Synergistic Photocatalytic Oxidation and Reductive Activation of Peroxymonosulfate by Bi-Based Heterojunction for Highly Efficient Organic Pollutant Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of BiVO4
2.2. Synthesis of CuBi2O4
2.3. Synthesis of CuBi2O4/BiVO4 Heterojunctions
2.4. CuBi2O4/BiVO4 Performance Evaluation Tests
3. Results
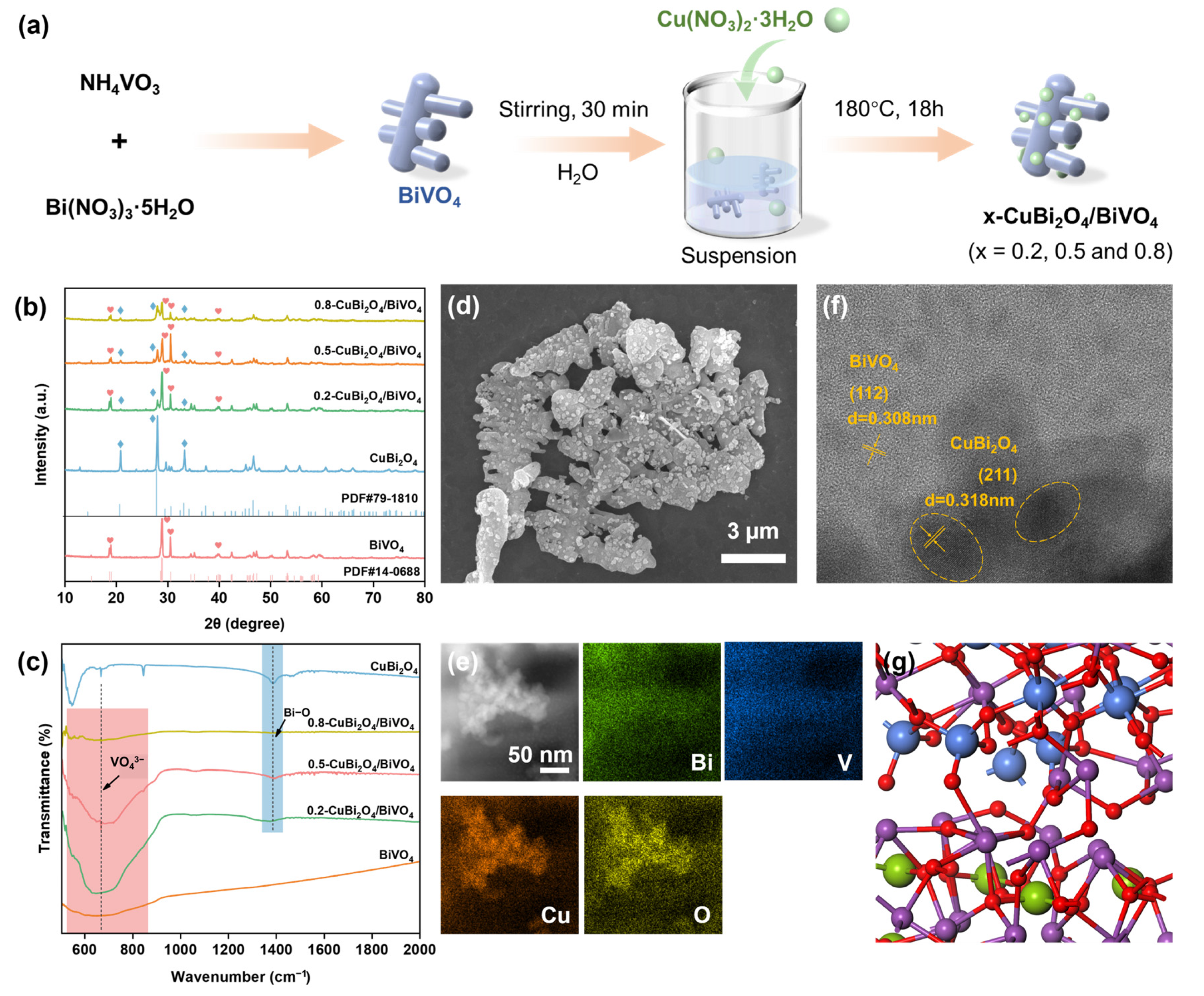
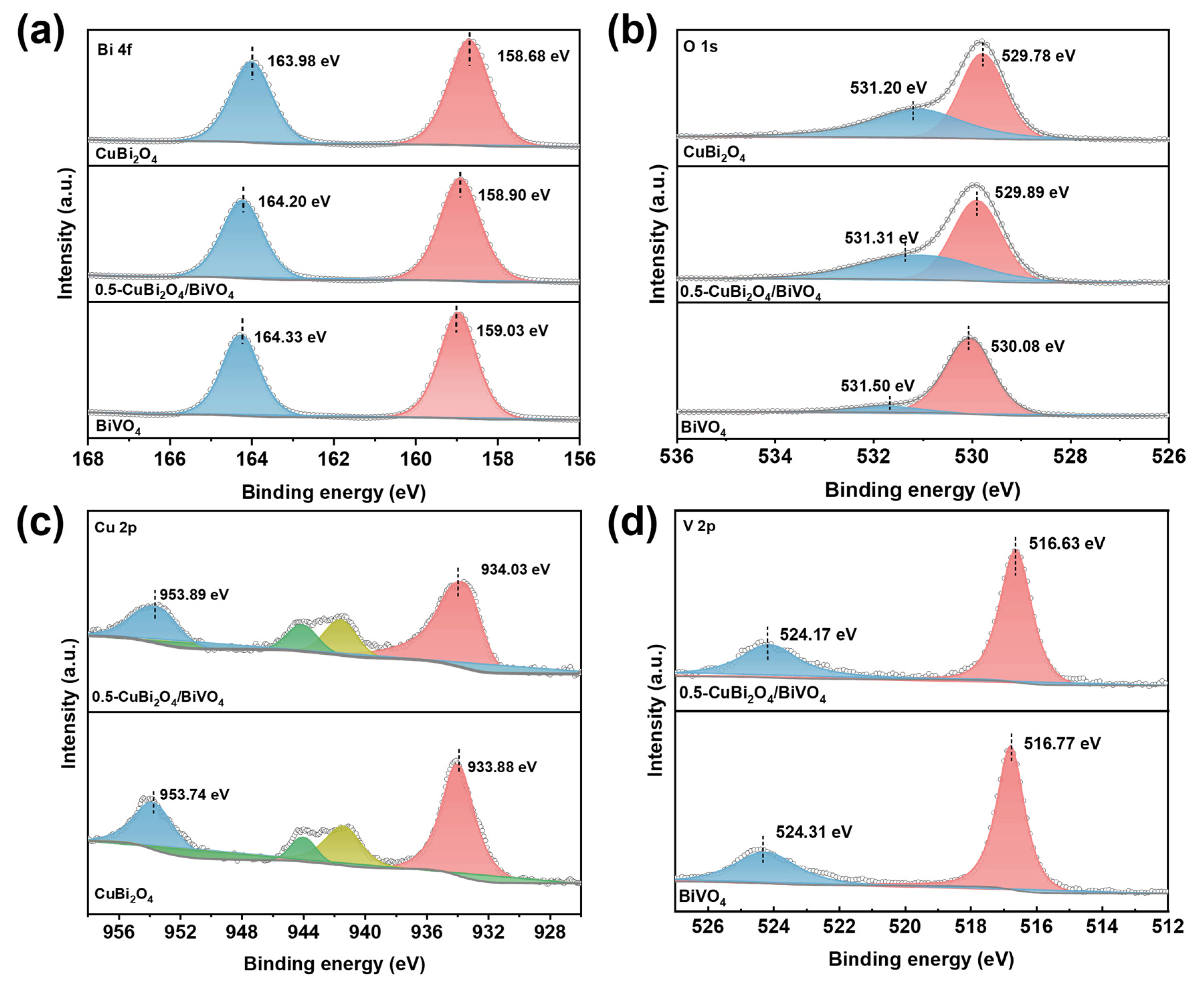
3.1. Structure and Morphology Analysis
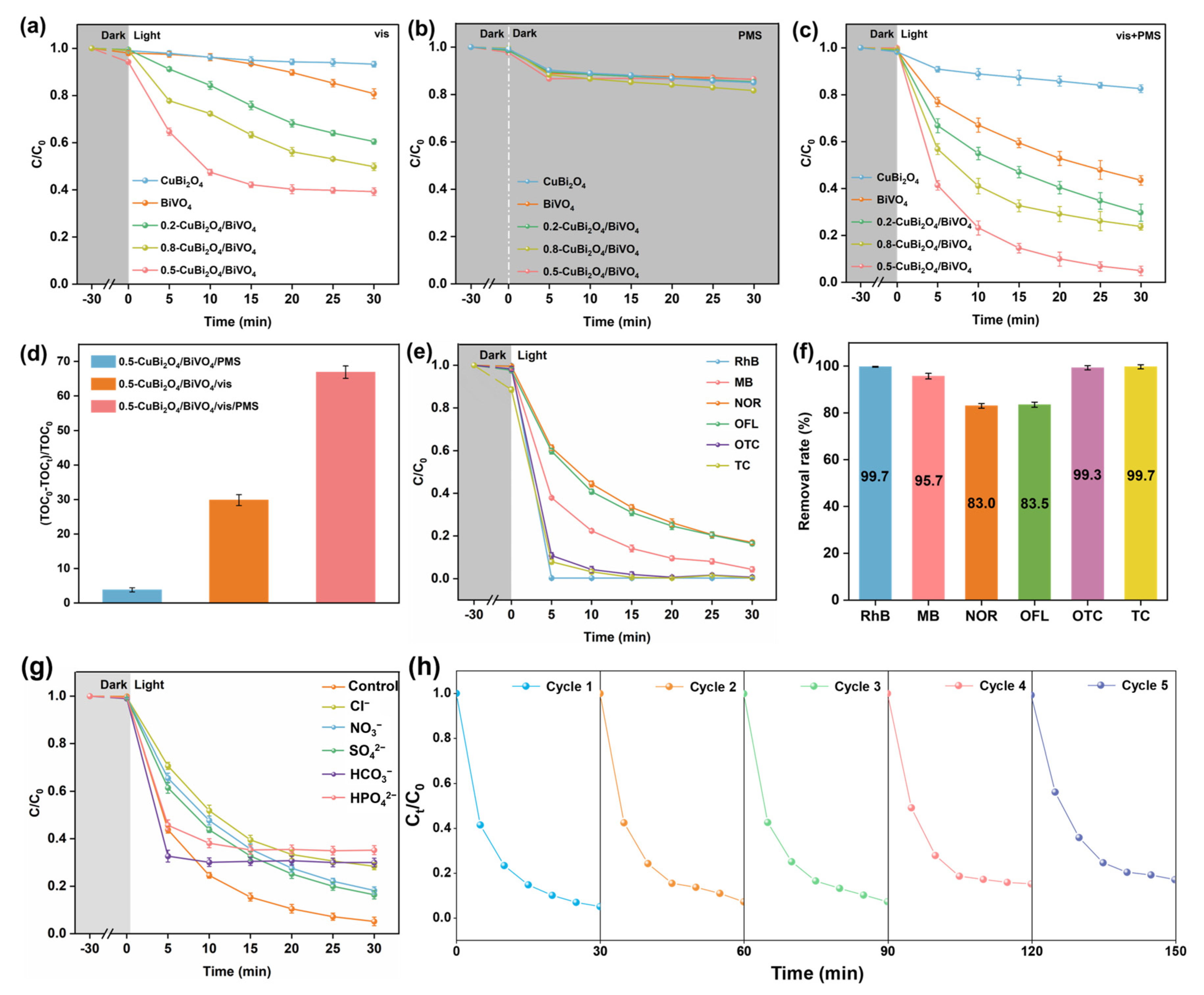
3.2. Analysis of Photocatalytic Performance
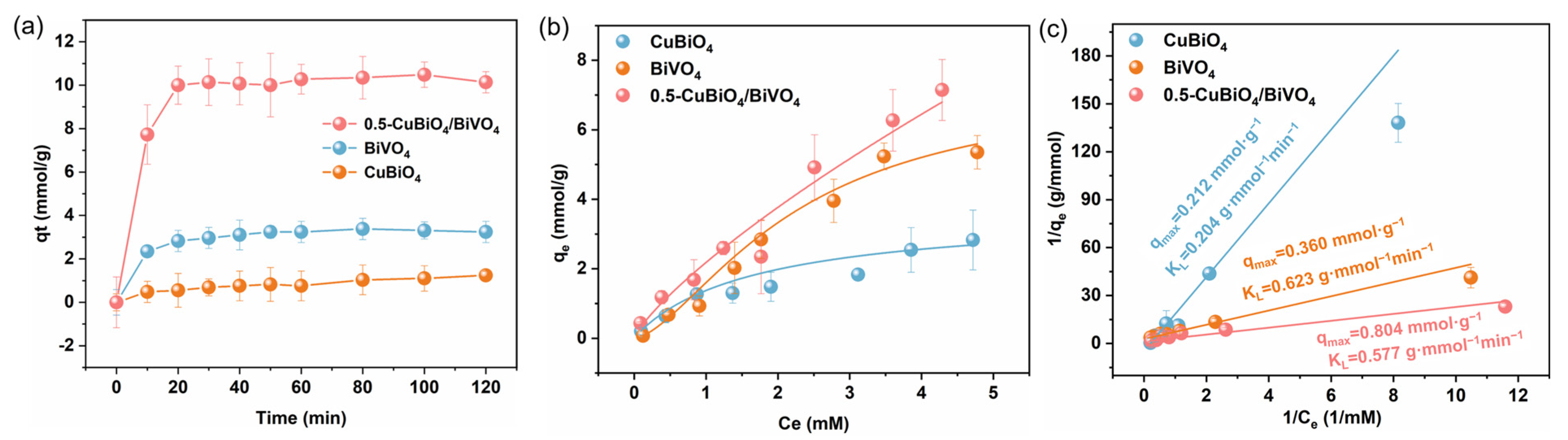
3.3. PMS Adsorption Test
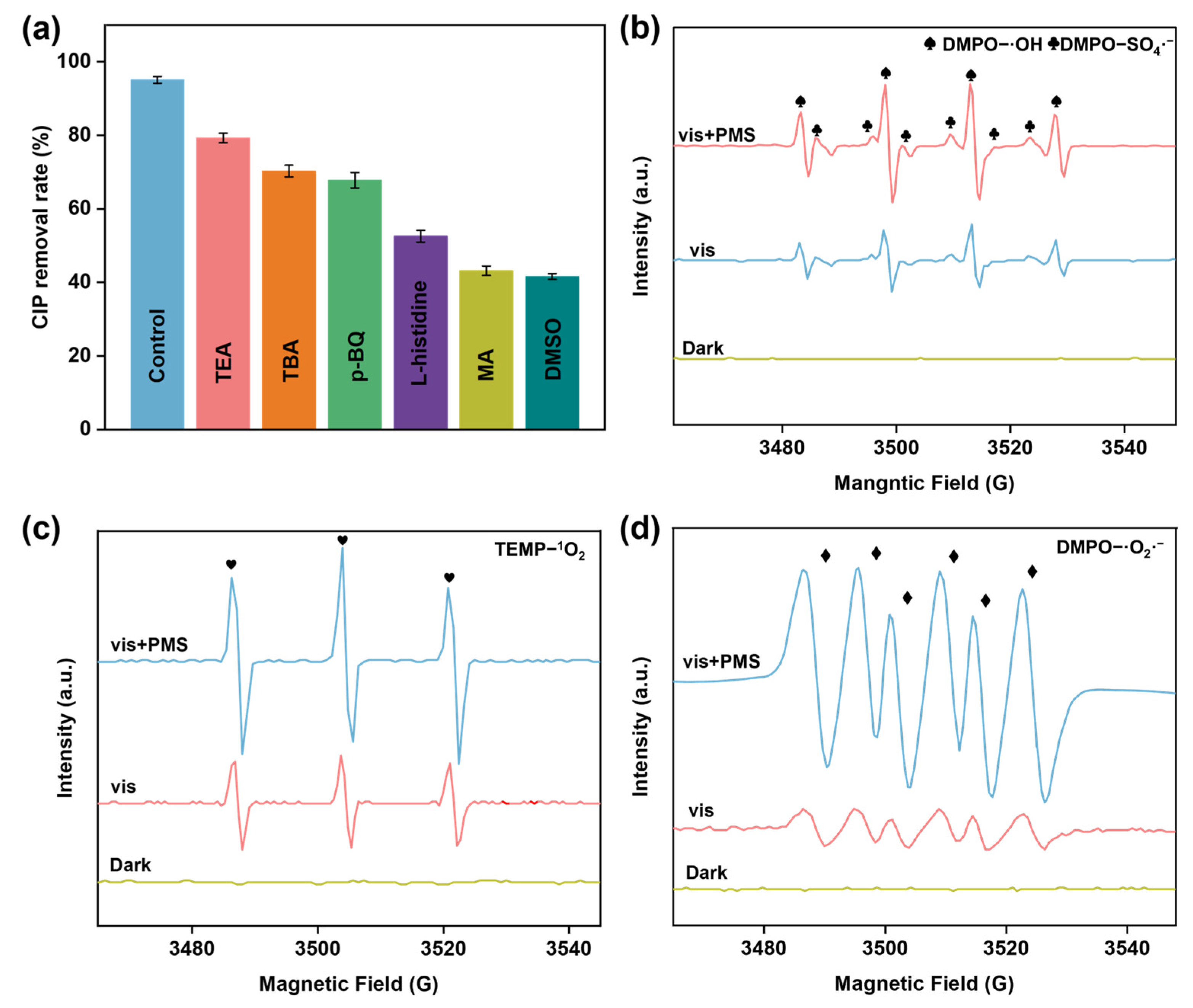
3.4. Analysis of PMS Activation Mechanism
3.5. Degradation Performance of CIP
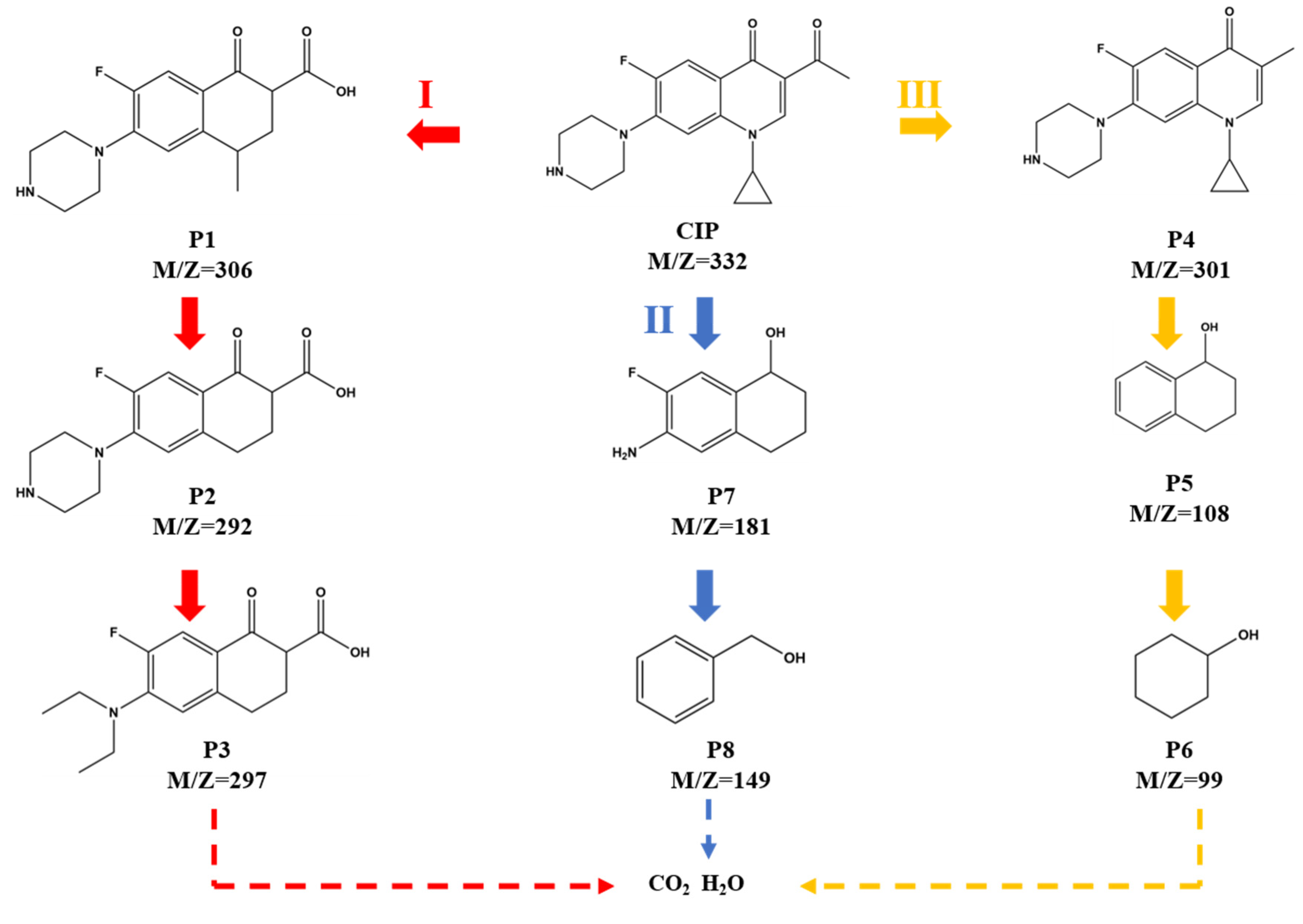
3.6. Degradation Pathway of CIP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, J.; Mi, X.; Chen, Z.; Xu, W.; Liu, W.; Ma, R.; Zhang, Y.; Du, Y.; Ni, B.-J. Efficient emerging contaminants (EM) decomposition via peroxymonosulfate (PMS) activation by Co3O4/carbonized polyaniline (CPANI) composite: Characterization of tetracycline (TC) degradation property and application for the remediation of EM-polluted water body. J. Clean. Prod. 2023, 405, 137023. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Oderinde, O.; Etafo, N.O.; Kifle, G.A.; Okeke, E.S.; Ejeromedoghene, O.; Mgbechidinma, C.L.; Oke, E.A.; Raheem, S.A.; Bakare, O.C.; et al. Recent perspective of antibiotics remediation: A review of the principles, mechanisms, and chemistry controlling remediation from aqueous media. Sci. Total Environ. 2023, 881, 163469. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Milakovic, M.; Švecová, H.; Ganjto, M.; Jonsson, V.; Grabic, R.; Udikovic-Kolic, N. Industrial wastewater treatment plant enriches antibiotic resistance genes and alters the structure of microbial communities. Water Res. 2019, 162, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, Y.; Li, J.; Cheng, X.; Dionysiou, D.D. Sulfamethoxazole degradation by visible light assisted peroxymonosulfate process based on nanohybrid manganese dioxide incorporating ferric oxide. Appl. Catal. B Environ. 2020, 278, 119297. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Du, Y.; Ma, W.; Liu, P.; Zou, B.; Ma, J. Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Ding, Z.; Zhao, Z.; Xu, X.; Fang, Z. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrason. Sonochem. 2017, 34, 953–959. [Google Scholar] [CrossRef]
- Fan, X.-Y.; Xu, S.-L.; Li, X.; Zhou, Z.-W.; Gao, Y.-X.; Zhao, J.-R.; Zhang, Z.-X. Efficient removal of size-fractionated antibiotic resistance genes (ARGs) in WWTPs secondary effluent by vacuum ultraviolet (VUV) activated potassium peroxymonosulfate (PMS). Chem. Eng. J. 2024, 487, 150779. [Google Scholar] [CrossRef]
- Song, H.; Yan, L.; Jiang, J.; Ma, J.; Zhang, Z.; Zhang, J.; Liu, P.; Yang, T. Electrochemical activation of persulfates at BDD anode: Radical or nonradical oxidation? Water Res. 2018, 128, 393–401. [Google Scholar] [CrossRef]
- Cho, D.-W.; Kwon, G.; Yoon, K.; Tsang, Y.F.; Ok, Y.S.; Kwon, E.E.; Song, H. Simultaneous production of syngas and magnetic biochar via pyrolysis of paper mill sludge using CO2 as reaction medium. Energy Convers. Manag. 2017, 145, 1–9. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Tian, D.; Zhou, H.; Zhang, H.; Zhou, P.; You, J.; Yao, G.; Pan, Z.; Liu, Y.; Lai, B. Heterogeneous photocatalyst-driven persulfate activation process under visible light irradiation: From basic catalyst design principles to novel enhancement strategies. Chem. Eng. J. 2022, 428, 131166. [Google Scholar] [CrossRef]
- Hasija, V.; Nguyen, V.-H.; Kumar, A.; Raizada, P.; Krishnan, V.; Khan, A.A.P.; Singh, P.; Lichtfouse, E.; Wang, C.; Thi Huong, P. Advanced activation of persulfate by polymeric g-C3N4 based photocatalysts for environmental remediation: A review. J. Hazard. Mater. 2021, 413, 125324. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Ghanbari, F.; Alvarez, A.; Silva Martinez, S. UV-LEDs assisted peroxymonosulfate/Fe2+ for oxidative removal of carmoisine: The effect of chloride ion. Korean J. Chem. Eng. 2017, 34, 2154–2161. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, Y.; Peng, J.; Yao, G.; Zhang, Y.; Lai, B. Efficient degradation of atrazine by LaCoO3/Al2O3 catalyzed peroxymonosulfate: Performance, degradation intermediates and mechanism. Chem. Eng. J. 2019, 372, 796–808. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Yao, J.; Song, Y.; Li, W.; Xuan, X. Coordination tuning of Fe2+ ions concentration in Fe-doped black phosphorus-carbonized cotton fiber (Fe-BP-CCF) composites to regulate photocatalysis and peroxymonosulfate (PMS) activation towards highly efficient degradation of organic pollutants. Chem. Eng. J. 2024, 483, 149326. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Guan, W.; Huang, H.; Liu, Y.; Kang, Z. Study on highly enhanced photocatalytic tetracycline degradation of type Ⅱ AgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard Mater. 2018, 349, 111–118. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Chen, Y.; Zhou, Y.; Ding, L.; Liang, H.; Li, X. Degradation of tetracycline hydrochloride by coupling of photocatalysis and peroxymonosulfate oxidation processes using CuO-BiVO4 heterogeneous catalyst. Process Saf. Environ. Prot. 2021, 145, 364–377. [Google Scholar] [CrossRef]
- Dou, X.; Chen, Y.; Shi, H. CuBi2O4/BiOBr composites promoted PMS activation for the degradation of tetracycline: S-scheme mechanism boosted Cu2+/Cu+ cycle. Chem. Eng. J. 2022, 431, 134054. [Google Scholar] [CrossRef]
- Sun, Q.M.; Xu, J.J.; Tao, F.F.; Ye, W.; Zhou, C.; He, J.H.; Lu, J.M. Boosted Inner Surface Charge Transfer in Perovskite Nanodots@Mesoporous Titania Frameworks for Efficient and Selective Photocatalytic CO2 Reduction to Methane. Angew. Chem. Int. Ed. 2022, 61, e202200872. [Google Scholar] [CrossRef]
- Sun, W.J.; Ji, H.Q.; Li, L.X.; Zhang, H.Y.; Wang, Z.K.; He, J.H.; Lu, J.M. Built-in Electric Field Triggered Interfacial Accumulation Effect for Efficient Nitrate Removal at Ultra-Low Concentration and Electroreduction to Ammonia. Angew. Chem. Int. Ed. 2021, 60, 22933–22939. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lai, K.; Li, N.; Gao, Y.; Ge, L. Efficient photocatalytic CO2 reduction to CH4 via electric field-regulated d-band center on Ga2S3/CuS S-type heterojunction interface structures. Appl. Catal. B Environ. Energy 2024, 357, 124302. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Shi, K.; Wu, Y.; Huang, W.; Min, Y.; Liu, Q.; Liang, Z. Nanoreactors Encapsulating Built-in Electric Field as a “Bridge” for Li–S Batteries: Directional Migration and Rapid Conversion of Polysulfides. Adv. Energy Mater. 2024, 14, 2303546. [Google Scholar] [CrossRef]
- Liu, M.; Xu, W.; Liu, S.; Liu, B.; Gao, Y.; Wang, B. Directional Polarization of a Ferroelectric Intermediate Layer Inspires a Built-In Field in Si Anodes to Regulate Li+ Transport Behaviors in Particles and Electrolyte. Adv. Sci. 2024, 11, 2402915. [Google Scholar] [CrossRef]
- Wu, M.; Jing, Q.; Feng, X.; Chen, L. BiVO4 microstructures with various morphologies: Synthesis and characterization. Appl. Surf. Sci. 2018, 427, 525–532. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Chai, J.; Xue, S.; Wang, R.; Jiang, L.; Wang, J.; Zhang, Z.; Dionysiou, D.D. Construction of novel symmetric double Z-scheme BiFeO3/CuBi2O4/BaTiO3 photocatalyst with enhanced solar-light-driven photocatalytic performance for degradation of norfloxacin. Appl. Catal. B Environ. 2020, 272, 119017. [Google Scholar] [CrossRef]
- Chenglin, Z.; Su, Z.; Feng, Z. Well-constructed CeO2-coated CuBi2O4 heterojunction: Enhanced charge carriers transportation. Surf. Interfaces 2025, 59, 105921. [Google Scholar] [CrossRef]
- Fang, C.; Su, H.; Hu, M.; Jiang, Z.; Xu, L.; Liu, C. Construction and performance of a novel CuBi2O4/In2O3 Z-scheme heterojunction photocatalyst. Mater. Sci. Semicond. Process. 2023, 160, 107464. [Google Scholar] [CrossRef]
- Baral, B.; Paramanik, L.; Parida, K. Functional facet isotype junction and semiconductor/r-GO minor Schottky barrier tailored In2S3@r-GO@(040/110)-BiVO4 ternary hybrid. J. Colloid Interface Sci. 2021, 585, 519–537. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Luo, N.; Shang, W.; Shi, S.; Li, H.; Liang, Y.; Zhou, A. Kinetic comparison of photocatalysis with H2O2-free photo-Fenton process on BiVO4 and the effective antibiotic degradation. Chem. Eng. J. 2022, 429, 132577. [Google Scholar] [CrossRef]
- Liu, M.; Xia, Y.; Zhao, W.; Jiang, R.; Fu, X.; Zimmerle, B.; Tian, L.; Chen, X. Modulating oxygen vacancy concentration on Bi4V2O11 nanorods for synergistic photo-driven plastic waste oxidation and CO2 reduction. J. Mater. Chem. A 2023, 11, 12770–12776. [Google Scholar] [CrossRef]
- Oropeza, F.E.; Feleki, B.T.; Zhang, K.H.L.; Hensen, E.J.M.; Hofmann, J.P. Influence of Reduced Cu Surface States on the Photoelectrochemical Properties of CuBi2O4. ACS Appl. Energy Mater. 2019, 2, 6866–6874. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, Y.; Huang, H.; Zhou, P.; Li, J.; Zhang, L.; Dai, B.; Xu, J.; Zhu, F.; Sheng, N.; et al. A novel Z-scheme Ag3VO4/BiVO4 heterojunction photocatalyst: Study on the excellent photocatalytic performance and photocatalytic mechanism. Appl. Catal. B Environ. 2019, 245, 448–458. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Hu, X.; Wu, X.; Chen, W.; Zhou, S.; Li, J.; Qi, C.; Ma, D.-K. Fluorine-doped CuBi2O4 nanorod arrays for enhanced photoelectrochemical oxygen reduction reaction toward H2O2 production. J. Catal. 2022, 410, 339–346. [Google Scholar] [CrossRef]
- Sekine, R.; Sato, T.; Zahran, Z.N.; Tsubonouchi, Y.; Chandra, D.; Hoshino, N.; Yagi, M. Visible-light-driven oxygen reduction by an anisotropically crystallized CuBi2O4 photocathode fabricated using a mixed metal-imidazole casting method. J. Mater. Chem. A 2024, 12, 2129–2139. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, J.; Tan, Y.; Deng, Y.; Li, C.; Sun, Z. Insight into peroxymonosulfate assisted photocatalysis over Fe2O3 modified TiO2/diatomite composite for highly efficient removal of ciprofloxacin. Sep. Purif. Technol. 2022, 293, 121123. [Google Scholar] [CrossRef]
- Wu, S.; Yu, X.; Zhang, J.; Zhang, Y.; Zhu, Y.; Zhu, M. Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal. Chem. Eng. J. 2021, 411, 128555. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, C.; Hu, Q.; Ding, L. 0D/3D NiCo2O4/defected UiO-66 catalysts for enhanced degradation of tetracycline in peroxymonosulfate/simulated sunlight systems: Degradation mechanisms and pathways. Chemosphere 2022, 299, 134322. [Google Scholar] [CrossRef]
- Cao, H.; Lu, Y.; Zhang, X.; Dong, W.; Shi, W.; Huang, Y. Engineering a simple multisignal-output probe for measuring residual peroxymonosulfate in advanced oxidation reactions. Chem. Eng. J. 2023, 471, 144663. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Tang, H.; Xie, C.; Zhao, Y.; Wu, P. Non-colorimetric sensing with 3,3′,5,5′-tetramethylbenzidine. Sens. Actuators B Chem. 2025, 422, 136643. [Google Scholar] [CrossRef]
- Cui, H.; Xing, N. A green method for the preparation of nitrogen-doped mesoporous carbon and its application in adsorptive desulfurization. New J. Chem. 2024, 48, 15802–15809. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q. An efficient one-step condensation and activation strategy to synthesize porous carbons with optimal micropore sizes for highly selective CO2 adsorption. Nanoscale 2014, 6, 4148–4156. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, A.B.; Valle-Vigón, P.; Sevilla, M. Synthesis of colloidal silica nanoparticles of a tunable mesopore size and their application to the adsorption of biomolecules. J. Colloid and Interface Sci. 2010, 349, 173–180. [Google Scholar] [CrossRef]
- Sierra-Trejo, P.V.; Guibal, E.; Louvier-Hernández, J.F. Arsenic Sorption on Chitosan-Based Sorbents: Comparison of the Effect of Molybdate and Tungstate Loading on As(V) Sorption Properties. J. Polym. Environ. 2020, 28, 934–947. [Google Scholar] [CrossRef]
- Tan, J.; Li, Z.; Li, J.; Meng, Y.; Yao, X.; Wang, Y.; Lu, Y.; Zhang, T. Visible-light-assisted peroxymonosulfate activation by metal-free bifunctional oxygen-doped graphitic carbon nitride for enhanced degradation of imidacloprid: Role of non-photochemical and photocatalytic activation pathway. J. Hazard. Mater. 2022, 423, 127048. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Ma, Y.; Song, Y.; Li, T.; Dong, S. Tetracycline hydrochloride degradation over manganese cobaltate (MnCo2O4) modified ultrathin graphitic carbon nitride (g-C3N4) nanosheet through the highly efficient activation of peroxymonosulfate under visible light irradiation. J. Colloid Interface Sci. 2021, 600, 449–462. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, C.; Tian, L.; Yuan, F.; Zheng, S.; Sun, Z. Fast and lasting electron transfer between γ-FeOOH and g-C3N4/kaolinite containing N vacancies for enhanced visible-light-assisted peroxymonosulfate activation. Chem. Eng. J. 2022, 429, 132374. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.-Y.; Li, J.; Lu, X.-T.; Yuan, L.-P. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015, 49, 12941–12950. [Google Scholar] [CrossRef]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef]
- Jia, J.; Liu, D.; Wang, S.; Li, H.; Ni, J.; Li, X.; Tian, J.; Wang, Q. Visible-light-induced activation of peroxymonosulfate by TiO2 nano-tubes arrays for enhanced degradation of bisphenol A. Sep. Purif. Technol. 2020, 253, 117510. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, X.; Dong, X.; Zhang, X.; Tan, Y.; Yuan, F.; Zheng, S.; Li, C. Synergistic activation of peroxymonosulfate via in situ growth FeCo2O4 nanoparticles on natural rectorite: Role of transition metal ions and hydroxyl groups. Chemosphere 2021, 263, 127965. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wang, Y.; Liu, F.; Ye, X.; Wei, S.; Sun, Y.; He, J. Synergistic Photocatalytic Oxidation and Reductive Activation of Peroxymonosulfate by Bi-Based Heterojunction for Highly Efficient Organic Pollutant Degradation. Nanomaterials 2025, 15, 471. https://doi.org/10.3390/nano15060471
Zhao X, Wang Y, Liu F, Ye X, Wei S, Sun Y, He J. Synergistic Photocatalytic Oxidation and Reductive Activation of Peroxymonosulfate by Bi-Based Heterojunction for Highly Efficient Organic Pollutant Degradation. Nanomaterials. 2025; 15(6):471. https://doi.org/10.3390/nano15060471
Chicago/Turabian StyleZhao, Xiaopeng, Yang Wang, Fangning Liu, Xiaobin Ye, Shangxiong Wei, Yilin Sun, and Jinghui He. 2025. "Synergistic Photocatalytic Oxidation and Reductive Activation of Peroxymonosulfate by Bi-Based Heterojunction for Highly Efficient Organic Pollutant Degradation" Nanomaterials 15, no. 6: 471. https://doi.org/10.3390/nano15060471
APA StyleZhao, X., Wang, Y., Liu, F., Ye, X., Wei, S., Sun, Y., & He, J. (2025). Synergistic Photocatalytic Oxidation and Reductive Activation of Peroxymonosulfate by Bi-Based Heterojunction for Highly Efficient Organic Pollutant Degradation. Nanomaterials, 15(6), 471. https://doi.org/10.3390/nano15060471






