
3.1.7. RPE-ATRA-Mediated Targeting
3.1.8. Integrin Receptor-Mediated Targeting
3.2. Biomimetic Strategies
3.2.1. Coating with Cancer Cell Membrane
3.2.2. Coating with Macrophage Membrane
3.3. Selective Organ-Targeting (SORT) Nanoparticles
4. The Controlled Release Strategies for CRISPR-Cas9 Delivery
4.1. pH-Responsive
4.2. Reactive Oxygen Species-Responsive
4.3. GSH-Responsive
4.4. Adenosine Triphosphate-Responsive
4.5. Near-Infrared Light-Responsive
4.6. Magnetic-Responsive
4.7. Dual Stimuli-Responsive and Multistage-Responsive
5. The Application of Delivery of the CRISPR-Cas9 by NPs
5.1. Gene Therapy
5.1.1. For Genetic Disease Therapy
5.1.2. For Cancer Therapy
5.2. Multimodal Synergistic Cancer Therapy
6. Challenges and Future Directions
6.1. Clinical Translation Challenges
6.1.1. Manufacturing Barriers
6.1.2. Regulatory Hurdles
6.1.3. Ongoing Clinical Trials and Technical Hurdles
6.2. Future Directions
6.2.1. Artificial Intelligence (AI)-Driven Nanoparticle Design
6.2.2. LNP: The Trailblazer in CRISPR Gene Therapy
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joung, J.K.; Sander, J.D. TALENs: A Widely Applicable Technology for Targeted Genome Editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome Editing with Engineered Zinc Finger Nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome Engineering Using the CRISPR-Cas9 System. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Sahel, D.K.; Vora, L.K.; Saraswat, A.; Sharma, S.; Monpara, J.; D’Souza, A.A.; Mishra, D.; Tryphena, K.P.; Kawakita, S.; Khan, S.; et al. CRISPR/Cas9 Genome Editing for Tissue-Specific In Vivo Targeting: Nanomaterials and Translational Perspective. Adv. Sci. 2023, 10, e2207512. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.; Yu, T. CRISPR/Cas9 Therapeutics: Progress and Prospects. Sig. Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, Z.; Zhang, Y.; Mu, W.; Han, X. Non-Viral Nanocarriers for CRISPR-Cas9 Gene Editing System Delivery. Chem. Eng. J. 2022, 435, 135116. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-Based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S.; Chakraborty, C. CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases. Mol. Ther. 2021, 29, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, Y.; Li, J.; Lu, A.; Liang, C. CRISPR/Cas Systems: Delivery and Application in Gene Therapy. Front. Bioeng. Biotechnol. 2022, 10, 942325. [Google Scholar] [CrossRef]
- Li, L.; Hu, S.; Chen, X. Non-Viral Delivery Systems for CRISPR/Cas9-Based Genome Editing: Challenges and Opportunities. Biomaterials 2018, 171, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Chew, W.L.; Tabebordbar, M.; Cheng, J.K.W.; Mali, P.; Wu, E.Y.; Ng, A.H.M.; Zhu, K.; Wagers, A.J.; Church, G.M. A Multifunctional AAV-CRISPR-Cas9 and Its Host Response. Nat. Methods 2016, 13, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, F.; Schmidt, K.; Kleinschmidt, J.A. A Viral Assembly Factor Promotes AAV2 Capsid Formation in the Nucleolus. Proc. Natl. Acad. Sci. USA 2010, 107, 10220–10225. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of Nucleic Acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Han, J.P.; Kim, M.; Choi, B.S.; Lee, J.H.; Lee, G.S.; Jeong, M.; Lee, Y.; Kim, E.-A.; Oh, H.-K.; Go, N.; et al. In Vivo Delivery of CRISPR-Cas9 Using Lipid Nanoparticles Enables Antithrombin Gene Editing for Sustainable Hemophilia A and B Therapy. Sci. Adv. 2022, 8, eabj6901. [Google Scholar] [CrossRef]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; Van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]
- Cho, E.Y.; Ryu, J.-Y.; Lee, H.A.R.; Hong, S.H.; Park, H.S.; Hong, K.S.; Park, S.-G.; Kim, H.P.; Yoon, T.-J. Lecithin Nano-Liposomal Particle as a CRISPR/Cas9 Complex Delivery System for Treating Type 2 Diabetes. J. Nanobiotechnol. 2019, 17, 19. [Google Scholar] [CrossRef]
- Liu, J.; Chang, J.; Jiang, Y.; Meng, X.; Sun, T.; Mao, L.; Xu, Q.; Wang, M. Fast and Efficient CRISPR/Cas9 Genome Editing In Vivo Enabled by Bioreducible Lipid and Messenger RNA Nanoparticles. Adv. Mater. 2019, 31, e1902575. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wilk, U.; Pöhmerer, J.; Hörterer, E.; Höhn, M.; Luo, X.; Mai, H.; Wagner, E.; Lächelt, U. Folate Receptor-Mediated Delivery of Cas9 RNP for Enhanced Immune Checkpoint Disruption in Cancer Cells. Small 2023, 19, 2205318. [Google Scholar] [CrossRef]
- Shao, M.; Qi, Y.; Sui, D.; Xu, F.-J. Phenylboronic Acid-Functionalized Polyaminoglycoside as an Effective CRISPR/Cas9 Delivery System. Biomater. Sci. 2021, 9, 7104–7114. [Google Scholar] [CrossRef]
- Cruz, L.J.; Van Dijk, T.; Vepris, O.; Li, T.M.W.Y.; Schomann, T.; Baldazzi, F.; Kurita, R.; Nakamura, Y.; Grosveld, F.; Philipsen, S.; et al. PLGA-Nanoparticles for Intracellular Delivery of the CRISPR-Complex to Elevate Fetal Globin Expression in Erythroid Cells. Biomaterials 2021, 268, 120580. [Google Scholar] [CrossRef]
- Tao, Y.; Yi, K.; Hu, H.; Shao, D.; Li, M. Coassembly of Nucleus-Targeting Gold Nanoclusters with CRISPR/Cas9 for Simultaneous Bioimaging and Therapeutic Genome Editing. J. Mater. Chem. B 2021, 9, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Bruhn, M.; de Ávila, B.E.; Beltrán-Gastélum, M.; Zhao, J.; Ramírez-Herrera, D.E.; Angsantikul, P.; Vesterager Gothelf, K.; Zhang, L.; Wang, J. Active Intracellular Delivery of a Cas9/sgRNA Complex Using Ultrasound-Propelled Nanomotors. Angew. Chem. Int. Ed. 2018, 57, 2657–2661. [Google Scholar] [CrossRef]
- Liu, J.; Li, G.; Guo, H.; Ni, C.; Gao, Y.; Cao, X.; Xia, J.; Shi, X.; Guo, R. Dual-Responsive Core–Shell Tecto Dendrimers Enable Efficient Gene Editing of Cancer Cells to Boost Immune Checkpoint Blockade Therapy. ACS Appl. Mater. Interfaces 2023, 15, 12809–12821. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Ray, M.; Yesilbag Tonga, G.; Lee, Y.-W.; Tay, T.; Sasaki, K.; Rotello, V.M. Direct Cytosolic Delivery of CRISPR/Cas9-Ribonucleoprotein for Efficient Gene Editing. ACS Nano 2017, 11, 2452–2458. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Wu, D.; Xin, H.; Chen, D.; Li, D.; Pan, H.; Zhou, C.; Ping, Y. Delivery of CRISPR/Cas9 Plasmids by Cationic Gold Nanorods: Impact of the Aspect Ratio on Genome Editing and Treatment of Hepatic Fibrosis. Chem. Mater. 2021, 33, 81–91. [Google Scholar] [CrossRef]
- Ju, E.; Li, T.; Ramos Da Silva, S.; Gao, S.-J. Gold Nanocluster-Mediated Efficient Delivery of Cas9 Protein through pH-Induced Assembly-Disassembly for Inactivation of Virus Oncogenes. ACS Appl. Mater. Interfaces 2019, 11, 34717–34724. [Google Scholar] [CrossRef]
- Lee, K.; Conboy, M.; Park, H.M.; Jiang, F.; Kim, H.J.; Dewitt, M.A.; Mackley, V.A.; Chang, K.; Rao, A.; Skinner, C.; et al. Nanoparticle Delivery of Cas9 Ribonucleoprotein and Donor DNA in Vivo Induces Homology-Directed DNA Repair. Nat. Biomed. Eng. 2017, 1, 889–901. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Xie, Y.; Wang, P.; Deng, S.; Qin, A.; Zhang, J.; Yu, X.; Zheng, W.; Jiang, X. Triple-Targeting Delivery of CRISPR/Cas9 To Reduce the Risk of Cardiovascular Diseases. Angew. Chem. Int. Ed. 2019, 58, 12404–12408. [Google Scholar] [CrossRef]
- Metzger, J.M.; Wang, Y.; Neuman, S.S.; Snow, K.J.; Murray, S.A.; Lutz, C.M.; Bondarenko, V.; Felton, J.; Gimse, K.; Xie, R.; et al. Efficient in Vivo Neuronal Genome Editing in the Mouse Brain Using Nanocapsules Containing CRISPR-Cas9 Ribonucleoproteins. Biomaterials 2023, 293, 121959. [Google Scholar] [CrossRef]
- Wang, Y.; Shahi, P.K.; Xie, R.; Zhang, H.; Abdeen, A.A.; Yodsanit, N.; Ma, Z.; Saha, K.; Pattnaik, B.R.; Gong, S. A pH-Responsive Silica–Metal–Organic Framework Hybrid Nanoparticle for the Delivery of Hydrophilic Drugs, Nucleic Acids, and CRISPR-Cas9 Genome-Editing Machineries. J. Control. Release 2020, 324, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shahi, P.K.; Wang, X.; Xie, R.; Zhao, Y.; Wu, M.; Roge, S.; Pattnaik, B.R.; Gong, S. In Vivo Targeted Delivery of Nucleic Acids and CRISPR Genome Editors Enabled by GSH-Responsive Silica Nanoparticles. J. Control. Release 2021, 336, 296–309. [Google Scholar] [CrossRef]
- Alsaiari, S.K.; Patil, S.; Alyami, M.; Alamoudi, K.O.; Aleisa, F.A.; Merzaban, J.S.; Li, M.; Khashab, N.M. Endosomal Escape and Delivery of CRISPR/Cas9 Genome Editing Machinery Enabled by Nanoscale Zeolitic Imidazolate Framework. J. Am. Chem. Soc. 2018, 140, 143–146. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Q.; Jiang, Y.; Zhang, M.; Wang, M.; Mao, L. Nanoscale ATP-Responsive Zeolitic Imidazole Framework-90 as a General Platform for Cytosolic Protein Delivery and Genome Editing. J. Am. Chem. Soc. 2019, 141, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Xie, R.; Burger, J.C.; Tong, Y.; Gong, S. Overcoming the Blood–Brain Barrier for Gene Therapy via Systemic Administration of GSH-Responsive Silica Nanocapsules. Adv. Mater. 2023, 35, 2208018. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, F.; Xu, Y.; He, C.; Luo, S.; Sun, X. Triple Functional Mild Photothermal Improves Gene Editing of PD-L1 for Enhanced Antitumor Immunity. J. Control. Release 2023, 354, 57–68. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Tong, S.; Lee, C.M.; Deshmukh, H.; Bao, G. Spatial Control of in Vivo CRISPR-Cas9 Genome Editing via Nanomagnets. Nat. Biomed. Eng. 2019, 3, 126–136. [Google Scholar] [CrossRef]
- Cho, H.; Yoo, M.; Pongkulapa, T.; Rabie, H.; Muotri, A.R.; Yin, P.T.; Choi, J.; Lee, K. Magnetic Nanoparticle-Assisted Non-Viral CRISPR-Cas9 for Enhanced Genome Editing to Treat Rett Syndrome. Adv. Sci. 2024, 11, 2306432. [Google Scholar] [CrossRef]
- Katti, A.; Diaz, B.J.; Caragine, C.M.; Sanjana, N.E.; Dow, L.E. CRISPR in Cancer Biology and Therapy. Nat. Rev. Cancer 2022, 22, 259–279. [Google Scholar] [CrossRef]
- Kugeratski, F.G.; McAndrews, K.M.; Kalluri, R. Multifunctional Applications of Engineered Extracellular Vesicles in the Treatment of Cancer. Endocrinology 2021, 162, bqaa250. [Google Scholar] [CrossRef]
- Happi Mbakam, C.; Lamothe, G.; Tremblay, G.; Tremblay, J.P. CRISPR-Cas9 Gene Therapy for Duchenne Muscular Dystrophy. Neurotherapeutics 2022, 19, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gao, M.; Yang, Y.; Li, W.; Bao, J.; Li, Y. The CRISPR/Cas9 System Delivered by Extracellular Vesicles. Pharmaceutics 2023, 15, 984. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, M.; Abbas, G.; Li, C.; Cui, M.; Zhang, X.-E.; Wang, D.-B. A Cancer Cell Membrane-Derived Biomimetic Nanocarrier for Synergistic Photothermal/Gene Therapy by Efficient Delivery of CRISPR/Cas9 and Gold Nanorods. Adv. Healthc. Mater. 2022, 11, e2201038. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, X.; Chen, Y.; Xin, H.; Wan, T.; Li, B.; Pan, H.; Li, D.; Ping, Y. Reprogramming the Tumor Microenvironment through Second-Near-Infrared-Window Photothermal Genome Editing of PD-L1 Mediated by Supramolecular Gold Nanorods for Enhanced Cancer Immunotherapy. Adv. Mater. 2021, 33, 2006003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-C.; Luo, B.-Y.; Zou, J.-J.; Wu, P.-Y.; Jiang, J.-L.; Le, J.-Q.; Zhao, R.-R.; Chen, L.; Shao, J.-W. Co-Delivery of Sorafenib and CRISPR/Cas9 Based on Targeted Core-Shell Hollow Mesoporous Organosilica Nanoparticles for Synergistic HCC Therapy. ACS Appl. Mater. Interfaces 2020, 12, 57362–57372. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Hu, S.; Li, K.; Shi, Y.; Pan, C.; Xu, Z.; Li, D.; Wang, H.; Li, B.; Chen, H. Breaking Osteoclast-Acid Vicious Cycle to Rescue Osteoporosis via an Acid Responsive Organic Framework-Based Neutralizing and Gene Editing Platform. Small 2024, 20, e2307595. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, C.; Wang, S.; Mäkilä, E.; Wang, J.; Koivisto, O.; Zhou, J.; Rosenholm, J.M.; Shu, Y.; Zhang, H. Microfluidic-Assisted Biomineralization of CRISPR/Cas9 in near-Infrared Responsive Metal–Organic Frameworks for Programmable Gene-Editing. Nanoscale 2022, 14, 15832–15844. [Google Scholar] [CrossRef]
- Zhai, L.-M.; Zhao, Y.; Xiao, R.-L.; Zhang, S.-Q.; Tian, B.-H.; Li, X.-X.; Zhang, R.; Ma, R.-S.; Liang, H.-X. Nuclear-Targeted Carbon Quantum Dot Mediated CRISPR/Cas9 Delivery for Fluorescence Visualization and Efficient Editing. Nanoscale 2022, 14, 14645–14660. [Google Scholar] [CrossRef]
- Liu, C.; Wan, T.; Wang, H.; Zhang, S.; Ping, Y.; Cheng, Y. A Boronic Acid–Rich Dendrimer with Robust and Unprecedented Efficiency for Cytosolic Protein Delivery and CRISPR-Cas9 Gene Editing. Sci. Adv. 2019, 5, eaaw8922. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, S.; Cheng, Z.; Ma, D.; Liu, T. A Glutathione-Sensitive Cationic Polymer Delivery of CRISPR-Cas9 RNA Plasmid for Targeting Nasopharyngeal Carcinoma Gene Therapy. Colloids Surf. B Biointerfaces 2023, 223, 113146. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.; Xie, R.; Wang, Y.; Xu, X.; Burger, J.; Gong, S. Guanidinium-Rich Lipopeptide-Based Nanoparticle Enables Efficient Gene Editing in Skeletal Muscles. ACS Appl. Mater. Interfaces 2023, 15, 10464–10476. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Wilson, D.R.; Choi, J.; Varanasi, M.; Sanders, K.; Karlsson, J.; Lim, M.; Green, J.J. Carboxylated Branched Poly(β-Amino Ester) Nanoparticles Enable Robust Cytosolic Protein Delivery and CRISPR-Cas9 Gene Editing. Sci. Adv. 2019, 5, eaay3255. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Jiao, M.; Xu, S.; Ismail, M.; Xie, X.; An, Y.; Guo, H.; Qian, R.; Shi, B.; Zheng, M. Brain-Targeted CRISPR/Cas9 Nanomedicine for Effective Glioblastoma Therapy. J. Control. Release 2022, 351, 739–751. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, X.; Yang, Q.; Zheng, M.; Shimoni, O.; Ruan, W.; Wang, Y.; Zhang, D.; Yin, J.; Huang, X.; et al. Blood-Brain Barrier–Penetrating Single CRISPR-Cas9 Nanocapsules for Effective and Safe Glioblastoma Gene Therapy. Sci. Adv. 2022, 8, eabm8011. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, L.; Li, C.; Long, Q.; Yang, Q.; Huang, A.; Tang, H. CRISPR/Cas9 Delivery by NIR-Responsive Biomimetic Nanoparticles for Targeted HBV Therapy. J. Nanobiotechnol. 2022, 20, 27. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Chen, C.; Li, Z.; Du, S.; Luan, X.; Gao, Y.; Han, X.; Song, Y. Multistage-Responsive Gene Editing to Sensitize Ion-Interference Enhanced Carbon Monoxide Gas Therapy. Small 2022, 18, 2204244. [Google Scholar] [CrossRef]
- Pu, Y.; Yin, H.; Dong, C.; Xiang, H.; Wu, W.; Zhou, B.; Du, D.; Chen, Y.; Xu, H. Sono-Controllable and ROS-Sensitive CRISPR-Cas9 Genome Editing for Augmented/Synergistic Ultrasound Tumor Nanotherapy. Adv. Mater. 2021, 33, 2104641. [Google Scholar] [CrossRef]
- Jo, A.; Ringel-Scaia, V.M.; McDaniel, D.K.; Thomas, C.A.; Zhang, R.; Riffle, J.S.; Allen, I.C.; Davis, R.M. Fabrication and Characterization of PLGA Nanoparticles Encapsulating Large CRISPR-Cas9 Plasmid. J. Nanobiotechnol. 2020, 18, 16. [Google Scholar] [CrossRef]
- Xie, R.; Wang, X.; Wang, Y.; Ye, M.; Zhao, Y.; Yandell, B.S.; Gong, S. pH-Responsive Polymer Nanoparticles for Efficient Delivery of Cas9 Ribonucleoprotein With or Without Donor DNA. Adv. Mater. 2022, 34, e2110618. [Google Scholar] [CrossRef]
- Wang, N.; Liu, C.; Lu, Z.; Yang, W.; Li, L.; Gong, S.; He, T.; Ou, C.; Song, L.; Shen, M.; et al. Multistage Sensitive NanoCRISPR Enable Efficient Intracellular Disruption of Immune Checkpoints for Robust Innate and Adaptive Immune Coactivation. Adv. Funct. Mater. 2020, 30, 2004940. [Google Scholar] [CrossRef]
- Qiao, L.; Gao, M.; Yi, X.; Peng, H.; Zhang, R.; Yao, W.; Sun, G.; He, X. Biomimetic Gene Editing System for Precise Tumor Cell Reprogramming and Augmented Tumor Therapy. J. Control. Release 2023, 356, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Alhazza, A.; Mahdipoor, P.; Hall, R.; Manda, A.; Lohan, S.; Parang, K.; Aliabadi, H.M. Modifying Peptide/Lipid-Associated Nucleic Acids (PLANAs) for CRISPR/Cas9 Ribonucleoprotein Delivery. Eur. J. Pharm. Sci. 2024, 195, 106708. [Google Scholar] [CrossRef]
- Yang, C.; Fu, Y.; Huang, C.; Hu, D.; Zhou, K.; Hao, Y.; Chu, B.; Yang, Y.; Qian, Z. Chlorin E6 and CRISPR-Cas9 Dual-Loading System with Deep Penetration for a Synergistic Tumoral Photodynamic-Immunotherapy. Biomaterials 2020, 255, 120194. [Google Scholar] [CrossRef]
- Zhao, L.; Li, D.; Zhang, Y.; Huang, Q.; Zhang, Z.; Chen, C.; Xu, C.-F.; Chu, X.; Zhang, Y.; Yang, X. HSP70-Promoter-Driven CRISPR/Cas9 System Activated by Reactive Oxygen Species for Multifaceted Anticancer Immune Response and Potentiated Immunotherapy. ACS Nano 2022, 16, 13821–13833. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Fan, X.; Guo, X.; Tang, J.; Li, H.; Tao, R.; Li, F.; Li, J.; Yang, D.; Yao, C.; et al. A DNA/Upconversion Nanoparticle Complex Enables Controlled Co-Delivery of CRISPR-Cas9 and Photodynamic Agents for Synergistic Cancer Therapy. Adv. Mater. 2024, 36, e2309534. [Google Scholar] [CrossRef]
- Wu, J.; Peng, H.; Lu, X.; Lai, M.; Zhang, H.; Le, X.C. Binding-Mediated Formation of Ribonucleoprotein Corona for Efficient Delivery and Control of CRISPR/Cas9. Angew. Chem. Int. Ed. 2021, 60, 11104–11109. [Google Scholar] [CrossRef]
- Farbiak, L.; Cheng, Q.; Wei, T.; Álvarez-Benedicto, E.; Johnson, L.T.; Lee, S.; Siegwart, D.J. All-In-One Dendrimer-Based Lipid Nanoparticles Enable Precise HDR-Mediated Gene Editing In Vivo. Adv. Mater. 2021, 33, e2006619. [Google Scholar] [CrossRef]
- Ma, L.; Ma, Y.; Gao, Q.; Liu, S.; Zhu, Z.; Shi, X.; Dai, F.; Reis, R.L.; Kundu, S.C.; Cai, K.; et al. Mulberry Leaf Lipid Nanoparticles: A Naturally Targeted CRISPR/Cas9 Oral Delivery Platform for Alleviation of Colon Diseases. Small 2024, 20, e2307247. [Google Scholar] [CrossRef]
- Li, B.; Manan, R.S.; Liang, S.-Q.; Gordon, A.; Jiang, A.; Varley, A.; Gao, G.; Langer, R.; Xue, W.; Anderson, D. Combinatorial Design of Nanoparticles for Pulmonary mRNA Delivery and Genome Editing. Nat. Biotechnol. 2023, 41, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, T.; Weng, Y.; Zhang, M.; Zhao, D.; Guo, S.; Hu, B.; Shao, W.; Wang, X.; Hussain, A.; et al. Ionizable Lipid-Assisted Efficient Hepatic Delivery of Gene Editing Elements for Oncotherapy. Bioact. Mater. 2022, 9, 590–601. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 Genome Editing Using Targeted Lipid Nanoparticles for Cancer Therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef] [PubMed]
- Moitra, P.; Skrodzki, D.; Molinaro, M.; Gunaseelan, N.; Sar, D.; Aditya, T.; Dahal, D.; Ray, P.; Pan, D. Context-Responsive Nanoparticle Derived from Synthetic Zwitterionic Ionizable Phospholipids in Targeted CRISPR/Cas9 Therapy for Basal-like Breast Cancer. ACS Nano 2024, 18, 9199–9220. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.; Chang, C.-H.; Tzeng, T.-Y.; Lin, A.M.-Y.; Lo, Y.-L. Gene-Editing by CRISPR-Cas9 in Combination with Anthracycline Therapy via Tumor Microenvironment-Switchable, EGFR-Targeted, and Nucleus-Directed Nanoparticles for Head and Neck Cancer Suppression. Nanoscale Horiz. 2021, 6, 729–743. [Google Scholar] [CrossRef]
- Gao, K.; Li, J.; Song, H.; Han, H.; Wang, Y.; Yin, B.; Farmer, D.L.; Murthy, N.; Wang, A. In Utero Delivery of mRNA to the Heart, Diaphragm and Muscle with Lipid Nanoparticles. Bioact. Mater. 2023, 25, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Zhuo, C.; Yi, K.; Zheng, C.; Mintz, R.L.; Lao, Y.-H.; Zhong, Q.; Ju, E.; Wang, H.; Shao, D.; et al. Hepatocyte-Confined CRISPR/Cas9-Based Nanocleaver Precisely Eliminates Viral DNA for Efficient and Safe Treatment of Hepatitis B Virus Infection. Nano Today 2023, 53, 102040. [Google Scholar] [CrossRef]
- Kenjo, E.; Hozumi, H.; Makita, Y.; Iwabuchi, K.A.; Fujimoto, N.; Matsumoto, S.; Kimura, M.; Amano, Y.; Ifuku, M.; Naoe, Y.; et al. Low Immunogenicity of LNP Allows Repeated Administrations of CRISPR-Cas9 mRNA into Skeletal Muscle in Mice. Nat. Commun. 2021, 12, 7101. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Zheng, W.; Cong, L.; Guo, Z.; Xie, Y.; Wang, L.; Tang, R.; Feng, Q.; Hamada, Y.; et al. Thermo-triggered Release of CRISPR-Cas9 System by Lipid-Encapsulated Gold Nanoparticles for Tumor Therapy. Angew. Chem. Int. Ed. 2018, 57, 1491–1496. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, J.; Jiang, Y.; Zhang, M.; Mao, L.; Wang, M. Cell-Selective Messenger RNA Delivery and CRISPR/Cas9 Genome Editing by Modulating the Interface of Phenylboronic Acid-Derived Lipid Nanoparticles and Cellular Surface Sialic Acid. ACS Appl. Mater. Interfaces 2019, 11, 46585–46590. [Google Scholar] [CrossRef] [PubMed]
- Soprano, E.; Migliavacca, M.; López-Ferreiro, M.; Pelaz, B.; Polo, E.; del Pino, P. Fusogenic Cell-Derived Nanocarriers for Cytosolic Delivery of Cargo inside Living Cells. J. Colloid. Interface Sci. 2023, 648, 488–496. [Google Scholar] [CrossRef]
- Xu, X.; Tang, H.; Guo, J.; Xin, H.; Ping, Y. A Dual-Specific CRISPR-Cas Nanosystem for Precision Therapeutic Editing of Liver Disorders. Sig. Transduct. Target. Ther. 2022, 7, 269. [Google Scholar] [CrossRef]
- Zhou, W.; Cui, H.; Ying, L.; Yu, X. Enhanced Cytosolic Delivery and Release of CRISPR/Cas9 by Black Phosphorus Nanosheets for Genome Editing. Angew. Chem. Int. Ed. 2018, 57, 10268–10272. [Google Scholar] [CrossRef]
- Yuan, C.; Chang, S.; Zhang, C.; Dong, D.; Ding, J.; Mahdavian, A.R.; Hu, Z.; Sun, L.; Tan, S. Post Cross-Linked ROS-Responsive Poly(β-Amino Ester)-Plasmid Polyplex NPs for Gene Therapy of EBV-Associated Nasopharyngeal Carcinoma. J. Mater. Chem. B 2024, 12, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Z.; Shen, M.; Wang, Y.; Wang, L.; Li, J.; Yang, W.; Li, J.; Li, H.; Wang, X.; et al. Programmable Unlocking Nano-Matryoshka-CRISPR Precisely Reverses Immunosuppression to Unleash Cascade Amplified Adaptive Immune Response. Adv. Sci. 2021, 8, 2100292. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, J.; Luan, X.; Liu, X.; Li, X.; Yang, J.; Huang, T.; Sun, L.; Wang, Y.; Lin, Y.; et al. Near-Infrared Upconversion–Activated CRISPR-Cas9 System: A Remote-Controlled Gene Editing Platform. Sci. Adv. 2019, 5, eaav7199. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, Y.; Du, S.; Wu, Y.; Shen, P.; Yan, T.; Li, X.; Song, Y.; Zha, Z.; Han, X. Controlled CRISPR-Cas9 Ribonucleoprotein Delivery for Sensitized Photothermal Therapy. Small 2021, 17, e2101155. [Google Scholar] [CrossRef]
- Yan, T.; Yang, K.; Chen, C.; Zhou, Z.; Shen, P.; Jia, Y.; Xue, Y.; Zhang, Z.; Shen, X.; Han, X. Synergistic Photothermal Cancer Immunotherapy by Cas9 Ribonucleoprotein-Based Copper Sulfide Nanotherapeutic Platform Targeting PTPN2. Biomaterials 2021, 279, 121233. [Google Scholar] [CrossRef]
- Wei, T.; Sun, Y.; Cheng, Q.; Chatterjee, S.; Traylor, Z.; Johnson, L.T.; Coquelin, M.L.; Wang, J.; Torres, M.J.; Lian, X.; et al. Lung SORT LNPs Enable Precise Homology-Directed Repair Mediated CRISPR/Cas Genome Correction in Cystic Fibrosis Models. Nat. Commun. 2023, 14, 7322. [Google Scholar] [CrossRef]
- Wei, T.; Cheng, Q.; Min, Y.-L.; Olson, E.N.; Siegwart, D.J. Systemic Nanoparticle Delivery of CRISPR-Cas9 Ribonucleoproteins for Effective Tissue Specific Genome Editing. Nat. Commun. 2020, 11, 3232. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, H.; Huang, X.; Chaurasiya, B.; Dong, D.; Shanley, T.P.; Zhao, Y.-Y. Robust Genome Editing in Adult Vascular Endothelium by Nanoparticle Delivery of CRISPR-Cas9 Plasmid DNA. Cell Rep. 2022, 38, 110196. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, F.; Chen, Y.; Liu, J.; Wang, X.; Chen, A.T.; Deng, G.; Zhang, H.; Liu, J.; Hong, Z.; et al. Targeted Delivery of CRISPR/Cas9-Mediated Cancer Gene Therapy via Liposome-Templated Hydrogel Nanoparticles. Adv. Funct. Mater. 2017, 27, 1703036. [Google Scholar] [CrossRef]
- Fang, N.; Wang, J.; Mao, H.-Q.; Leong, K.W.; Chan, V. BHEM-Chol/DOPE Liposome Induced Perturbation of Phospholipid Bilayer. Colloids Surf., B 2003, 29, 233–245. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Xu, C.-F.; Luo, Y.-L.; Lu, Z.-D.; Wang, J. Systemic Delivery of CRISPR/Cas9 with PEG-PLGA Nanoparticles for Chronic Myeloid Leukemia Targeted Therapy. Biomater. Sci. 2018, 6, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ji, W.; Hall, J.M.; Hu, Q.; Wang, C.; Beisel, C.L.; Gu, Z. Self-Assembled DNA Nanoclews for the Efficient Delivery of CRISPR–Cas9 for Genome Editing. Angew. Chem. Int. Ed. 2015, 54, 12029–12033. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key Principles and Methods for Studying the Endocytosis of Biological and Nanoparticle Therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Rouet, R.; Thuma, B.A.; Roy, M.D.; Lintner, N.G.; Rubitski, D.M.; Finley, J.E.; Wisniewska, H.M.; Mendonsa, R.; Hirsh, A.; De Oñate, L.; et al. Receptor-Mediated Delivery of CRISPR-Cas9 Endonuclease for Cell-Type-Specific Gene Editing. J. Am. Chem. Soc. 2018, 140, 6596–6603. [Google Scholar] [CrossRef]
- Chen, G.; Ma, B.; Wang, Y.; Gong, S. A Universal GSH-Responsive Nanoplatform for the Delivery of DNA, mRNA, and Cas9/sgRNA Ribonucleoprotein. ACS Appl. Mater. Interfaces 2018, 10, 18515–18523. [Google Scholar] [CrossRef]
- Hanif, S.; Muhammad, P.; Chesworth, R.; Rehman, F.U.; Qian, R.; Zheng, M.; Shi, B. Nanomedicine-Based Immunotherapy for Central Nervous System Disorders. Acta Pharmacol. Sin. 2020, 41, 936–953. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Actis Dato, V.; Chiabrando, G.A. The Role of Low-Density Lipoprotein Receptor-Related Protein 1 in Lipid Metabolism, Glucose Homeostasis and Inflammation. Int. J. Mol. Sci. 2018, 19, 1780. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M.; Arnold, K.S.; Newhouse, Y.M.; Innerarity, T.L.; Weisgraber, K.H.; Segall, M.L.; Phillips, M.C.; Lund-Katz, S. Apolipoprotein E;-Low Density Lipoprotein Receptor Interaction. Influences of Basic Residue and Amphipathic Alpha-Helix Organization in the Ligand. J. Lipid Res. 2000, 41, 1087–1095. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Li, W.; Zhu, G.; Sun, S.; Chi, H.; Yin, Y.; Diao, H.; Xing, X.-J.; Guo, Z.; Wang, L.; et al. Functionalized PDA/DEX-PEI@HA Nanoparticles Combined with Sleeping-Beauty Transposons for Multistage Targeted Delivery of CRISPR/Cas9 Gene. Biomed. Pharmacother. 2021, 142, 112061. [Google Scholar] [CrossRef]
- Francis, C.; Wroblewska, L.; Pegman, P.; Amiji, M. Systemic Biodistribution and Hepatocyte-Specific Gene Editing with CRISPR/Cas9 Using Hyaluronic Acid-Based Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102488. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Johnsen, K.B.; Kucharz, K.; Lauritzen, M.; Moos, T. Blood-Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics 2022, 14, 2237. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Curtis, S.; Bai, A.; Young, A.; Derosier, D.; Ripley, S.; Bai, S. CRISPR/Cas9 Targeting Liposomes Knocked down Multidrug Resistance Proteins in Brain Endothelial Cells as a Model to Predict Potential Pharmacoresistance. Colloids Surf. B Biointerfaces 2023, 222, 113103. [Google Scholar] [CrossRef]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the Folate Receptor α in Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Inoue, M.; Muta, K.; Mohammed, A.F.A.; Onodera, R.; Higashi, T.; Ouchi, K.; Ueda, M.; Ando, Y.; Arima, H.; Jono, H.; et al. Feasibility Study of Dendrimer-Based TTR-CRISPR pDNA Polyplex for Ocular Amyloidosis in Vitro. Biol. Pharm. Bull. 2022, 45, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, X.; Wang, M. Intracellular Delivery of mRNA for Cell-Selective CRISPR/Cas9 Genome Editing Using Lipid Nanoparticles. Chembiochem 2023, 24, e202200801. [Google Scholar] [CrossRef]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.-J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, X.; Wang, R.; Zeng, Y.; Zeng, Z.; Xie, T. Recent Research Progress of RGD Peptide–Modified Nanodrug Delivery Systems in Tumor Therapy. Int. J. Pept. Res. Ther. 2023, 29, 53. [Google Scholar] [CrossRef]
- Zhang, K.; Chooi, W.H.; Liu, S.; Chin, J.S.; Murray, A.; Nizetic, D.; Cheng, D.; Chew, S.Y. Localized Delivery of CRISPR/dCas9 via Layer-by-Layer Self-Assembling Peptide Coating on Nanofibers for Neural Tissue Engineering. Biomaterials 2020, 256, 120225. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-H.; Ye, P.-J.; Zhou, Y.-C.; He, D.-X.; Wei, H.; Yu, C.-Y. Cell Membrane-Camouflaged Nanoparticles as Drug Carriers for Cancer Therapy. Acta Biomater. 2020, 105, 1–14. [Google Scholar] [CrossRef]
- Alyami, M.Z.; Alsaiari, S.K.; Li, Y.; Qutub, S.S.; Aleisa, F.A.; Sougrat, R.; Merzaban, J.S.; Khashab, N.M. Cell-Type-Specific CRISPR/Cas9 Delivery by Biomimetic Metal Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 1715–1720. [Google Scholar] [CrossRef]
- Vijayan, V.; Uthaman, S.; Park, I.-K. Cell Membrane Coated Nanoparticles: An Emerging Biomimetic Nanoplatform for Targeted Bioimaging and Therapy. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Noh, I., Ed.; Springer: Singapore, 2018; pp. 45–59. ISBN 9789811304453. [Google Scholar]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective Organ Targeting (SORT) Nanoparticles for Tissue-Specific mRNA Delivery and CRISPR-Cas Gene Editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Sun, Y.; Yu, X.; Lee, S.M.; Cheng, Q.; Wei, T.; Gong, J.; Robinson, J.; Zhang, D.; et al. Preparation of Selective Organ-Targeting (SORT) Lipid Nanoparticles (LNPs) Using Multiple Technical Methods for Tissue-Specific mRNA Delivery. Nat. Protoc. 2023, 18, 265–291. [Google Scholar] [CrossRef] [PubMed]
- Dilliard, S.A.; Cheng, Q.; Siegwart, D.J. On the Mechanism of Tissue-Specific mRNA Delivery by Selective Organ Targeting Nanoparticles. Proc. Natl. Acad. Sci. USA 2021, 118, e2109256118. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Han, H.; Zhao, S.; Xu, B.; Yin, B.; Lawanprasert, A.; Trinidad, M.; Burgstone, B.W.; Murthy, N.; Doudna, J.A. Lung and Liver Editing by Lipid Nanoparticle Delivery of a Stable CRISPR–Cas9 Ribonucleoprotein. Nat. Biotechnol. 2024, 1–13. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Ma, Y.; Hou, Y.-C.; Pan, J.; Chen, S.-Y.; Chien, M.-H.; Zhang, Z.-X.; Hsu, W.-H.; Wang, X.; Zhang, J.; et al. Dual Targeted Extracellular Vesicles Regulate Oncogenic Genes in Advanced Pancreatic Cancer. Nat. Commun. 2023, 14, 6692. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, Q.; Wei, T.; Yu, X.; Johnson, L.T.; Farbiak, L.; Siegwart, D.J. Membrane-Destabilizing Ionizable Phospholipids for Organ-Selective mRNA Delivery and CRISPR–Cas Gene Editing. Nat. Mater. 2021, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Glass, Z.; Chen, J.; Haas, M.; Jin, X.; Zhao, X.; Rui, X.; Ye, Z.; Li, Y.; Zhang, F.; et al. Lipid Nanoparticle-Mediated Codelivery of Cas9 mRNA and Single-Guide RNA Achieves Liver-Specific in Vivo Genome Editing of Angptl3. Proc. Natl. Acad. Sci. USA 2021, 118, e2020401118. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Luo, T.; Mao, L.; Wang, M. Spatiotemporal Delivery of CRISPR/Cas9 Genome Editing Machinery Using Stimuli-Responsive Vehicles. Angew. Chem. Int. Ed. 2021, 60, 8596–8606. [Google Scholar] [CrossRef]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. pH-Responsive Nanoparticles for Drug Delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Liang, Y.; Chang, H.; Cai, T.; Feng, B.; Gordon, K.; Zhu, Y.; Shi, H.; He, Y.; Xie, L. Targeting Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9): From Bench to Bedside. Sig. Transduct. Target. Ther. 2024, 9, 13. [Google Scholar] [CrossRef]
- Stower, H. Gene Editing for Duchenne Muscular Dystrophy. Nat. Med. 2018, 24, 1491. [Google Scholar] [CrossRef]
- Liang, J.; Liu, B. ROS-responsive Drug Delivery Systems. Bioeng. Transla. Med. 2016, 1, 239–251. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, T.; DiSanto, R.; Tai, W.; Gu, Z. ATP-Triggered Anticancer Drug Delivery. Nat. Commun. 2014, 5, 3364. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, N.; Zhou, J.K.; Xu, C.; Quek, C.H.; Zhu, Y.; Tang, D.; Hung, L.Y.; Najjar, S.A.; Shiu, C.Y.A.; Margolis, K.G.; et al. Phenylboronic Acid-Functionalized Polyplexes Tailored to Oral CRISPR Delivery. Nano Lett. 2023, 23, 757–764. [Google Scholar] [CrossRef]
- Long, C.; McAnally, J.R.; Shelton, J.M.; Mireault, A.A.; Bassel-Duby, R.; Olson, E.N. Prevention of Muscular Dystrophy in Mice by CRISPR/Cas9–Mediated Editing of Germline DNA. Science 2014, 345, 1184–1188. [Google Scholar] [CrossRef]
- Cavazzana, M.; Antoniani, C.; Miccio, A. Gene Therapy for β-Hemoglobinopathies. Mol. Ther. 2017, 25, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Chatterjee, S.; Sun, Y.; Dilliard, S.A.; Moore, S.; Xiao, Y.; Bian, X.; Yamada, K.; Sung, Y.-C.; Levine, R.M.; et al. Bone-Marrow-Homing Lipid Nanoparticles for Genome Editing in Diseased and Malignant Haematopoietic Stem Cells. Nat. Nanotechnol. 2024, 19, 1409–1417. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, R.; Yang, Z.; Chen, Z. Genome Editing Using CRISPR/Cas9 to Treat Hereditary Hematological Disorders. Gene Ther. 2022, 29, 207–216. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Castiglione, V.; Rapezzi, C.; Franzini, M.; Panichella, G.; Vergaro, G.; Gillmore, J.; Fontana, M.; Passino, C.; Emdin, M. RNA-Targeting and Gene Editing Therapies for Transthyretin Amyloidosis. Nat. Rev. Cardiol. 2022, 19, 655–667. [Google Scholar] [CrossRef]
- Kotit, S. Lessons from the First-in-Human in Vivo CRISPR/Cas9 Editing of the TTR Gene by NTLA-2001 Trial in Patients with Transthyretin Amyloidosis with Cardiomyopathy. Glob. Cardiol. Sci. Pract. 2023, 2023, e202304. [Google Scholar] [CrossRef] [PubMed]
- Strebhardt, K.; Ullrich, A. Targeting Polo-like Kinase 1 for Cancer Therapy. Nat. Rev. Cancer 2006, 6, 321–330. [Google Scholar] [CrossRef]
- Kieser, A.; Sterz, K.R. The Latent Membrane Protein 1 (LMP1). In Epstein Barr Virus Volume 2: One Herpes Virus: Many Diseases; Münz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 119–149. ISBN 978-3-319-22834-1. [Google Scholar]
- Li, X.; Pan, Y.; Chen, C.; Gao, Y.; Liu, X.; Yang, K.; Luan, X.; Zhou, D.; Zeng, F.; Han, X.; et al. Hypoxia-Responsive Gene Editing to Reduce Tumor Thermal Tolerance for Mild-Photothermal Therapy. Angew. Chem. Int. Ed. 2021, 60, 21200–21204. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef] [PubMed]
- Kafetzis, K.N.; Papalamprou, N.; McNulty, E.; Thong, K.X.; Sato, Y.; Mironov, A.; Purohit, A.; Welsby, P.J.; Harashima, H.; Yu-Wai-Man, C.; et al. The Effect of Cryoprotectants and Storage Conditions on the Transfection Efficiency, Stability, and Safety of Lipid-Based Nanoparticles for mRNA and DNA Delivery. Adv. Healthc. Mater. 2023, 12, 2203022. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.S.; Butler, K.S.; Brinker, C.J.; Azzawi, M.; Conlan, S.; Dufés, C.; Owen, A.; Rannard, S.; Scott, C.; Chen, C.; et al. On the Issue of Transparency and Reproducibility in Nanomedicine. Nat. Nanotechnol. 2019, 14, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Tavridou, A.; Rogers, D.; Farinelli, G.; Gravanis, I.; Jekerle, V. Genome-Editing Medicinal Products: The EMA Perspective. Nat. Rev. Drug Discov. 2024, 23, 242–243. [Google Scholar] [CrossRef] [PubMed]
- DepoCyte—Withdrawal of Application for Variation to Marketing Authorisation|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/variation/depocyte (accessed on 28 March 2025).
- Human Genome Editing: A Framework for Governance. Available online: https://www.who.int/publications/i/item/9789240030060 (accessed on 25 March 2025).
- Quality, Non-Clinical and Clinical Aspects of Medicinal Products Containing Genetically Modified Cells—Scientific Guideline | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/quality-non-clinical-clinical-aspects-medicinal-products-containing-genetically-modified-cells-scientific-guideline (accessed on 26 March 2025).
- Drug Products, Including Biological Products, That Contain Nanomaterials—Guidance for Industry | FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-products-including-biological-products-contain-nanomaterials-guidance-industry (accessed on 29 March 2025).
- Hemmrich, E.; McNeil, S. Active Ingredient vs Excipient Debate for Nanomedicines. Nat. Nanotechnol. 2023, 18, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. The World’s First CRISPR Therapy Is Approved: Who Will Receive It? Nat. Biotechnol. 2023, 42, 3–4. [Google Scholar] [CrossRef]
- Bluebird Bio Announces Temporary Suspension on Phase 1/2 and Phase 3 Studies of LentiGlobin Gene Therapy for Sickle Cell Disease (Bb1111)—Bluebird Bio, Inc. Available online: https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-announces-temporary-suspension-phase-12-and-phase-3 (accessed on 29 March 2025).
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, X.; Wang, Y.; Yu, Y.; Huang, N.; Li, G.; Li, X.; Wu, J.C.; Yang, S. Artificial Intelligence in Drug Development. Nat. Med. 2025, 31, 45–59. [Google Scholar] [CrossRef]
- Luttens, A.; Cabeza De Vaca, I.; Sparring, L.; Brea, J.; Martínez, A.L.; Kahlous, N.A.; Radchenko, D.S.; Moroz, Y.S.; Loza, M.I.; Norinder, U.; et al. Rapid Traversal of Vast Chemical Space Using Machine Learning-Guided Docking Screens. Nat. Comput. Sci. 2025. [Google Scholar] [CrossRef]
- Witten, J.; Raji, I.; Manan, R.S.; Beyer, E.; Bartlett, S.; Tang, Y.; Ebadi, M.; Lei, J.; Nguyen, D.; Oladimeji, F.; et al. Artificial Intelligence-Guided Design of Lipid Nanoparticles for Pulmonary Gene Therapy. Nat. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Fia, W. Reflection Paper on the Use of Artificial Intelligence (AI) in the Medicinal Product Lifecycle_240903; European Medicines Agency: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Cullis, P.R.; Felgner, P.L. The 60-Year Evolution of Lipid Nanoparticles for Nucleic Acid Delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Solomon, S.D.; Kachadourian, J.; Walsh, L.; Rocha, R.; Lebwohl, D.; Smith, D.; Täubel, J.; Gane, E.J.; Pilebro, B.; et al. CRISPR-Cas9 Gene Editing with Nexiguran Ziclumeran for ATTR Cardiomyopathy. N. Engl. J. Med. 2024, 391, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
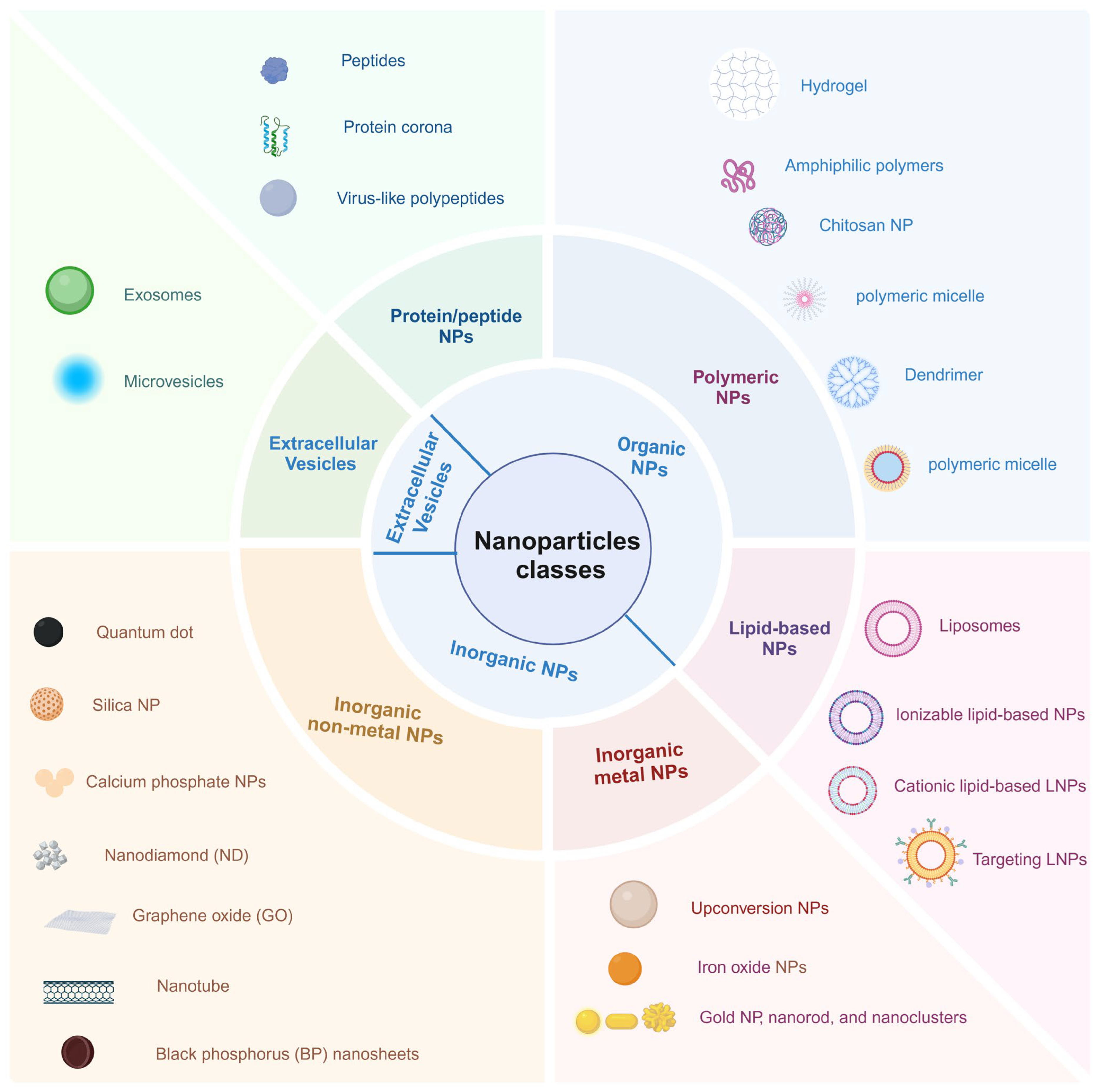
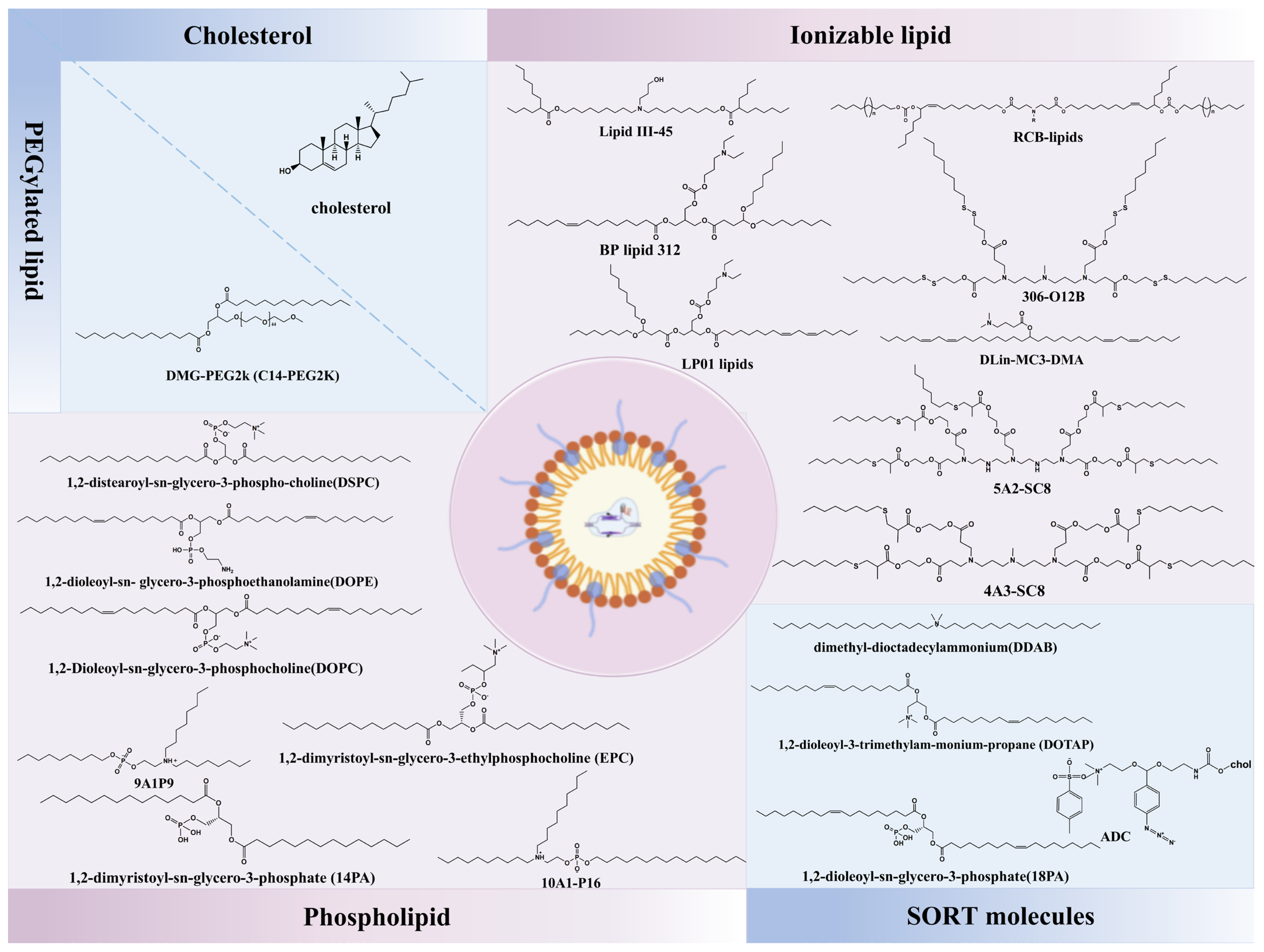
| Materials | Hydrodynamic Diameter | Cas9 Form and Encapsulation Efficiency (or Loading Content) | Target Cell; Gene; Efficiency and Analysis of Gene Editing In Vitro | Refs. |
|---|---|---|---|---|
| Gold nanorods (AuNRs) | 150 nm | plasmids (1.35 μg/μg) | AAVS1 in 293T cells (15.37% knock-out) and PLK1(15.50% knock-out) in A549 cells by analyzing gel bands of the digestion products of T7E1, Fas gene (10.5% knock-out) in Hepa1-6 cells by Sanger sequencing | [26] |
| Gold nanoparticles (GNPs) | 546 nm | RNP and donor DNA (61.5%) | CXCR4 in hES cells, hiPS cells, BMDCs, and the dystrophin gene (HDR efficiency 3~4%) in myoblasts by PCR | [28] |
| Au nanoparticle-loaded core–shell tecto dendrimers (Au CSTDs) | 108.0~131.2 nm | plasmids | PD-L1 (59.8% knock-out) in B16F10 cells by T7 endonuclease I (T7EI) assay | [24] |
| Protamine-capped gold nanoclusters | 3.5 nm | plasmids | EGFP gene (30% knock-out) in U2OS-EGFP cells by flow cytometry, human papillomavirus (HPV) type 18 (HPV18)-E7 oncogene (29% knock-out) in HeLa by T7 endonuclease I (T7EI) assay and Western blotting | [22] |
| Cancer cell membrane-derived nanocarrier (mCas9-sGNRs) | length ≈ 60 nm, width ≈ 15 nm | RNP (60%) | Survivin gene (33% knock-out) in MDA-MB-231 cells by T7 endonuclease I (T7EI) assay | [43] |
| Cationic gold nanorod | (length 60.14 ± 3.56 nm, width 8.02 ± 0.59 nm) | plasmids (ANP/plasmids = 0.15) | PD-L1 (39.7% indel) in B16F10 cells by T7 endonuclease I (T7EI) assay | [44] |
| Silica–metal–organic framework hybrid nanoparticles (SMOF NPs) consisting of both silica and zeolitic imidazole framework (ZIF) | 110 nm | RNP (>9 wt% and loading efficiency > 90%) | HEK 293-GFP cells (~60% knock-in) by flow cytometry | [31] |
| Zeolitic imidazolate framework-8 (ZIF-8) | 100 nm | RNP (1.2%, w/v and loading efficiency of 17%) | EGFP gene (37% knock-down) in Chinese hamster ovary (CHO) cells by qRT-PCR | [33] |
| Core–shell hollow mesoporous organosilica nanoparticles | 156.6 ± 1.8 nm | plasmids (76.65%) | EGFR gene (66.3% knock-out) in HepG2 cells by T7 endonuclease I (T7EI) assay | [45] |
| ZIF8-NaHCO3 @Cas9 (ZNC) | 40~70 nm | plasmids | RANKL (71.12% knock-out) in MC3T3-E1 cells by flow cytometry analysis | [46] |
| PB@RNP-EuMOFs | ~150 nm | RNP (60%) | GFP (47% knock-out) in HeLa-GFP cells by Sanger sequencing assay | [47] |
| Polyethylenimine (PEI) and polyethylene glycol (PEG) conjugated carbon quantum dots (CQDs-PP) | 2.4 nm | plasmids | EFHD1 gene (34.2% knock-out) in HeLa cells by PCR and sequencing | [48] |
| PBA-rich cationic polymer | 300 nm | RNP | EGFP (40% knock-out) in HEK293-EGFP reporter cells by flow cytometry | [49] |
| Hyperbranched polyamide amine (HPAA) | 500 nm | plasmids | SGK3 gene (13% knock-out) in HNE-1 cells by flow cytometry | [50] |
| Lipopeptide (GD-LP) | 231.3 nm | RNP (20 wt%) | EGFP gene (72.6% knock-down) in GFP-HEK 293 cells by flow cytometry | [51] |
| Carboxylated branched poly (b-amino ester) | ~200 nm | RNP (30 w/w) | GFP gene (77% knock-out) in HEK293 cells and GFP gene (47% knock-out) in GL261 murine glioma cells | [52] |
| Angiopep-2 decorated, guanidinium and fluorine functionalized polymeric nanoparticle | ~143 nm | RNP (50 w/w) | PLK1 gene (32% knock-out) in U87MG cells by restriction enzyme (BstAP I) digestion assay | [53] |
| Angiopep-2-functionalized, disulfide-cross-linked nanocapsules | ~30 nm | RNP (almost 100%) | PLK1 gene (38.1% knock-down) in U87MG cells T7E1 assay | [54] |
| Phenylboronic acid (PBA)-functionalized, disulfide-bonded branched polyaminoglycoside (SS-HPT-P) | 200 nm | plasmids (SS-HPT-P2/pDNA (w/w = 30)) | Survivin gene (20% knock-out) in A549 cells by flow cytometry | [20] |
| CuS-RNP@PEI | 28 nm | RNP (CuS/RNP (w/w = 8)) | GFP (40.7% knock-out) in GFP- MDA-MB-231 cells by flow cytometry; PTPN2 gene (36.5% knock-out) in HEK 293 cells by T7 endonuclease I (T7E1) assay | [20] |
| UCNPs-Cas9@CM | ~140 nm | RNP (~33.3%) | HBV DNA (33.75% knock-out) in HepG2.2.15 and HepAD38 cells by sequence analysis | [55] |
| MG-RNP@CaCO3 | < 100 nm | RNP | Nrf2 gene (36.03% knock-out) in EGFP-A549 cells by T7 Endonuclease I (T7E1) assay | [56] |
| P/M@CasMTH1 | ~100 nm | RNP (MTK/sgRNA = 1:6) | EGFP gene (42.6% knock-out) in EGFP-A549 cells by CLMS, MTH1 gene (39.9% knock-out) in A549 cells by T7 endonuclease I (T7EI) assay | [57] |
| PLGA | ~350 nm | 49–75% for Cas9 and 69–89% for sgRNA | EGFP gene (70% knock-down) in EGFP-HUDEP-2 cells by RT-qPCR | [21] |
| PLGA | 210~350 nm | plasmids (1.6 wt%) | Cas9 protein expression in wild-type mouse bone marrow-derived macrophages (BMDMs) by Western Blots and gene-editing efficiency was comparable to Lipofectamine | [58] |
| Methoxy-poly(ethyleneglycol)-b-poly(2-(azepan-1-yl) ethyl Methacrylate) (mPEG-PC7A) | ~30 nm | RNP (17 wt%) | HDR efficiency of HDR-NP to 7.0% in BFP-expressing human embryonic stem cells (hESCs) by flow cytometry | [59] |
| Multistage-sensitive nanocomplex (MUSE) | 138 ± 3 nm | plasmids | CD47 (35% indel) and PD-L1 (47% indel) in B16-F10 cells by T7 endonuclease I (T7EI) assay | [60] |
| pCas9-loaded nanocore (PRTM/pCas9/Ca; NP) | 187 ± 3 nm | plasmids (90%) | HIF-1α (~75% knock-out) in H1299 cells by qPCR | [61] |
| Cas9En-ArgNP nano-assemblies | 475 ± 60 nm | RNP (ArgNP: Cas9En = 2:1) | AAVS1 gene (29% knock-out) in HeLa cells byT7 endonuclease I (T7EI) assay | [25] |
| Peptide/lipid-associated nucleic acids (PLANAs) | ~100 nm | RNP (89%) | HPRT protein (35% indel) in HEK293 cells by T7 endonuclease I (T7EI) assay | [62] |
| Multifunctional nanosystem (HPR@CCP) | 51.8 ± 10.3 | plasmids (99.8 ± 2.36%) | EGFP gene (74.12% expression) in mouse skin melanoma cells B16F10 cell line by flow cytometry | [63] |
| F-PC/pHCP | ∼50 nm | plasmids (N/P = 2) | PD-L1 gene (58.4% knock-out) in B16F10 cells by flow cytometry | [64] |
| Silica nanoparticle (SNP) | 52 ± 4 nm | RNP (>90%) | GFP gene (~72% knock-out) in GFP- HEK293 cells by T7 endonuclease I (T7EI) assay | [32] |
| Silica nanocapsules (SNCs) | ∼50 nm | RNP (>90%) | GFP gene (~70% knock-out) in GFP- HEK293 cells by T7 endonuclease I (T7EI) assay | [35] |
| Magnetic core–shell nanoparticle (MCNP) | 98.84 ± 3.96 nm | plasmids (>80%) | MeCP2 gene (42.95% repair) in induced pluripotent stem cell-derived neural progenitor cells (iPSC-NPCs) from a Rett syndrome patient by confocal laser scanning microscopy (CLSM) | [38] |
| Fe3O4 @mPDA-mPEG-Ni | 260 nm | RNP (92.8%) | EGFP gene (45–50% knock-out) in EGFP-293 T cells by flow cytometry and PD-L1 gene (42.1% knock-out) in B16F10 cells by confocal laser scanning microscopy (CLSM) | [36] |
| Lipid-modified oligoamino amides and folic acid (FolA)-PEG | ~50 nm | RNP (89.5%) | PD-L1 (60.7% knock-out) and PVR (58.7% knock-out) in CT26 cells by Sanger sequencing | [19] |
| Rolling circle amplification (RCA)-based multifunctional DNA/UCNP complex | ~45 nm | plasmids (DNA layer ≈4.80 nm thick) | Nuclear factor E2-related factor 2 (Nrf2) gene (18.7% knock-out) in MCF-7 cells by agarose gel electrophoresis | [65] |
| Binding-mediated protein corona (BMPC) | 24.2 nm | RNP corona (25:1) | EGFP (12.8% indel) and EMX1 gene (10.5% indel) in EGFP-HEK293 cells by T7 endonuclease I (T7EI) assay | [66] |
| 4A3-SC8 dLNPs | 100 nm | mRNA (>92%) | GFP (≈18% HDR efficiency) in HEK293 B/GFP cells by flow cytometry | [67] |
| LNP-INT01 (LP01+PEG-DMG) | <100 nm | mRNA (>95%) | TTR gene (>97%) in mouse primary hepatocytes by next-generation sequencing (NGS) analysis | [16] |
| LNP (P127 M@pCD98) | 267.2 nm | plasmids (100%) | CD98 (61.3% knock-down) in CT-26 cells by RT-qPCR | [68] |
| LNP (RCB-4-8) | 85.7 ± 1.6 nm | mRNA (87.1 ± 2.3%) | GFP (~95% knock-out) in HEK 293 cells by flow cytometry | [69] |
| LNP (iLP181) | 98.43 ± 6.00 nm | plasmids | PKL-1(>30% knock-out) in HepG2-Luc cells by RT-qPCR | [70] |
| LNP(BAMEA-O16B) | 233.6 ± 2.3 nm | mRNA | GFP (>90% knock-out) in HEK-GFP cells by confocal laser scanning microscopy (CLSM) | [18] |
| LNP | 75.3 nm | mRNA (>90%) | SERPINC1 gene (~60 knock-out) in mouse C2C12 cell by targeted deep sequencing | [15] |
| LNP | 80 nm | mRNA (>90%) | EGFP gene (94% knock-out) in GFP-HEK293 cells by next-generation sequencing (NGS), PKL-1(98% knock-out) in GFP-HEK293 cells by next-generation sequencing (NGS) | [71] |
| LNP | 90 ± 4 nm | plasmids (N/P = 6) | FOXC1 gene (~80% knock-out) in MDA-MB-468 by confocal laser scanning microscopy (CLSM) | [72] |
| Peptide-conjugated lipids | 159.80 ± 3.87 nm | plasmids (95.47 ± 4.38%) | HuR gene (48.94 ± 0.68% knock-out) in SAS cells by confocal laser scanning microscopy (CLSM) | [73] |
| LNP | 112.5~144.0 nm | mRNA (74.6~82.9%) | CRE (53.2%~61.8% td-tomato positive cells) in NIH 3T3 CRE reporter cells by flow cytometry | [74] |
| Nano-cleaver (HepCCCleaver) | 215.3 ± 1.1 nm | plasmids (wt/wt = 50) | HBV DNA (89.1% knock-out) in HepAD38 cells by T7 endonuclease I (T7E1) assay and DNA sequencing | [75] |
| LNP | 79.1 nm | mRNA (96%) | DMD gene (43.6% knock-out) in DMD patient myoblasts by T7 endonuclease I (T7E1) assay | [76] |
| Lipid/AuNPs complex | 101.2 ± 5.6 nm | plasmids (97%) | PLK-1 gene (65% knock-out) in A375 cells by Western blot assay | [77] |
| PBA- BADP/Cas9 mRNA NPs | 111 ± 2 nm | mRNA | EGFP gene (~25% knock-out) in EGFP- HEK293 cells and (~50% knock-out) in EGFP-HeLa cells by T7 endonuclease I (T7E1) assay | [78] |
| PEGylated nanocapsules (NCs) | 36 ± 3 nm | RNP (~40 wt%) | GFP gene (~70% knock-out) in GFP- HEK293 cells by flow cytometry | [30] |
| Fusogenic cancer cell-derived nanocarriers | ~200 nm | RNP | EGFP (knock-out 35%) in 293-T-HEK-dEGFP reporter cells by flow cytometry | [79] |
| US-propelled Cas9/sgRNA@ AuNWs | 400 nm | RNP | GFP (80% knock-out) in GFP-B16F10 cells by confocal laser scanning microscopy (CLSM) | [23] |
| Materials | Model | Target Gene | Target Disease | The Modes of Administration | CRISPR Cas9 Dose | Duration | Outcome | Refs. |
|---|---|---|---|---|---|---|---|---|
| Lipid-modified oligo amino amides and folic acid (FolA)-PEG | CT26 in Balb/c tumor model | PD-L1 and PVR gene | CT26 tumor | intra-tumoral injection | plasmids (12.5 μg) | every 2 days for 3 days | dual PD-L1/PVR immune checkpoint disruption, and suppressed tumor growth in vivo | [19] |
| Phenylboronic acid (PBA)-functionalized, disulfide-bonded branched polyaminoglycoside (SS-HPT-P) | tumor-bearing mouse model | survivin gene | tumor | tail-vein injection | plasmids (25 μg) | every 2 days for 12 days | inhibited tumor proliferation and migration and enhanced the sensitivity of cancer cells to anti-tumor drugs | [20] |
| Cancer cell membrane-derived nanocarrier (mCas9-sGNRs) | MDA-MB-231-tumor-bearing mice model | BIRC5 gene | Adenocarcinoma of breast | intravenous injection | / | every 3 days for 18 days | enhanced antitumor efficacy and reduced tumor thermal tolerance | [43] |
| Macrophage membrane-coated polyplexes (PD/P@M) | hepatic ischemia-reperfusion injury (IRI) in mice | Alox12 | hepatic ischemia-reperfusion injury (IRI) | tail-vein injection | plasmids (30 µg) | day 1, day 3, and day 5 for 3 times | the level of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) reduced tumor necrosis factor-α (TNF-α) and interferon-gamma (IFN-γ) level | [80] |
| concanavalin-A (ConA) induced hepatic fibrosis in mice | Fas gene | hepatic fibrosis | tail-vein injection | plasmids (30 µg) | weekly for 4 weeks | validated the amelioration of the hepatic inflammation and fibrosis | ||
| concanavalin-A (ConA) induced fulminant hepatic failure in mice | Fas gene | fulminant hepatic failure | tail-vein injection | plasmids (30 µg) | day 1, day 3, and day 5 for 3 times | reduced hyperemia and prolonged the survival time of the model mice | ||
| Carboxylated branched poly (β-amino ester) | mouse glioma model | ReNL reporter gene | murine glioma tumors | intracranial injection | 10 µL with RNP (15 w/w%) | single | bright ReNL fluorescence within the tumor bulk, the brightest ReNL signal was localized in closest proximity to the injection site | [52] |
| Core–shell hollow mesoporous organosilica nanoparticles | H22 tumor-bearing mice model | EGFR gene | hepatocellular carcinoma (HCC) | intravenous injection | plasmids (2.5 mg/kg) | every 2 days for 22 days | achieved efficient EGFR gene therapy and caused 85% tumor inhibition in a mouse model, showed high accumulation at the tumor site in vivo, and exhibited good safety with no damage to major organs | [45] |
| P/M@CasMTH1 | A549 tumor-bearing Balb/c nude mice | MTH1 gene | tumor | tail-vein injection | 20 mg/kg | single | destroyed the self-defense system of tumor cells and led to the inhibition of tumor progression in vivo | [57] |
| Gold nanoclusters (GNCs) | WT-C57BL/6 mice | Pcsk9 gene | cardiovascular Diseases | intravenous injection | / | single | achieved efficient gene editing of Pcsk9 in the liver and the down-regulation of serum LDL-C | [29] |
| Gold nanorods (AuNRs) | ConA-Induced Hepatic Fibrosis | Fas gene | chronic hepatitis | intravenous injection | plasmids (2.0 μg/kg) | for 4 weeks after 48 h of each injection of ConA | delivered CMV-Cas9-Fas to the hepatocytes and rescued the mice from chronic hepatitis | [26] |
| MG-RNP@CaCO3 | A549 tumor-bearing BALB/c nude mice | Nrf2 gene | tumor | intravenous injection | MG-RNP@CaCO3 (16 mg/kg) | every 3 days for 7 days | reduced the Nrf2 expression and suppressed tumor growth | [56] |
| Glutathione (GSH)-responsive silica nanocapsules (SNCs) | wild-type mice | App- and Th- gene | central nervous system (CNS) disorders | intravenous injection | total RNA (5 mg/kg) | every 5 days for 3 times | led to 19.1% reduction in the expression level of intact APP and 30.3% reduction in the expression level of TH | [35] |
| Angiopep-2-functionalized, disulfide-cross-linked nanocapsules | GBM tumor-bearing mice | PLK1 gene | glioblastoma | intravenous injection | RNP (1.5 mg/kg) | single | marked inhibition of GBM tumor growth and the approximate trebling of median survival time | [54] |
| Angiopep-2 decorated, guanidinium and fluorine functionalized polymeric nanoparticle | orthotopic U87MG-Luc glioma-bearing nude mice | PLK1 gene | glioblastoma | intravenous injection | Cas9 (15 μ g) | every 2 days for 5 times | suppressed tumor growth and improved the median survival time of mice bearing orthotopic glioblastoma to 40 days | [53] |
| Black phosphorus nanosheets (BPs) | A549/EGFP tumor-bearing nude mice model | EGFP | tumor | intra-tumoral injection | 50 μL of Cas9N3-BPs (100 μg/mL BPs and 800 nM Cas9N3 in PBS | single | reduced the EGFP signals around the site of injection | [81] |
| Guanidium-rich lipopeptide GD-LP | Duchenne muscular dystrophy (DMD) mouse model | DMD gene | Duchenne muscular dystrophy (DMD) | intramuscular injection | 20 µL (0.6 μg/μL RNP and 0.18 μg/μL ssODN) | single | restored dystrophin expression, reduced skeletal muscle fibrosis, and significantly improved muscle strength | [51] |
| pCas9-loaded nanocore (PRTM/pCas9/Ca; NP) | H1299-Luc xenograft model | HIF-1α | tumor | intravenous injection | plasmids (10 ug) | single | augmented the therapeutic efficacy of PTX, causing distinct apoptosis and noticeable tumor suppression | [61] |
| P-aP-DTT-LMP-g4 polyplex NP | C666-1 xenograft tumor model | Lmp1 gene | nasopharyngeal carcinoma (NPC) | peritumoral injection | plasmids (10 mg) | on days 1, 4, 7, and 10 | achieved good tumor penetration and tumor growth inhibition | [82] |
| Hyperbranched polyamide amine (HPAA) | HNE-1 cells-bearing mice | SGK3 gene | nasopharyngeal carcinoma (NPC) | intravenous injection | / | every 2 days for 21 days | inhibited angiogenesis and tumor cell proliferation | [50] |
| Methoxy-poly(ethyleneglycol)-b-poly(2-(azepan-1-yl) ethyl methacrylate) (mPEG-PC7A) | Mdx Mice | DMD gene | Duchenne muscular dystrophy | intramuscular injection | 1.2 µg/µL RNP and 0.36 µg/µL ssODN | single | exhibited less muscle fibrosis and fewer pathological characteristics of muscular dystrophy, as well as interstitial fibrosis | [59] |
| Programmable unlocking of the nano-matryoshka-CRISPR system (PUN) | B16-F10 xenograft tumor model. | PD-L1 and PTPN2 gene | melanoma | tail-vein injection | plasmids (5 µg) | every 2 days | activated cascade amplified adaptive immunity and induced long-term immune memory effect. | [83] |
| Au nanoparticle-loaded core–shell tecto dendrimers (Au CSTDs) | B16F10 tumor-bearing mice | PD-L1 gene | melanoma | intra-tumoral injection | Cas9-PD-L1 (10 μg) | every 3 days for 12 days | increased the distribution of CD4+/CD8+ T cells, reduced the proportion of immunosuppressive cells, and upregulated the cytokines TNF-α/IFN-γ/IL-6 | [24] |
| F-PC/pHCP | B16F10 tumor-bearing mice | PD-L1 gene | melanoma | peritumoral injection | plasmids (1 mg/kg) | single | potentiated immunotherapy efficacy through a combination of PD-1/PD-L1 checkpoint blockade and tumor immune microenvironment reprogramming and triggered an immune memory response to increase the proportions of Tem and Tcm cells in the spleen, achieving efficient suppression of distant tumor growth and lung metastasis | [64] |
| Fe3O4 @mPDA-mPEG-Ni | B16F10 tumor-bearing mice | PD-L1 gene | tumor | tail-vein injection | / | single | the mild photothermal stimulated anti-tumor immune responses without causing damage to normal tissues like skin, and also could promote the specific release of RNP in tumors, and the gene knock-out efficiency on the PD-L1 gene in melanoma cells in vivo was about 25.1%, | [36] |
| UCNPs-Cas9@PEI | A549-tumor-bearing mice | PKL1 | tumor | intratumor injection | 100 uL, 3.5 mg/mL | every 3 days for 20 days | targeted PKL-1gene and inhibited the proliferation of tumor cell | [84] |
| UCNPs-Cas9@CM | HBV-Tg mice | HBV | chronic hepatitis B virus (HBV) infection | tail-vein injection | RNP (40 μg) | every day for 14 days | decreased serum levels of HBV DNA, HBsAg and HBeAg, as well as the HBsAg and HBcAg levels in hepatocytes | [55] |
| CuS-RNP/DOX@PEI | A375-tumor-bearing BALB/c mice | Hsp90 | tumor | intra-tumoral injection | nanocomposites (5 mM) | every 3 days for 3 weeks | potentially tumor synergistic therapy, including GT, mild-PTT, and CT. Photothermal controlled gene editing to disrupt Hsp90α provides a potential strategy to reduce tumor thermal tolerance for enhanced mild-PTT effects. | [85] |
| ZIF8-NaHCO3@Cas9 (ZNC) | OVX-induced osteoporosis mice model | nuclear factor kappa-B ligand (RANKL) | osteoporosis | femoral marrow cavity injection | / | single | released the carried NaHCO3 to achieve acid neutralization and reduce ROS level, inhibiting RANKL expression and osteoclast activity and suppressing the expression of RANKL, reducing the formation of osteoclasts and effectively cutting off the source of acidic micro-environment formation. | [46] |
| MG-RNP@CaCO3 | A549 tumor-bearing BALB/c nude mice | Nrf2 gene | / | intravenous injection | 16 mg/kg | every 3 days for 7 times | inhibited tumor growth and protected normal surrounding tissue from oxidative stress. | [56] |
| CuS-RNP@PEI | B16F10 tumor-bearing orthotopic mouse model | PTPN2 | malignant neoplasm | peritumor injection | / | every 2 days for 3 times | tumor tissues with indicated treatments and with no noticeable abnormality nor appreciable organ damage | [86] |
| Multistage-sensitive nanocomplex (MUSE) | B16F11 tumor-bearing mice model | CD47, PD-L1 | tumor | intravenous injection | 0.25 mg/kg | once every 3 days for 8 times | activated robust CD8+ T cells and M1 macrophage-mediated adaptations and root ions anti-tumor immune response and triggered long-lasting immune memory action, led to significant inhibition of tumor growth and improved survival with virtually undetectable off-target delivery effects | [60] |
| LNP | cystic fibrosis (CF) mouse model | CFTR | cystic fibrosis | tail-vein injection | 2 mg/kg | once a week for 3 times | 2.34% of CFTR gene extracted from whole lung tissue was corrected, corrected G542X mutation in mouse lungs | [87] |
| LNP | ΔEx44 DMD mice model | DMD gene | Duchenne muscular dystrophy (DMD) | tail-vein injection | sg DMD (1 mg/kg) | once a week for 3 times | 4.2% of dystrophin protein in TA muscles | [88] |
| LNP | OV8-bearing mice | PLK1 gene | ovarian tumors | intraperitoneal injection | 0.75 mg/kg | single | targeted treatment of disseminated tumors and increased overall survival by ~80% | [71] |
| 005 GBM-bearing mice | PLK1 gene | glioblastoma | intracerebral injection | 0.05 mg/kg | single | reduced tumor growth and increased median survival from 32.5 to >48 days | ||
| LNP-INT01 | / | transthyretin (Ttr) gene | TTR amyloidosis (ATTR) | tail-vein injection | 1 mg/kg | single | editing in the liver reached nearly ~70%, while serum levels of TTR were reduced more than 90% | [16] |
| LNP-NTLA-2001 | patients with hereditary ATTR amyloidosis with polyneuropathy | transthyretin (Ttr) gene | TTR amyloidosis (ATTR) | intravenous injection | total RNA (0.1 mg/kg or 0.3 mg/kg) | within an ongoing phase 1 clinical study | led to a decrease in serum TTR protein concentrations with only mild adverse events | [89] |
| LNP (ZAPL75C) | MDA-MB-468 cells tumor-bearing mice | FOXC1 gene | tumor | intravenous injection | plasmids (20 μg) | two injections | ∼42.2% and ∼82.2% reduction in tumor volume | [72] |
| Peptide-conjugated lipids | SAS/luc-bearing mouse model | HuR gene | tumor | intravenous injection | 10 mg/kg | single | targeted to tumor sites and induced apoptosis in more tumor cells | [73] |
| LNP | hemophilia A and B mouse model | SERPINC1 gene | hemophilia A and B | intracerebral injection | 1.2 mg/kg | three times with 2-week | reduced spontaneous bleeding and secondary hemophilia complications by enhanced thrombosis potential | [15] |
| Nano-cleaver (HepCCCleaver) | HBV replication mouse models | HBV | hepatitis B virus infection | intravenous injection | 20 mg/kg | single | resulted in decreased levels of HBsAg by 48.6%, HBeAg by 58.7%, HBV DNA by 53.5%, and HBV RNA by 56.3% and achieved efficient virus elimination to treat HBV infection in vivo | [75] |
| LNP | DMD exon 45 knock-in (hEx45KI) mice | DMD gene | Duchenne muscular dystrophy (DMD) | intramuscular injection | mRNA (10 μg) | single | generated a mouse model of DMD | [76] |
| LNP (P127 M@pCD98) | IL-10 knock-out C57/BL6 mice | CD98 | Chronic Colitis | oral injection | plasmids (1 μg) | every other day for 15 days | decreased CD98 expression, down-regulated pro-inflammatory cytokines (TNF-α and IL-6), up-regulated anti-inflammatory factors (IL-10), and polarized macrophages to M2 phenotype | [68] |
| LNP (iLP181) | HepG2-Luc tumor-bearing mice | PLK1 | tumor | intra-tumoral injection | plasmids (0.5 mg/kg) | every 2–4 days for 21 days | achieved tumor growth suppression by gene abolishment of PLK1 | [70] |
| 4A3-SC8 dLNPs | HEK293 B/GFP tumor-bearing mice | GFP | tumor | intra-tumoral injection | 0.5 mg/kg. | single | >20% HDR-mediated gene correction in vivo | [67] |
| Represents Liposomes | Target Organ | Formulation (Molar Ratio) | Refs. |
|---|---|---|---|
| 9A1P9-5A2-SC8 | liver | 9A1P9:5A2-SC8:cholesterol:DMG-PEG2000 = 25:30:30:1 | [122] |
| 9A1P9-DDAB iPLNPs | lung | 9A1P9:DDAB:cholesterol:DMG-PEG2000 = 60:30:40:0.4 | |
| 10A1P16-MDOA iPLNPs | spleen | 10A1P16:MDOA:cholesterol:DMG-PEG2000 = 25:30:30:1 | |
| 18:1 DAP (DODAP) | liver | 5A2-SC8:DOPE: cholesterol:C14PEG:DODAP = 19:19:38:4:20 | [119] |
| 18:1 TAP (DOTAP) | lung | 5A2-SC8:DOPE:cholesterol:C14PEG:DOTAP = 11.9:11.9:23.8:2.4:50 | |
| 18:1 PA (18PA) | spleen | 5A2-SC8:DOPE:cholesterol:C14PEG:18PA = 16.7:16.7:33.3:3.3:30 | |
| 306-O12B LNP | liver | 306-O12B:cholesterol:DOPC:DMG-PEG = 50:38.5:10:1.5 | [123] |
| DOTAP40 | lung | 5A2-SC8:DOPE:cholesterol:DMG-PEG = 24:24:47:5 | [87] |
| mDLNP-2 | liver | 5A2-SC8:DOPE:cholesterol:DMG-PEG = 36:20:40:4 | |
| 20% DODAP 4A3-SC8 | lung | 4A3-SC8:DOPE:cholesterol:DMG-PEG:DODAP = 15:15:30:3:16 | [118] |
| 50% DOTAP 4A3-SC8 | liver | 4A3-SC8:DOPE: cholesterol:DMG-PEG:DOTAP = 15:15:30:3:63 | |
| 10% 18PA 4A3-SC8 | spleen | 4A3-SC8:DOPE:cholesterol:DMG-PEG:18PA = 15:15:30:3:7 | |
| FX12m | liver | BP lipid 312:DOPE: cholesterol:DMG-PEG-2000:cholesterol-PEG-2000 = 46.0:12.4:40.0:1.2:0.4 | [120] |
| FC8m | lung | ADC:Lipid III-45:DOPE:cholesterol:DMG-PEG-2000 = 46.0:24.0:12.5:16.0:1.5 | |
| LNP-INT01 | liver | LP01 lipid:cholesterol:DSPC:PEG2000-DMG = 45:44:9:2 | [16] |
| RCB-4-8 LNPs | lung | ionizable lipids (RCB):DOTAP:cholesterol:C14-PEG2000 = 30:39:30:1 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Gao, M.; Bao, J. Precisely Targeted Nanoparticles for CRISPR-Cas9 Delivery in Clinical Applications. Nanomaterials 2025, 15, 540. https://doi.org/10.3390/nano15070540
Liu X, Gao M, Bao J. Precisely Targeted Nanoparticles for CRISPR-Cas9 Delivery in Clinical Applications. Nanomaterials. 2025; 15(7):540. https://doi.org/10.3390/nano15070540
Chicago/Turabian StyleLiu, Xinmei, Mengyu Gao, and Ji Bao. 2025. "Precisely Targeted Nanoparticles for CRISPR-Cas9 Delivery in Clinical Applications" Nanomaterials 15, no. 7: 540. https://doi.org/10.3390/nano15070540
APA StyleLiu, X., Gao, M., & Bao, J. (2025). Precisely Targeted Nanoparticles for CRISPR-Cas9 Delivery in Clinical Applications. Nanomaterials, 15(7), 540. https://doi.org/10.3390/nano15070540







