Recent Advances in Nanostructured Conversion-Type Cathodes: Fluorides and Sulfides
Abstract
1. Introduction
2. Brief Overview of Conversion-Type Cathode Materials
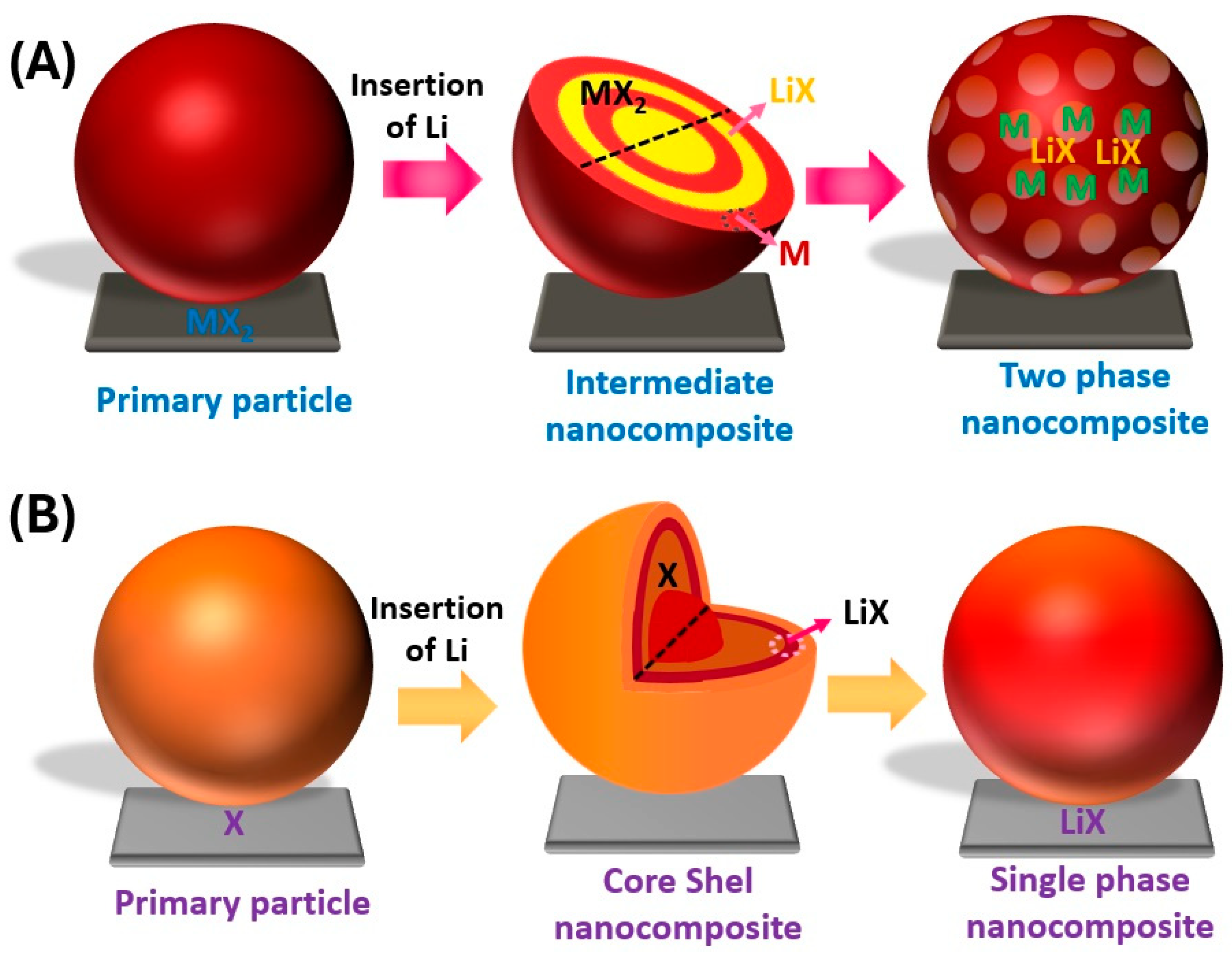
2.1. Limitations of Conversion Cathode
2.2. Strategies to Improve the Limitations of Conversion Cathode
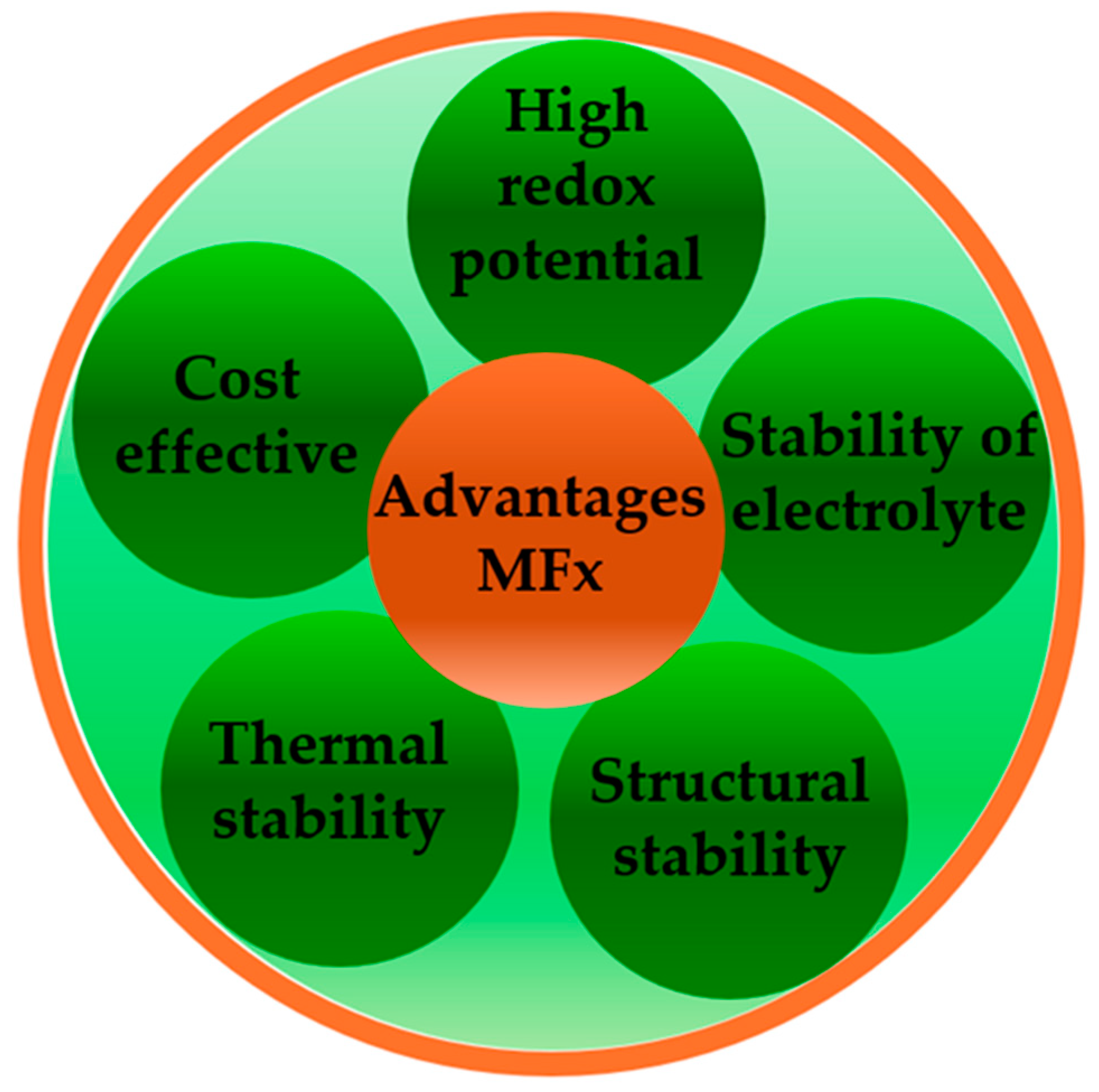
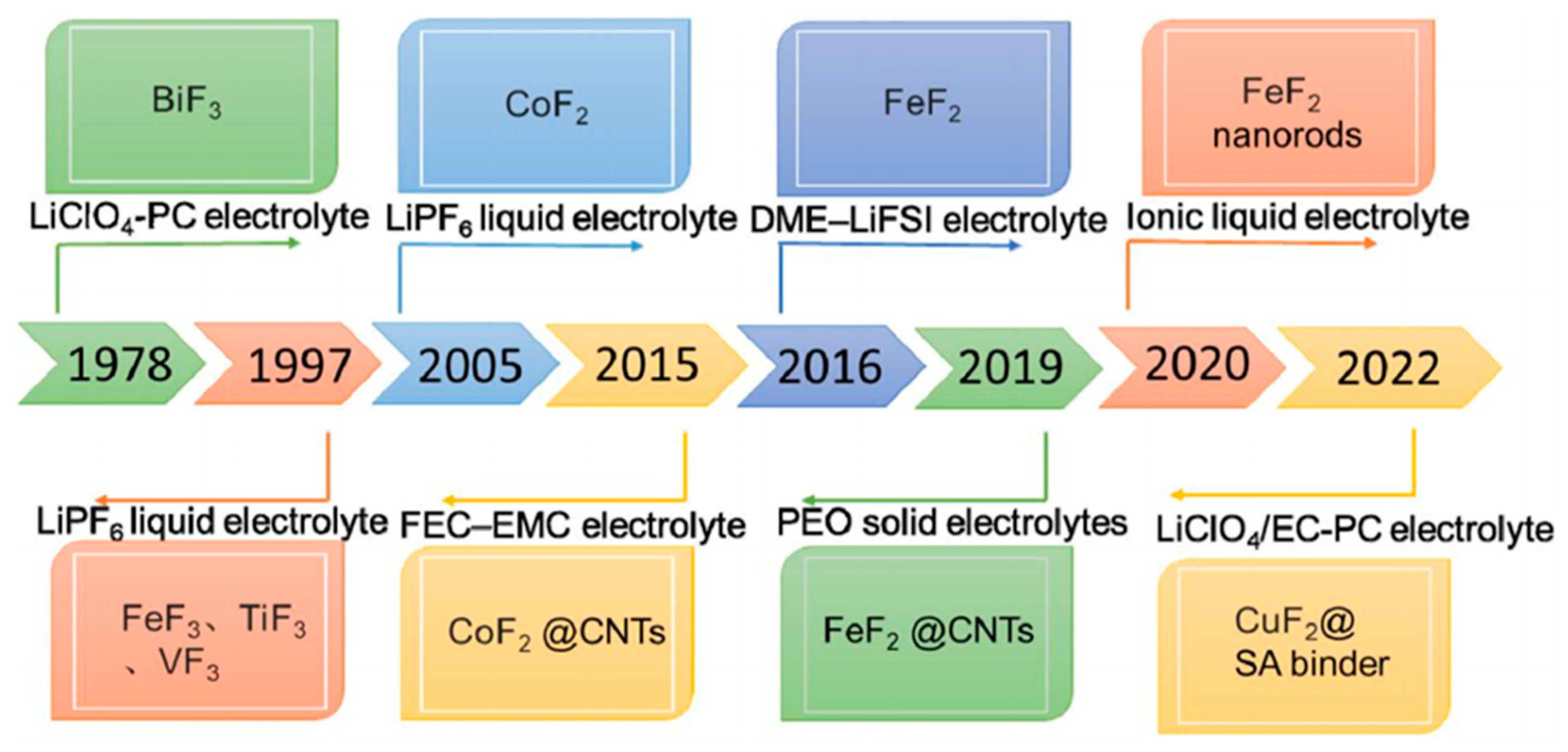
3. Fluoride Compounds
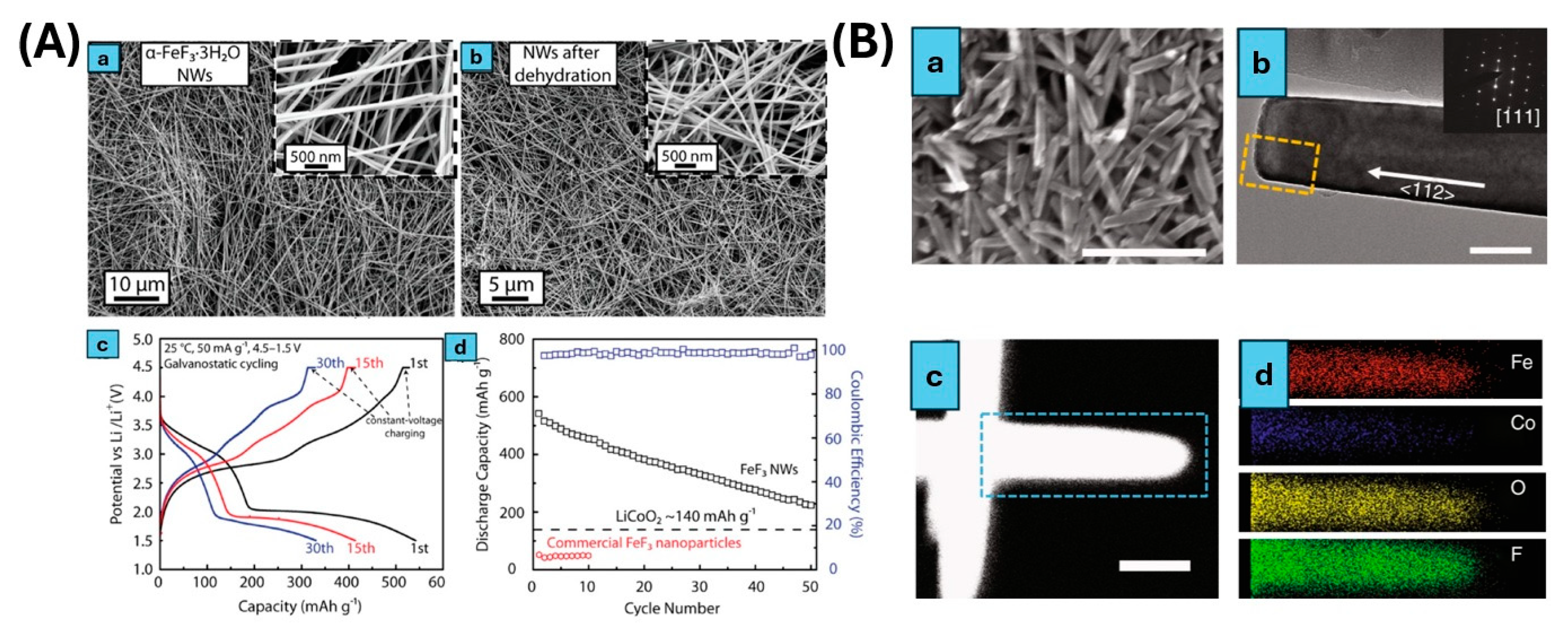
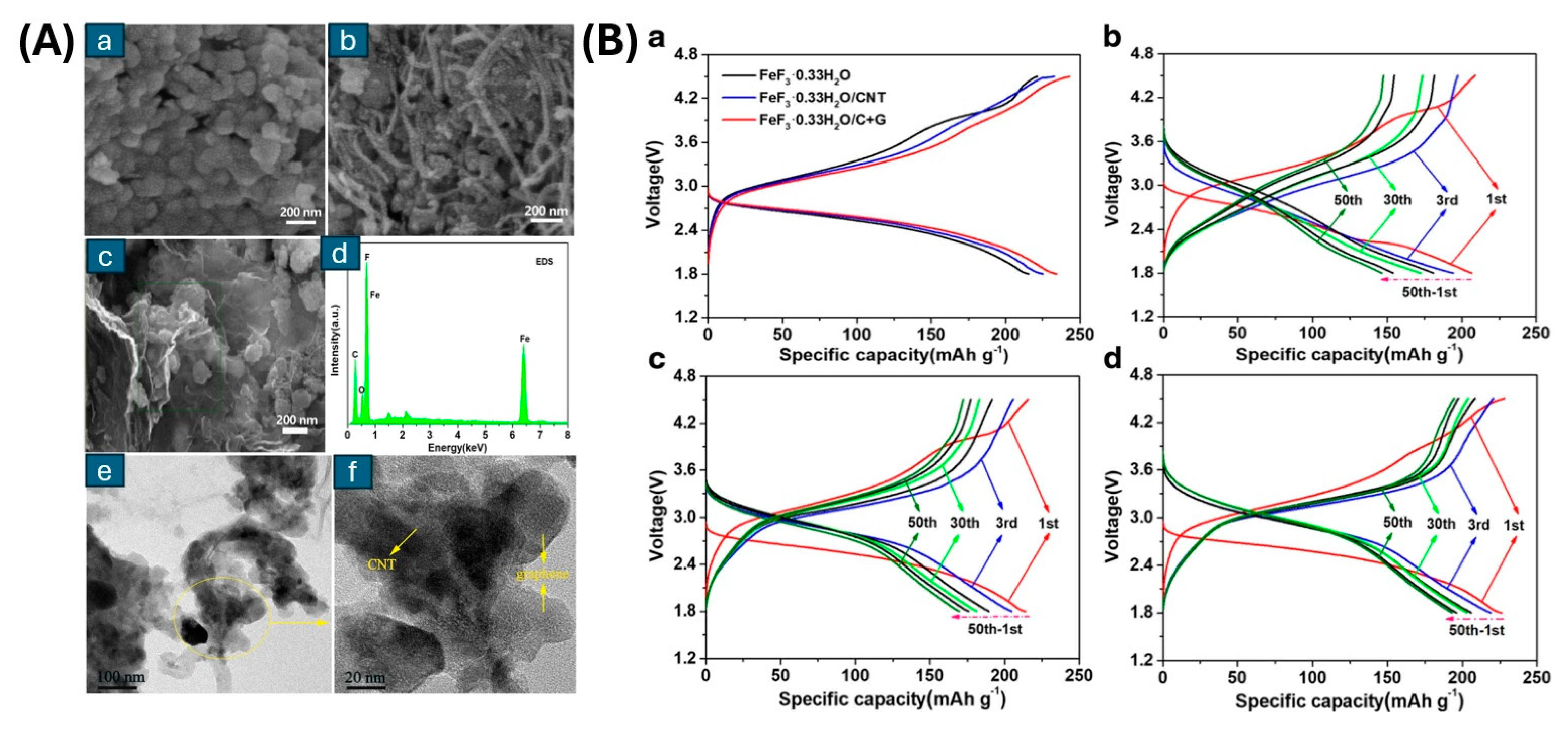
3.1. Iron Trifluoride (FeF3)
3.2. Iron Fluoride (FeF2)
3.3. Cupper Fluoride (CuF2)
3.4. Cobalt Fluoride
3.5. Other Metal Fluorides
4. Sulphur and Sulfide Compounds
- Polysulfide Confinement
- Enhanced Conductivity
- Increased Surface Area and Porosity
- Carbon Nanotubes (CNTs): These can be utilized as conductive cathode additives to develop a network that enhances the overall performance and helps in polysulfide confinement.
- Graphene: The 2D structural properties of graphene offer a wide surface area and high conductivity, which can trap polysulfides effectively and also provide efficient electron pathways.
- Mesoporous Carbon: These materials have a large surface area and can offer a host for sulfur, helping to confine polysulfides and protect them from dissolving in electrolytes.
- Metal–Organic Frameworks (MOFs): These can offer a highly porous structure and have the potential to adsorb and confine polysulfides.
4.1. Sulfur and Carbon-Based Composites
4.2. Sulfur and Graphene Composites
4.3. Sulfur and CNT Composites
4.4. Sulfur and Metallic Oxide Composites
4.5. Metal Oxide-Based Nanocomposite
5. A Realistic Look at Conversion Cathode
5.1. Scalability of the Nano Structured Conversion Cathode
5.2. Long Time Cyclability of Conversion Cathode
6. Conclusions and Future Direction
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Thomas Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Hasan, K.; Tom, N.; Yuce, M.R. Navigating Battery Choices in IoT: An Extensive Survey of Technologies and Their Applications. Batteries 2023, 9, 580. [Google Scholar] [CrossRef]
- Vasant Kumar, R.; Sarakonsri, T. A Review of Materials and Chemistry for Secondary Batteries. In High Energy Density Lithium Batteries: Materials Engineering Applications; Wiley-VCH: Weinheim, Germany, 2010; Volume 53, pp. 53–80. [Google Scholar]
- Comanescu, C. Ensuring Safety and Reliability: An Overview of Lithium-Ion Battery Service Assessment. Batteries 2025, 11, 6. [Google Scholar] [CrossRef]
- Kaur, G.; Gates, B.D. Review—Surface Coatings for Cathodes in Lithium-Ion Batteries: From Crystal Structures to Electrochemical Performance. J. Electrochem. Soc. 2022, 169, 043504. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Eshetu, G.G.; Laruelle, S.; Grugeon, S.; Zaghib, K.; Julien, C.; Mauger, A.; Guyomard, D.; Rojo, T.; et al. From Solid-Solution Electrodes and the Rocking-Chair Concept to Today’s Batteries. Angew. Chem. 2020, 132, 542–546. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Massé, R.C.; Liu, C.; Li, Y.; Mai, L.; Cao, G. Energy storage through intercalation reactions: Electrodes for rechargeable batteries. Natl. Sci. Rev. 2017, 4, 26–53. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Liga, B.; Mario, M.; Gints, K. A review of the degradation mechanisms of NCM cathodes and corresponding mitigation strategies. J. Energy Storage 2023, 73, 108875. [Google Scholar]
- Wang, L.; Chen, H.; Zhang, Y.; Liu, J.; Peng, L. Research Progress in Strategies for Enhancing the Conductivity and Conductive Mechanism of LiFePO4 Cathode Materials. Molecules 2024, 29, 5250. [Google Scholar] [CrossRef]
- Islam, M.; Ur, S.C.; Yoon, M.S. Improved performance of porous LiFePO4/C as lithium battery cathode processed by high energy milling comparison with conventional ball milling. Curr. Appl. Phys. 2015, 15, 541–546. [Google Scholar] [CrossRef]
- Pal, U.; Roy, B.; Hasanpoor, M.; Ilbeygi, H.; Mendes, T.; Kerr, R.; Vazhapully, L.; Song, C.; Wang, D.; Boot-Handford, M.; et al. Developing a High-Performing Spinel LiMn2O4 Cathode Material with Unique Morphology, Fast Cycling and Scaled Manufacture. Bateries Supercaps 2024, 7, e202400072. [Google Scholar] [CrossRef]
- Ma, Y.; Qing, S.; Liu, H.; Ma, C.; Yu, Y.; Yu, C.; Wang, L. Conversion-type cathode materials for high energy density solid-state lithium batteries. J. Energy Chem. 2025, 100, 409–425. [Google Scholar] [CrossRef]
- Wu, F.; Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Zhao, W.; Choi, W.; Yoon, W.-S. Nanostructured Electrode Materials for Rechargeable Lithium-Ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 195–219. [Google Scholar] [CrossRef]
- Afia, S.E.; Cano, A.; Arévalo, P.; Jurado, F. Rechargeable Li-Ion Batteries, Nanocomposite Materials and Applications. Batteries 2024, 10, 413. [Google Scholar] [CrossRef]
- Pitchai, R.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Nanostructured cathode materials: A key for better performance in Li-ion batteries. J. Mater. Chem. 2011, 21, 11040. [Google Scholar] [CrossRef]
- Uddin, M.J.; Alaboina, P.K.; Cho, S.-J. Nanostructured cathode materials synthesis for lithium-ion batteries. Mater. Today Energy 2017, 5, 138–157. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Zhang, X.; Li, L.; Wu, F. Advanced cathode materials for lithium-ion batteries using nanoarchitectonics. Nanoscale Horiz. 2016, 1, 423–444. [Google Scholar] [CrossRef]
- Mahmood, N.; Tang, T.Y.; Hou, Y.L. Nanostructured anode materials for lithium-ion batteries: Progress, challenge and perspective. Adv. Energy Mater. 2016, 6, 1600374. [Google Scholar] [CrossRef]
- Reddy, M.V.; Rao, G.V.S.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Chen, G.; Yan, L.T.; Luo, H.M.; Guo, S.J. Nanoscale engineering of heterostructured anode materials for boosting lithium-ion storage. Adv. Mater. 2016, 28, 7580–7602. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Z.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, L.; Lou, X.W. Nanostructured Conversion-type Anode Materials for Advanced Lithium-Ion Batteries. Chem 2018, 4, 972–996. [Google Scholar] [CrossRef]
- Olbrich, L.F.; Xiao, A.W.; Pasta, M. Conversion-type fluoride cathodes: Current state of the art. Curr. Opin. Electroche. 2021, 30, 100779. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Hua, Y.; Yang, Q.; Holze, R.; Mijowska, E.; Chu, P.K.; Chen, X. Recent advances of metal fluoride compounds cathode materials for lithium-ion batteries: A review. Mater. Futures 2024, 3, 032101. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Chen, G.; Liu, H.; Han, Y.; Zhai, S.; Zhang, L.; Pan, Y.; Li, Q.; Liu, Q. Pseudocapacitance-Enhanced Storage Kinetics of 3D Anhydrous Iron (III) Fluoride as a Cathode for Li/Na-Ion Batteries. Nanomaterials 2022, 12, 4041. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Z.; Zou, J.; Gao, P.; Niu, X.; Li, H.; Chen, L. Li-free Cathode Materials for High Energy Density Lithium Batteries. Joule 2019, 3, 2086–2102. [Google Scholar] [CrossRef]
- Klein, F.; Jache, B.; Bhide, A.; Adelhelm, P. Conversion reactions for sodium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 15876–15887. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X.; et al. Approaching the capacity limit of lithium cobalt oxide in lithium-ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Zhou, H.; Xin, F.; Pei, B.; Whittingham, M.S. What Limits the Capacity of Layered Oxide Cathodes in Lithium Batteries? ACS Energy Lett. 2019, 4, 1902–1906. [Google Scholar] [CrossRef]
- Liu, S.; Su, J.; Zhao, J.; Chen, X.; Zhang, C.; Huang, T.; Wu, J.; Yu, A. Unraveling the capacity fading mechanisms of LiNi0.6Co0.2Mn0.2O2 at elevated temperatures. J. Power Sources 2018, 393, 92–98. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Wang, F.; Robert, R.; Chernova, N.A.; Pereira, N.; Omenya, F.; Badway, F.; Hua, X.; Ruotolo, M.; Zhang, R.; Wu, L.; et al. Conversion Reaction Mechanisms in Lithium-Ion Batteries: Study of the Binary Metal Fluoride Electrodes. J. Am. Chem. Soc. 2011, 133, 18828–18836. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Liu, Y.; Huang, Y. Confined selenium within porous carbon nanospheres as cathode for advanced Li–Se batteries. Nano Energy 2014, 9, 229–236. [Google Scholar] [CrossRef]
- Wang, X.; Gu, W.; Lee, J.T.; Nitta, N.; Benson, J.; Magasinski, A.; Schauer, M.W.; Yushin, G. Carbon Nanotube–CoF2 Multifunctional Cathode for Lithium-Ion Batteries: Effect of Electrolyte on Cycle Stability. Small 2015, 11, 5164–5173. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y. Flexible Tellurium-Based Electrode for High-Performance Lithium-Tellurium Battery. Nanomaterials 2021, 11, 2903. [Google Scholar] [CrossRef]
- Badway, F.; Cosandey, F.; Pereira, N.; Amatucci, G.G. Carbon Metal Fluoride Nanocomposites: High-Capacity Reversible Metal Fluoride Conversion Materials as Rechargeable Positive Electrodes for Li Batteries. J. Electrochem. Soc. 2003, 150, A1318–A1327. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Misra, S.; Nelson, J.; Toney, M.F.; Cui, Y. High-Capacity Micrometer-Sized Li2S Particles as Cathode Materials for Advanced Rechargeable Lithium-Ion Batteries. J. Am. Chem. Soc. 2012, 134, 15387–15394. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Xu, Y.; Zhu, Y.; Bigio, D.; Wang, C. Lithium–tellurium batteries based on tellurium/porous carbon composite. J. Mater. Chem. A 2014, 2, 12201–12207. [Google Scholar] [CrossRef]
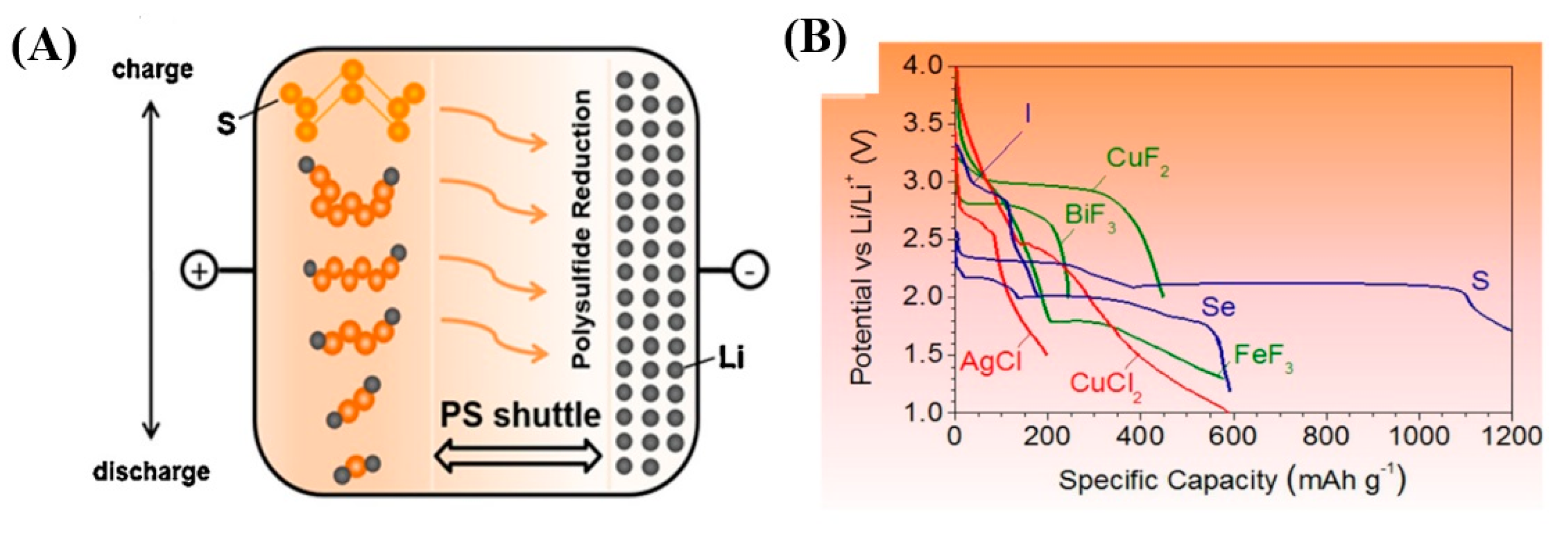
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Hua, X.; Robert, R.; Du, L.-S.; Wiaderek, K.M.; Leskes, M.; Chapman, K.W.; Chupas, P.J.; Grey, C.P. Comprehensive Study of the CuF2 Conversion Reaction Mechanism in a Lithium Ion Battery. J. Phys. Chem. C 2014, 118, 15169–15184. [Google Scholar] [CrossRef]
- Felker, L.K.; Kelmers, A.D. Chemistry of Metal Chloride Complexes in Aprotic Systems. Sep. Sci. Technol. 1983, 18, 1439–1453. [Google Scholar] [CrossRef]
- Shimoda, K.; Shikano, M.; Murakami, M.; Sakaebe, H. Capacity fading mechanism of conversion-type FeF3 electrode: Investigation by electrochemical operando nuclear magnetic resonance spectroscopy. J. Power Sources 2020, 477, 228772. [Google Scholar] [CrossRef]
- Gao, J.; Lowe, M.A.; Kiya, Y.; Abruna, H.D. Effects of Liquid Electrolytes on the Charge–Discharge Performance of Rechargeable Lithium/Sulfur Batteries: Electrochemical and in-Situ X-ray Absorption Spectroscopic Studies. J. Phys. Chem. C 2011, 115, 25132–25137. [Google Scholar] [CrossRef]
- Liu, P.; Vajo, J.J.; Wan, J.S.; Li, W.; Liu, J.Z. Thermodynamics and Kinetics of the Li/FeF3 Reaction by Electrochemical Analysis. J. Phys. Chem. C. 2012, 116, 6467–6473. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Kang, Y.; Zhao, Y.; Tian, Y.; Wang, X.; Tan, Y.; Liang, Z.; Wozny, J.; Li, T.; et al. Side Reactions/Changes in Lithium-Ion Batteries: Mechanisms and Strategies for Creating Safer and Better, Batteries. Adv. Mater. 2024, 36, 2401482. [Google Scholar] [CrossRef]
- Shi, Y.-L.; Shen, M.-F.; Xu, S.-D.; Zhuang, Q.-C.; Jiang, L.; Qiang, Y.-H. Electrochemical impedance spectroscopy investigation of the FeF3/C cathode for lithium-ion batteries. Solid State Ion. 2012, 222–223, 23–30. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; O’Neill, L.; Cleaver, T.; Walus, S. Lithium-Sulfur Battery Technology Readiness and Applications—A Review. Energies 2017, 10, 1937. [Google Scholar] [CrossRef]
- Zoua, M.; Wena, W.; Lia, J.; Lina, Y.; Laia, H.; Huang, Z. Nano-crystalline FeOOH mixed with SWNT matrix as a superior anode material for lithium batteries. J. Energy Chem. 2014, 23, 513–518. [Google Scholar] [CrossRef]
- Wu, F.; Kim, H.; Magasinski, A.; Lee, J.T.; Lin, H.-T.; Yushin, G. Harnessing Steric Separation of Freshly Nucleated Li2S Nanoparticles for Bottom-Up Assembly of High-Performance Cathodes for Lithium-Sulfur and Lithium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1400196. [Google Scholar] [CrossRef]
- Badway, F.; Mansour, A.N.; Pereira, N.; Al-Sharab, J.F.; Cosandey, F.; Plitz, I.; Amatucci, G.G. Structure and electrochemistry of copper fluoride nanocomposites utilizing mixed conducting matrices. Chem. Mater. 2007, 19, 4129–4141. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Geder, J.; Hoster, H.E.; Jossen, A.; Garche, J.; Yu, D.Y.W. Impact of active material surface area on thermal stability of LiCoO2 cathode. J. Power Sources 2014, 257, 286–292. [Google Scholar] [CrossRef]
- Ma, R.; Lu, Z.; Wang, C.; Wang, H.E.; Yang, S.; Xi, L.; Chung, J.C. Large-scale fabrication of graphene-wrapped FeF3nanocrystals as cathode materials for lithium-ion batteries. Nanoscale 2013, 5, 6338–6343. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Gwon, H.; Kim, J.; Kang, K. Fabrication of FeF3 Nanoflowers on CNT branches and their application to high power lithium rechargeable batteries. Adv. Mater. 2010, 22, 5260–5264. [Google Scholar] [CrossRef]
- Fan, X.; Luo, C.; Lamb, J.; Zhu, Y.; Xu, K.; Wang, C. PEDOT Encapsulated FeOF Nanorod Cathodes for High Energy Lithium-Ion Batteries. Nano Lett. 2015, 15, 7650–7656. [Google Scholar] [CrossRef]
- Zhou, H.; Ruther, R.E.; Adcock, J.; Zhou, W.; Dai, S.; Nanda, J. Controlled Formation of Mixed Nanoscale Domains of High Capacity Fe2O3–FeF3 Conversion Compounds by Direct Fluorination. ACS Nano 2015, 9, 2530–2539. [Google Scholar] [CrossRef]
- Tao, X.; Wang, J.; Ying, Z.; Cai, Q.; Zheng, G.; Gan, Y.; Huang, H.; Xia, Y.; Liang, C.; Zhang, W.; et al. Strong Sulfur Binding with Conducting Magnéli-Phase TinO2n–1 Nanomaterials for Improving Lithium–Sulfur Batteries. Nano Lett. 2014, 14, 5288–5294. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Ge, X.; Ma, J.; Zhang, Z.; Li, Q.; Wang, C.; Yin, L. Reduced graphene oxide wrapped MOFs-derived cobalt-doped porous carbon polyhedrons as sulfur immobilizers as cathodes for high performance lithium sulfur batteries. Nano Energy 2016, 23, 15–26. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Yi, L.; Guo, H.; Tan, J.; Shu, H.; Yang, X.; Yang, Z.; Wang, X. Excellent cycle performance of Co-doped FeF3/C nanocomposite cathode material for lithium-ion batteries. J. Mater. Chem. 2012, 22, 17539. [Google Scholar] [CrossRef]
- Su, J.; Nong, W.; Song, H.; Li, Y.; Wang, C. Enhanced Li-storage capability and cyclability of iron fluoride cathodes by non-equivalent cobalt doping. J. Alloys Compd. 2021, 870, 159395. [Google Scholar] [CrossRef]
- Ali, G.; Rahman, G.; Chung, K.Y. Cobalt-doped pyrochlore-structured iron fluoride as a highly stable cathode material for lithium-ion batteries. Electrochim. Acta 2017, 238, 49–55. [Google Scholar] [CrossRef]
- Fan, X.; Hu, E.; Ji, X.; Zhu, Y.; Han, F.; Hwang, S.; Liu, J.; Bak, S.; Ma, Z.; Gao, T.; et al. High energy-density and reversibility of iron fluoride cathode enabled via an intercalation-extrusion reaction. Nat. Commun. 2018, 9, 2324. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Turcheniuk, K.; Ren, X.; Magasinski, A.; Song, A.Y.; Xiao, Y.; Kim, D.; Yushin, G. Cycle stability of conversion-type iron fluoride lithium battery cathode at elevated temperatures in polymer electrolyte composites. Nat. Mater. 2019, 18, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Nam, K.-W.; Seo, D.-H.; Hong, J.; Kim, H.; Gwon, H.; Kang, K. Energy storage in composites of a redox couple host and a lithium-ion host. Nano Today 2012, 7, 168–173. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, Y.; Luo, C.; Gao, T.; Suo, L.; Liou, S.-C.; Xu, K.; Wang, C. In situ lithiated FeF3/C nanocomposite as high energy conversion-reaction cathode for lithium-ion batteries. J. Power Sources 2016, 307, 435–442. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, Y.; Luo, C.; Suo, L.; Lin, Y.; Gao, T.; Xu, K.; Wang, C. Pomegranate-Structured Conversion-Reaction Cathode with a Built-in Li Source for High-Energy Li-Ion Batteries. ACS Nano 2016, 10, 5567–5577. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Luo, L.; Chen, L.; Cheng, H.; Orenstein, R.; Zhang, X. Nanofiber Materials for Lithium-Ion Batteries. Adv. Fiber Mater. 2023, 5, 1141–1197. [Google Scholar] [CrossRef]
- Lee, K.T.; Cho, J. Roles of nanosize in lithium reactive nanomaterials for lithium ion batteries. Nano Today 2011, 6, 28–41. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, R.; Hu, X.; Chen, Y.; Xiao, C.; He, F.; Zhang, S.; Chen, J.; Hu, G. Insights into the electrochemical performance of metal fluoride cathodes for lithium batteries. Energy Mater. 2022, 2, 200027. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, S.; Wen, J.; Song, Z.; Wang, T.; Zhang, R.; Fan, X.; Luo, W. Metal chloride cathodes for next-generation rechargeable lithium batteries. iScience 2024, 27, 109557. [Google Scholar] [CrossRef] [PubMed]
- Gmitter, A.J.; Badway, F.; Rangan, S.; Bartynski, R.A.; Halajko, A.; Pereira, N.; Amatucci, G.G. Formation, dynamics, and implication of solid electrolyte interphase in high voltage reversible conversion fluoride nanocomposites. J. Mater. Chem. 2010, 20, 4149–4161. [Google Scholar] [CrossRef]
- Gmitter, A.J.; Gural, J.; Amatucci, G.G. Electrolyte development for improved cycling performance of bismuth fluoride nanocomposite positive electrodes. J. Power Sources 2012, 217, 21–28. [Google Scholar] [CrossRef]
- Liu, A.; Yuan, H.; Wang, Y.; Liu, Y.; Luo, J.; Nai, J.; Tao, X. Reviewing metal fluorides as the cathode materials for high performance Li batteries. Inf. Funct. Mater. 2024, 1, 26–67. [Google Scholar] [CrossRef]
- Li, L.; Meng, F.; Jin, S. High-Capacity Lithium-Ion Battery Conversion Cathodes Based on Iron Fluoride Nanowires and Insights into the Conversion Mechanism. Nano Lett. 2012, 12, 6030–6037. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Wei, S.; Wang, X.; Liu, M.; Hu, H. Iron fluoride microspheres by titanium dioxide surface modification as high-capacity cathode of Li-ion batteries. J. Alloys Comp. 2017, 719, 331–340. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, H.; Ru, H.; Shi, Y.; Zhuang, Q.; Cui, Y.; Ju, Z.; Cui, Y. Nanosized FeF3·0.33H2O as Cathode Material for High-Performance Li-Ion Batteries. J. Electrochem. Soc. 2021, 168, 030501. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Reversible Three-Electron Redox Behaviors of FeF3 Nanocrystals as High-Capacity Cathode-Active Materials for Li-Ion Batteries. J. Phys. Chem. C 2010, 114, 3190–3195. [Google Scholar] [CrossRef]
- Fu, L.; Xu, Z.; Zhu, J.; Yang, W.; Li, D.; Zhou, L. Electrochemical properties of carbon-wrapped FeF3 nanocomposite as cathode material for lithium-ion battery. Electrochim. Acta 2018, 281, 88–98. [Google Scholar]
- Lu, L.; Li, S.; Li, J.; Lan, L.; Lu, Y.; Xu, S.; Huang, S.; Pan, C.; Zhao, F. High-Performance Cathode Material of FeF3·0.33H2O Modified with Carbon Nanotubes and Graphene for Lithium-Ion Batteries. Nanoscale Res. Lett. 2019, 14, 100. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Feng, W. Metal Fluoride Cathode Materials for Lithium Rechargeable Batteries: Focus on Iron Fluorides. Small Methods 2023, 7, 2201152. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Yoon, H.; Kang, S.; Kim, D.I.; Hong, J.; Shin, M.; Yoo, D.J.; Kim, M. Achieving High Stability and Capacity in Micron-Sized Conversion-Type Iron Fluoride Li-Metal Batteries. Adv. Sci. 2024, 11, 2410114. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; He, X.; Wang, L.; Wang, J.; Dong, L.; Xie, Y.; Hao, Y. A Novel Sugar-Assisted Solvothermal Method for FeF2 Nanomaterial and Its Application in LIBs. Materials 2023, 16, 1437. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.W.; Lee, H.J.; Capone, I.; Robertson, A.; Wi, T.U.; Fawdon, J.; Wheeler, S.; Lee, H.W.; Grobert, N.; Pasta, M. Understanding the conversion mechanism and performance of monodisperse FeF2 nanocrystal cathodes. Nat. Mater. 2020, 19, 644–654. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Li, J.; Wen, L.; He, X. A One-Pot Approach Toward FeF2–Carbon Core–Shell Composite and Its Application in Lithium-Ion Batteries. J. Alloys Compd. 2014, 606, 226–230. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, D.; Zhang, X.; Song, H.; Chen, X. Carbon-Nanotube-Encapsulated FeF2 Nanorods for High-Performance Lithium-Ion Cathode Materials. ACS Appl. Mater. Interfaces 2014, 6, 21223–21229. [Google Scholar] [CrossRef]
- Wygant, B.R.; Schorr, N.B.; Kolesnichenko, I.V.; Lambert, T.N. Nanoparticulate FeF2@C as a Li Battery Conversion Cathode. ACS Appl. Energy Mater. 2022, 5, 13346–13355. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, W.; Huang, J.; Tang, X.; Wang, H.; Liu, W.; Xiao, D.; Guo, Y.; Zhang, Y. Pressure-induced growth of coralloid-like FeF2 nanocrystals to enable high-performance conversion cathode. J. Energy Chem. 2023, 79, 291–300. [Google Scholar] [CrossRef]
- Su, Y.; Liu, S.; Zhu, D.; Luo, Y.; Zhang, X.; Yan, J.; Chen, J.; Geng, L.; Guo, B.; Li, H.; et al. Cryo-TEM studies of binder free high performance FeF2 cathode based full cells enabled by surface engineering. Energy Storage Mater. 2023, 59, 102779. [Google Scholar] [CrossRef]
- Krahl, T.; Marroquin; Winkelmann, F.; Martin, A.; Pinna, N.; Kemnitz, E. Novel synthesis of anhydrous and hydroxylated CuF2 nanoparticles and their potential for lithium-ion batteries. Chem.—Eur. J. 2018, 24, 7177–7187. [Google Scholar] [CrossRef]
- Cui, Y.H.; Xue, M.Z.; Zhou, Y.N.; Peng, S.M.; Wang, X.L.; Fu, Z.W. The investigation on electrochemical reaction mechanism of CuF2 thin film with lithium. Electrochem. Acta 2011, 56, 2328–2335. [Google Scholar] [CrossRef]
- Seo, J.K.; Cho, H.M.; Takahara, K.; Chapman, K.W.; Borkiewicz, O.J.; Sina, M.; Shirley Meng, Y. Revisiting the conversion reaction voltage and the reversibility of the CuF2 electrode in Li-ion batteries. Nano Res. 2017, 10, 4232–4244. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, Z.; Tian, L.; Zhao, Y.; Liu, H.; Lai, C.; Yuan, Z. Porous anhydrous CuF2 with a micro-nano-hierarchical structure as high-performance cathode material for Li-ion battery. J. Mater. Sci. 2023, 58, 10120–10130. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, S.; Yang, D.; Li, Y.; Yao, R.; Lang, X.; Tan, H.; Li, Y.; Jiang, Q. High-Performance Pomegranate-Like CuF2 Cathode Derived from Spent Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202409255. [Google Scholar] [CrossRef]
- Guan, Q.; Cheng, J.; Li, X.; Ni, W.; Wang, B. Porous CoF2 spheres synthesized by a one-pot solvothermal method as high-capacity cathode materials for lithium-ion batteries. Chin. J. Chem. 2017, 35, 48–54. [Google Scholar] [CrossRef]
- Li, J.; Li, X.F.; Jiang, Q.T.; Duan, R.X.; Cao, G.Q.; Wang, J.J.; Li, W.B. Construction of CoF2 nanoconfined in N-doped carbon matrix as high-capacity cathodes to boost reversibility of lithium-ion batteries. Rare Met. 2024. [Google Scholar] [CrossRef]
- Park, H.; Jang, I.S.; Song, B.-Y.; Kang, Y.C.; Kim, S.; Chun, J. Facile synthesis of cobalt fluoride (CoF2)/multi-walled carbon nanotube (MWCNT) nanocomposites and improvement of their electrochemical performance as cathode materials for Li-ion batteries. J. Mater. Chem. A 2023, 11, 15319–15328. [Google Scholar] [CrossRef]
- Meng, X.; Zhao, Y.; Zhang, T.; Pan, Y.; Jiang, Y.; Yang, D.; Li, Q.; Li, Q. Understanding the CEI Evolution of CoF2 Cathode in Lithium-Ion Batteries by Operando Magnetometry. Mater. Today Energy, 2025; under review. [Google Scholar]
- Fu, Z.W.; Li, C.L.; Liu, W.Y.; Ma, J.; Wang, Y.; Qin, Q.Z. Electrochemical reaction of lithium with cobalt fluoride thin film electrode. J. Electrochem Soc. 2005, 152, E50. [Google Scholar] [CrossRef]
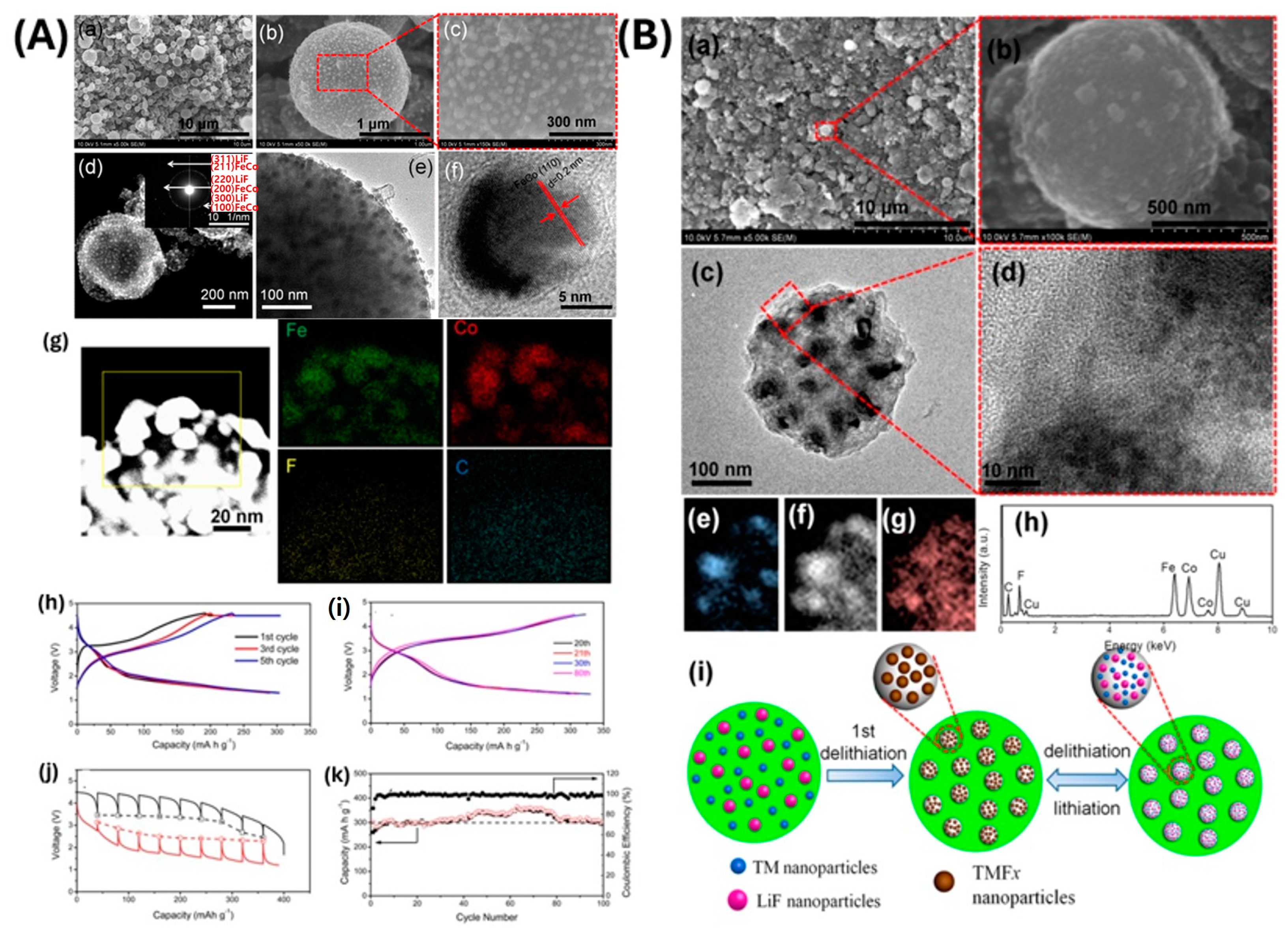
- Zhao, Y.; Wei, K.; Wu, H.; Ma, S.; Li, J.; Cui, Y.; Dong, Z.; Cui, Y.; Li, C. LiF Splitting Catalyzed by Dual Metal Nanodomains for an Efficient Fluoride Conversion Cathode. ACS Nano 2019, 13, 2490–2500. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Zeng, Q.; Zhou, G.; Yin, L.; Li, F.; Cheng, H.M.; Gentle, I.R.; Lu, G.Q.M. Carbon–sulfur composites for Li–S batteries: Status and prospects. J. Mater. Chem. A 2013, 1, 9382. [Google Scholar] [CrossRef]
- Safdar, T.; Huang, C. Sulfur/carbon cathode material chemistry and morphology optimisation for lithium-sulfur batteries. RSC Adv. 2024, 14, 30743–30755. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ma, W.; Zhang, S.; Tang, B. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019, 23, 707–732. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, N.; Yan, J.; Kang, W.; Ju, J.; Ruan, Y.; Wang, X.; Zhuang, X.; Li, Q.; Cheng, B. A review on anode for lithium-sulfur batteries: Progress and prospects. Chem. Eng. J. 2018, 347, 343–365. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhou, S. Linear active disturbance rejection control for pressurized water reactor power. Ann. Nucl. Energy 2018, 111, 22–30. [Google Scholar] [CrossRef]
- Sun, L.; Li, Z.; Zhai, L.; Moon, H.; Song, C.; Oh, K.S.; Kong, X.; Han, D.; Zhu, Z.; Wu, Y.; et al. Electrostatic Polarity-Regulated, Vinylene-Linked Cationic Covalent Organic Frameworks as an Ionic Sieve Membrane for Long-Cyclable Lithium-Sulfur Batteries. Energy Storage Mater. 2024, 66, 103222. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Z.; Han, X.; Huang, S.; Mei, P.; Zhang, Q. A conjugated porous triazine-linked polyimide host with dual confinement of polysulfides for high-performance lithium-sulfur batteries. J. Colloid Interface Sci. 2025, 682, 599–607. [Google Scholar] [CrossRef]
- Pan, W.; Watanabe, T.; Matsunaga, T.; Kumar, M.; Thakur, N.; Yamamoto, K.; Uesugi, M.; Takeuchi, A.; Sakuda, A.; Hayashi, A.; et al. Tuning the ionic and electronic paths in Li2S-based cathode for high-rate performance all-solid-state lithium-sulfur batteries. Solid State Ion. 2024, 406, 116479. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, C.; Wang, L.; Yang, H.; Lv, W.; Yang, Q.H. Design and modification of metal sulfide-based catalysts for lithium-sulfur batteries. Particuology 2024, 86, 86–100. [Google Scholar] [CrossRef]
- Koh, J.Y.; Kim, S.; Park, M.S.; Yang, H.J.; Yang, T.H.; Jung, Y. The Role of the Carbon Framework in Sulfur-Carbon Composite Cathodes in Li-S Batteries. Electrochim. Acta 2016, 212, 212–216. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Xu, J.; Cao, F.; Li, C. Carbon-based derivatives from metal-organic frameworks as cathode hosts for Li–S batteries. J. Energy Chem. 2019, 38, 94–113. [Google Scholar] [CrossRef]
- Ding, X.; Pan, Z.; Liu, N.; Li, L.; Wang, X.; Xu, G.; Yang, J.; Yang, J.; Yu, N.; Liu, M.; et al. Freestanding Carbon Nanotube Film for Flexible Straplike Lithium/Sulfur Batteries. Chem.—Eur. J. 2019, 25, 3775–3780. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Li, P.; Fu, F.; Wang, Z.; Zhang, W. Three-dimensional CNT/graphene-sulfur hybrid sponges with high sulfur loading as superior-capacity cathodes for lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 18605–18610. [Google Scholar] [CrossRef]
- Guo, Z.; Nie, H.; Yang, Z.; Hua, W.; Ruan, C.; Chen, D.; Ge, M.; Chen, X.; Huang, S. 3D CNTs/Graphene-S-Al3Ni2 Cathodes for High-Sulfur-Loading and Long-Life Lithium–Sulfur Batteries. Adv. Sci. 2018, 5, 1800026. [Google Scholar] [CrossRef]
- Nulu, A.; Nulu, V.; Sohn, K.Y. N-doped CNTs wrapped sulfur-loaded hierarchical porous carbon cathode for Li-sulfur battery studies. RSC Adv. 2024, 14, 2564–2576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Peng, H.J.; Li, B.Q.; Huang, J.Q. Kinetic Promoters for Sulfur Cathodes in Lithium-Sulfur Batteries. Acc. Chem. Res. 2024, 57, 545–557. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, G.L.; Yu, Z.; Zhang, L.; Hwang, I.; Mo, Y.X.; Ren, Y.; Cheng, L.; Sun, C.J.; Ren, Y.; et al. A high-energy and long-cycling lithium–sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites. Nat. Nanotechnol. 2021, 16, 166–173. [Google Scholar] [CrossRef]
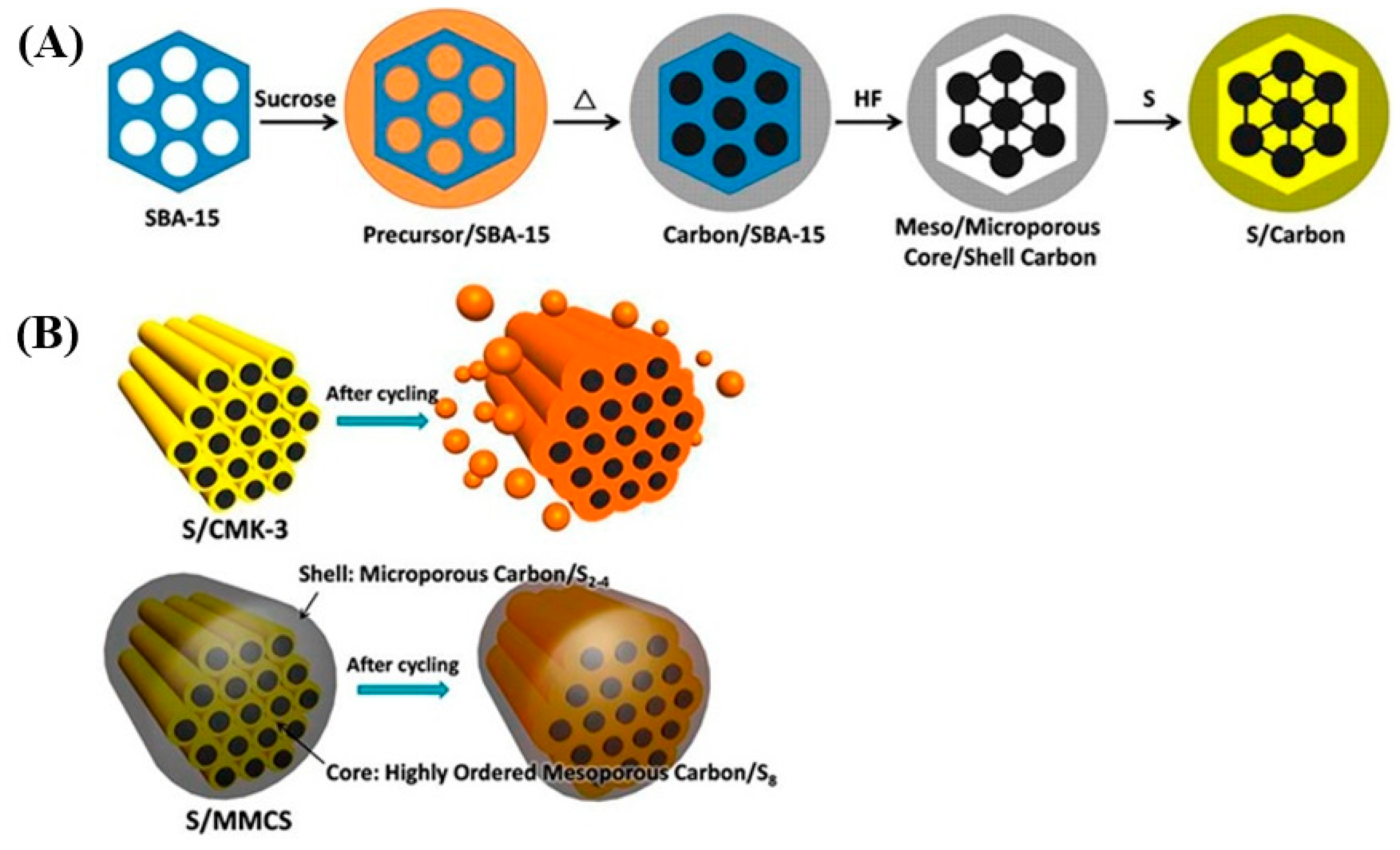
- Li, Z.; Jiang, Y.; Yuan, L.; Yi, Z.; Wu, C.; Liu, Y.; Strasser, P.; Huang, Y. A Highly Ordered Meso@Microporous Carbon-Supported Sulfur@Smaller Sulfur Core Shell Structured Cathode for Li S Batteries. ACS Nano 2014, 8, 9295–9303. [Google Scholar] [CrossRef]
- Nan, C.; Lin, Z.; Liao, H.; Song, M.K.; Li, Y.; Cairns, E.J. Durable Carbon-Coated Li 2 S Core–Shell Spheres for High Performance Lithium/Sulfur Cells. J. Am. Chem. Soc. 2014, 136, 4659–4663. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Ma, C.; Wang, S.; Shi, Y.; Koratkar, N.; Ren, W.; Li, F.; Cheng, H.M. A graphene foam electrode with high sulfur loading for flexible and high energy Li-S batteries. Nano Energy 2015, 11, 356–365. [Google Scholar] [CrossRef]
- Zhou, F.; Meng, Y.; Wang, T.; Sun, D.; Gao, L.; Sun, Z.; Wang, Y.; Zeng, J.; Wang, B.; Zhang, R.; et al. Strutted graphene foam loading sulfur for high-rate long-lifetime Li-S batteries. Nano Energy 2024, 127, 109755. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, B.; Li, X.; He, X.; Sun, Z.; Zhang, W.; Gao, Z.; Zhang, L.; Xhen, X.; Chen, Z.; et al. A high-energy-density long-cycle lithium–sulfur battery enabled by 3D graphene architecture. Carbon Energy 2024, 6, e599. [Google Scholar] [CrossRef]
- Abdul Razzaq, A.; Yao, Y.; Shah, R.; QI, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-performance lithium sulfur batteries enabled by a synergy between sulfur and carbon nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Wu, J.; Ding, S.; Ye, S.; Lai, C. Grafting polymeric sulfur onto carbon nanotubes as highly-active cathode for lithium–sulfur batteries. J. Energy Chem. 2020, 42, 27–33. [Google Scholar] [CrossRef]
- Zheng, S.; Yi, F.; Li, Z.; Zhu, Y.; Xu, Y.; Luo, C.; Yang, J.; Wang, C. Copper-stabilized sulfur-microporous carbon cathodes for Li-S batteries. Adv. Funct. Mater. 2014, 24, 4156–4163. [Google Scholar] [CrossRef]
- Kim, S.J.; Ahn, M.; Park, J.; Jeoung, Y.; Yu, S.H.; Min, D.H. Enhancing the of Performance of Lithium-Sulfur Batteries through Electrochemical Impregnation of Sulfur in Hierarchical Mesoporous Carbon Nanoparticles. ChemElectroChem 2020, 7, 3653–3655. [Google Scholar] [CrossRef]
- Dong, W.; Guo, Y.; Wang, W.; Hong, X.; Xu, X.; Yang, F.; Zhao, M.; Zhang, X.; Shen, D.; Yang, S. Synthesis of recrystallized g-C3N3/CNTs composites as sulfur hosts for lithium-sulfur batteries with enhanced cycling stability via a dissolution-precipitation approach. J. Energy Storage 2025, 110, 115256. [Google Scholar] [CrossRef]
- Gu, X.; Lai, C. Recent development of metal compound applications in lithium-sulphur batteries. J. Mater Res. 2018, 33, 16–31. [Google Scholar] [CrossRef]
- Ma, F.; Liang, J.; Wang, T.; Chen, X.; Fan, Y.; Hultman, B.; Xie, H.; Han, J.; Wu, G.; Li, Q. Efficient entrapment and catalytic conversion of lithium polysulfides on hollow metal oxide submicro-spheres as lithium-sulfur battery cathodes. Nanoscale 2018, 10, 5634–5641. [Google Scholar] [CrossRef]
- Cao, K.; Liu, H.; Li, Y.; Wang, Y.; Jiao, L. Encapsulating sulfur in δ-MnO2 at room temperature for Li-S battery cathode. Energy Storage Mater. 2017, 9, 78–84. [Google Scholar] [CrossRef]
- Li, Q.; Ma, Z.; Li, J.; Liu, Z.; Fan, L.; Qin, X.; Shao, G. Core-Shell-Structured Sulfur Cathode: Ultrathin Î-MnO2Nanosheets as the Catalytic Conversion Shell for Lithium Polysulfides in High Sulfur Content Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2020, 12, 35049–35057. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, K.; Liu, Y.; Li, Y.; Wei, M. Rutile TiO2 Mesocrystals as Sulfur Host for High-Performance Lithium–Sulfur Batteries. Chem.—Eur. J. 2017, 23, 16312–16318. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Moon, J.H. Polyhedral TiO2 particle-based cathode for Li-S batteries with high volumetric capacity and high performance in lean electrolyte. Chem. Eng. J. 2020, 399, 125670. [Google Scholar] [CrossRef]
- Lin, J.X.; Mo, Y.X.; Zhang, P.F.; Li, Y.Y.; Wu, Y.J.; Zhang, S.J.; Gao, Z.G.; Chen, J.D.; Ren, W.F.; Li, J.T.; et al. Ultrahigh sulfur content up to 93 wt% encapsulated in multilayer nanoshell of V/V2O5 composite to suppress shuttle effect of lithium–sulfur battery with high-performance. Mater. Today Energy 2019, 13, 267–276. [Google Scholar] [CrossRef]
- Dai, C.; Lim, J.M.; Wang, M.; Hu, L.; Chen, Y.; Chen, Z.; Chen, H.; Bao, S.J.; Shen, B.; Li, Y.; et al. Honeycomb-Like Spherical Cathode Host Constructed from Hollow Metallic and Polar Co9S8 Tubules for Advanced Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1704443. [Google Scholar] [CrossRef]
- Yue, B.; Wang, L.; Zhang, N.; Xie, Y.; Yu, W.; Ma, Q.; Wang, J.; Liu, G.; Dong, X. Dual-Confinement Effect of Nanocages@Nanotubes Suppresses Polysulfide Shuttle Effect for High-Performance Lithium–Sulfur Batteries. Small 2024, 20, 2308603. [Google Scholar] [CrossRef]
- Shao, Q.; Xu, L.; Guo, D.; Su, Y.; Chen, J. Atomic level design of single iron atom embedded mesoporous hollow carbon spheres as multi-effect nanoreactors for advanced lithium-sulfur batteries. J. Mater. Chem. A 2020, 8, 23772–23783. [Google Scholar] [CrossRef]
- Liu, Y.; Kou, W.; Li, X.; Huang, C.; Shui, R.; He, G. Constructing Patch-Ni-Shelled Pt@Ni Nanoparticles within Confined Nanoreactors for Catalytic Oxidation of Insoluble Polysulfides in Li-S Batteries. Small 2019, 15, 1902431. [Google Scholar] [CrossRef]
- Lin, N.; Chen, K.; Chen, S.; Wang, F.; Wang, D.; Gan, F.; He, X.; Huang, Y. Manipulating the redox kinetics of Li–S chemistry by porous hollow cobalt-B, N codoped-graphitic carbon polyhedrons for high performance lithium-sulfur batteries. Carbon 2019, 149, 564–571. [Google Scholar]
- Ye, H.; Sun, J.; Zhang, S.; Lin, H.; Zhang, T.; Yao, Q.; Lee, J.Y. Stepwise Electrocatalysis as a Strategy against Polysulfide Shuttling in Li-S Batteries. ACS Nano 2019, 13, 14208–14216. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huan, Y.; Wang, D.; Li, M.; Cheng, X.; Bai, Z.; Wu, P.; Peng, W.; Zhang, R.; Ji, Z. Hierarchical VS2 Nano-Flowers as Sulfur Host for Lithium Sulfur Battery Cathodes. J. Electrochem. Soc. 2019, 166, A188–A194. [Google Scholar] [CrossRef]
- Chen, X.; Du, G.; Zhang, M.; Kalam, A.; Diang, S.; Su, Q.; Xu, B.; Al-Sehemi, A.G. Vanadium Sulfide@Sulfur Composites as High-Performance Cathode for Advanced Lithium–Sulfur Batteries. Energy Technol. 2020, 8, 1901163. [Google Scholar] [CrossRef]
- Hong, X.; Li, S.; Tang, X.; Sun, Z.; Li, F. Self-supporting porous CoS2/rGO sulfur host prepared by bottom-up assembly for lithium-sulfur batteries. J. Alloys Compd. 2018, 749, 586–593. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Nazar, L.F. A graphene-like metallic cathode host for long-life and high-loading lithium-sulfur batteries. Mater. Horiz. 2016, 3, 130–136. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, L.; Guo, C.; Li, D.; Vasileff, A.; Wang, H.; Qiao, S.Z. A 3D Hybrid of Chemically Coupled Nickel Sulfide and Hollow Carbon Spheres for High Performance Lithium–Sulfur Batteries. Adv. Funct. Mater. 2017, 27, 1702524. [Google Scholar] [CrossRef]
- Majumder, S.; Shao, M.; Deng, Y.; Chen, G. Ultrathin sheets of MoS2/g-C3N4 composite as a good hosting material of sulfur for lithium–sulfur batteries. J. Power Sources 2019, 431, 93–104. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Zheng, J.; Zhang, B.; Ling, M.; Hou, Y.; Liang, C. Electrospinning MoS2-Decorated Porous Carbon Nanofibers for High-Performance Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 11893–11899. [Google Scholar] [CrossRef]
- Lei, T.; Chen, W.; Huang, J.; Yan, C.; Sun, H.; Wang, C.; Zhang, W.; Li, Y.; Xiong, J. Multi-Functional Layered WS2 Nanosheets for Enhancing the Performance of Lithium–Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1601843. [Google Scholar] [CrossRef]
- Guo, B.; Bandaru, S.; Dai, C.; Chen, H.; Zhang, Y.; Xu, Q.; Bao, S.; Chen, M.; Xu, M. Self-Supported FeCo2S4 Nanotube Arrays as Binder-Free Cathodes for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 43707–43715. [Google Scholar] [CrossRef]
- Ci, H.; Cai, J.; Ma, H.; Shi, Z.; Cui, G.; Wang, M.; Jin, J.; Wei, N.; Lu, C.; Zhao, W.; et al. Defective VSe2-Graphene Heterostructures Enabling in Situ Electrocatalyst Evolution for Lithium-Sulfur Batteries. ACS Nano 2020, 14, 11929–11938. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Kim, J.T.; Hao, X.; Wang, C.; Sun, X. Cathode materials for single-phase solid-solid conversion Li-S batteries. Matter 2023, 6, 316–343. [Google Scholar] [CrossRef]
- Liu, C.; Wu, M.; Liu, Y.; Lu, Z.; Yang, Y.; Shi, S.; Yang, G. Effect of ball milling conditions on microstructure and lithium storage properties of LiNi0.5Mn1.5O4 as cathode for lithium-ion batteries. Mater. Res. Bull. 2018, 99, 436–443. [Google Scholar] [CrossRef]
- Marichy, C.; Bechelany, M.; Pinna, N. Atomic Layer Deposition of Nanostructured Materials for Energy and Environmental Applications. Adv. Mater. 2012, 24, 1017–1032. [Google Scholar] [CrossRef]
- Islam, M.; Ahmed, M.S.; Faizan, M.; Ali, B.; Bhuyan, M.M.; Bari, G.A.K.M.R.; Nam, K.-W. Review on the Polymeric and Chelate Gel Precursor for Li-Ion Battery Cathode Material Synthesis. Gels 2024, 10, 586. [Google Scholar] [CrossRef]
- Wu, X.J.; Zhang, Q.; Tang, G.; Cao, Y.L.; Yang, H.X.; Li, H.; Ai, X.P. A solid-phase conversion sulfur cathode with full capacity utilization and superior cycle stability for lithium-sulfur batteries. Small 2022, 18, e2106144. [Google Scholar] [CrossRef]
- Zou, J.; Zhao, J.; Wang, B.J.; Chen, S.L.; Chen, P.Y.; Ran, Q.W.; Li, L.; Wang, X.; Yao, J.M.; Li, H.; et al. Unraveling the reaction mechanism of FeS2 as a Li-ion battery cathode. ACS Appl. Mater. Interfaces 2020, 12, 44850–44857. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Raut, B.; Yun, S.; Kim, H.Y.; Nam, K.-W. A Comprehensive Review of Functional Gel Polymer Electrolytes and Applications in Lithium-Ion Battery. Gels 2024, 10, 563. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, G.; Li, S.; Liu, H.; Wang, L.; Li, H. Unlocking cycling longevity in micro-sized conversion-type FeS2 cathodes. Joule 2023, 7, 2609–2621. [Google Scholar] [CrossRef]



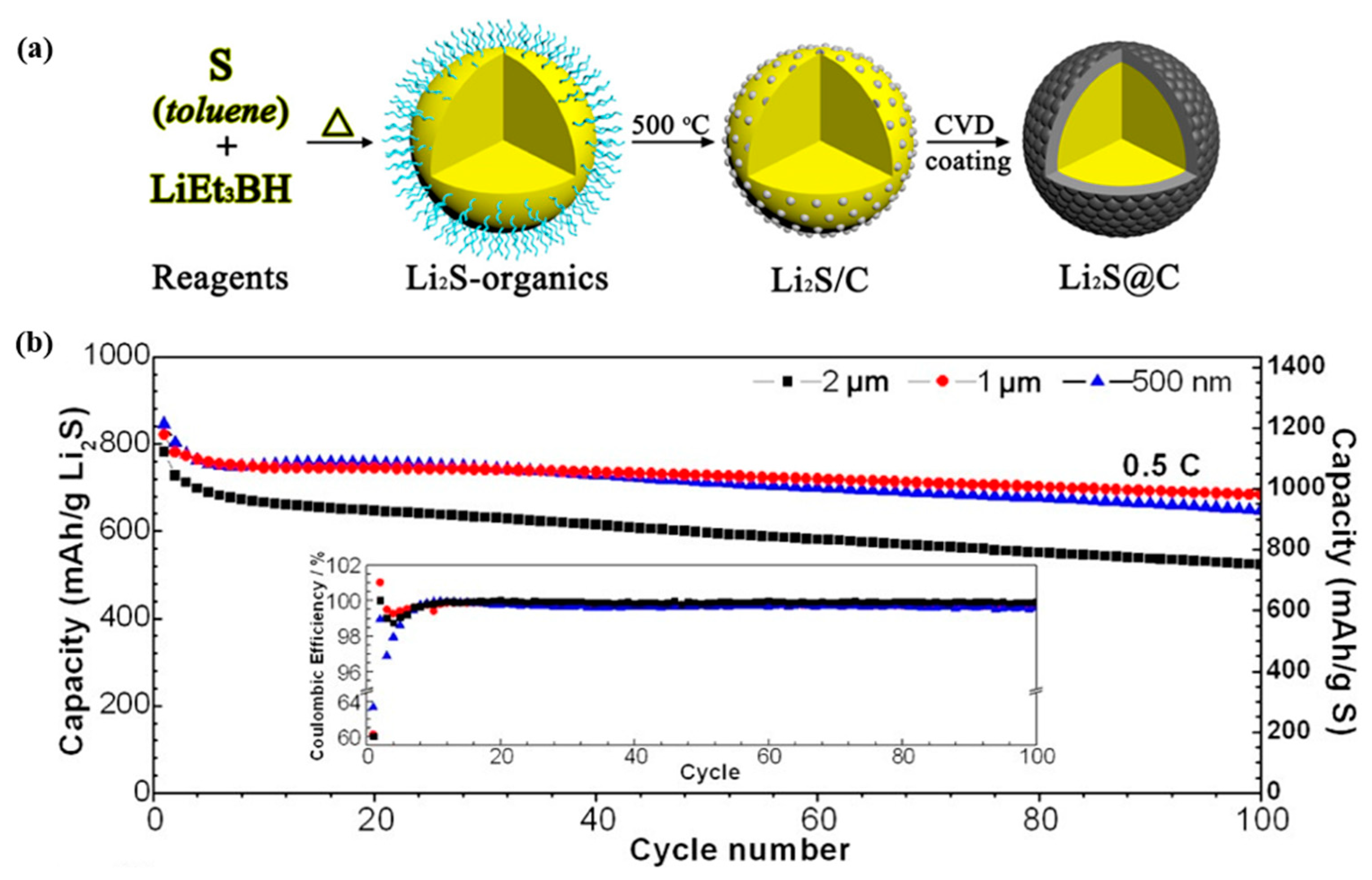

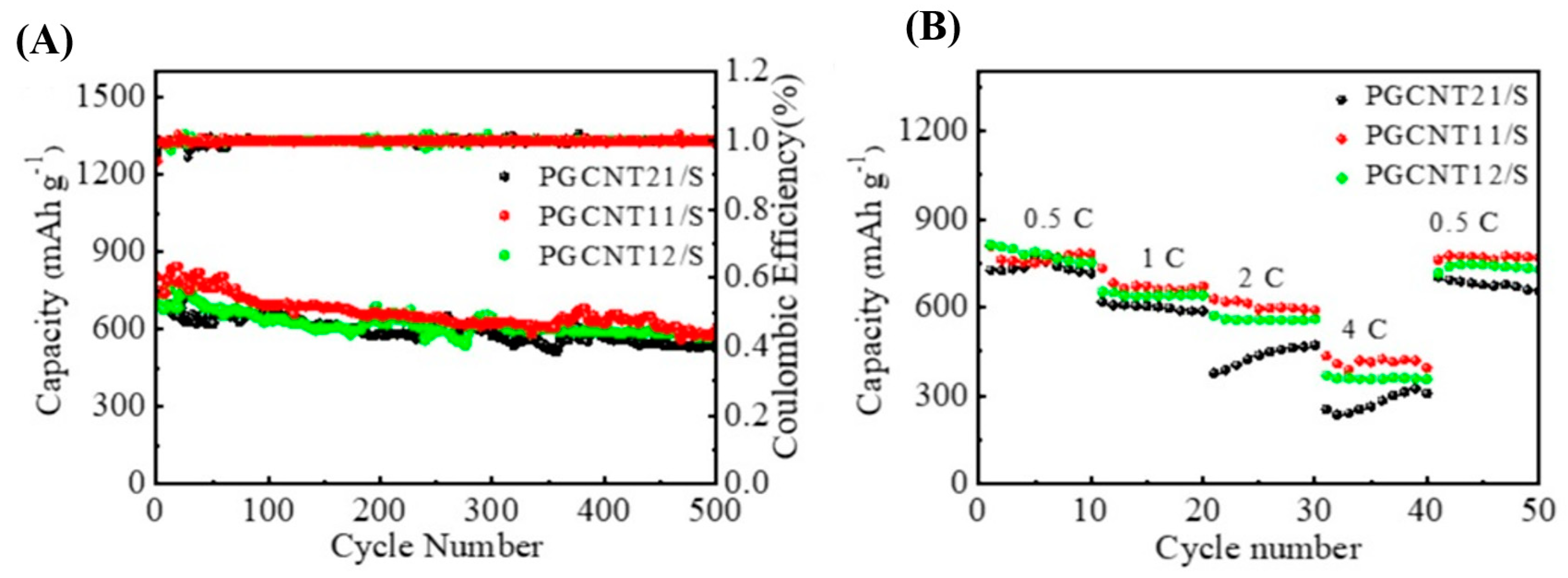

| Metal/Metal Oxide/Sulfides | Morphology | Final Form of Cathode for LiS | Initial Discharge Capacity [mA h g−1] | References |
|---|---|---|---|---|
| Fe | Mesoporous hollow carbon spheres | Fe-N/MHCS | 1236_0.1 C | [140] |
| Pt-Ni | Nanoparticles | PtNi_C | 1093.8_0.2 C | [141] |
| B-Co | Nanoparticles | Hollow-Co-BN-GC | 1089_0.5 C | [142] |
| Fe-Co | Single atom on carbon nanosheets | Fe-N_C/S on Co- N_C/S (dual layer electrode) | 1343_0.2 C | [143] |
| VS2 | Nanoflowers | VS2/S | 1026.6_0.2 C | [144] |
| Nanoparticles | VS2_S | 1275_0.2 C | [145] | |
| CoS2 | Nanoparticles | CoS2/rGO-S | 993.5_0.5 C | [146] |
| Co9S8 | Nanosheet interconnected to form 3D | Co9S8/S75 | 863_2 C | [147] |
| NiS | Nanoparticles | S/NiS-HS | 723_0.5 C | [148] |
| MoS2 | Nanosheet | MoS2/g-C3N4/S | 966_0.5 C | [149] |
| Nanoparticles on carbon nanofiber (CNF) | S_MoS2_CNF | 1398_0.2 C | [150] | |
| WS2 | Nanosheet | C_WS2/S | 563_2 C | [151] |
| FeCo2S4 | Hollow nanotubes | FeCo2S4/CC_S | 969_0.5 C | [152] |
| VSe2 | Nanoflakes | VSe2-VG_CC/S | 1025_0.5 C | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.; Ahmed, M.S.; Yun, S.; Ali, B.; Kim, H.-Y.; Nam, K.-W. Recent Advances in Nanostructured Conversion-Type Cathodes: Fluorides and Sulfides. Nanomaterials 2025, 15, 420. https://doi.org/10.3390/nano15060420
Islam M, Ahmed MS, Yun S, Ali B, Kim H-Y, Nam K-W. Recent Advances in Nanostructured Conversion-Type Cathodes: Fluorides and Sulfides. Nanomaterials. 2025; 15(6):420. https://doi.org/10.3390/nano15060420
Chicago/Turabian StyleIslam, Mobinul, Md. Shahriar Ahmed, Sua Yun, Basit Ali, Hae-Yong Kim, and Kyung-Wan Nam. 2025. "Recent Advances in Nanostructured Conversion-Type Cathodes: Fluorides and Sulfides" Nanomaterials 15, no. 6: 420. https://doi.org/10.3390/nano15060420
APA StyleIslam, M., Ahmed, M. S., Yun, S., Ali, B., Kim, H.-Y., & Nam, K.-W. (2025). Recent Advances in Nanostructured Conversion-Type Cathodes: Fluorides and Sulfides. Nanomaterials, 15(6), 420. https://doi.org/10.3390/nano15060420








