Enhancing Visible-Light Photocatalytic Activity of AgCl Photocatalyst by CeO2 Modification for Degrading Multiple Organic Pollutants
Abstract
1. Introduction
2. Experiments
2.1. Preparation of CeO2
2.2. Synthesis of CeO2/AgCl Photocatalyst
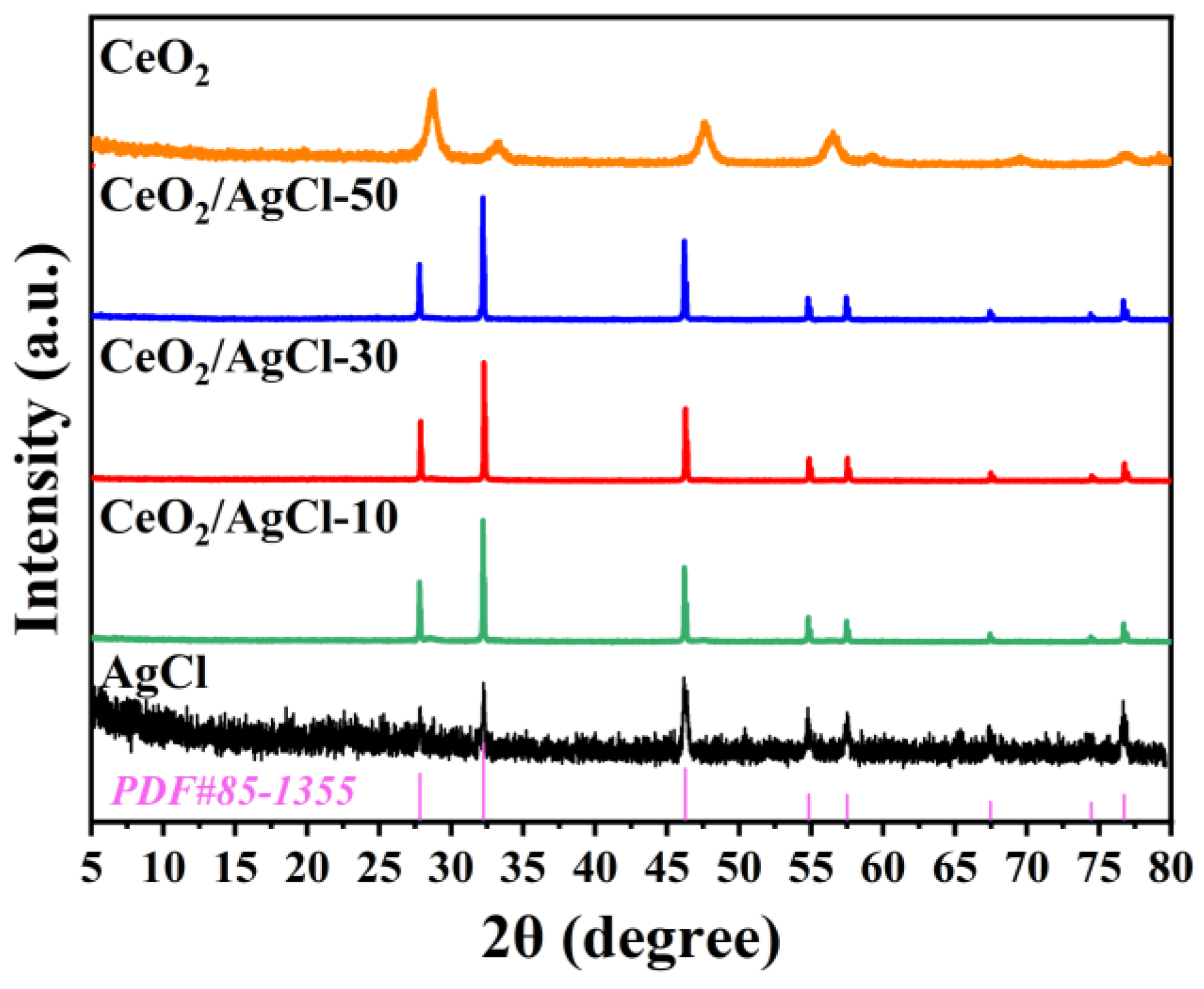
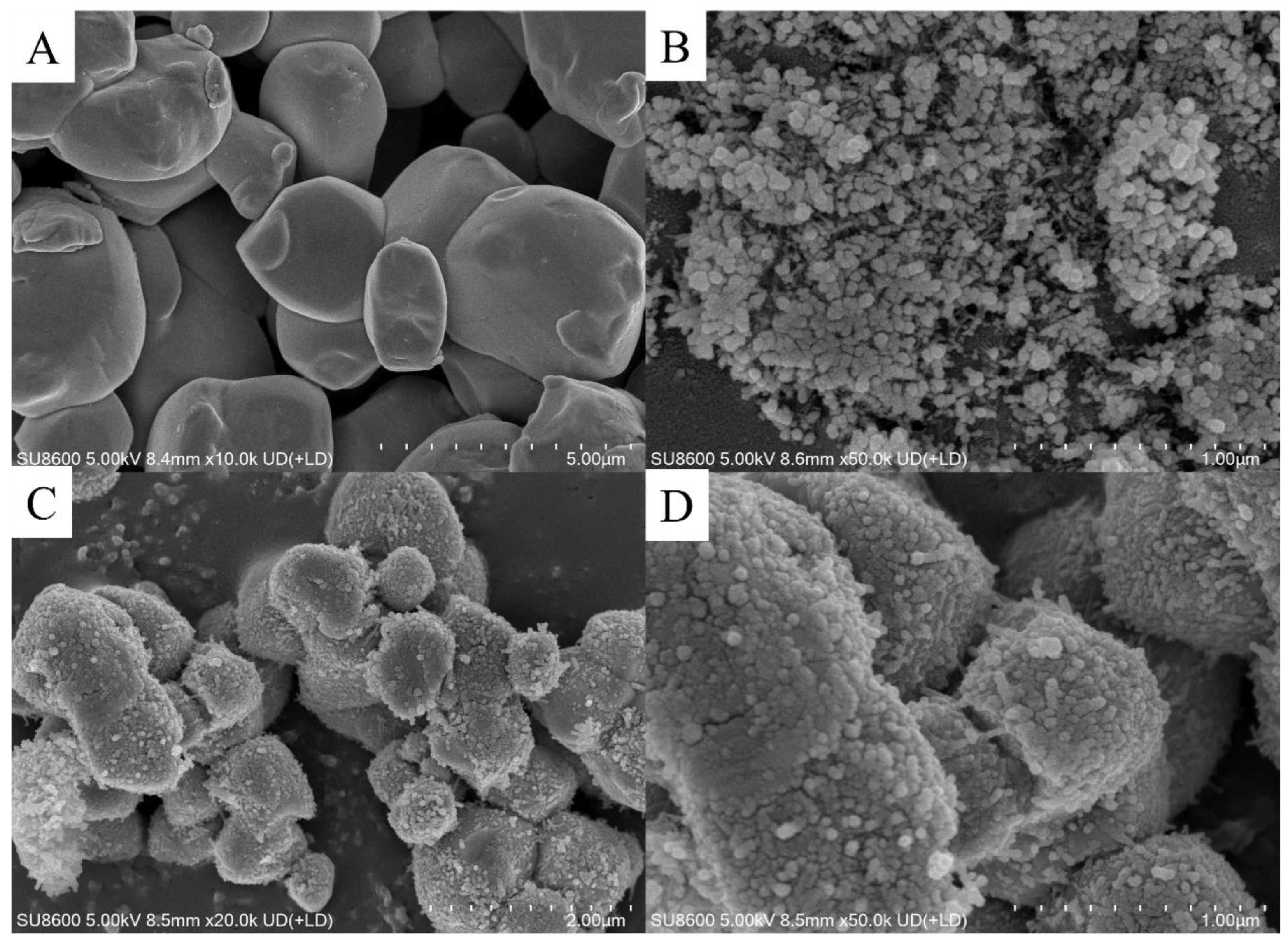
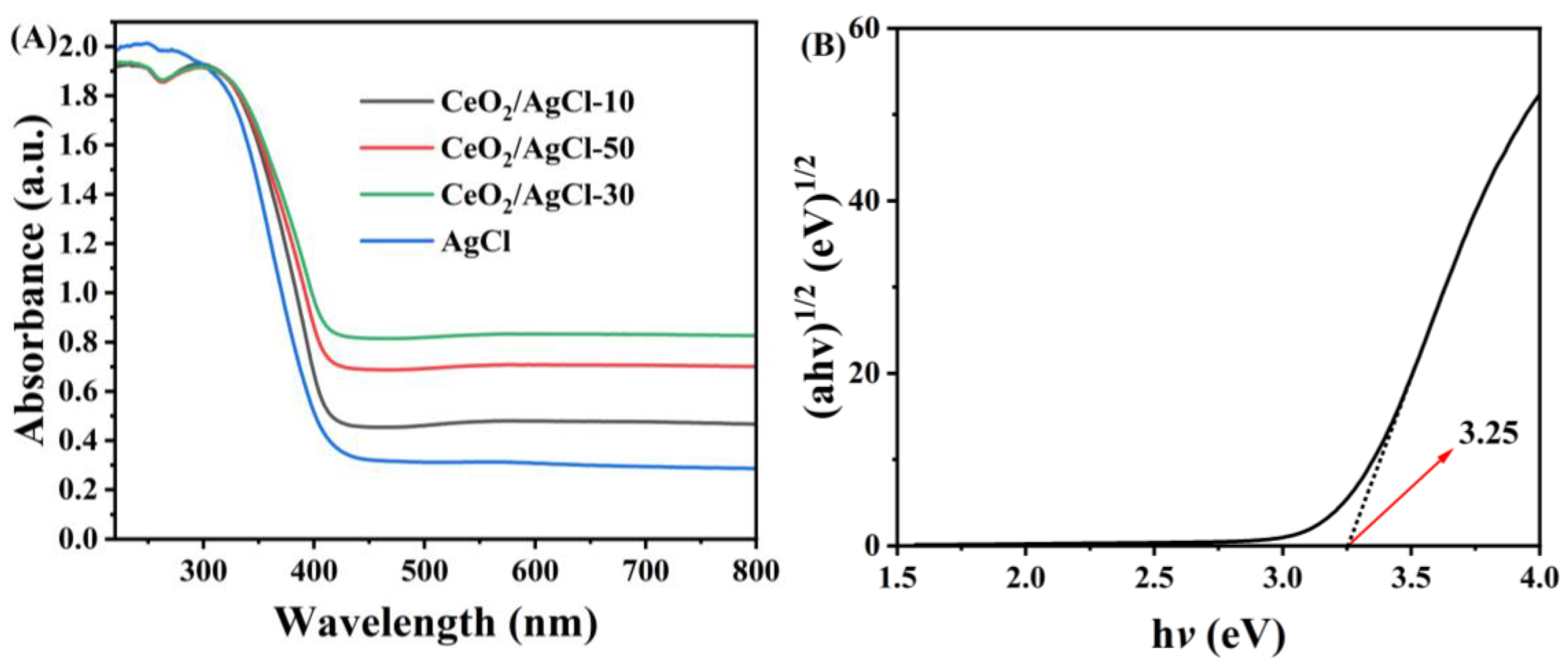
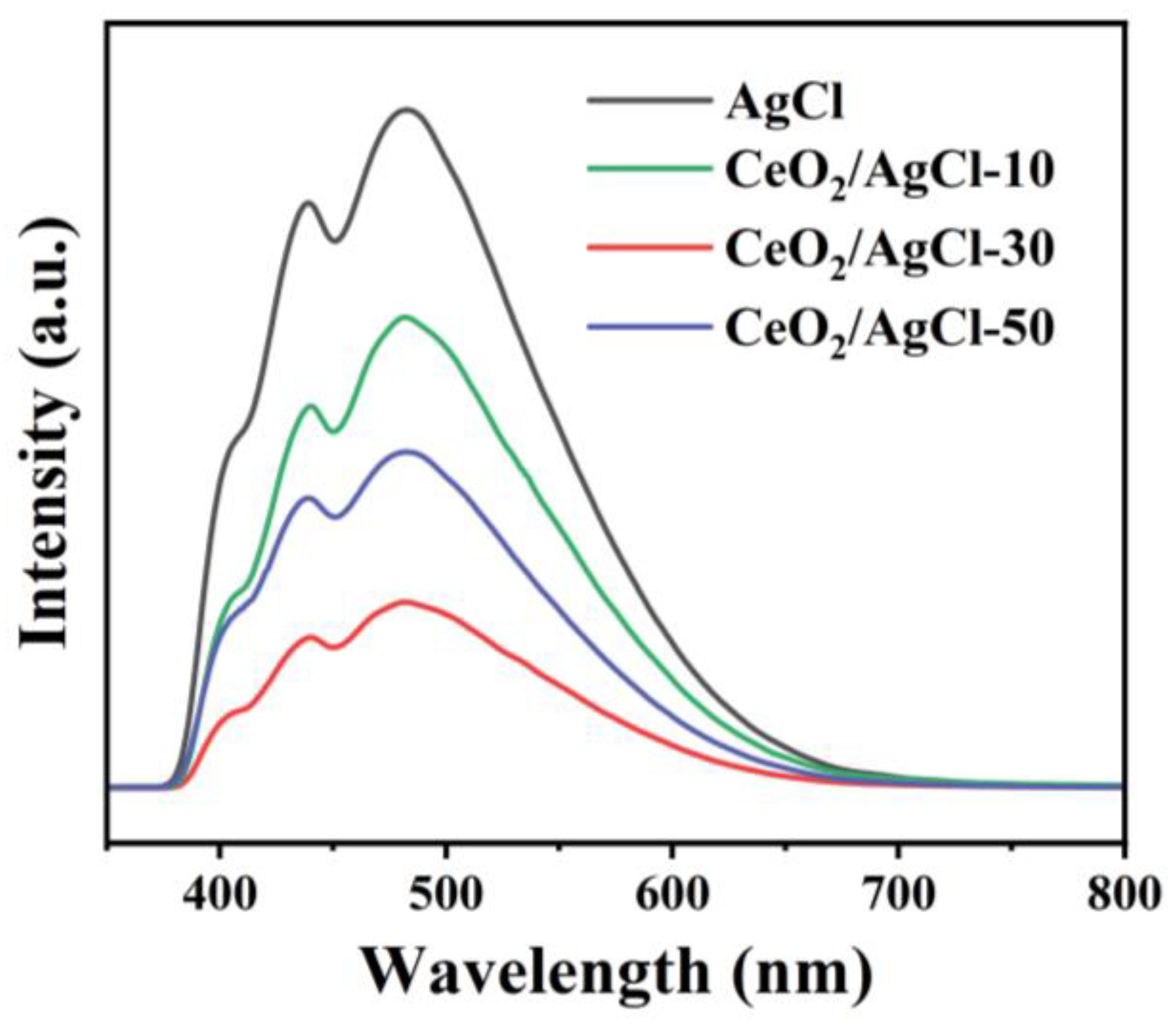
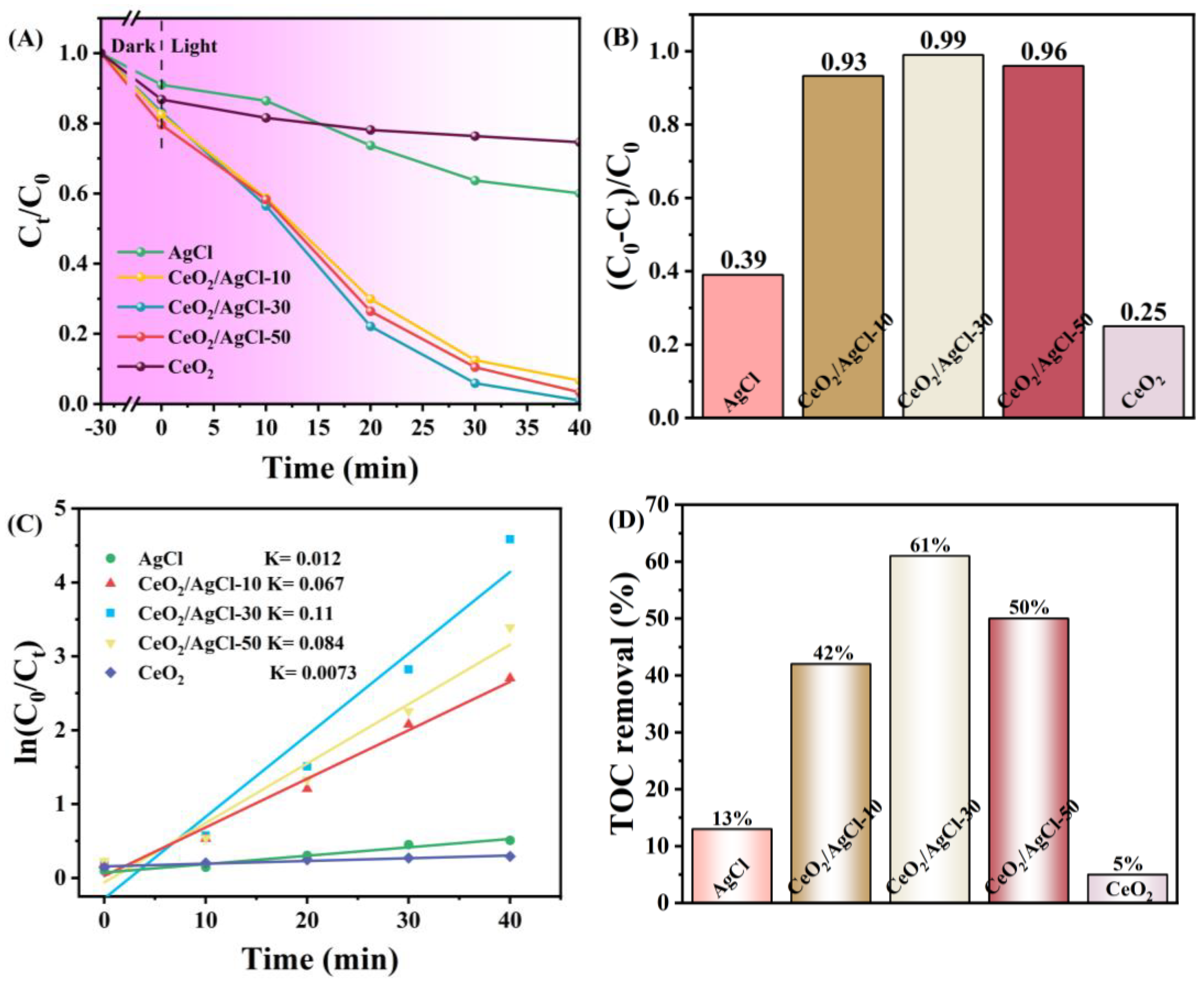
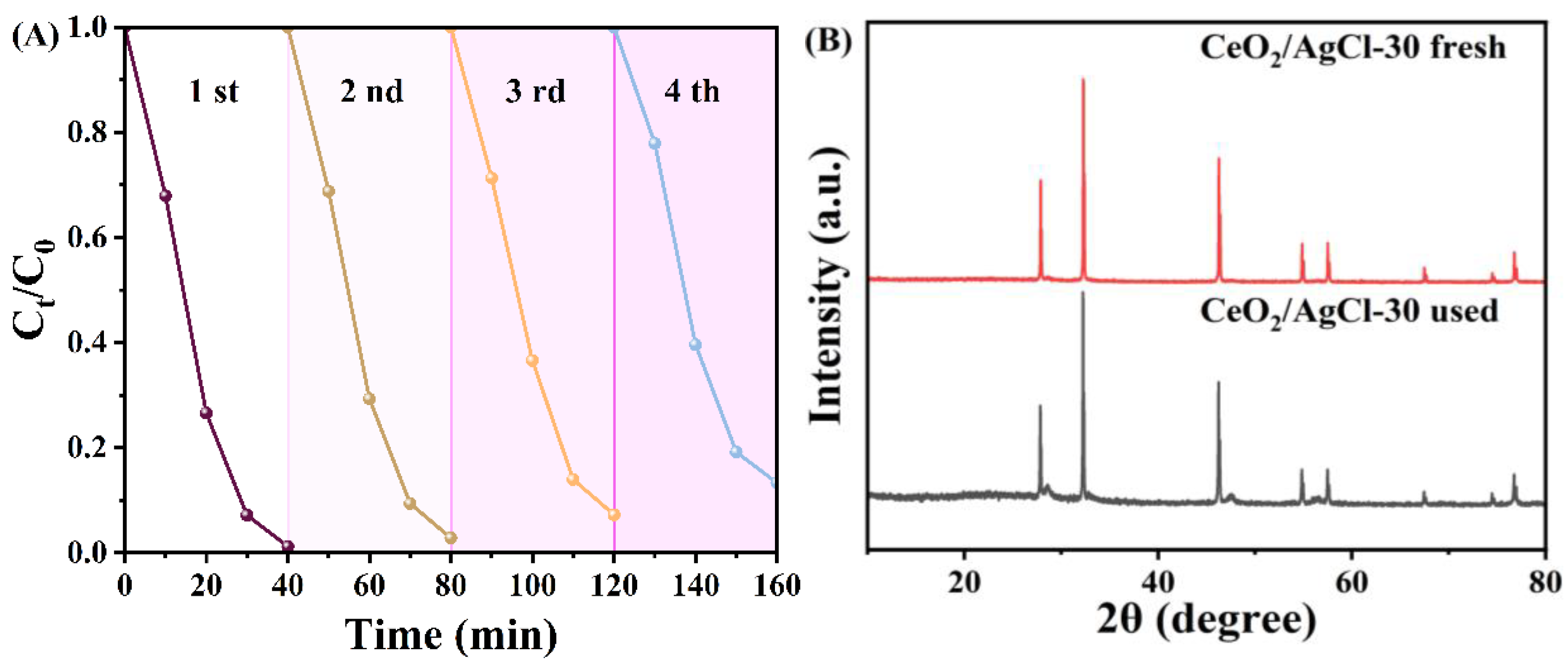
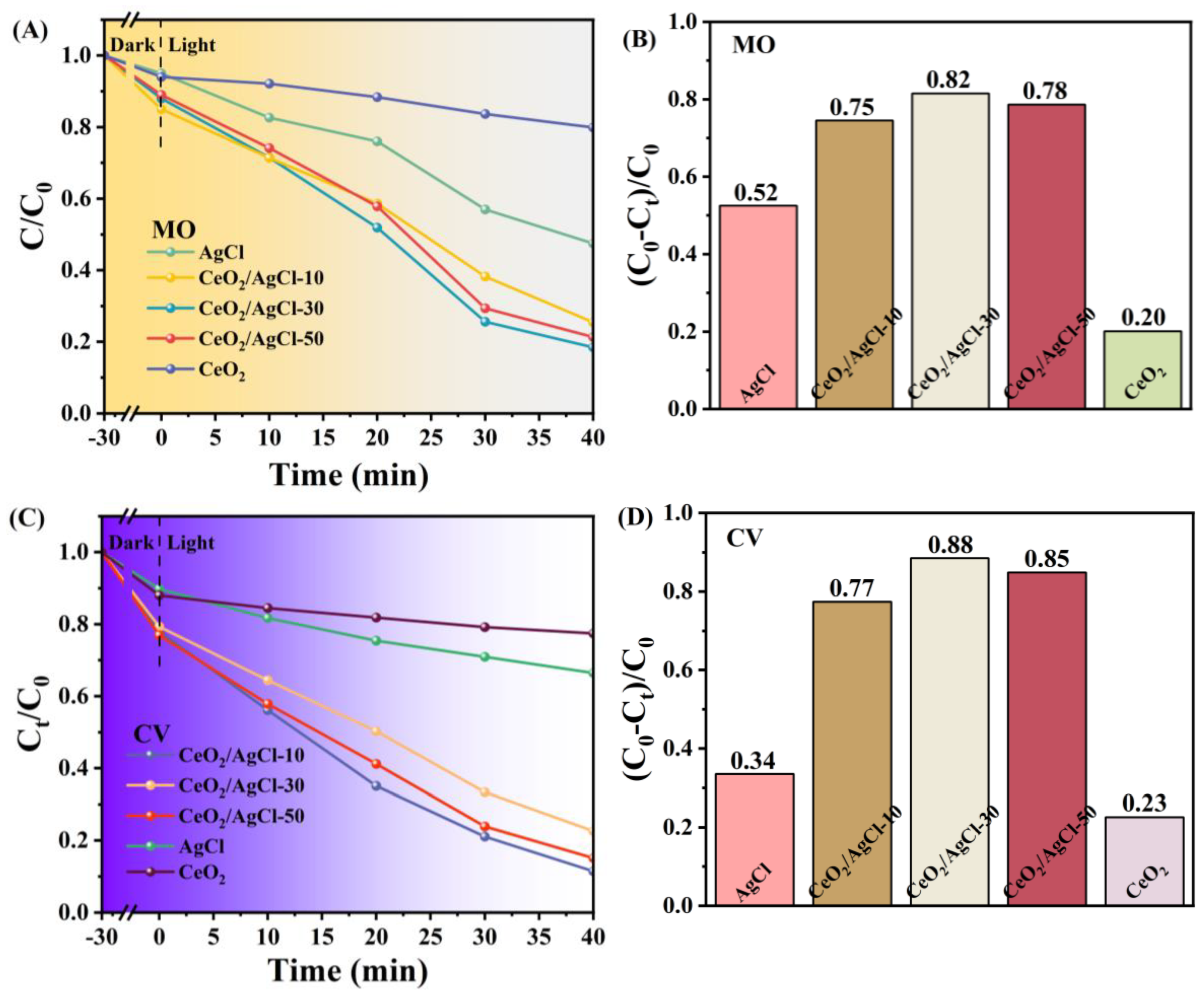
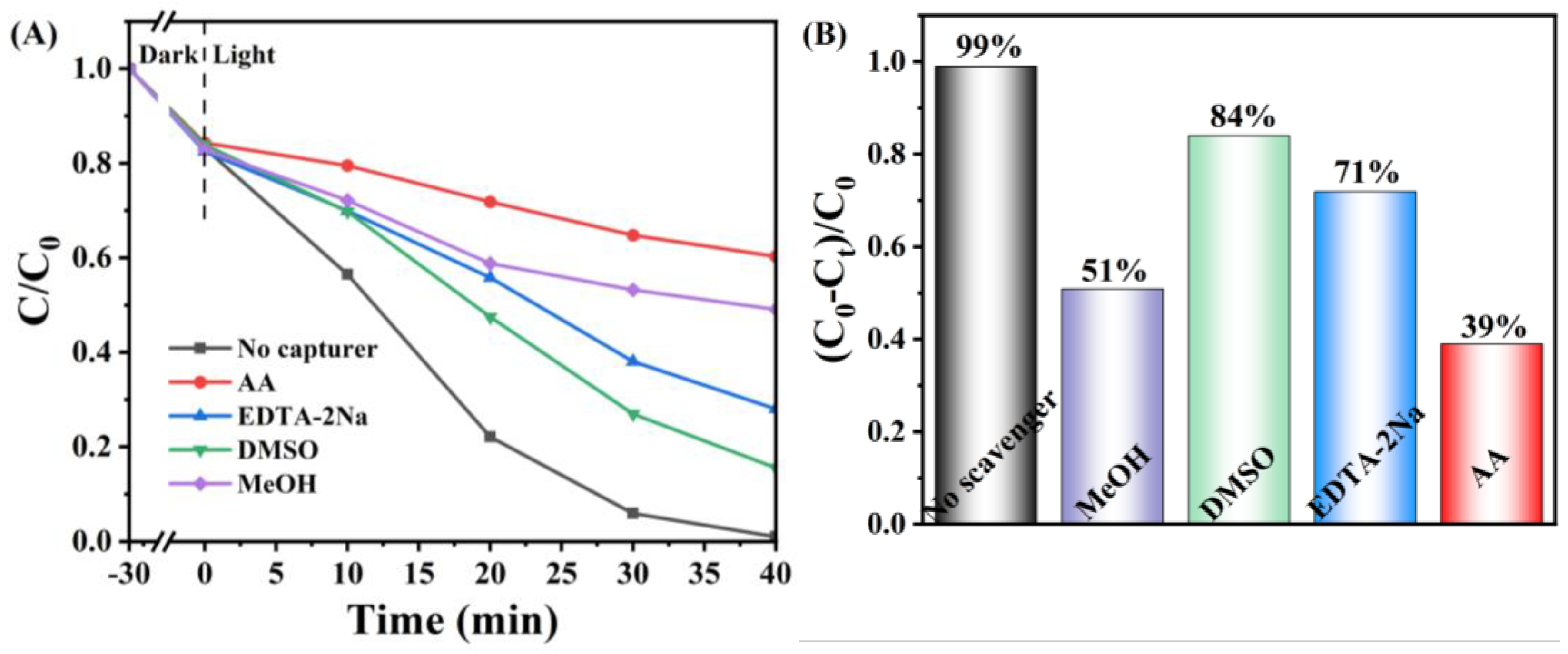
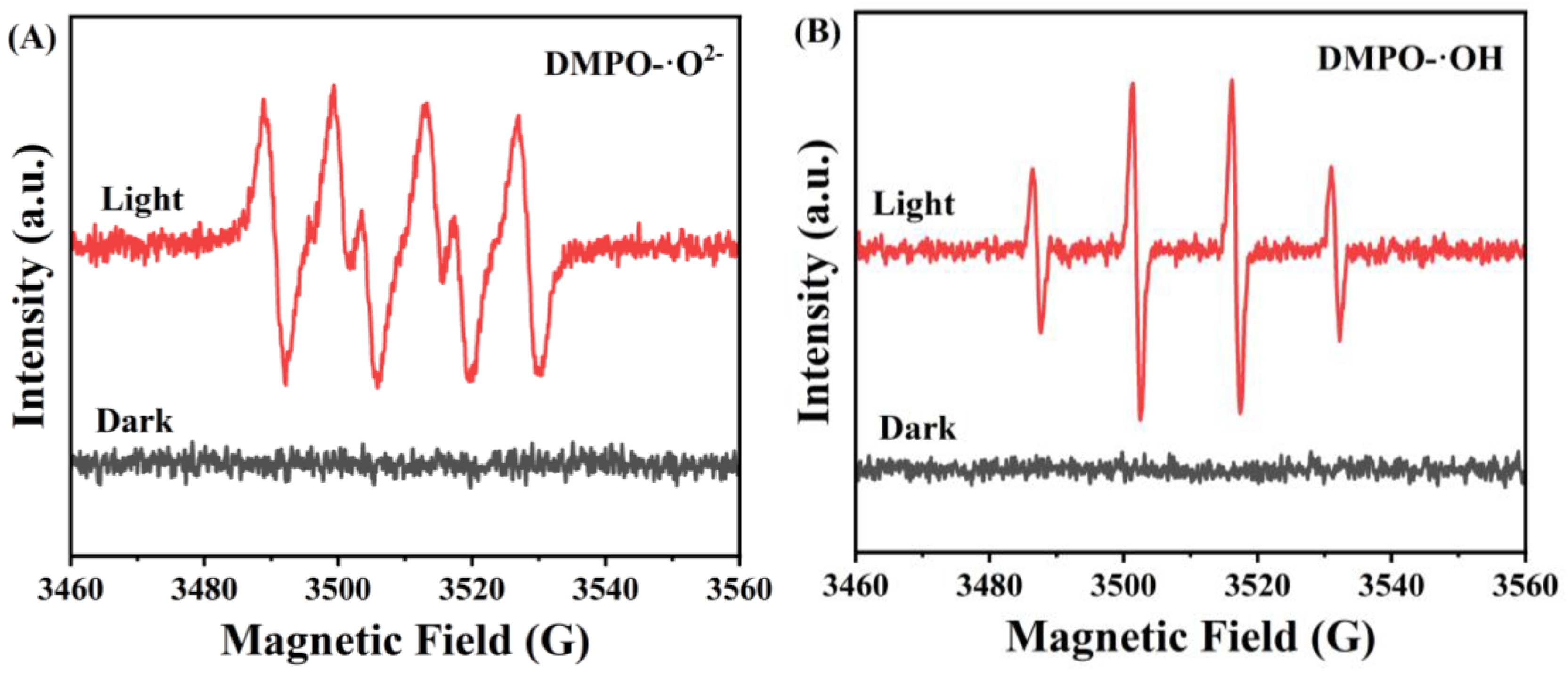
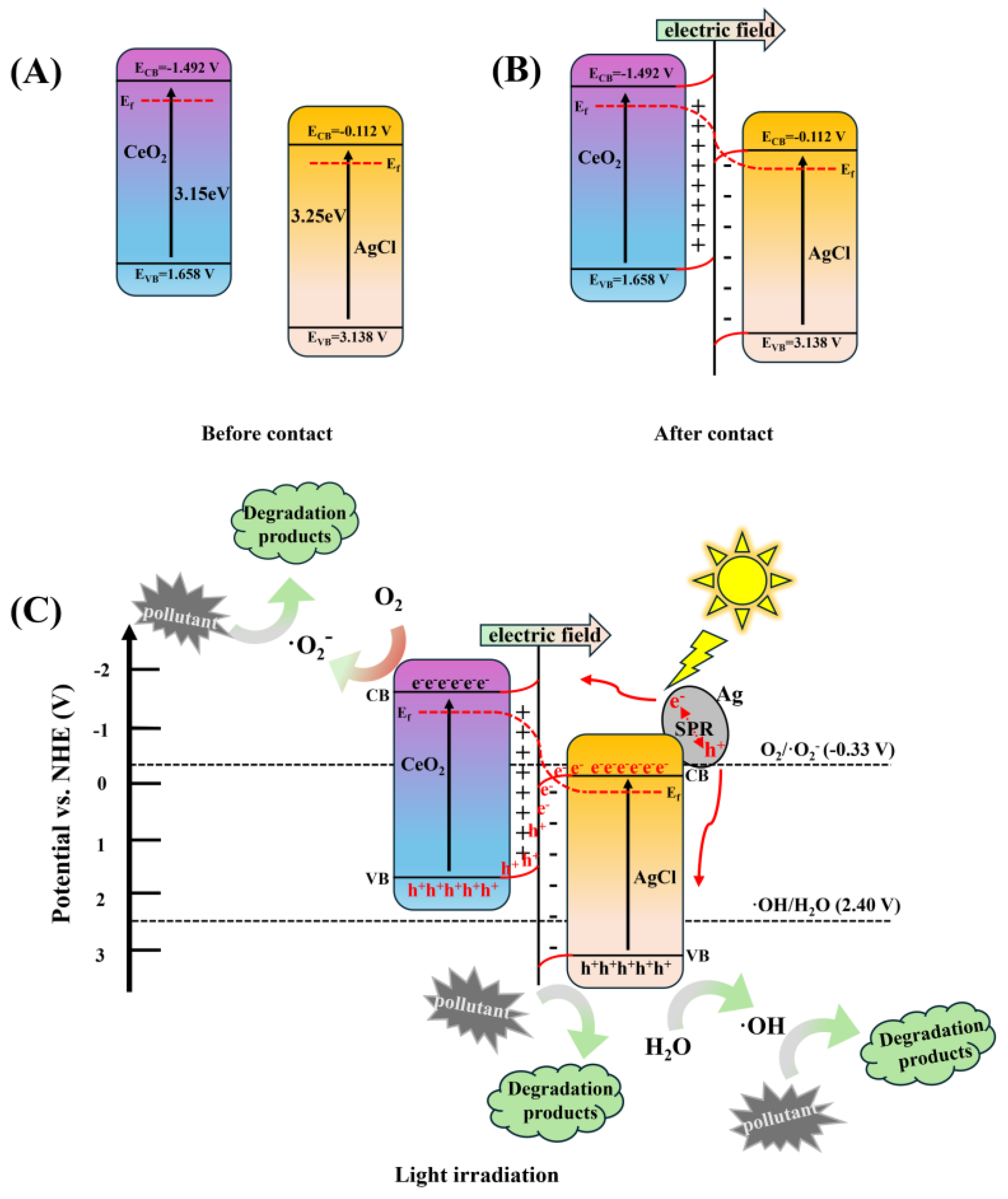
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [PubMed]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical development and prospects of photocatalysts for pollutant removal in water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, F. Recent advances in molecular oxygen activation via photocatalysis and its application in oxidation reactions. Chem. Eng. J. 2021, 421, 129915. [Google Scholar]
- Shi, L.; Ma, J.; Yao, L.Z.; Cui, L.S.; Qi, W. Enhanced photocatalytic activity of Bi12O17Cl2 nano-sheets via surface modification of carbon nanotubes as electron carriers. J. Colloid. Interf. Sci. 2018, 519, 1–10. [Google Scholar]
- Wang, Y.; Liu, H.; Wu, B.; Zhou, T.; Wang, J.; Zhou, J.; Li, S.; Cao, F.; Qin, G. Preparation and visible-light-driven photocatalytic property of AgX (X= Cl, Br, I) nanomaterials. J. Alloy Compd. 2019, 776, 948–953. [Google Scholar] [CrossRef]
- An, C.; Wang, S.; Sun, Y.; Zhang, Q.; Zhang, J.; Wang, C.; Fang, J. Plasmonic silver incorporated silver halides for efficient photocatalysis. J. Mater. Chem. A 2016, 4, 4336–4352. [Google Scholar]
- Zhao, X.; Lv, X.; Cui, H.; Wang, T. Preparation of bismuth stannate/silver@ silver chloride film samples with enhanced photocatalytic performance and self-cleaning ability. J. Colloid. Interf. Sci. 2017, 507, 260–270. [Google Scholar] [CrossRef]
- Weng, B.; Qi, M.Y.; Han, C.; Tang, Z.R.; Xu, Y.J. Photocorrosion inhibition of semiconductor-based photocatalysts: Basic principle, current development, and future perspective. ACS Catal. 2019, 9, 4642–4687. [Google Scholar]
- Yu, J.; Sun, D.; Wang, T.; Li, F. Fabrication of Ag@ AgCl/ZnO submicron wire film catalyst on glass substrate with excellent visible light photocatalytic activity and reusability. Chem. Eng. J. 2018, 334, 225–236. [Google Scholar] [CrossRef]
- Mohseni, N.; Haghighi, M.; Shabani, M. Bimetallic Co2+ Cr3+ LDH anchored AgCl as excellent solar-light-responsive Ag-decorated type II nano-heterojunction for photodegradation of various dyes. Process Saf. Environ. 2022, 168, 668–688. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energ. Environ. Sci. 2012, 5, 8475–8505. [Google Scholar]
- Das, H.T.; Dutta, S.; Das, N.; Das, P.; Mondal, A.; Imran, M. Recent trend of CeO2-based nanocomposites electrode in supercapacitor: A review on energy storage applications. J. Energy Storage 2022, 50, 104643. [Google Scholar]
- Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar]
- Kim, J.Y.; Jang, B.; Lim, M.; Park, J.Y.; Choa, Y.H. Enhanced PtRu by CeO2 hollow nanofibers: Hydrogen gas sensing with CO-resistant in fuel cell. J. Power Sources 2024, 613, 234842. [Google Scholar]
- Agarwal, U.P.; Ralph, S.A.; Baez, C.; Reiner, R.S.; Verrill, S.P. Effect of sample moisture content on XRD-estimated cellulose crystallinity index and crystallite size. Cellulose 2017, 24, 1971–1984. [Google Scholar]
- Yin, H.; Wang, X.; Wang, L.; Nie, Q.; Zhang, Y.; Yuan, Q.; Wu, W. Ag/AgCl modified self-doped TiO2 hollow sphere with enhanced visible light photocatalytic activity. J. Alloy Compd. 2016, 657, 44–52. [Google Scholar]
- Wang, P.; Huang, B.; Lou, Z.; Zhang, X.; Qin, X.; Dai, Y.; Zheng, Z.; Wang, X. Synthesis of highly efficient Ag@ AgCl plasmonic photocatalysts with various structures. Chem-Asianj 2010, 16, 538–544. [Google Scholar]
- Danish, M.; Sandhu, Z.A.; Sharif, S.; Raza, M.A.; Elahi, M.; Aslam, M.; Zain, M.; Batoo, K.M.; Ijaz, M.F. Excellence of Engineered Ce2O3 and Bi2O3@ Ce2O3 Nanomaterials for Unveiling the Pseudocapacitive and Catalytic Applications. J. Inorg. Organomet P 2024, 1–21. [Google Scholar] [CrossRef]
- Qiao, R.; Mao, M.; Hu, E.; Zhong, Y.; Ning, J.; Hu, Y. Facile formation of mesoporous BiVO4/Ag/AgCl heterostructured microspheres with enhanced visible-light photoactivity. Inorg. Chem. 2015, 54, 9033–9039. [Google Scholar]
- Brant, P.; Stephenson, T.A. X-ray photoelectron spectra of mixed-valence diruthenium complexes. Distinguishing between strongly and weakly interacting metals in delocalized class III complexes. Inorg. Chem. 1987, 26, 22–26. [Google Scholar]
- Alonso, J.A. Electronic and atomic structure, and magnetism of transition-metal clusters. Chem. Rev. 2000, 100, 637–678. [Google Scholar] [PubMed]
- Winter, B.; Faubel, M. Photoemission from liquid aqueous solutions. Chem. Rev. 2006, 106, 1176–1211. [Google Scholar] [PubMed]
- Jaffari, G.H.; Imran, A.; Bah, M.; Ali, A.; Bhatti, A.S.; Qurashi, U.S.; Shah, S.I. Identification and quantification of oxygen vacancies in CeO2 nanocrystals and their role in formation of F-centers. Appl. Surf. Sci. 2017, 396, 547–553. [Google Scholar]
- Li, X.; Yao, C.; Lu, X.; Hu, Z.; Yin, Y.; Ni, C. Halloysite–CeO2–AgBr nanocomposite for solar light photodegradation of methyl orange. Appl. Clay Sci. 2015, 104, 74–80. [Google Scholar]
- Su, F.; Li, P.; Huang, J.; Gu, M.; Liu, Z.; Xu, Y. Photocatalytic degradation of organic dye and tetracycline by ternary Ag2O/AgBr–CeO2 photocatalyst under visible-light irradiation. Sci. Rep-UK 2021, 11, 85. [Google Scholar]
- Lin, X.; Liu, H.; Guo, B.; Zhang, X. Synthesis of AgCl/Ag/AgCl core-shell microstructures with enhanced photocatalytic activity under sunlight irradiation. J. Environ. Chem. Eng. 2016, 4, 4021–4028. [Google Scholar]
- Han, S.H.; Liu, H.M.; Sun, C.C.; Jin, P.J.; Chen, U. Photocatalytic performance of AgCl@ Ag core–shell nanocubes for the hexavalent chromium reduction. J. Mater. Sci. 2018, 53, 12030–12039. [Google Scholar]
- Shi, L.; Huang, Y.; Seo, H.J. Emission red shift and unusual band narrowing of Mn2+ in NaCaPO4 phosphor. J. Phys. Chem. A 2010, 114, 6927–6934. [Google Scholar]
- Haddad, R.E.; Gazeau, S.; Pécaut, J.; Marchon, J.C.; Medforth, C.J.; Shelnutt, J.A. Origin of the red shifts in the optical absorption bands of nonplanar tetraalkylporphyrins. J. Am. Chem. Soc. 2003, 125, 1253–1268. [Google Scholar]
- Dai, W.; Hu, X.; Wang, T.; Xiong, W.; Luo, X.; Zou, J. Hierarchical CeO2/Bi2MoO6 heterostructured nanocomposites for photoreduction of CO2 into hydrocarbons under visible light irradiation. Appl. Surf. Sci. 2018, 434, 481–491. [Google Scholar]
- Jakimińska, A.; Macyk, W. Photochemical transformations of AgCl in the context of its eventual photocatalytic applications. J. Photoch Photobio A 2023, 445, 115048. [Google Scholar]
- Fan, Y.; Bao, Y.; Song, Z.; Sun, Z.; Wang, D.; Han, D.; Niu, L. Controllable synthesis of coloured Ag 0/AgCl with spectral analysis for photocatalysis. Rsc. Adv. 2018, 8, 24812–24818. [Google Scholar]
- Wang, X.; Li, S.; Ma, Y.; Yu, H.; Yu, J. H2WO4·3H2O/Ag/AgCl composite nanoplates: A plasmonic Z-scheme visible-light photocatalyst. J. Phys. Chem. C 2011, 115, 14648–14655. [Google Scholar]
- Chen, D.; Li, T.; Chen, Q.; Gao, J.; Fan, B.; Li, J.; Li, X.; Zhang, R.; Sun, J.; Gao, L. Hierarchically plasmonic photocatalysts of Ag/AgCl nanocrystals coupled with single-crystalline WO3 nanoplates. Nanoscale 2012, 4, 5431–5439. [Google Scholar]
- Myrick, M.L.; Simcock, M.N.; Baranowski, M.; Brooke, H.; Morgan, S.L.; McCutcheon, J.N. The Kubelka-Munk diffuse reflectance formula revisited. Appl. Spectrosc. Rev. 2011, 46, 140–165. [Google Scholar]
- Landi, S., Jr.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid. State Commun. 2022, 341, 114573. [Google Scholar]
- Han, C.; Ge, L.; Chen, C.; Li, Y.; Zhao, Z.; Xiao, X.; Li, Z.; Zhang, J. Site-selected synthesis of novel Ag@ AgCl nanoframes with efficient visible light induced photocatalytic activity. J. Mater. Chem. A 2014, 2, 12594–12600. [Google Scholar]
- Majher, J.D.; Gray, M.B.; Strom, T.A.; Woodward, P.M. Cs2NaBiCl6: Mn2+—A new orange-red halide double perovskite phosphor. Chem. Mater. 2019, 31, 1738–1744. [Google Scholar]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar]
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406. [Google Scholar]
- Bi, L.; Gao, X.; Zhang, L.; Wang, D.; Zou, X.; Xie, T. Enhanced photocatalytic hydrogen evolution of NiCoP/g-C3N4 with improved separation efficiency and charge transfer efficiency. ChemSusChem 2018, 11, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chang, Y.; Shen, X.; Liu, Z.; Zhu, B.; Macharia, D.K.; Wang, Z.; Chen, Z.; Zhang, L. Construction of Ag/AgCl-CN heterojunctions with enhanced photocatalytic activities for degrading contaminants in wastewater. J. Colloid. Interf. Sci. 2019, 543, 25–33. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Z.; Feng, Y.; Lin, S.; Li, H.; Gao, X. Surface oxygen vacancy modified Bi2MoO6/MIL-88B (Fe) heterostructure with enhanced spatial charge separation at the bulk & interface. Appl. Catal. B-Environ. 2020, 268, 118740. [Google Scholar]
- Yu, S.; Li, J.; Zhang, Y.; Li, M.; Dong, F.; Zhang, T.; Huang, H. Local spatial charge separation and proton activation induced by surface hydroxylation promoting photocatalytic hydrogen evolution of polymeric carbon nitride. Nano Energy 2018, 50, 383–392. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Zhang, X.; Dou, J.; Yan, S.; Yao, L.; Fu, Y.; Shi, L. Enhanced Photocatalytic Activity of AgI/BiO2-x Heterojunction Photocatalyst. ChemistrySelect 2021, 6, 13434–13442. [Google Scholar] [CrossRef]
- He, C.; Jing, P.; Wang, P.; Ji, J.; Ouyang, T.; Cui, Y.; Pu, Y. A novel hierarchical BaTiO3/AgI heterojunction with boosting spatial charge kinetics for photocatalytic degradation of organic pollutant. Ceram. Int. 2021, 47, 33426–33434. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, F.; Zhan, S.; He, Q.; Song, Y.; Zhang, C.; Lai, J. Morphology modulation and performance optimization of nanopetal-based Ag-modified Bi2O2CO3 as an inactivating photocatalytic material. Environ. Res. 2021, 198, 111256. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; De Witte, B.; Van Langenhove, H. Titanium dioxide mediated heterogeneous photocatalytic degradation of gaseous dimethyl sulfide: Parameter study and reaction pathways. Appl. Catal. B-Environ. 2005, 60, 93–106. [Google Scholar]
- Chang, C.R.; Wang, Y.G.; Li, J. Theoretical investigations of the catalytic role of water in propene epoxidation on gold nanoclusters: A hydroperoxyl-mediated pathway. Nano Res. 2011, 4, 131–142. [Google Scholar] [CrossRef]
- Liu, L.M.; Crawford, P.; Hu, P. The interaction between adsorbed OH and O2 on TiO2 surfaces. Prog. Surf. Sci. 2009, 84, 155–176. [Google Scholar] [CrossRef]
- Padma, R.; Sreenu, K.; Reddy, V.R. Electrical and frequency dependence characteristics of Ti/polyethylene oxide (PEO)/p-type InP organic-inorganic Schottky junction. J. Alloy Compd. 2017, 695, 2587–2596. [Google Scholar]
- Lee, M.H.; Takei, K.; Zhang, J.; Kapadia, R.; Zheng, M.; Chen, Y.Z.; Nah, J.; Matthews, T.; Chueh, Y.L.; Ager, J.; et al. p-Type InP nanopillar photocathodes for efficient solar-driven hydrogen production. Angew. Chem. Int. Edit 2012, 51, 10760. [Google Scholar]
- Wang, J.; Zhang, J.; Peh, S.B.; Liu, G.; Kundu, T.; Dong, J.; Ying, Y.; Qian, Y.; Zhao, D. Cobalt-containing covalent organic frameworks for visible light-driven hydrogen evolution. Sci. China Chem. 2020, 63, 192–197. [Google Scholar]
- Xu, Q.; Wageh, S.; Al-Ghamdi, A.A.; Li, X. Design principle of S-scheme heterojunction photocatalyst. J. Mater. Sci. Technol. 2022, 124, 171–173. [Google Scholar]
- Zhang, X.; Zhang, H.; Yu, J.; Wu, Z.; Zhou, Q. Preparation of flower-like Co3O4 QDs/ Bi2WO6 p-n heterojunction photocatalyst and its degradation mechanism of efficient visible-light-driven photocatalytic tetracycline antibiotics. Appl. Surf. Sci. 2022, 585, 152547. [Google Scholar]
- Quan, Y.; Liu, M.; Wu, H.; Tian, X.; Dou, L.; Wang, Z.; Ren, C. Rational design and construction of S-scheme CeO2/AgCl heterojunction with enhanced photocatalytic performance for tetracycline degradation. Appl. Surf. Sci. 2024, 642, 158601. [Google Scholar]
- Pal, A.; Jana, A.; Bhattacharya, S.; Datta, J. SPR effect of AgNPs decorated TiO2 in DSSC using TPMPI in the electrolyte: Approach towards low light trapping. Electrochim. Acta 2017, 243, 33–43. [Google Scholar]
- He, Z.; Sun, C.; Yang, S.; Ding, Y.; He, H.; Wang, Z. Photocatalytic degradation of rhodamine B by Bi2WO6 with electron accepting agent under microwave irradiation: Mechanism and pathway. J. Hazard. Mater. 2009, 162, 1477–1486. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Yang, N.; Xu, T.; Yang, Y.; Lv, Y. Enhancing Visible-Light Photocatalytic Activity of AgCl Photocatalyst by CeO2 Modification for Degrading Multiple Organic Pollutants. Nanomaterials 2025, 15, 537. https://doi.org/10.3390/nano15070537
Xu L, Yang N, Xu T, Yang Y, Lv Y. Enhancing Visible-Light Photocatalytic Activity of AgCl Photocatalyst by CeO2 Modification for Degrading Multiple Organic Pollutants. Nanomaterials. 2025; 15(7):537. https://doi.org/10.3390/nano15070537
Chicago/Turabian StyleXu, Li, Ning Yang, Tong Xu, Yang Yang, and Yanfei Lv. 2025. "Enhancing Visible-Light Photocatalytic Activity of AgCl Photocatalyst by CeO2 Modification for Degrading Multiple Organic Pollutants" Nanomaterials 15, no. 7: 537. https://doi.org/10.3390/nano15070537
APA StyleXu, L., Yang, N., Xu, T., Yang, Y., & Lv, Y. (2025). Enhancing Visible-Light Photocatalytic Activity of AgCl Photocatalyst by CeO2 Modification for Degrading Multiple Organic Pollutants. Nanomaterials, 15(7), 537. https://doi.org/10.3390/nano15070537




