Densely Stacked CoCu-MOFs Coated with CuAl/LDH Enhance Sulfamethoxazole Degradation in PMS-Activated Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of CoCu-MOFs and CoCu/LDH
2.3. Characterization
2.4. Degradation Experimental Procedure and Analytical Methods
3. Results and Discussion
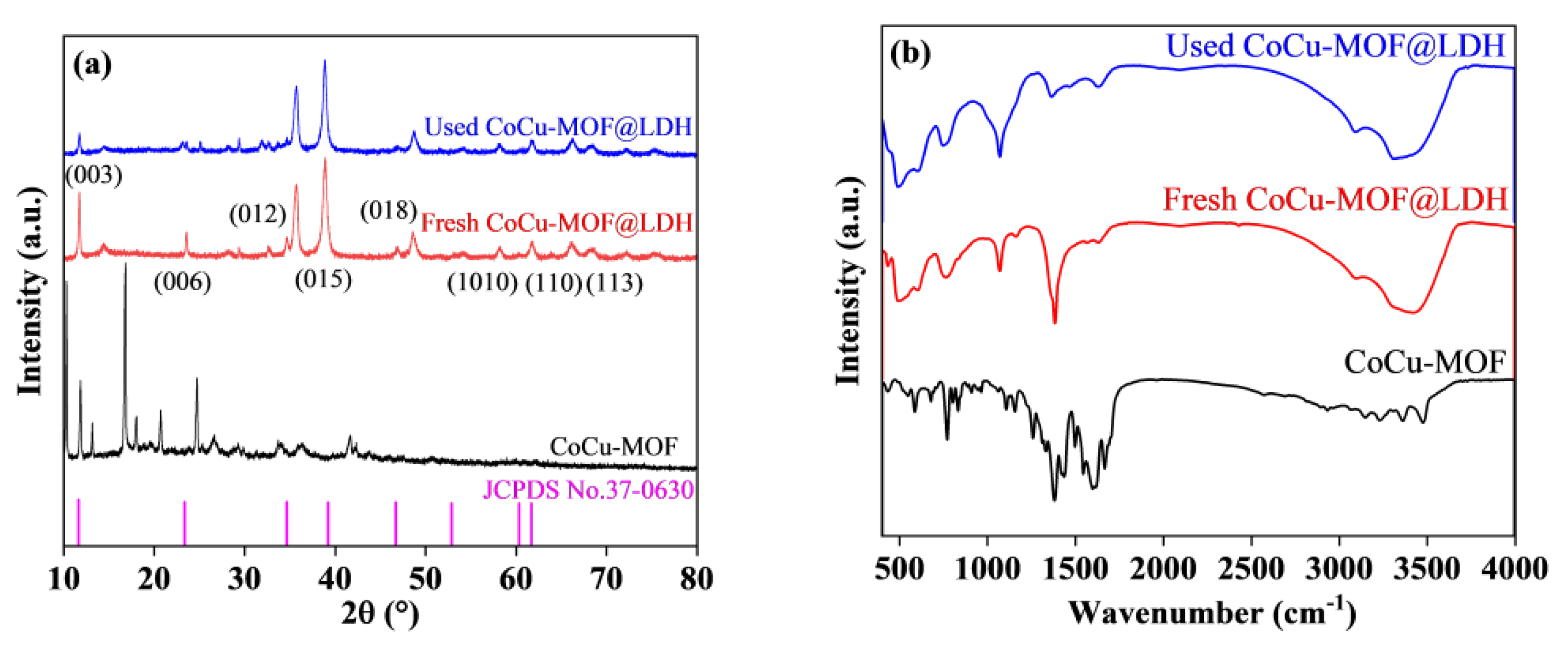
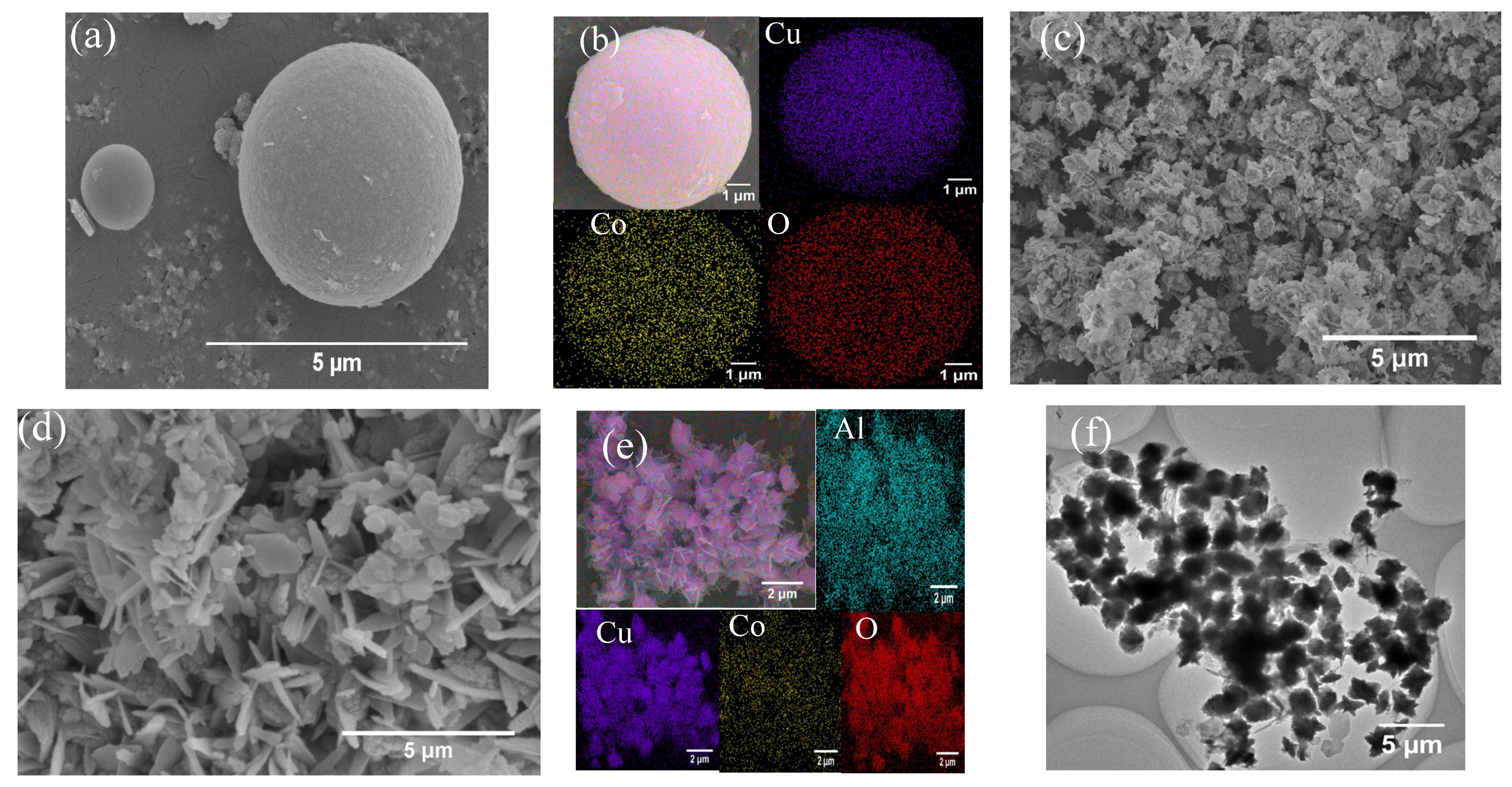
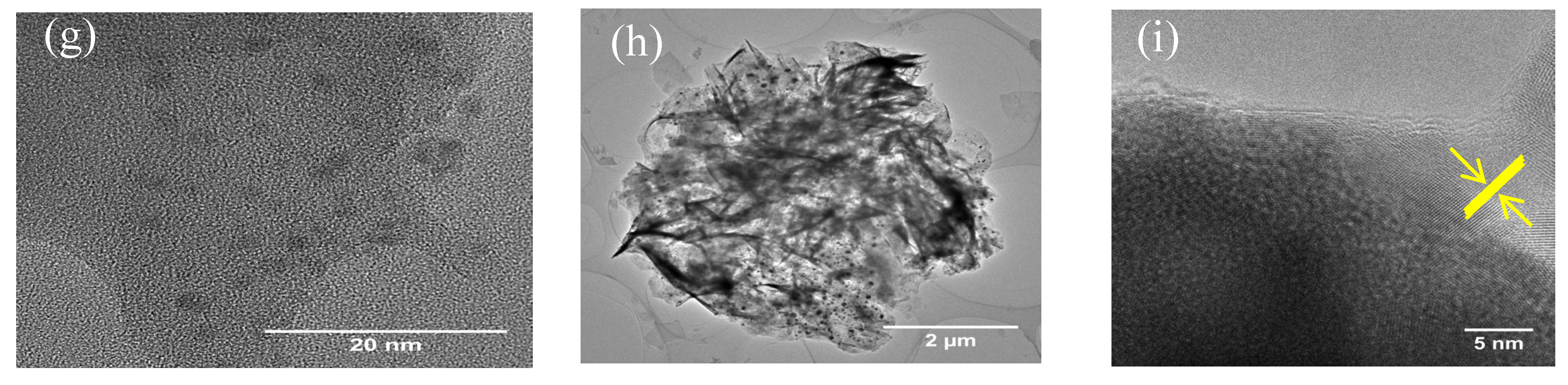
3.1. Catalyst Characterization
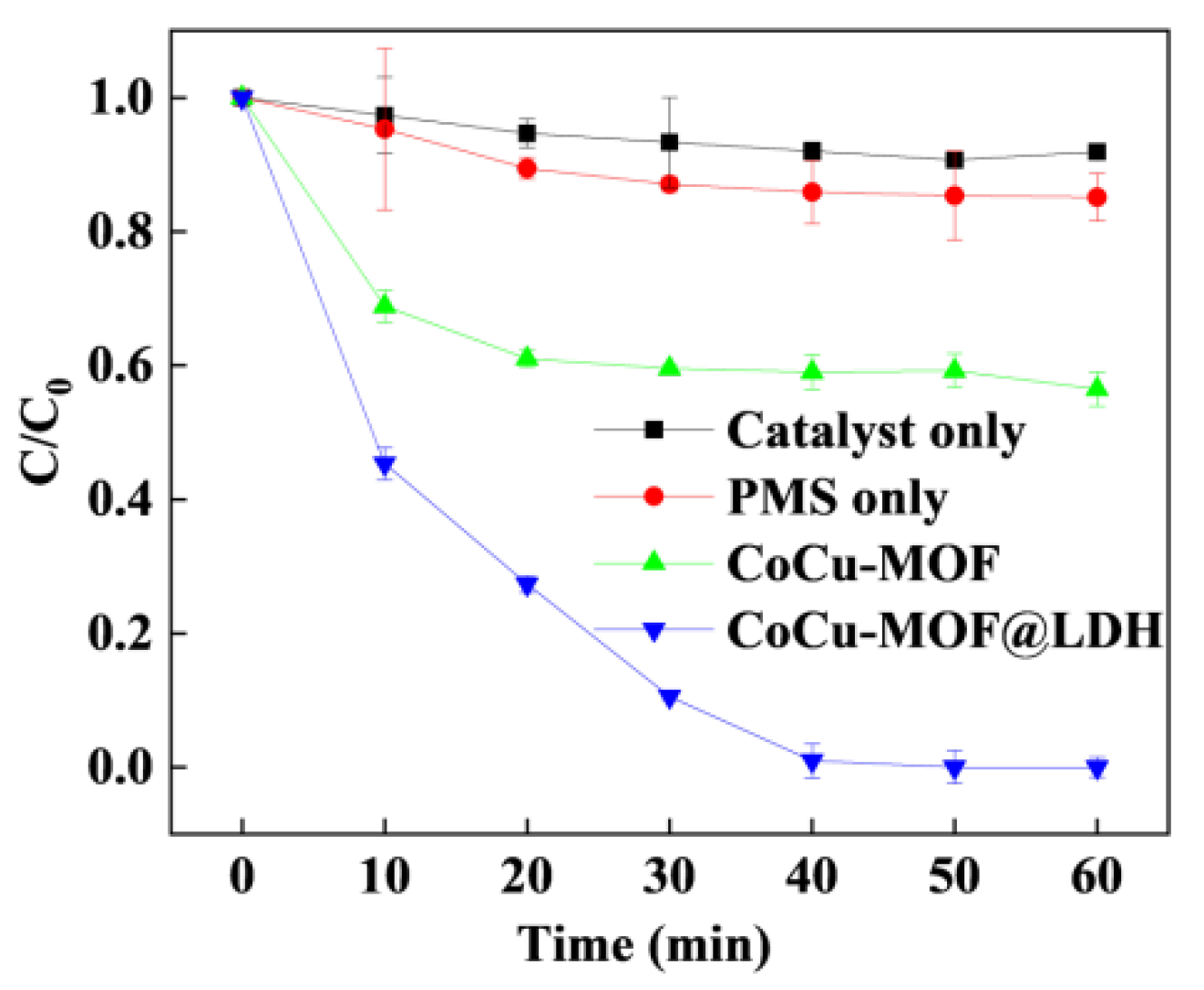
3.2. Catalytic Degradation Activity and Properties
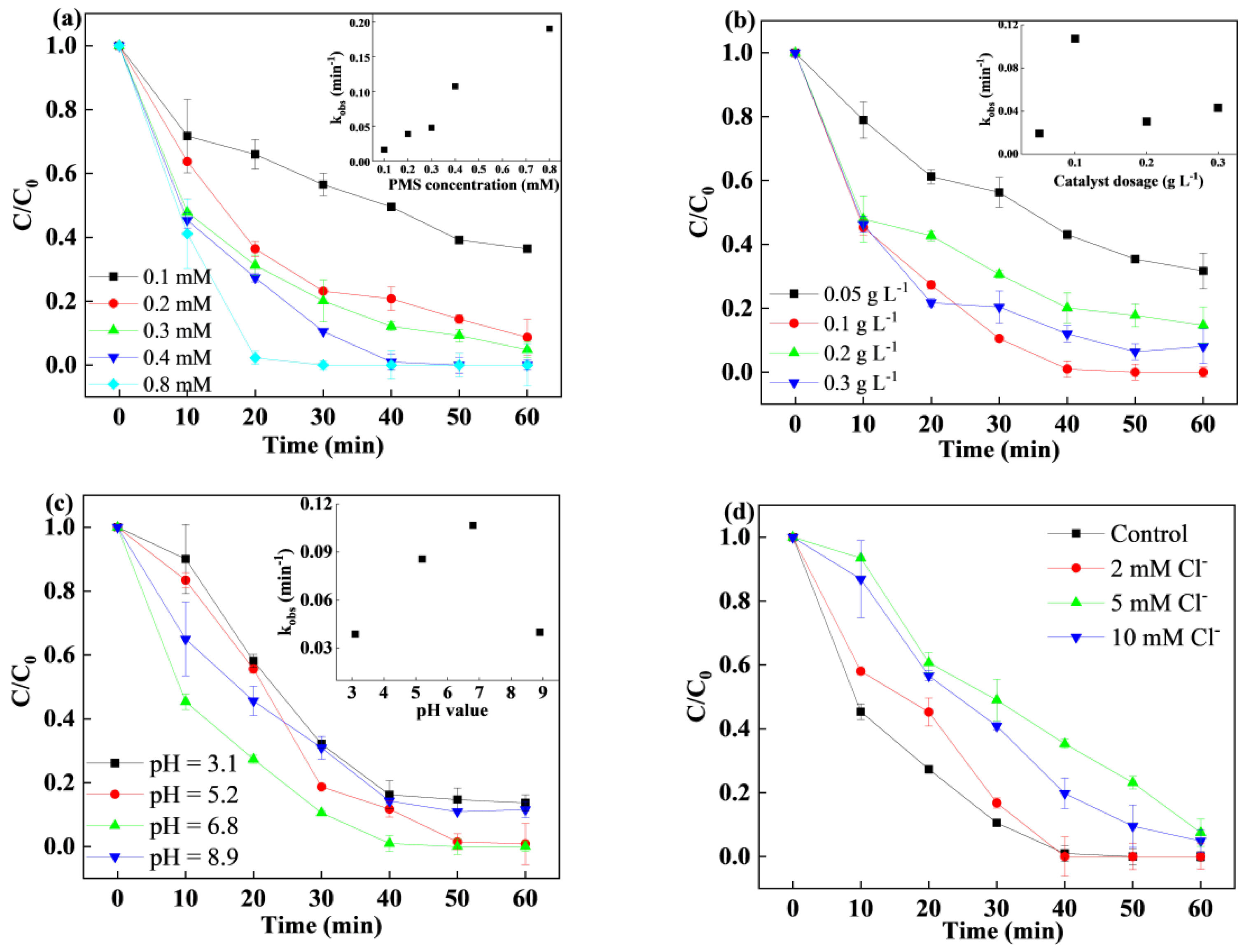
3.3. Effects of Different Parameters on SMX Degradation
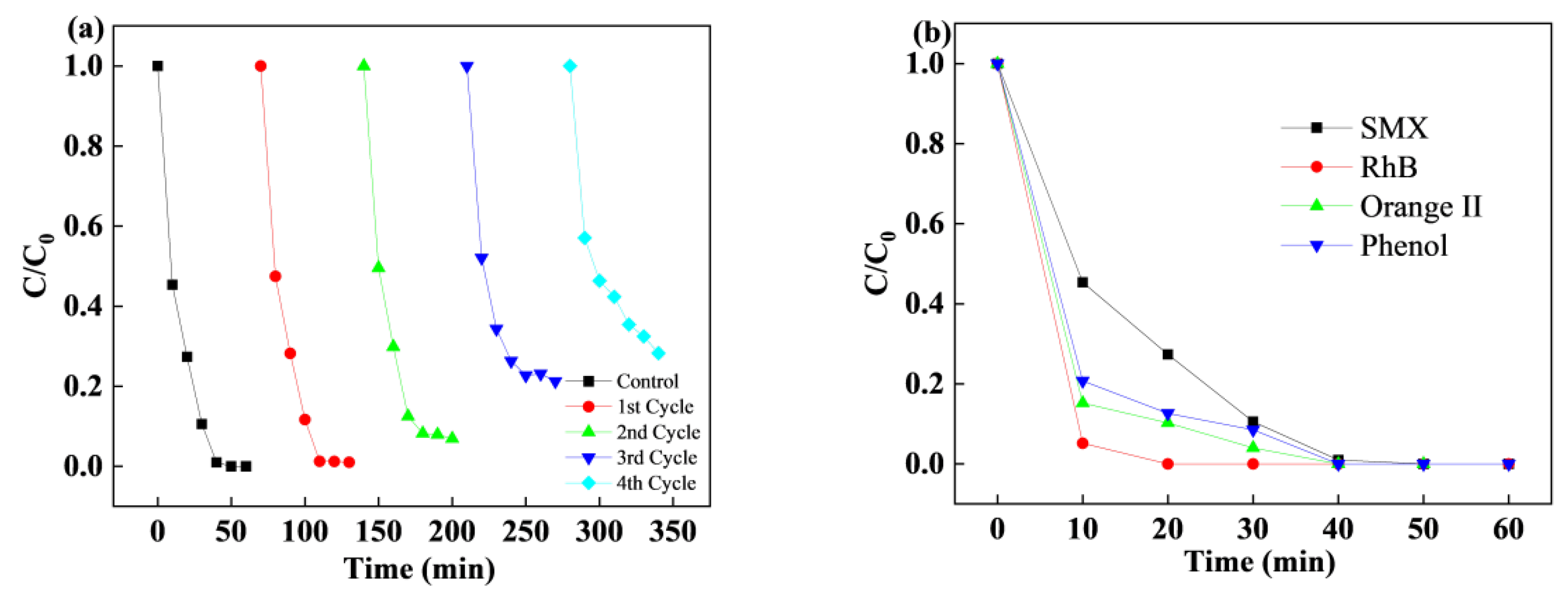
3.4. Stability and Reusability of CoCu/LDH
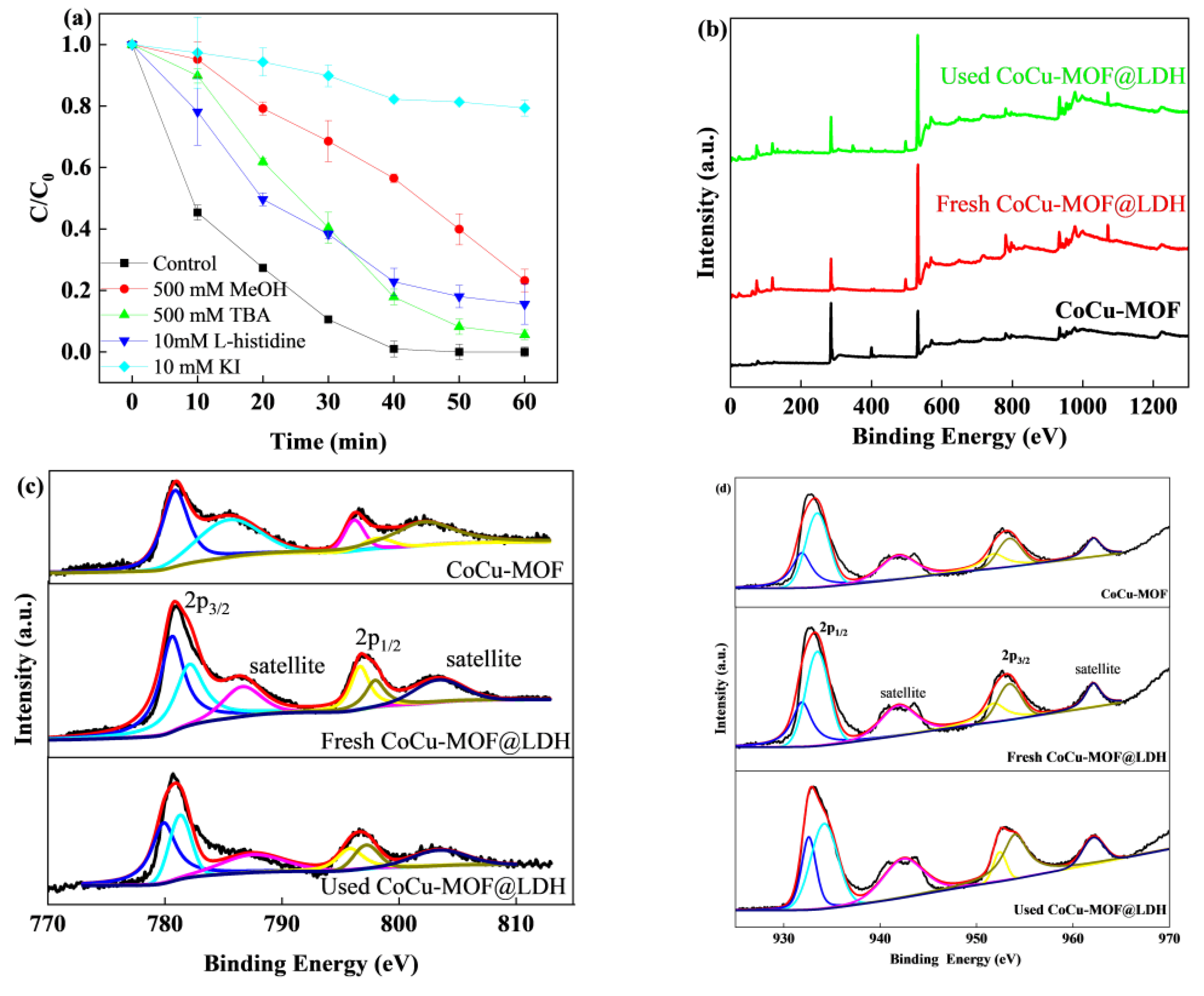
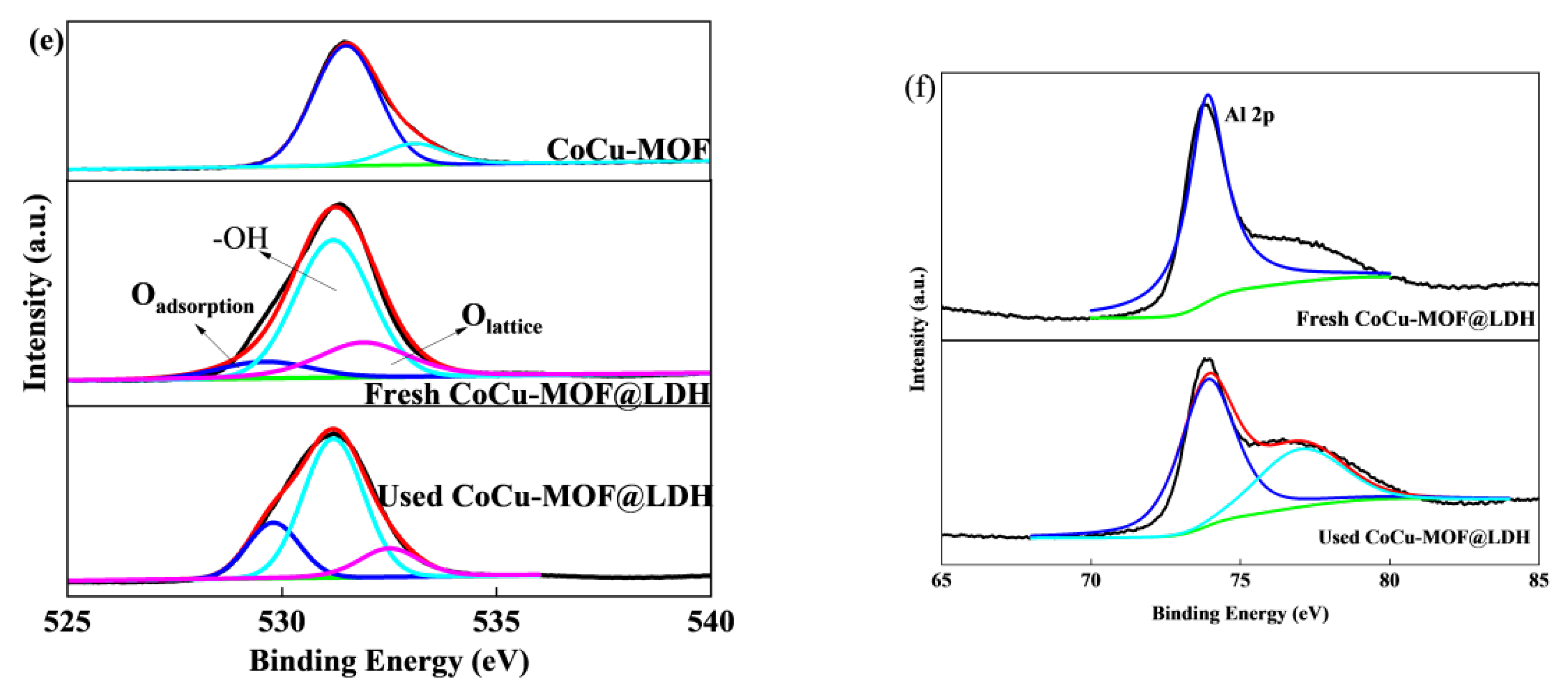
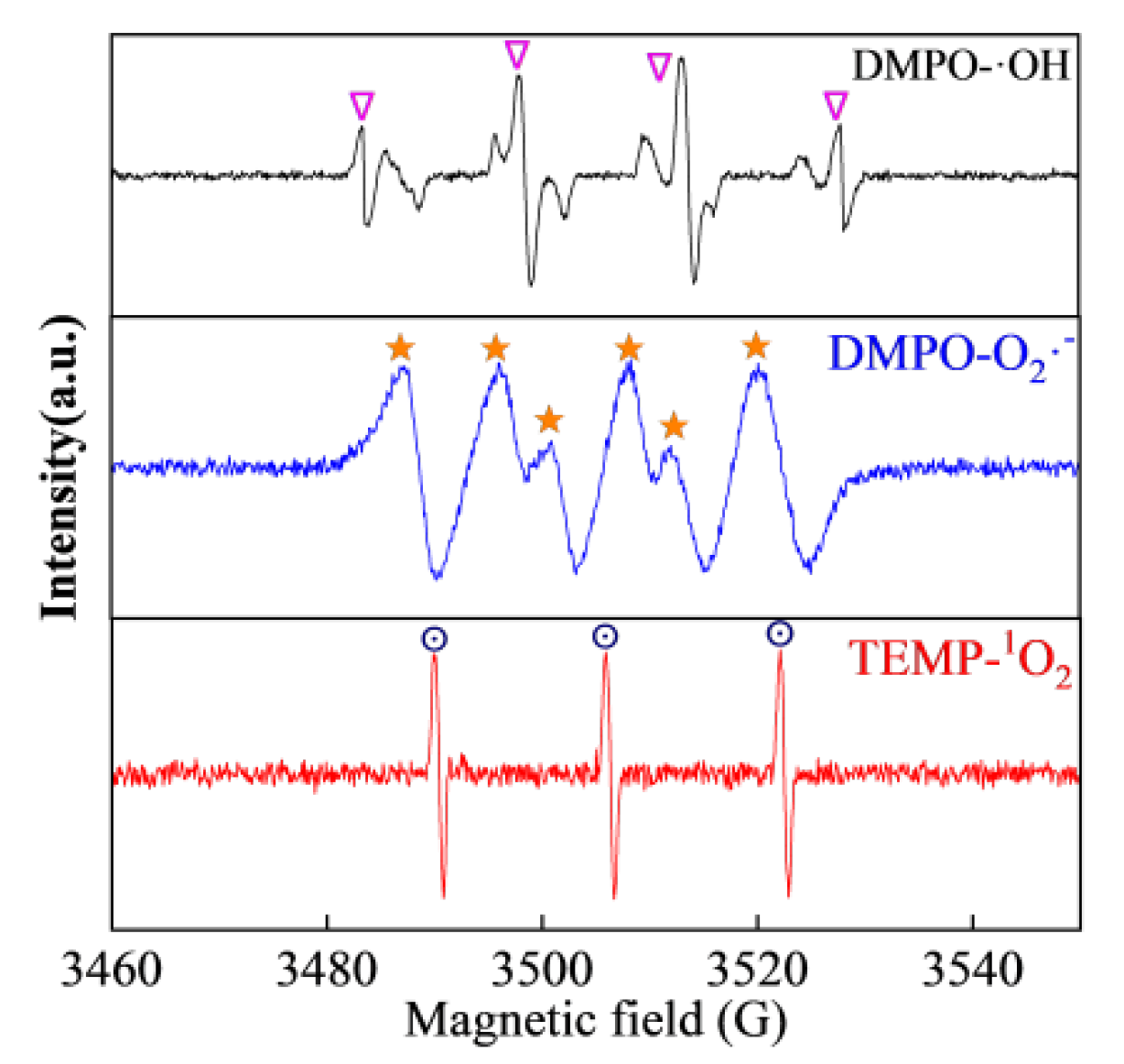
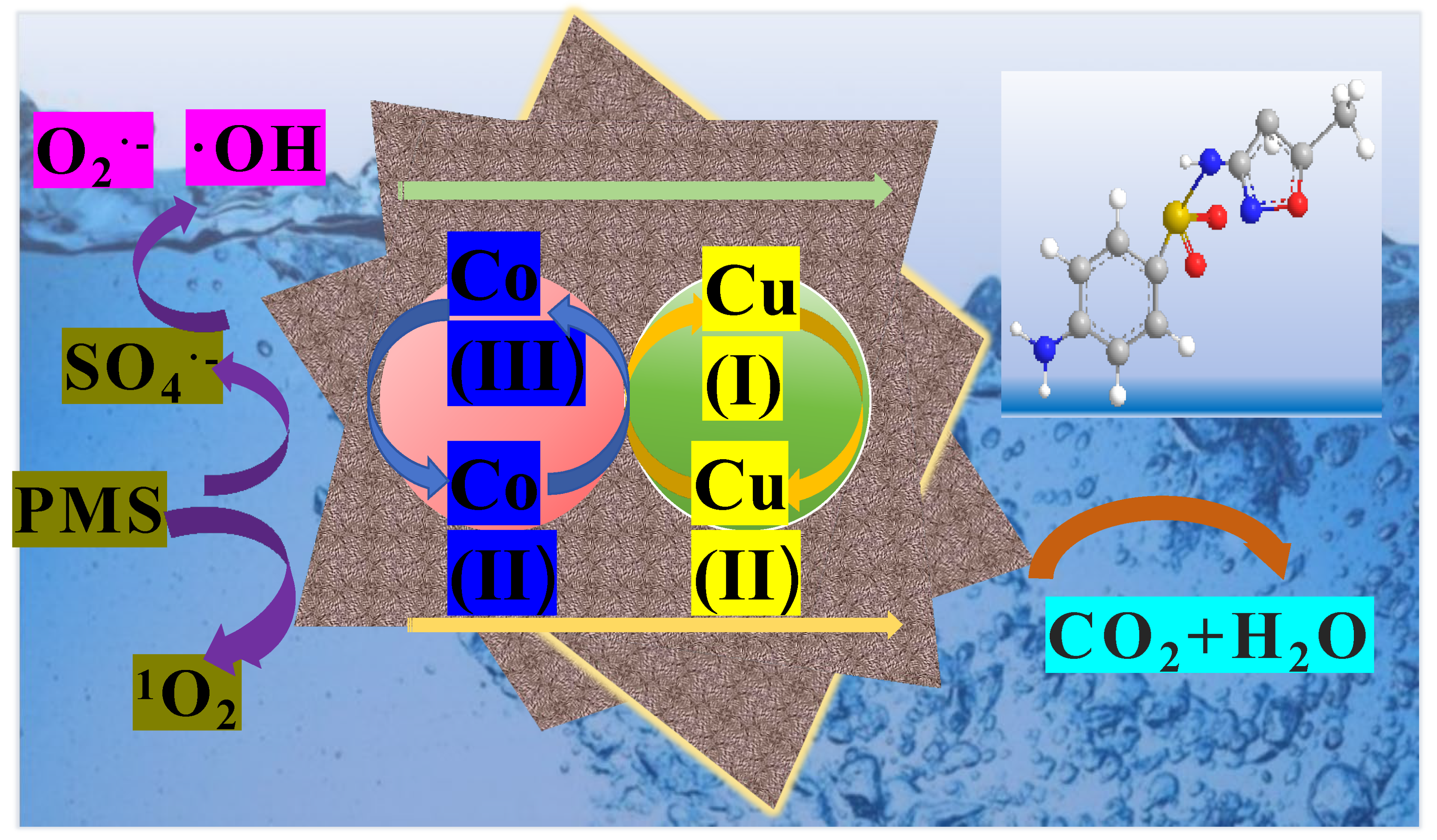
3.5. Degradation Mechanism
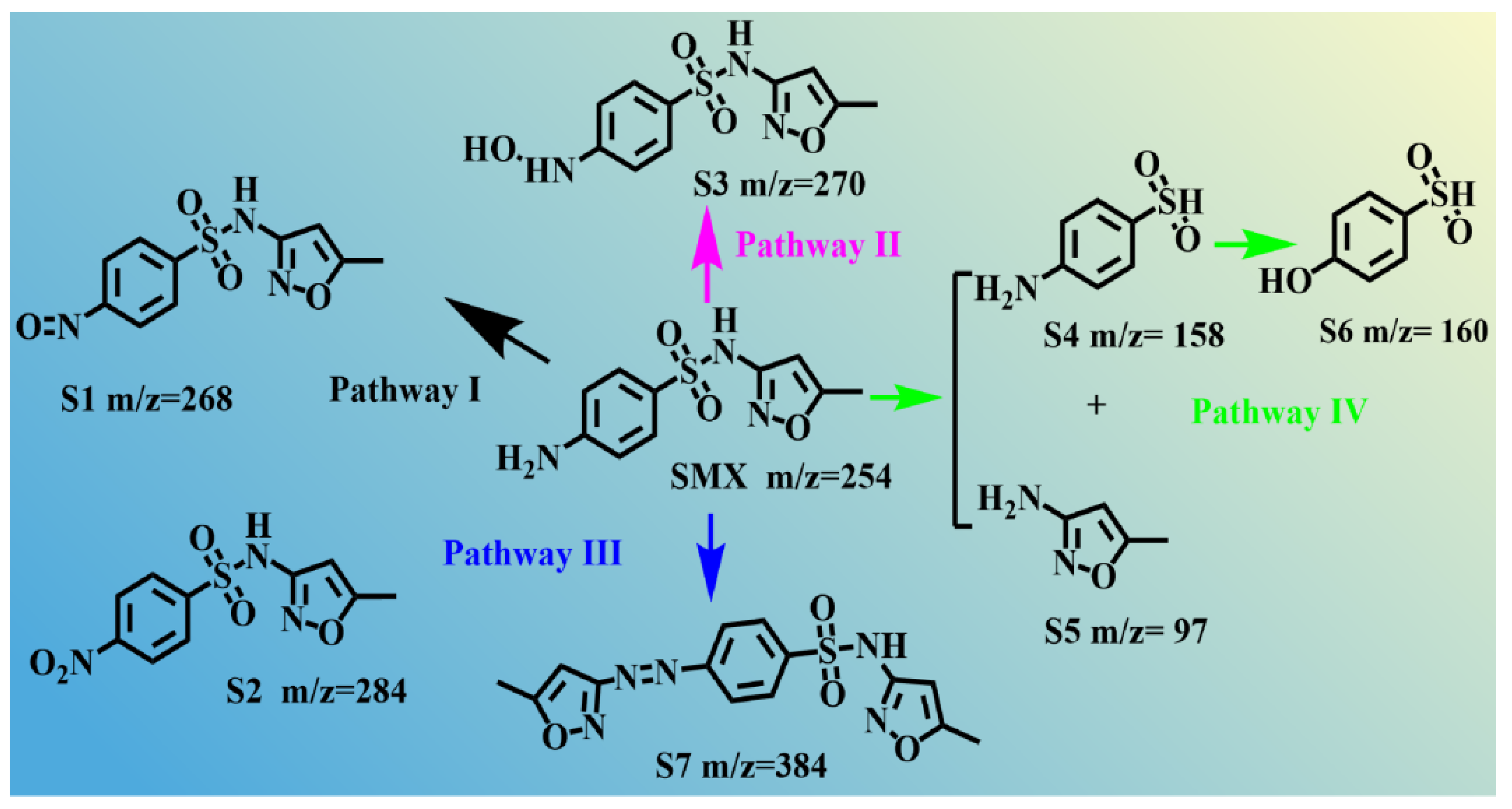
3.6. Possible Degradation Pathways of SMX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, R.; Wang, T.; Wang, Z.; Cheng, W.; Li, L.; Wang, Y.; Xie, X. Activation of peroxymonosulfate with natural pyrite-biochar composite for sulfamethoxazole degradation in soil: Organic matter effects and free radical conversion. J. Hazard. Mater. 2024, 469, 133895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Sun, H.; Li, K.; Wu, Z. Catalytic micro-structured ceramic beads and efficacy evaluation through SMX degradation in PMS-activated systems. Sep. Purif. Technol. 2025, 354, 129060. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; An, X.; Wang, Y.; Gao, M.; Wang, R.; Ke, P.; Cheng, X. Defects and oxygen functional groups on sludge biochar synergistic activation of PMS for degradation of sulfamethoxazole and practical application. J. Environ. Chem. Eng. 2024, 12, 114958. [Google Scholar] [CrossRef]
- Zhang, B.; He, Y.; Shi, W.; Liu, L.; Li, L.; Liu, C.; Lens, P.N.L. Biotransformation of sulfamethoxazole (SMX) by aerobic granular sludge: Removal performance, degradation mechanism and microbial response. Sci. Total. Environ. 2023, 858, 159771. [Google Scholar] [CrossRef]
- He, P.; Gu, C.; Tang, B.; Zhou, Y.; Gan, M.; Zhu, J. Expeditious degradation of SMX by high-valent cobalt-oxo species derived from cobalt-doped C3N5-activated peroxymonosulfate with the assistance of visible light. Sep. Purif. Technol. 2022, 301, 122009. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, G.; Shao, P.; Ren, W.; Li, M.; Hu, Y.; Yang, L.; Shi, H.; Luo, X. A comparison of SMX degradation by persulfate activated with different nanocarbons: Kinetics, transformation pathways, and toxicity. Appl. Catal. B-Environ. 2022, 310, 121345. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Guo, Q.; Wang, X.; Zhang, L.; Gao, X.; Luo, Y. Highly efficient degradation of sulfamethoxazole (SMX) by activating peroxymonosulfate (PMS) with CoFe2O4 in a wide pH range. Sep. Purif. Technol. 2021, 276, 119403. [Google Scholar] [CrossRef]
- Liu, Y.; Han, M.; Li, F.; Zhang, N.; Lu, S.; Liu, X.; Wu, F. Performance and mechanism of SMX removal by an electrolysis-integrated ecological floating bed at low temperatures: A new perspective of plant activity, iron plaque, and microbial functions. J. Hazard. Mater. 2024, 463, 132802. [Google Scholar] [CrossRef]
- Gao, W.; Chen, H.; Liu, X.; Zhang, L.; Ding, H.; Deng, C. Enhanced sulfamethoxazole degradation in aqueous media using a synergistic system with dielectric barrier discharge plasma, biochar-enriched iron concentrate, and persulfate. J. Water. Process. Eng. 2025, 69, 106579. [Google Scholar] [CrossRef]
- Peng, X.; Li, J.; Liu, S.; Zeng, P.; Shan, M. Bimetallic FeCo-MOF as peroxymonosulfate activator for efficient degradation of sulfamethoxazole: Performance and mechanism. Mol. Catal. 2025, 572, 114751. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Wu, L.; Xue, J.; He, X.; Huang, M.; Yang, L. Mechanistic insights into activation of peracetic acid by sludge biogas residue biochar for efficient sulfamethoxazole degradation in aqueous solution. Bio. Technol. 2025, 418, 131857. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, C.; Zhang, L.; Zhang, J.; Cao, X.; Li, H.; Ren, C.; Xu, X.; Shang, Y. Gallic acid enhanced sulfamethoxazole degradation in Cu(II)/peracetic acid system: Key roles of HO• and Cu(III). J. Environ. Chem. Eng. 2024, 12, 114838. [Google Scholar] [CrossRef]
- Du, L.; Huang, D.; Cheng, M.; Xiao, R.; Wang, G.; Zhou, C.; Li, R.; Xu, W.; Huang, H. Construction of biochar supported single cobalt atom catalysts from coffee grounds on peroxymonosulfate activation for sulfamethoxazole degradation. Chem. Eng. J. 2025, 504, 158889. [Google Scholar] [CrossRef]
- Li, S.; Yan, S.; Tong, Z.; Yong, X.; Zhang, X.; Zhou, J. Assessment of photocatalytic activities of layered double hydroxide@petrochemical sludge biochar for sulfamethoxazole degradation. Sep. Purif. Technol. 2025, 355, 129732. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial waste waters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Wang, C.; Ren, S.; Cao, T.; Wang, J.; Peng, R.; Lv, Z.; Zhu, X.; Song, Y.; Na, J.; Mao, Y. Sulfate radicals-based advanced oxidation process (SR-AOPs): The future enabled by MOFs-based nanofibers. Chem. Eng. J. 2024, 496, 154352. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Zheng, H.; Dong, X.; Leong, Y.K.; Chang, J.S. Advances and challenges in the removal of organic pollutants via sulfate radical-based advanced oxidation processes by Fe-based metal-organic frameworks: A review. Sci. Total. Environ. 2024, 926, 171885. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Zhao, G.; Zhang, Z.; Su, P.; Li, Y.; Mu, Y.; Zhou, W. A critical review on the activation of peroxymonosulfate by MOFs for antibiotics degradation: Affecting factor, performance and mechanism. J. Environ. Chem. Eng. 2024, 12, 113634. [Google Scholar] [CrossRef]
- Manickavasagam, G.; He, C.; Lin, K.Y.A.; Saaid, M.; Oh, W.D. Recent advances in catalyst design, performance, and challenges of metal-heteroatom-co-doped biochar as peroxymonosulfate activator for environmental remediation. Environ. Res. 2024, 252, 118919. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Xu, X.; He, S.; Qian, Y. Activation mechanism and strengthening strategies of perovskites activated peroxymonosulfate for organic pollutants degradation: Recent advances and perspective. J. Alloys Compd. 2024, 973, 172898. [Google Scholar]
- Wang, J.; Hu, Z.; Zheng, Z.; Wang, C.; Tang, X. Different performances of zinc/cobalt-based zeolite imidazolate frameworks derivatives to effectively activate peroxymonosulfate for degradation of organic pollutants. Sep. Purif. Technol. 2024, 340, 126783. [Google Scholar] [CrossRef]
- Zhao, W.; Shen, Q.; Nan, T.; Zhou, M.; Xia, Y.; Hu, G.; Zheng, Q.; Wu, Y.; Bian, T.; Wei, T.; et al. Cobalt-based catalysts for heterogeneous peroxymonosulfate (PMS) activation in degradation of organic contaminants: Recent advances and perspectives. J. Alloys Compd. 2023, 958, 170370. [Google Scholar] [CrossRef]
- Brillas, E. Activation of persulfate and peroxymonosulfate for the removal ofherbicides from synthetic and real waters and wastewaters. J. Environ. Chem. Eng. 2023, 11, 110380. [Google Scholar] [CrossRef]
- Wei, J.; Li, F.; Zhou, L.; Han, D.; Gong, J. Strategies for enhancing peroxymonosulfate activation by heterogenous metal-based catalysis: A review. Chinese J. Chem. Eng. 2022, 50, 12–28. [Google Scholar] [CrossRef]
- Lee, J.; Ly, Q.V.; Cui, L.L.; Truong, H.B.; Park, Y.; Hwang, Y. Singlet oxygen dominant-activation by hollow structural cobalt-based MOF/peroxymonosulfate system for micropollutant removal. Chemosphere 2024, 364, 143250. [Google Scholar] [CrossRef]
- Dai, L.; Yan, J.; Jiang, S.; Wang, W.; Tang, H.; Guo, R. Stratified nanoflower bimetallic cobalt/iron alloy derived from CoFe-MOFs as efficient peroxymonosulfate activator for antibiotics degradation: Performance, mechanism, and toxicity. J. Alloys Compd. 2025, 1010, 177031. [Google Scholar] [CrossRef]
- Wang, L.; Ding, X.; Yang, X.; Quan, Y.; Li, P.; Yan, F. Efficient activation of peroxymonosulfate over stable MOF-derived cobalt manganese oxides for rapid removal of bisphenol compounds. Catal. Today. 2024, 435, 114707. [Google Scholar] [CrossRef]
- Shi, Q.; Hou, Y.; Zhu, Q.; Hao, Y. MOF-derived CuCo carbon microspheres assembled with nitrogen-dopedcarbon nanotubes as PMS activator for the efficient degradation of p-nitrophenol. Sep. Purif. Technol. 2025, 354, 129107. [Google Scholar] [CrossRef]
- Su, C.; Zhang, N.; Zhu, X.; Sun, Z.; Hu, X. pH adjustable MgAl@LDH-coated MOFs-derived Co2.25Mn0.75O4 for SMX degradation in PMS activated system. Chemosphere 2023, 339, 139672. [Google Scholar] [CrossRef]
- Liu, D.; Gu, W.; Zhou, L.; Wang, L.; Zhang, J.; Liu, Y.; Lei, J. Recent advances in MOF-derived carbon-based nanomaterials for environmental applications in adsorption and catalytic degradation. Chem. Eng. J. 2022, 427, 131503. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Li, X.; Huo, P.; Shi, W. Design of metal-organic frameworks (MOFs)-based photocatalyst for solar fuel production and photo-degradation of pollutants. Chinese J. Catal. 2021, 42, 872–903. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, Y.R.; Yoon, S.; Choi, J.; Kim, H.; Lim, K.S.; Ha, S.J.; Park, J.A.; Kim, H.O. Application of metal-organic frameworks for photocatalytic degradation of microplastics: Design, challenges, and scope. Chemosphere 2024, 366, 143518. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, I.; Bukhari, A.; Shaheen, A.; Nazir, A.; Gilani, E.; Zain, H.; Muhammad, S.; Hussain, S. Activation of persulfate, peroxymonosulfate, and peroxydisulfate using metal-organic framework catalysts for degradation of antibiotics: Identification, quantification, interconversion, and transformation of reactive species. J. Environ. Chem. Eng. 2024, 12, 112838. [Google Scholar] [CrossRef]
- Sharmin, A.; Bhuiyan, M.A.; Pramanik, B.K. A hybrid LMO MOF catalytic membrane with PMS activation for efficient degradation of pharmaceutical micropollutants and nanoplastics removal. Sep. Purif. Technol. 2025, 360, 130961. [Google Scholar] [CrossRef]
- Lv, G.; Ye, H.; Lai, C.; Ma, D.; Zhou, X.; Huo, X.; Deng, H.; Tang, L.; Tong, T.; Yan, M.; et al. Ultrathin LDH-coated SnO2 catalysts linked by hydrogen bonding for PMS activation via interlayer high-speed electron transfer channels: Performance and mechanism. J. Environ. Chem. Eng. 2025, 13, 115029. [Google Scholar] [CrossRef]
- Mi, X.; Ma, R.; Pu, X.; Fu, X.; Geng, M.; Qian, J. FeNi-layered double hydroxide (LDH)@biochar composite for activation of peroxymonosulfate (PMS) towards enhanced degradation of doxycycline (DOX): Characterizations of the catalysts, catalytic performances, degradation pathways and mechanisms. J. Clean. Prod. 2022, 378, 134514. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, X.; Yang, Q.; Wang, D.; Duan, A.; Pan, S.; Zheng, J. Ultrathin In2O3-Ov/ZnAl-LDH-Ov S-scheme heterojunctions with oxygen-rich vacancies for efficient photocatalytic peroxymonosulfate activation for the degradation of nitenpyram. J. Environ. Chem. Eng. 2025, 13, 115193. [Google Scholar] [CrossRef]
- Xin, Q.; Liu, S.; Lu, S.; Chen, Z.; Han, P.; Xin, S.; Wang, Q.; Liu, G.; Zhou, C.; Xin, Y.; et al. Surface-bound sulfate radical-dominated degradation of sulfamethoxazole in the CuFeAl-LDH/peroxymonosulfate system: The abundant hydroxyl groups enhancing efficiency mechanism. Chem. Eng. J. 2023, 471, 144453. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, D.; Yan, X.; Wang, Y.; Zhang, J.; Hu, X.; Zhou, M.; Liu, P. Enhanced peroxymonosulfate activation for antibiotic and heavy metal removal using ZIF-67-derived magnetic Ni/Co-LDH@NC: Bimetallic electronic synergy and oxygen vacancy effects. Appl. Catal. B-Environ. 2025, 362, 124753. [Google Scholar] [CrossRef]
- Gu, J.; Wei, G.; He, Y.; Zhang, Y.; Xiong, D.; Zhang, L.; Zhou, Y.; He, S. Red mud-based Fe2O3/Cu-Al LDH prepared through mechanochemical synthesis as effective peroxymonosulfate activator for lomefloxacin hydrochloride degradation via radical-nonradical cooperation mechanisms. J. Water. Process. Eng. 2024, 68, 106517. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, H.; Wu, D.; Shang, X.; Lv, M.; Yu, H. Single Co atoms incorporated on Fe-Ni-LDH with enriched oxygen vacancies for efficient peroxymonosulfate activation: Investigation of electron transfer mechanism. J. Environ. Chem. Eng. 2024, 12, 113723. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Hu, C.; Wang, L.; Wang, Q.; Li, L.; Hu, X. High-performance, stable CuCo-LDH/sepiolite composite with innovative peroxymonosulfate activation for norfloxacin destruction. J. Environ. Chem. Eng. 2024, 12, 114128. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Hou, X.; Bu, W.; Lu, S.; Song, X.; Zhou, C.; Wang, Q.; Xin, S.; Liu, G.; et al. Insights into the efficient removal and mechanism of NiFeAl-LDH with abundant hydroxyl to activate peroxymonosulfate for sulfamethoxazole wastewater. J. Colloid. Interface Sci. 2025, 678, 920–936. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, M.; Zhang, L.; Song, J.; Huang, P.; Zhang, W.; Gao, D.; Hu, X.; Wang, L.; Guo, Q. Efficient degradation of norfloxacin via MnCo-LDH/sepiolite activating peroxymonosulfate: Performance, mechanism and degradation pathway. Mater. Res. Bull. 2024, 179, 112974. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Do, Q.H.; Chen, C.W.; Chen, W.H.; Bui, X.T.; Dong, C.D. Decoration of marigold flower-like CoMo LDH on reinforced cow manure-derived biochar as an effective peroxymonosulfate activator for degradation of Bisphenol A in water. J. Environ. Chem. Eng. 2024, 12, 114699. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, Y.; Li, D. Efficient degradation of aniline by Fe@LDHs activating persulfate process: Performance, mechanism and application potential. J. Water. Process. Eng. 2024, 64, 105497. [Google Scholar] [CrossRef]
- Lee, E.; Jagan, G.; Choi, J.U.; Cha, B.; Yoon, Y.; Saravanakumar, K.; Park, C.M. Peroxymonosulfate-activated photocatalytic degradation of norfloxacin via a dual Z-scheme g-C3N5/BiVO4/CoFe-LDH heterojunction: Operation and mechanistic insights. Chem. Eng. J. 2024, 494, 152961. [Google Scholar] [CrossRef]
- Yin, Y.; Pan, S.; Lu, J.; Asif, A.H.; Cui, S.; Wang, S.; Sun, H. Ruthenium decorated nickel-iron layered double hydroxides (NiFe-LDH) for promoting peroxymonosulfate activation and atrazine degradation. J. Environ. Chem. Eng. 2024, 12, 112996. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Z.; Sun, W.; Li, T.; Zhang, J.; Qiu, Z.; Younas, M. Degradation of Tetracycline through peroxymonosulfate activation with Co/Fe-LDH modified magnetic hydrochar: Synergistic effect and low toxicity. Sep. Purif. Technol. 2024, 351, 128023. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, S.; Chen, D.; Feng, Y.; Zhang, W.; Sun, S.; Lv, G.; Zhang, W.; Zhang, J.Z.; Ding, H. Ultrathin BiOCl-OV/CoAl-LDH S-scheme heterojunction for efficient photocatalytic peroxymonosulfate activation to boost Co (IV)=O generation. Water Res. 2024, 258, 121774. [Google Scholar] [CrossRef]
- Xie, M.; Liang, M.; Liu, C.; Xu, Z.; Yu, Y.; Xu, J.; You, S.; Wang, D.; Rad, S. Peroxymonosulfate activation by CuMn-LDH for the degradation of bisphenol A: Effect, mechanism and pathway. Ecotoxicol. Environ. Saf. 2024, 270, 115929. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Gan, X.; Shang, J.; Cheng, X. High-performance, stable CoNi LDH@Ni foamcomposite membrane with innovative peroxymonosulfate activation for 2,4-dichlorophenol destruction. J. Environ. Sci. 2024, 141, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Jiang, B.; Zhang, L.; Sun, Y.; Yang, X.; Zhang, L. 1D/2D CoNi-LDH/ZnIn2S4 S-scheme heterojunction for effectively tetracycline degradation under photocatalytic-peroxymonosulfate activation system: DFT calculations and mechanism insights. Chem. Eng. J. 2023, 478, 147535. [Google Scholar] [CrossRef]
- Du, M.; Zhang, H.; Dong, S.; Wang, B.; Zhang, Y. S doping in CoOHS modified hollow fiber membranes boosting electron transfer between activated PMS and pollutants in a continuous flow reactor. Sep. Purif. Technol. 2024, 335, 126175. [Google Scholar] [CrossRef]
- Wang, R.; Ren, X.; Guo, W. CeO2@LDH decorated 3D porous module with mortise-tenon structure: Activation of peroxymonosulfate for ultra fast removal of tetracycline. J. Clean. Prod. 2023, 428, 139452. [Google Scholar] [CrossRef]
- Shang, K.; Morent, R.; Geyter, N.D.; Wang, Y.; Yang, Z. Plasma catalytic degradation of sulfamethoxazole in water with Fe/Mn-LDO catalyst: Performance and mechanism. Sep. Purif. Technol. 2025, 360, 131145. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, X.; Chang, L.; Chai, H.; Huang, Y. The MOF/LDH derived heterostructured Co3O4/MnCo2O4 composite for enhanced degradation of levofloxacin by peroxymonosulfate activation. Sep. Purif. Technol. 2022, 294, 121182. [Google Scholar] [CrossRef]
- Ramachandran, R.; Sakthivel, T.; Li, M.; Shan, H.; Xu, Z.; Wang, F. Efficient degradation of organic dye using Ni-MOF derived NiCo-LDH as peroxymonosulfate activator. Chemosphere 2021, 271, 128509. [Google Scholar] [CrossRef]
- Li, H.; Su, L.; Zheng, J.; Lu, S.; Yang, Z.; Wang, C.; Xu, S.; Zhou, Q.; Tang, J.; Huang, M.; et al. MOFs derived carbon supporting CuCo nanospheres as efficient catalysts of peroxymonosulfate for rapid removal of organic pollutant. Chem. Eng. J. 2023, 451, 139114. [Google Scholar] [CrossRef]
- García, A.; Sanchez, N.C.; Palomino, G.T.; Cabello, C.P. MOF derived porous Fe-Cu@carbon catalyst for the degradation of bisphenol A through a persulfate-based advanced oxidation process. Microporous Mesoporous Mater. 2025, 381, 113366. [Google Scholar] [CrossRef]
- Li, B.; Hu, X.; Gu, Y.; Zhang, W.; Xu, W.; Yang, H.; Ye, S.; Yang, Z.; Liu, N.; Tan, X. Complexation between butyl xanthate and Cu enhanced peroxydisulfate activation and cation redox cycle by Fe-Cu-LDH/biochar. J. Environ. Chem. Eng. 2024, 12, 112466. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Liu, X.; Ji, M.; Jiang, F. Densely Stacked CoCu-MOFs Coated with CuAl/LDH Enhance Sulfamethoxazole Degradation in PMS-Activated Systems. Nanomaterials 2025, 15, 432. https://doi.org/10.3390/nano15060432
Zhong X, Liu X, Ji M, Jiang F. Densely Stacked CoCu-MOFs Coated with CuAl/LDH Enhance Sulfamethoxazole Degradation in PMS-Activated Systems. Nanomaterials. 2025; 15(6):432. https://doi.org/10.3390/nano15060432
Chicago/Turabian StyleZhong, Xin, Xiaojun Liu, Meihuan Ji, and Fubin Jiang. 2025. "Densely Stacked CoCu-MOFs Coated with CuAl/LDH Enhance Sulfamethoxazole Degradation in PMS-Activated Systems" Nanomaterials 15, no. 6: 432. https://doi.org/10.3390/nano15060432
APA StyleZhong, X., Liu, X., Ji, M., & Jiang, F. (2025). Densely Stacked CoCu-MOFs Coated with CuAl/LDH Enhance Sulfamethoxazole Degradation in PMS-Activated Systems. Nanomaterials, 15(6), 432. https://doi.org/10.3390/nano15060432





